Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Association Between Weight-Adjusted Waist Index and Albuminuria in Type 2 Diabetes Mellitus in the Chinese Population
Authors Qin Y, Ye J, Li H, Wu X, Xia Y, Deng X
Received 16 April 2024
Accepted for publication 13 September 2024
Published 24 September 2024 Volume 2024:17 Pages 3585—3592
DOI https://doi.org/10.2147/DMSO.S474007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Rebecca Conway
Yu Qin, Jingjing Ye, Haoxiang Li, Xunan Wu, Yue Xia, Xia Deng
Department of Endocrinology, Affiliated Hospital of Jiangsu University, Zhenjiang, People’s Republic of China
Correspondence: Xia Deng, Department of Endocrinology, Affiliated Hospital of Jiangsu University, Zhenjiang, 212000, People’s Republic of China, Email [email protected]
Purpose: This study aimed to investigate the relationship between weight-adjusted waist index (WWI) and albuminuria in patients with type 2 diabetes mellitus (T2DM) in the Chinese population.
Patients and Methods: A total of 860 adult patients in the Department of Endocrinology of the Affiliated Hospital of Jiangsu University were retrospectively analyzed from June 2018 to September 2023. Correlations between WWI and albuminuria (albumin-to-creatinine ratio (UACR) ≥ 30 mg/g were defined as albuminuria) were analyzed using the Pearson and Spearman methods. The associations between albuminuria and Age, gender, body mass index (BMI), waist circumference/ hip circumference (WHR), systolic blood pressure(SBP), diastolic blood pressure (DBP), fasting plasma glucose (FPG), 2-hour postprandial plasma glucose (2h PG), fasting plasma insulin (FIns), 2-h postprandial insulin (2hINS), glycosylated hemoglobin (HbA1c), WWI, homeostasis model assessment of insulin resistance (HOMA-IR) were analyzed via binary logistic regression.
Results: Compared with the normal albumin group, serum urea nitrogen, serum creatinine, UACR, and WWI levels in the albuminuria group were significantly increased, while estimated glomerular filtration rate (eGFR) levels were significantly decreased (P < 0.05). Correlation analyses revealed that WWI was positively correlated with UACR but negatively correlated with urea nitrogen, serum creatinine, and eGFR (P < 0.05). Binary logistic regression analyses indicated that WWI was an independent risk factor for albuminuria in T2DM patients. Receiver operating characteristic curve results showed that the area under the curve for albuminuria as predicted by WWI was 0.605 [95% CI = (0.563– 0.646), P < 0.001].
Conclusion: WWI is independently associated with albuminuria in the Chinese patients with type 2 diabetes and may serve as a simple indicator for albuminuria risk assessment.
Keywords: weight-adjusted waist index, kidney function, albuminuria, type 2 diabetes
Introduction
Diabetic kidney disease (DKD) is one of the most common microvascular complications in diabetic patients and one of the common causes of end-stage renal disease and death.1 The main feature of DKD is an impaired glomerular filtration barrier, which is usually evaluated based on the eGFR.2 In the early stages of DKD, clinical symptoms are typically not obvious and only manifest as glomerular hypertrophy, an increased glomerular filtration rate, slight morphological changes in the glomeruli, and microalbuminuria. However, as the disease progresses, glomerulosclerosis and tubulointerstitial fibrosis manifest, which then gradually lead to the deterioration of renal function and massive macroalbuminuria, followed by end-stage renal disease.3,4 Therefore, UACR is often used as an evaluation indicator for early-stage DKD.5,6 Several studies have shown that obesity can lead to renal dysfunction and the aggravated deterioration of renal function, seriously affecting patient quality of life.7,8 Albuminuria and chronic kidney disease (CKD) progression can be significantly alleviated by drugs, surgery, alimentary control, exercise, and weight loss.9–11
BMI is the traditional index used to assess obesity, but it cannot distinguish between fat-free weight and fat weight.12 Therefore, Park et al13 first proposed the WWI, which can be readily measured by normalizing waist circumference (WC) to body weight, as an alternative index for obesity. This index leverages the benefits of WC while weakening its correlation with BMI, allowing it to assess both fat mass and muscle mass. Previous studies have reported that WWI is closely related to the risk of hyperuricemia,14 diabetes,15 and cardiovascular disease.16 However, no relevant studies have reported the relationship between WWI and albuminuria in patients with T2DM in the Chinese population. Therefore, the purpose of this study was to investigate the relationship between WWI and albuminuria in the Chinese T2DM patients.
Materials and Methods
Study Participants
This was a cross-sectional study based on a population of T2DM patients in Zhenjiang, Jiangsu, China. A total of 860 patients with T2DM, aged 52.92 ± 11.71 years old, were retrospectively selected from the Department of Endocrinology of the Affiliated Hospital of Jiangsu University from June 2018 to September 2023, comprising 505 men and 355 women.This study was divided into groups according to UACR ≥ 30 mg/g. This research complies with the principle of the Helsinki Declaration. To be eligible for inclusion, patients had to have been diagnosed as per the diagnostic criteria for diabetes established by the American Diabetes Association.17 Hypertension was defined as SBP ≥ 140 mmHg and/or DBP≥90 mmHg.18
The exclusion criteria were as follows: 1) other types of diabetes; 2) acute complications of diabetes mellitus (diabetic ketoacidosis, diabetic lactic acidosis); 3) chronic viral or bacterial infections (severe infection of respiratory system, urinary system, etc); 4) other serious kidney diseases or drug-induced kidney diseases; 5) severe liver disease; 6) asthma or other autoimmune diseases; 7) tumors; or 8) mental illnesses (such as schizophrenia and depression). In addition, to reduce the influence of renal failure on outcomes, we excluded all T2DM patients with an eGFR of less than or equal to 30 mL/min/1.72m2. This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Jiangsu University. All participants provided informed consent to participate.
Collection of Clinical and Biochemical Data
General clinical data were collected, including age, sex, WC (measured by circling the abdomen through the midpoint of the line connecting the twelfth rib of the mid-axillary line on both sides and the anterior superior iliac spine), hip circumference (HC; measured using a soft ruler to circle the pubic symphysis and the most convex portion of the buttocks), blood pressure (Systolic and diastolic blood pressure were measured after 15 minutes of rest), height, and weight.
After 8 hours of overnight fasting, venous blood was collected, and both fasting and 2-hour postprandial blood glucose levels were detected via the glucose oxidase method. In addition, fasting and 2-hour postprandial insulin levels were detected using a chemiluminescence method, while HbA1c levels were detected through high-performance liquid chromatography. An automatic biochemical testing instrument was used to detect liver and kidney function and to conduct lipid analyses, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA); triglyceride (TG); total cholesterol (TC); high-density lipoprotein cholesterol (HDL-c); low-density lipoprotein cholesterol (LDL-C), blood urea nitrogen (BUN); serum creatinine (Scr). Urine was collected in the middle of the morning, and the albumin and creatinine levels in these urine samples were detected with an automated biochemical detection instrument, allowing for the calculation of UACR. T2DM patients with a UACR of < 30 mg/g were defined as being in the normal albumin group (n = 584), while T2DM patients with a UACR of ≥ 30 mg/g were defined as being in the albuminuria group (n = 276).19
WWI was determined by dividing WC (cm) by the square root of body weight (kg).
The glomerular filtrate rate was calculated using the CKD-EPI formula:
eGFR = a × (Scr/b)c × (0.993) age, eGFR < 90 is abnormal20
a = 141 for men and 144 for women; b = 0.9 for men and 0.7 for women; and c = −0.411 for men with Scr ≤ 0.9mg/dL, −1.209 for men with Scr > 0.9mg/dL, −0.329 for women with Scr ≤ 0.7mg/dL, and −1.209 for women with Scr > 0.7mg/dL.21
HOMA − IR = fasting plasma glucose(FPG) (mmol∕L) × fasting plasma insulin(FIns),(U∕mL)∕22.5,HOMA-IR>2.7 is abnormal22
WHR=WC/HC;
BMI = Weight (kg)/[Height (m)]2
Statistical Analysis
Statistical analyses were performed using SPSS version 22.0. For normally distributed data, continuous variables were expressed as mean ± SD; for skewed data, continuous variables were expressed as median [quartile (IQR)]; and for categorical variables, the data were expressed as frequencies (n) and percentages (%). One-way ANOVA was used to compare data between groups. Spearman/Pearson correlations were used to analyze the correlations between WWI and clinical parameters. The factors influencing renal function and albuminuria were analyzed through binary logistic regression analyses. P < 0.05 was considered statistically significant. To evaluate the predictive performance of WWI for the risk of albuminuria in T2DM, receiver operating characteristic (ROC) curves were generated. Optimal cut-off values were derived from the Youden index (maximum [sensitivity + specificity − 1]). All significance tests were two-tailed, with P < 0.05 as the threshold for statistical significance.
Results
Characteristics of the Study Population According to Albuminuria Status
All participants (860) were divided into a normal albumin group and an albuminuria group. The prevalence of increased albuminuria among participants was (n=276) 32.1%. Compared with the normal albumin group, the albuminuria group exhibited a higher prevalence of hypertension, a longer course of disease, and significantly increased BUN, Scr, UACR, WWI, blood pressure, BMI, WHR, FIns, 2hIns, UA, TG, and HOMA-IR levels, whereas eGFR and HDL-c levels were significantly decreased (P < 0.05) (Table 1).
 |
Table 1 Baseline Clinical Characteristics of All Patients |
Correlations Between WWI and Other Parameters
In all participants, WWI was positively correlated with age, SBP, WHR, BMI, FIns, 2hIns, TC, LDL-c, HOMR-IR, and UACR levels, whereas it was negatively correlated with BUN, Scr, and eGFR levels (P < 0.05) (Table 2).
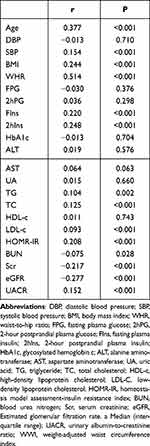 |
Table 2 Correlation of WWI with Clinical Parameters |
Binary Logistic Regression Analysis of UACR in T2DM Patients
To assess the effects of WWI on the risk of albuminuria in T2DM, we used a binary logistic regression model. The dependent variable was whether patients had albuminuria (normal UACR = 0; abnormal UACR = 1). Age, gender, BMI, WHR, SBP, DBP, FPG, 2h PG, FIns, 2h Ins, HbA1c, WWI, and HOMA-IR were independent variables, and a binary logistic regression analysis was performed. The results showed that WWI, SBP, Scr, UA, and HDL-c were independent risk factors for an abnormal UACR in T2DM patients (Table 3).
 |
Table 3 Results of Binary Logistic Regression Analysis for UACR in T2DM Patients |
Assessment of the Predictive Value of WWI for Albuminuria in T2DM
Finally, we analyzed the value of WWI for the diagnosis of albuminuria in T2DM. ROC curves yielded an AUC of 0.605 [95% CI = (0.563–0.646), P < 0.001], indicating that WWI offered diagnostic value for albuminuria in T2DM patients. The optimal cut-off for WWI was 11, with sensitivity and specificity values of 48.2% and 74.3%, respectively (Figure 1).
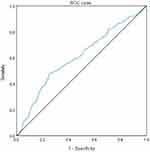 |
Figure 1 ROC curves of WWI indices in predicting albuminuria risk in T2DM patients. |
Discussion
In this study, the relationship between WWI and albuminuria in T2DM patients was investigated for the first time in a Chinese population. The results showed that WWI values in T2DM patients with albuminuria were significantly higher than those in T2DM patients in the normal albumin group. Correlation analyses indicated that WWI was positively correlated with UACR and negatively correlated with eGFR. Regression analyses showed that WWI was independently associated with albuminuria, even after adjusting for multiple confounders. These results indicate that WWI, a simple indicator, can be used for the early prediction and evaluation of albuminuria.
Obesity is a condition caused by excessive accumulation of body fat.23 Traditional measures of obesity such as BMI, WC, and WHR are associated with DKD risk, but the results are still controversial. For example, one study reported that patients who were overweight or obese rather than exhibiting abdominal obesity were more likely to develop DKD.24 However, in another study, abdominal obesity was more closely associated with DKD than general obesity.25 In addition, diabetic patients with a higher BMI have been shown to exhibit a lower risk of DKD and decreased kidney function.26 As a new obesity-related indicator, WWI leverages the advantages of WC while weakening its correlation with BMI.13 WWI is positively correlated with total fat mass and abdominal fat mass and negatively correlated with skeletal muscle mass.27 In addition, WWI levels are reportedly significantly higher in metabolically unhealthy individuals relative to healthy subjects, even with similar levels of obesity.28 Thus, WWI can reflect “true obesity” that is metabolically unhealthy. The results of this study revealed that the WWI levels in the albuminuria group were significantly higher than those in the normal albumin group. Correlation analyses indicated that WWI was positively correlated with UACR and negatively correlated with eGFR levels. ROC curve results further showed that the risk of albuminuria increased significantly after WWI was greater than 11. Studies of American adults have also found that WWI is closely related to albuminuria, and the correlation is stronger than that between BMI and WC.29 Li et al found that WWI was positively correlated with CKD and albuminuria, and the correlation was better than that for other obesity indicators (BMI, WC, height, or weight), such that WWI was considered to be the best obesity indicator for predicting CKD and albuminuria.19,29 The cut-off of WWI was 11.3446, and its prediction value for albuminuria was 0.5889 in Li’study.The cut-off of WWI was 11, and its prediction value for albuminuria was 0.605 in ours.Our study was based on a Chinese population. These two studies were based on an American population. Thus, WWI was a better predictor of urinary albumin in the Chinese population.Previous studies have also shown that visceral obesity, as assessed by visceral adiposity index(VAI) is significantly correlated with the increase in UACR levels in Chinese pre-diabetic subjects, indicating that abdominal obesity may be closely related to albuminuria.30
The results of this study also found that WWI was positively correlated with FIns, 2hIns, TG, TC, LDL-c, and HOMR-IR levels, among other indicators. An elevated WWI reflects a state of excessive body fat accumulation and increased muscle mass loss.27 Muscle-fat imbalance leads to dysregulation of adipocytokine release, inflammation, dysregulated lipid metabolism, and insulin resistance.31 Previous studies have shown that dyslipidemia, insulin resistance, and chronic inflammation play a crucial role in the development of DKD. When the reserve capacity of the adipose tissue is lower than the production of lipids, lipids will be deposited in the kidneys and other tissues and organs, leading to insulin resistance. Moreover, large amounts of lipid accumulation can promote lipopolysaccharide-induced inflammation through the 5’ -adenylate activated protein kinase or peroxisome proliferator-activated receptor-α mediated signaling pathways, resulting in the extensive proliferation of glomerular basement membrane cells and aggravated glomerular sclerosis and tubulointerstitial damage.32
This study has some limitations. First, due to the cross-sectional nature of this study, we were unable to draw a causal relationship between WWI and albuminuria. Second, we adjusted for some potential covariates, but we could not completely exclude the influence of other possible confounding factors. Nevertheless, our results still show that WWI is closely related to the occurrence and development of albuminuria. For people with high WWI levels, early assessment of target organ damage and timely intervention may reduce the risk of DKD and improve prognosis. Moreover, WWI is simple to calculate, economical, and generally applicable to different populations. It may be a superior indicator for assessing obesity. Further longitudinal follow-up studies are needed to verify whether WWI can be used as an early screening tool to prevent early kidney disease in patients.
Conclusion
WWI is independently associated with albuminuria in the Chinese patients with type 2 diabetes and may serve as a simple indicator for albuminuria risk assessment.
Funding
The Projects from Social Development of Zhenjiang (SH2023029), the Key Research and Development Project for Social Development in Jiangsu Province (BE2018692), the Youth Science and Technology Talent Recruitment Project of Zhenjiang city (ZJTJ2101), the Affiliated Hospital of Jiangsu University Beigu Talent Cultivation Plan Project (BGYCB202206), the Affiliated Hospital of Jiangsu University Doctoral Initiation Fund Project (jdfykc2021005).
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Xu C, Ha X, Yang S, Tian X, Jiang H. Advances in understanding and treating diabetic kidney disease: focus on tubulointerstitial inflammation mechanisms. Front Endocrinol. 2023;14:1232790. doi:10.3389/fendo.2023.1232790
2. Doshi SM, Friedman AN. Diagnosis and management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol. 2017;12(8):1366–1373. doi:10.2215/CJN.11111016
3. Sinha SK, Nicholas SB. Pathomechanisms of diabetic kidney disease. J Clin Med. 2023;12(23). doi:10.3390/jcm12237349
4. Fang Z, Liu R, Xie J, He JC. Molecular mechanism of renal lipid accumulation in diabetic kidney disease. J Cell & Mol Med. 2024;28(11):e18364. doi:10.1111/jcmm.18364
5. Dattani R, Ul-Haq Z, Shah M, et al. Association and progression of multi-morbidity with chronic kidney disease stage 3a secondary to type 2 diabetes mellitus, grouped by albuminuria status in the multi-ethnic population of northwest London: a real-world study. PLoS One. 2023;18(8):e0289838. doi:10.1371/journal.pone.0289838
6. Rojano Toimil A, Ciudin A. GLP-1 receptor agonists in diabetic kidney disease: from physiology to clinical outcomes. J Clin Med. 2021;10(17). doi:10.3390/jcm10173955
7. Nagayama D, Fujishiro K, Tsuda S, et al. Enhanced prediction of renal function decline by replacing waist circumference with ”A Body Shape Index (ABSI)” in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obesity. 2022;46(3):564–573. doi:10.1038/s41366-021-01026-7
8. Landecho MF, Alegría-Murillo L, López-Fidalgo J, et al. Unravelling gender-specific factors that link obesity to albuminuria. Eur J Clin Invest. 2020;50(11):e13307. doi:10.1111/eci.13307
9. Rossing P, Persson F, Frimodt-Møller M. Improvements in albuminuria and chronic kidney disease progression with the appetite suppressant lorcaserin. Kidney Int. 2019;95(6):1287–1288. doi:10.1016/j.kint.2019.03.002
10. Salminen P, Nuutila P. Gastric bypass vs medical treatment in renoprotection for patients with class 1 obesity, type 2 diabetes, and albuminuria. JAMA Surgery. 2020;155(8):e200421. doi:10.1001/jamasurg.2020.0421
11. Ren W, Gong Y, Zhen Q, et al. Effect of weight loss on proteinuria in adults with type 2 diabetes: a real-world study. Diabetes Res Clin Pract. 2023;206:111021. doi:10.1016/j.diabres.2023.111021
12. Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol. 2021;12:706978. doi:10.3389/fendo.2021.706978
13. Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. 2018;8(1):16753. doi:10.1038/s41598-018-35073-4
14. Ding Y, Xu Z, Zhou X, Luo Y, Xie R, Li Y. Association between weight-adjusted-waist index and the risk of hyperuricemia in adults: a population-based investigation. Front Endocrinol. 2023;14:1236401. doi:10.3389/fendo.2023.1236401
15. Liu Y, Liu X, Zhang S, et al. Association of anthropometric indices with the development of diabetes among hypertensive patients in china: a cohort study. Front Endocrinol. 2021;12:736077. doi:10.3389/fendo.2021.736077
16. Ding C, Shi Y, Li J, et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: a prospective cohort study. Nut Metab Cardiovasc Dis. 2022;32(5):1210–1217. doi:10.1016/j.numecd.2022.01.033
17. Diabetes Care. 2. Diagnosis and classification of diabetes: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S20–s42. doi:10.2337/dc24-S002
18. Dzau VJ, Hodgkinson CP. Precision Hypertension. Hypertension. 2024;81(4):702–708. doi:10.1161/HYPERTENSIONAHA.123.21710
19. Li X, Wang L, Zhou H, Xu H. Association between weight-adjusted-waist index and chronic kidney disease: a cross-sectional study. BMC Nephrol. 2023;24(1):266. doi:10.1186/s12882-023-03316-w
20. Hu J, Yang S, Zhang A, et al. Abdominal obesity is more closely associated with diabetic kidney disease than general obesity. Diabetes Care. 2016;39(10):e179–180. doi:10.2337/dc16-1025
21. Cusumano AM, Tzanno-Martins C, Rosa-Diez GJ. The glomerular filtration rate: from the diagnosis of kidney function to a public health tool. Front Med. 2021;8:769335. doi:10.3389/fmed.2021.769335
22. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419.
23. Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circul Res. 2020;126(11):1477–1500. doi:10.1161/CIRCRESAHA.120.316101
24. Man REK, Gan ATL, Fenwick EK, et al. The relationship between generalized and abdominal obesity with diabetic kidney disease in type 2 diabetes: a multiethnic asian study and meta-analysis. Nutrients. 2018;10(11). doi:10.3390/nu10111685
25. Lu F, Fan J, Li F, et al. Abdominal adipose tissue and type 2 diabetic kidney disease: adipose radiology assessment, impact, and mechanisms. Abdom Radiol. 2024;49(2):560–574. doi:10.1007/s00261-023-04062-1
26. Huang WH, Chen CY, Lin JL, Lin-Tan DT, Hsu CW, Yen TH. High body mass index reduces glomerular filtration rate decline in type II diabetes mellitus patients with stage 3 or 4 chronic kidney disease. Medicine. 2014;93(7):e41. doi:10.1097/MD.0000000000000041
27. Kim NH, Park Y, Kim NH, Kim SG. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 2021;50(3):780–786. doi:10.1093/ageing/afaa208
28. Abolnezhadian F, Hosseini SA, Alipour M, et al. Association metabolic obesity phenotypes with cardiometabolic index, atherogenic index of plasma and novel anthropometric indices: a link of FTO-rs9939609 polymorphism. Vasc Health Risk Manag. 2020;16:249–256. doi:10.2147/VHRM.S251927
29. Qin Z, Chang K, Yang Q, Yu Q, Liao R, Su B. The association between weight-adjusted-waist index and increased urinary albumin excretion in adults: a population-based study. Frontiers in Nutrition. 2022;9:941926. doi:10.3389/fnut.2022.941926
30. Wang J, Jin X, Chen K, et al. Visceral adiposity index is closely associated with urinary albumin-creatinine ratio in the Chinese population with prediabetes. Diabetes/Metab Res Rev. 2021;37(7):e3424. doi:10.1002/dmrr.3424
31. Li CW, Yu K, Shyh-Chang N, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cach Sarcop Mus. 2022;13(2):781–794. doi:10.1002/jcsm.12901
32. Watanabe K, Sato E, Mishima E, Miyazaki M, Tanaka T. What’s new in the molecular mechanisms of diabetic kidney disease: recent advances. Int J Mol Sci. 2022;24(1). doi:10.3390/ijms24010570
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

