Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Association of Monocyte Chemoattractant Protein-1 (MCP-1) 2518 A/G Polymorphism with Obesity in Korean Type 2 Diabetes Mellitus
Authors Park S, Lee DH , Lee S, Jeon HJ
Received 29 June 2024
Accepted for publication 15 October 2024
Published 21 October 2024 Volume 2024:17 Pages 3917—3924
DOI https://doi.org/10.2147/DMSO.S484860
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Sangshin Park,1,* Dong-Hwa Lee,2,* Shinyoung Lee,1 Hyun Jeong Jeon2
1Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea; 2Department of Internal Medicine, Chungbuk National University College of Medicine and Chungbuk National University Hospital, Cheongju, Korea
*These authors contributed equally to this work
Correspondence: Hyun Jeong Jeon, Department of Internal Medicine, Chungbuk National University College of Medicine 1 Chungbuk Na-Tional University Hospital, 776, 1Sunhwan-ro, Seowon-gu, Cheongju-si, Chungcheongbuk-do, 28644, South Korea, Tel +82-43-269-6352, Email [email protected]
Purpose: Monocyte chemoattractant protein-1 (MCP-1) is a member of the CC chemokine family, and the MCP-1 2518 A/G gene polymorphism is reported to be correlated with chronic inflammatory diseases, including insulin resistance and metabolic syndrome. However, few studies have investigated the association between MCP-1 gene polymorphisms and obesity in patients with type 2 diabetes mellitus (T2DM). We conducted a retrospective case-control study and evaluated the association between the MCP-1 2518 A/G polymorphism and obesity in Korean patients with T2DM.
Patients and Methods: This single-center, retrospective, case-control study enrolled 526 Korean patients with T2DM. Obesity was defined using the body mass index (BMI) with a cutoff level of 25 kg/m2. Polymerase chain reaction-restriction fragment length polymorphism was used to analyze MCP-1 2518 A/G polymorphism; the genotypes was presented as GG, AG, or AA. We compared the MCP-1 2518 A/G polymorphism with the prevalence of diabetic complications, as well as clinical and biochemical characteristics.
Results: The obese group had a higher number of females and higher C-peptide, insulin, triglycerides, aspartate transaminase (AST), and alanine transaminase (ALT) levels. The obese group also had a higher prevalence of cardiovascular disease than the non-obese group. The obese group had a higher frequency of the MCP-1 2518 AA genotype and the A allele than the non-obese group. The results of multiple logistic regression analysis showed that the non-G allele of MCP-1 was significantly associated with obesity (odds ratio (OR), 1.888; P=0.016).
Conclusion: This study demonstrates that the MCP-1 2518 A/G polymorphism is associated with obesity in Korean patients with T2DM. Further studies involving various ethnic groups are required to validate our results.
Keywords: obesity, MCP-1 protein, polymorphism, diabetes mellitus
Introduction
Obesity is increasing at an alarming rate worldwide owing to changes in lifestyle and eating habits. According to the World Health Organization, approximately 30% of the world’s population was considered overweight or obese in 2020.1 The prevalence of obesity in Korea is comparable to the global prevalence, and increased from 31.4% in 2009 to 38.3% in 2020. In addition, the prevalence of obese individuals with a body mass index (BMI) of 35kg/m2 or higher tripled to 26.3% in 2021. The prevalence of obesity also differs by sex, with 48% in the male population and 27.7% in the female population.2 The obesity rate among patients with type 2 diabetes mellitus (T2DM) is reported to be approximately 50%. The obesity rate of patients with T2DM in Korea is 54.4%: 12.9% for class II obesity, with a BMI of 30kg/m2 or higher, and 1.9% for class III obesity, with a BMI of 35 kg/m2 or higher.3
Obesity induces a chronic inflammatory response by increasing the production of adipokines and various inflammatory cytokines owing to the excessive accumulation of adipocytes in the body. Leptin, resistin, and adiponectin are adipokines that are secreted by adipocytes. Adiponectin is closely associated with obesity and is markedly reduced in obese patients. Adiponectin is responsible for anti-inflammatory reactions, such as reducing the expression of adhesion molecules, and plays a role in inhibiting atherosclerosis.4 Therefore, atherosclerosis may occur more easily in obese patients with reduced adiponectin levels than in healthy individuals. Obesity in diabetes causes intravascular atherosclerosis due to reduced adiponectin and increased leptin production, which increases the incidence of chronic diabetic complications.5 Therefore, active weight management is necessary for patients with diabetes.
Monocyte chemoattractant protein-1 (MCP-1) is a CC chemokine that was discovered in 1989 and plays a pivotal role in the development of inflammation.6 Key factors in MCP-1 production are cytokines, such as tumor necrosis factor-alpha (TNF-α) and inter-leukin (IL)-1β, which are secreted from excessively accumulated adipocytes. In addition, an increase in oxidative stress, such as high blood glucose levels and ischemia, is known to result in an increase in MCP-1 production.7 MCP-1 has been reported to be involved in major pathophysiological mechanisms of chronic inflammatory diseases, such as cerebral infarction, myocardial infarction, diabetes, and chronic arthritis.8 The mechanism through which MCP-1 causes chronic inflammatory diseases involves continuous inflammatory induction and responses in the body. In patients with cerebral and myocardial infarctions, the serum MCP-1 concentration is increased, and there is an inverse correlation between the severity of the disease and the concentration of serum MCP-1, indicating that MCP-1 plays an important role in chronic inflammatory disease.9,10
Obesity is a state in which chronic inflammatory reactions occur in the body, and several studies have reported an association between MCP-1 and obesity. It has been reported that the expression of MCP-1 in visceral and subcutaneous fat tissues increase and the concentration of MCP-1 in the serum increases in obese patients.11,12 Youngce et al suggested that adipocyte generation increases when MCP-1 is administered.13 In addition, the direct administration of MCP-1 in animal models has been reported to increase insulin resistance and adipocyte activation.14
Meanwhile, an association between the serum concentration of MCP-1 and the MCP-1 2518 A/G polymorphism has been reported, suggesting an association between MCP-1 and chronic inflammatory diseases. In particular, studies have reported an association between insulin resistance and MCP-1 gene polymorphisms in diseases that are associated with major pathophysiological mechanisms. In Korea, a correlation between poly-cystic ovarian syndrome and MCP-1 2518 A/G polymorphism has been reported.15 However, a study demonstrated that MCP-1 gene polymorphisms may differ according to race.16 MCP-1 gene polymorphisms exhibit a correlation with insulin resistance in both non-diabetic and diabetic patients; however, the results indicating its association with weight differ among studies.17 Several studies have suggested that MCP-1 gene poly-morphism is related to insulin resistance; however, very few studies related to obesity have investigated the related MCP-1 serum concentrations. Obesity can lead to or worsen diabetic complications; therefore, the evaluation of screening factors is essential.
In this study, we aimed to evaluate the relationship between MCP-1 2518 A/G poly-morphism and obesity in Korean patients with T2DM.
Materials and Methods
Study Design and Participants
This single-center, retrospective, case-control study enrolled 526 Korean patients with T2DM. Among the enrolled patients, 262 were female and 264 were male. The average duration of diabetes was approximately 12 years. Obesity was evaluated based on the BMI. The BMI was defined as the value obtained by dividing the patient’s weight (kg) by the square of the patient’s height (m2). We placed patients with a BMI of 25 kg/m2 or more in the obese group and those with a BMI of 25 kg/m2 in the non-obese group based on the Asia-Pacific guidelines. A total of 241 participants were classified as obese, and 285 were classified as non-obese. We compared the MCP-1 2518 A/G polymorphism with the prevalence of diabetic complications, as well as clinical and biochemical characteristics.
Genotyping of MCP-1 Gene Polymorphic Variants
DNA samples were collected from each participant and PCR was performed. Approximately 100 ng genomic DNA was mixed with 0.2 mmol/L primers (Forward primer: 5’-GCTCCGGGCCCAGTATCT-3’ and reverse primer: 5’-ACAGGGAAGGTGAAGGGTATGA-3’), 1.5 mmol/L MgCl2, 0.2 mmol/L dNTPs, and 1 U GoTaq Hot Start Polymerase (Promega Corporation, Madison, WI, USA). The process had 3 main steps: Denaturation; heating the solution to 95 °C. Annealing: The sample was cooled to 59 °C for 30s and 72 °C for 50s. Extension: This cycle was repeated approximately 36 times. The 236 bp PCR products that were fully digested into 182 and 54 bp fragments (homozygous cut) using the Fermentas restriction enzymes were interpreted as the GG genotype. The 236 bp PCR fragments that were incompletely digested due to a lack of recognition sequences were interpreted as the AA genotype (no cut).
Statistical Analyses
Statistical analyses were performed using SPSS for Windows (version 22.0; IBM Corp., Armonk, NY, USA). The Chi-square test was used to calculate the probability of Hardy-Weinberg equilibrium. To evaluate the differences between the two groups, the Stu-dent’s t-test was used for continuous variables and the Chi-square test was used for categorical variables. Multiple logistic regression analysis was used to investigate the relationships between obesity and genotype, sex, age, diabetes duration, hypertension (HTN), dyslipidemia, and hemoglobin A1c (HbA1c). A P-value of <0.05 was considered statistically significant.
Ethics Statement
Written informed consent was obtained from all participants. This study was approved by the Institutional Review Board of the Chungbuk National University Hospital (IRB No. 2017–10-009-009). The current study was conducted according the guidelines administered by the Declaration of Helsinki.
Results
Baseline Characteristics of the Patients
The demographic and biochemical characteristics of the patients according to their BMI (non-obese vs obese) are presented in Table 1. The average BMI of the non-obese group was 22.6 ± 1.7 kg/m2, whereas that of the obese group was 27.8 ± 2.8 kg/m2. The proportion of female patients was higher in the obese group than in the non-obese group (56.4% vs 44.2%, P=0.005). The prevalence of HTN (68% vs 55.4%, P=0.003) was statistically higher in the obese group. According to the laboratory findings, the aspartate aminotransferase (AST) (28.6±15.2 vs 25.5±13.4, P=0.013), alanine aminotransferase (ALT) (33.9±24.1, 27.5±22.0, P=0.002), and triglyceride levels (171.9±107.2 vs 150.6±87.4, P=0.017) were higher in the obese group than those in the non-obese group. C-peptide and insulin concentrations were relatively high in the obese group, but no statistical difference was observed between the two groups in terms of the homeostatic model assistance for insulin resistance (HOMA-IR), which indicates insulin resistance. There were also no significant differences in the blood sugar, HbA1c, or cholesterol levels between the two groups.
 |
Table 1 Baseline Characteristics of Enrolled Patients |
Diabetic Complications According to BMI
Diabetic neuropathy, retinopathy, and nephropathy, which are diabetic microvascular complications, were not significantly different between the two groups (Table 2). Among the macrovascular complications of diabetes, cardiovascular disease was significantly more common in the obese group (17.8% vs 10.2%; P=0.011), while there was no significant difference in the prevalence of cerebrovascular disease.
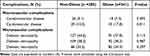 |
Table 2 Prevalence of Diabetic Complications According to BMI |
Distribution of MCP-1 2518 A/G Polymorphism
The distribution of the MCP-1 2518 A/G polymorphism comprised the AA genotype (14.1%, n=74), AG genotype (47.5%, n=250), and GG genotype (38.4%, n=202) (Table 3). The obese group exhibited a relatively high rate of the AA genotype (17.8% vs 10.9%; P=0.022) compared to the non-obese group (Figure 1A). The MCP-1 2518 A/G polymorphism distribution followed the Hardy-Weinberg equilibrium principle. The frequency of MCP-1 gene alleles exhibited different patterns relative to BMI. The frequency of the A allele was higher in the obese group than that in the non-obese group (P =0.046) (Figure 1B).
 |
Table 3 Association Between Genotypes of MCP-1 Polymorphism and Obesity |
Multiple Logistic Regression Analysis: Risk Factors of Obesity
Multiple logistic regression analysis was performed to determine the effects of various risk factors, including genotypes, on obesity (Table 4). The non-G allele of MCP-1 was significantly associated with obesity (odds ratio (OR), 1.888; P=0.016). Among the other parameters, significant associations with obesity were observed in females, patients with HTN, and patients with dyslipidemia ((OR): 1.728, 1.943, and 1.529, respectively; P=0.003, P=0.001, and P=0.022, respectively).
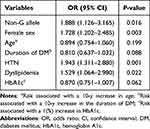 |
Table 4 Risk Factors of Obesity |
Discussion
In this study, the MCP-1 AA genotype was frequently observed in obese patients, confirming that the MCP-1 2518 A/G polymorphism is related to obesity in Korean patients with T2DM. To date, no studies have reported the association between obesity and the MCP-1 2518 A/G polymorphism in patients with T2DM.
One of the key findings of this study is the association between the MCP-1 AA genotype and obesity. The frequency of the AA genotype was significantly higher in the obese group compared to the non-obese group, consistent with previous studies that demonstrated a relationship between MCP-1 polymorphisms and obesity-related metabolic conditions. The role of MCP-1 in mediating inflammatory processes and adipocyte accumulation may explain this association. MCP-1 has been shown to influence the development of insulin resistance and promote adipose tissue inflammation, both of which are central to the pathophysiology of obesity.
Insulin resistance associated with the MCP-1 2518 A/G polymorphism has been demonstrated in obese Japanese patients with T2DM and in a cohort study of German patients with T2DM.17,18 However, in these studies, the C-peptide and insulin levels were significantly higher in the obese group, while HOMA-IR levels were not statistically significant; therefore, the association between the MCP-1 2518 A/G polymorphism and insulin resistance could not be revealed. These studies were not sufficient to demonstrate the association between the number of recruited patient groups, and HOMA-IR levels may have been affected by differences in age and sex. In addition, considering that genotyping may vary between races, a large cohort study on the association between the MCP-1 2518 A/G polymorphism and insulin resistance is necessary in various ethnic groups.
MCP-1, also known as CCL2, is a key CC chemokine involved in recruiting monocytes and other immune cells to sites of inflammation by binding to the CCR2 receptor.6 It is produced by various cells, including adipocytes, in response to inflammatory stimuli like TNF-α and IL-1β. In obesity, excessive adipocyte accumulation leads to increased MCP-1 production, contributing to chronic inflammation and metabolic complications such as T2DM and cardiovascular disease. Elevated MCP-1 levels are linked to insulin resistance, atherosclerosis, and diabetic complications.9,10 Polymorphisms in the MCP-1 gene, such as the 2518 A/G variant, are associated with differences in MCP-1 expression and susceptibility to inflammatory conditions.
Obesity is a low-grade chronic inflammatory state characterized by activated macro-phages. MCP-1 released from adipocytes is known to be a key mediator in this inflammatory process. Serum MCP-1 is highly expressed in chronic inflammatory diseases, such as cerebral infarction, myocardial infarction, diabetes, chronic arthritis, and obesity.8 Previously, Kouyama and Simeoni et al confirmed that the MCP-1 2518 A/G poly-morphism is related to a low serum MCP-1 concentration, but this study did not directly measure the serum MCP-1 concentration; therefore, it was not possible to confirm the relationship between the MCP-1 2518 A/G polymorphism and serum MCP-1 concentrations in T2DM.17,18 Therefore, measurement of the concentration of MCP-1 should be further considered to describe the role of serum MCP-1 in MCP-1 2518 A/G polymorphism and obesity.
A comparison of the prevalence of diabetic complications according to obesity in patients with T2DM demonstrated that diabetic neuropathy, retinopathy, nephropathy, and cerebrovascular disease did not differ between the two groups; however, the prevalence of coronary artery disease was significantly higher in the obese groups. According to a 2022 study published by Mousaie et al, T1DM is associated with obesity, cardiovascular disease, diabetic neuropathy, retinopathy, and nephropathy, while T2DM is associated with obesity, cardiovascular disease, and nephropathy. In particular, diabetic retinopathy is strongly associated with obesity in T1DM and diabetic neuropathy is strongly associated with obesity in T2DM, which is consistent with the results of this study in terms of diabetic retinopathy and cardiovascular disease.19
There are some limitations in this study. The clinical factors associated with obesity, such as physical activity and dietary habits, were not assessed in this study. Additionally, other important parameters related to obesity, including waist circumference and body composition, could not be obtained due to the retrospective nature of the study. The absence of a control group consisting of individuals without T2DM represents another limitation. Finally, not all polymorphisms of MCP-1 were investigated in this analysis. Therefore, a prospective study that corrects for these factors is needed.
Conclusion
In conclusion, this study demonstrates that the non-G allele of MCP-1 2518 A/G polymorphism is significantly associated with obesity in Korean patients with T2DM. Additionally, hypertension, dyslipidemia, and female sex were identified as independent risk factors for obesity in this population. These findings underscore the complex interplay between genetic and metabolic factors in the pathogenesis of obesity, highlighting the need for personalized approaches to obesity management in patients with T2DM. Further studies involving various ethnic groups are required to confirm our results.
Data Sharing Statement
Data may be available from the corresponding author with a reasonable request.
Ethics Declarations
Written informed consent was obtained from all participants. This study was approved by the Institutional Review Board of the Chungbuk National University Hospital (IRB No. 2017-10-009-009). The current study was conducted according the guidelines administered by the Declaration of Helsinki.
Funding
This work was supported by Chungbuk National University BK21 program (2022).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. doi:10.1007/978-3-319-48382-5_1
2. Yang YS, Jung JH, Son JW. Taskforce team of the obesity fact sheet of the Korean society for the study of obesity. Obesity fact sheet in Korea. Trends in obesity prevalence and obesity-related comorbidity incidence stratified by age from 2009 to 2019. J Obes Metab Syndr. 2021;31(2):169–177. doi:10.7570/jomes22024
3. Bae JH, Han KD, Ko SH, et al. Diabetes fact sheet in Korea 2021. Dia-Betes Metab J. 2022;46:417–426. doi:10.4093/dmj.2022.0106
4. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18:1321. doi:10.3390/ijms18061321
5. Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometabolic Synd. 2009;4:44–49. doi:10.1111/j.1559-4572.2008.00030.x
6. Rollins BJ. Monocyte chemoattractant Protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi:10.1016/1357-4310(96)88772-7
7. Singh S, Anshita D, Ravichandiran V. MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101:107598. doi:10.1016/j.intimp.2021.107598
8. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant Protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi:10.1089/jir.2008.0027
9. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112. doi:10.1161/CIRCRESAHA.116.303577
10. Georgakis MK, Gill D, Rannikmäe K, et al. Genetically determined levels of circulating cytokines and risk of stroke. Circulation. 2019;139:256–268. doi:10.1161/CIRCULATIONAHA.118.035905
11. Huber J, Kiefer FW, Zeyda M, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–3221. doi:10.1210/jc.2007-2630
12. Catalán V, Gómez-Ambrosi J, Ramirez B, et al. Proinflammatory cytokines in obesity: impact of T2DMMellitus and gastric bypass. Obes Surg. 2007;17:1464–1474. doi:10.1007/s11695-008-9424-z
13. Younce CW, Azfer A, Kolattukudy PE. MCP-1(monocyte chemotactic Protein-1)-induced protein, a recently identified zinc finger protein, induces adipogenesis in 3T3-L1 pre-adipocytes without peroxisome proliferator-activated receptor gamma. J Biol Chem. 2009;284:27620–27628. doi:10.1074/jbc.M109.025320
14. Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. doi:10.1182/blood.V96.1.34
15. Li L, Ryoo JE, Lee KJ, Choi BC, Baek KH. Genetic variation in the MCP-1 gene promoter associated with the risk of polycystic ovary syndrome. PLoS One. 2015;10:e0123045. doi:10.1371/journal.pone.0123045
16. Chen W, Cui J, Xiang G, Zhang J, Gao H. Association between MCP-1-2518 A>G polymorphism and asthma susceptibility: a meta-analysis. Braz J Med Biol Res. 2019;52:e8549. doi:10.1590/1414-431x20198549
17. Kouyama K, Miyake K, Zenibayashi M, et al. Association of serum MCP-1 concentration and MCP-1 polymorphism with insulin resistance in Japanese individuals with ObeseT2DM. Kobe J Med Sci. 2008;53:345–354.
18. Simeoni E, Hoffmann MM, Winkelmann BR, et al. Association between the A-2518G polymorphism in the monocyte chemoattractant Protein-1 gene and insulin resistance and T2DMMellitus. Diabetolo-gia. 2004;47:1574–1580. doi:10.1007/s00125-004-1494-4
19. Moosaie F, Ghaemi F, Mechanick JI, et al. Obesity and diabetic complications: a study from the nationwide diabetes report of the national program for prevention and control of diabetes(NPPCD-2021) implications for action on multiple scales. Prim Care Dia-betes. 2022;16:422–429. doi:10.1016/j.pcd.2022.03.009
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


