Back to Journals » Drug Design, Development and Therapy » Volume 19
Association of NR1I2 Polymorphism with Midazolam Clearance in Mechanically Ventilated ICU Patients: A Population Pharmacokinetic and Pharmacogenetic Study
Authors Xie H, Zheng Y, Zhang H, Guo Y, Liu M, Weng Q, Wu X
Received 10 September 2024
Accepted for publication 4 February 2025
Published 4 March 2025 Volume 2025:19 Pages 1527—1541
DOI https://doi.org/10.2147/DDDT.S495647
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Helin Xie,1,* You Zheng,1,2,* Hui Zhang,3 Yanmei Guo,3 Maobai Liu,1 Qinyong Weng,3 Xuemei Wu1
1Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, Fujian, 350001, People’s Republic of China; 2College of Pharmacy, Fujian Medical University, Fuzhou, Fujian, 350000, People’s Republic of China; 3Department of Critical Care Medicine, Fujian Medical University Union Hospital, Fuzhou, 350001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qinyong Weng, Department of Critical Care Medicine, Fujian Medical University Union Hospital, Gulou District, Fuzhou, Fujian, 350001, People’s Republic of China, Email [email protected]; Xuemei Wu, Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, Fujian, 350001, People’s Republic of China, Email [email protected]
Background: Significant variability in the metabolism of midazolam (MDZ) exists among mechanically ventilated (MV) patients in the intensive care unit (ICU) due to complex clinical conditions and genetic factors. The NR1I2 gene (PXR), which encodes a nuclear receptor that regulates drug-metabolizing enzymes like CYP3A4, plays a critical role in MDZ metabolism. Polymorphisms in NR1I2, along with variations in genes such as CYP3A4, CYP3A5, and ABCB1, may influence enzyme activity and MDZ pharmacokinetics (PK). Understanding these factors is essential for optimizing MDZ dosing in high-risk patient populations.
Methods: We studied 61 MV ICU patients receiving continuous MDZ infusion. A population pharmacokinetic (PopPK) model was used to assess MDZ PK, with genetic factors (NR1I2 rs2461817, CYP3A4, CYP3A5, ABCB1, and other PXR polymorphisms) and clinical covariates (body weight (BW), aspartate aminotransferase (AST) levels) evaluated for their impact on MDZ clearance (CL).
Results: The PK of MDZ and its metabolite, 1-hydroxymidazolam (1-OH-MDZ), were accurately described using a one-compartment model. The estimated population means for MDZ and 1-OH-MDZ CL were 22.6 L/h (inter-individual variability [IIV] 59.4%) and 67.1 L/h (IIV 57.7%), respectively. MDZ CL was significantly associated with the NR1I2 rs2461817 polymorphism and AST levels, accounting for 11.3% of the variability. MDZ CL decreased by 32.7% as AST increased from 22 IU/L to 60 IU/L, and by 40.7% in patients homozygous for the NR1I2 rs2461817 variant. BW also influenced the CL of 1-OH-MDZ, demonstrating a 34.2% increase as weight increased from 54 kg to 65 kg. Simulations confirmed the significant impact of NR1I2 rs2461817 on MDZ CL.
Conclusion: The PopPK model highlights the significant impact of NR1I2 rs2461817 polymorphism on MDZ CL in Chinese MV patients, emphasizing the need to consider genetic and clinical factors for optimizing MDZ dosing in ICU settings.
Keywords: midazolam, 1-hydroxymidazolam, mechanically ventilated, population pharmacokinetics, NR1I2 polymorphism
Introduction
Sedation is crucial in managing mechanically ventilated (MV) patients in the intensive care unit (ICU), as it reduces restlessness and facilitates invasive procedures.1,2 Inadequate sedation occurs in over 20% of ICU patients, with some studies reporting rates as high as 75%.3 Insufficient and excessive sedation pose considerable risks: inadequate sedation can cause patient-ventilator asynchrony, restlessness, and unplanned extubation, whereas excessive sedation may lead to prolonged mechanical ventilation, extended ICU stays, and an increased incidence of delirium.1,4
Midazolam (MDZ), a commonly used sedative in the ICU, is favored for its rapid onset, especially in scenarios requiring quick sedation, such as anesthesia induction or acute seizure management.5–7 Although it is typically considered to have a short half-life (t1/2),7 midazolam’s pharmacokinetics (PK) can vary significantly in ICU patients due to factors such as organ failure.5,8,9 For instance, its t1/2 can extend from 2.2 h to 35.5 h, and in cases of liver or renal dysfunction, it may even reach up to 140 h.10 While a prolonged t1/2 can help maintain stable sedation and reduce the frequency of dose adjustments, it also raises the risk of drug accumulation, over-sedation, delayed awakening, slow cognitive recovery, and extended ICU stays.5 In patients with morbid obesity, an increased volume (Vd) can further prolong drug clearance (CL), necessitating careful dose adjustments to prevent both under- and over-sedation.11
Given these PK challenges, an individualized drug management strategy is essential when using MDZ. Clinical decisions should not only rely on monitoring sedation depth and physiological parameters but should also account for the patient’s specific pathophysiological state, genetic factors influencing drug metabolism, and other PK changes. These factors collectively contribute to MDZ’s PK variability, emphasizing the need for personalized dosing strategies to optimize therapeutic outcomes, reduce side effects, and improve patient prognosis.
The PK of MDZ is influenced by both genetic and physiological factors, underscoring the necessity of understanding these variables for personalized dosing strategies.12–14 Traditional statistical methods often fail to capture the complex interactions among these factors. In contrast, population pharmacokinetics (PopPK) offers a more effective approach to identify clinical factors contributing to MDZ PK variability. Recent PopPK studies on MDZ predominantly focused on pediatric groups11,15–21 with limited studies conducted on adults.18,22–25 Key predictors of MDZ CL and its metabolite, 1-hydroxymidazolam (1-OH-MDZ), include alcohol misuse, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, age, albumin levels, interleukin-6 (IL-6) levels, body weight (BW), and creatinine clearance (CLcr).18,23,25 CYP3A4 and CYP3A5, Phase I enzymes, primarily metabolize MDZ to 1-OH-MDZ.22 However, the impact of CYP3A4 and CYP3A5 polymorphisms on MDZ PK remains unclear.
The Pregnane X receptor (PXR; NR1I2) plays a crucial role in drug metabolism and significantly influences MDZ metabolism.23,26–29 Variations in PXR expression or activity, along with single nucleotide polymorphisms (SNPs) in the PXR gene, contribute to inter-individual variability in drug metabolism.29 While SNPs in the PXR locus have been shown to affect the PK of drugs like atazanavir, cyclosporine, and carbamazepine, their impact on MDZ metabolism, especially in Chinese ICU patients, remains underexplored and warrants further investigation.29–31
This study aims to develop the first PopPK model specifically tailored for MDZ in Chinese ICU patients, integrating both physiological covariates and NR1I2 genetic polymorphisms. By integrating these variables, the model will offer insights into optimizing MDZ dosing, minimizing inadequate sedation, and improving patient safety in critically ill populations.
Materials and Methods
Study Design and Study Population
The study was conducted in the ICU of Fujian Medical University Union Hospital, Fuzhou, China, between April 2020 and August 2022. The study received approval from the hospital’s Institutional Review Board (IRB No.: 2021YF003-01). Written informed consent was obtained from the patient or their legal representative, ensuring compliance with ethical protocols. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting criteria. The criteria for inclusion were: (1) Administered a continuous intravenous infusion of MDZ; (2) Participants aged 18 years or older, irrespective of gender; (3) Required mechanical breathing for a minimum duration of 24 hours. The exclusion criteria consisted of the following: (1) Hepatic coma or cirrhosis; (2) Inability to evaluate sedation levels due to underlying neurological problems; (3) Hemodynamic instability necessitating frequent modifications of sedative medicine doses; (4) Pregnant and lactating women, or anyone with an allergy to MDZ.
The starting dosage and method of administration were set according to the manufacturer’s package insert for MDZ. 50 mg MDZ solution was diluted into 40 mL of 0.9% normal saline or 5% glucose solution, forming a final concentration of 1 mg/mL. It was administered through a pump system with a rate of 2–4 mL per hour. Nurses evaluated the level of sedation using the Richmond Agitation-Sedation Scale (RASS), adjusting the infusion rate to achieve a specific RASS score, with each dose adjustment being documented. Data collected during the treatment with MDZ were extracted from the hospital’s electronic medical record system in a standardized format. The variables collected in this study included demographic information such as age, sex, body weight (BW), and height. Other data points recorded were the date of MDZ initiation, disease diagnosis, RASS, APACHE II score, serum creatinine (SCr), C-reactive protein (CRP), IL-6, alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), albumin (ALB), prothrombin time (PT), and co-medications such as propofol and methylprednisolone. The body mass index (BMI) and creatinine clearance (CLcr) were calculated using their respective formulas.
CLcr were estimated by Cockcroft and Gault equation:
Blood Sampling and Analytical Assays
The arterial blood sampling schedule included the following time points: before the initial MDZ dose (t = 0), followed by sampling at t = 0‒0.5 h, t = 1‒3 h, t = 4‒6 h, and t = 10‒12 h. The samples were subsequently centrifuged at 15,000 × g for 10 minutes at 4°C, and the plasma was stored at −80°C until required.
The concentrations of MDZ and 1-OH-MDZ in plasma were measured using a validated high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The analysis was performed on a Shimadzu LC-20 system (Nishinokyo-Kuwabaracho, Japan) coupled with an AB SCIEX Triple Quad 4500 MD mass spectrometer (Framingham, MA, USA). Midazolam-d4 maleate was used as the internal standard. Gradient elution was carried out at a flow rate of 0.6 mL/min. The linear ranges for MDZ and 1-OH-MDZ were 0.5–1000 ng/mL and 0.25–500 ng/mL, respectively. The method demonstrated intra-day and inter-day precision below 9%, and accuracy ranged from 92.11% to 111.52%. The limits of quantification for MDZ and 1-OH-MDZ were 0.5 ng/mL and 0.25 ng/mL, respectively.
Furthermore, the handling of missing data and outliers was addressed in the following manner:32 Missing covariate data was imputed using the median value of the population. Concentration values that fell below the lower limit of quantitation (<5%) were excluded from the pharmacokinetic evaluation.
Genotyping
Genomic DNA was obtained from whole blood using the TIANamp Blood DNA Kit (Tiangen, Beijing, China). Research has demonstrated that variations in NR1I2 can impact the expression of other genes, including CYP3A and ABCB1. To explore these variations, we analyzed 23 loci across four genes (CYP3A4, CYP3A5, ABCB1, and NR1I2) . The specific loci analyzed included rs2242480 and rs2246709 (CYP3A4); rs776746 and rs15524 (CYP3A5); rs2032582, rs1128503 and rs1045642 (ABCB1); and rs1464603, rs1464602, rs3732357, rs6785049, rs2276707, rs10934498, rs3814055, rs2472677, rs3732359, rs3814058, rs3732360, rs4688040, rs2276706, rs1523130, rs2461817, and rs1523127 (NR1I2).
Population Pharmacokinetic Modelling
Structural Model and Choice of Statistical Model
The PopPK of MDZ and its active metabolite (1-OH-MDZ) were analyzed using NONMEM 7.5.0 (ICON Development Solutions, MD, USA). A simultaneous modeling approach was applied, in which MDZ disposition was described by a one-compartment model, and 1-OH-MDZ was characterized by a separate one-compartment model for its formation and elimination. The dose, infusion rate, and plasma concentrations of MDZ (Mw = 325.77 g/mol) and 1-OH-MDZ (Mw = 341.77 g/mol) were converted to molar equivalents, and the conversion rate of MDZ to 1-OH-MDZ was fixed at 0.6 based on prior literature.23 Individual variability (IIV) was described using an exponential model, while residual variability (RUV) was evaluated using additive, proportional, and combined error models. The final model was selected based on the objective function value (OFV), parameter precision, error estimates, shrinkage values, and goodness-of-fit (GOF) plots. Model outputs and diagnostics were analyzed using R (version 3.6.1) and Perl-Speaks-NONMEM (PsN, version 5.0.0) within RStudio.
Covariate Analysis
Covariate selection was performed using stepwise forward inclusion and backward elimination, with OFV reductions >3.84 (p < 0.05) for inclusion and >6.63 (p < 0.01) for elimination. In addition, multicollinearity among the continuous covariates was evaluated using the variance inflation factor (VIF), with a threshold of VIF < 10 indicating no significant multicollinearity.33 Covariates included demographic factors (eg, age, sex, BW), biochemical parameters (eg, AST, ALT, CRP), concomitant medications, and genetic polymorphisms. Concomitant medications included drugs commonly used in the ICU, such as propofol and methylprednisolone, with a usage rate exceeding 10%.
Model Evaluation
The accuracy and consistency of the final PopPK model were evaluated using GOF plots, bootstrap resampling, and visual prediction checks (VPC). Model adequacy was assessed using GOF plots. The final model’s internal stability and validity were assessed using a non-parametric bootstrap method, which involved generating 1000 bootstrap datasets. The median and 95% confidence intervals (ranging from 2.5% to 97.5%) of the parameter estimations were compared between the 1000 bootstrap replicates and the original dataset. The predictive performance was assessed using VPC on 1000 simulated datasets. The plots were used to compare the observed data with the 5th, 50th, and 95th percentiles of the simulated data.
Simulation
Simulations were conducted to assess the impact of key covariates, including AST levels and NR1I2 rs2461817 genotypes, on the CL of MDZ and its metabolite, 1-OH-MDZ. To simulate plasma concentrations in a typical patient, IIV and RUV were set to zero. A standard dosing regimen of MDZ was applied, consisting of a loading dose of 3 mg and a maintenance dose of 4 mg/h, derived from the recommended dose range (0.01–0.05 mg/kg for loading and 0.02–0.1 mg/(kg·h) for maintenance) based on the median weight (62 kg) of the study population. AST levels were stratified at the 25th, 50th, and 75th percentiles (22, 37, and 60 IU/L), and patients were grouped by the NR1I2 rs2461817 genotype into wild-type homozygous, mutant heterozygous, and mutant homozygous categories.
Results
Patients and Samples
We assessed 69 patients for eligibility based on the inclusion and exclusion criteria. Eight patients were excluded due to incomplete data (n=3) or failure to meet inclusion criteria (n=5) (Figure 1). Table 1 provides a concise summary of the clinical characteristics exhibited by these patients. Out of the 61 patients, 237 blood samples were analyzed, with both MDZ and 1-OH-MDZ measured in each sample. Samples with concentrations below the quantification limit (3 for MDZ and 3 for 1-OH-MDZ, each amounting to 1.3%) were excluded. The plasma concentrations of MDZ ranged from 1.53 to 1622.36 ng/mL, while the plasma concentrations of 1-OH-MDZ ranged from 0.28 to 166.19 ng/mL. The initial infusion rate for all patients ranged from 2 to 6 mg/h. The genotyping findings for the patients can be found in Supplementary Table 1. All the 22 SNP genotypes, except for NR1I2 rs1464603, had P-values larger than 0.05, indicating that they all adhered to Hardy-Weinberg equilibrium.
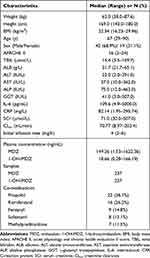 |
Table 1 Demographic and Clinical Characteristics of the Patient |
 |
Figure 1 Flowchart of patient inclusion for the modeling population. |
Development of the Population Pharmacokinetic Model
Figure 2 illustrates the structure of the PopPK model that describes the pharmacokinetic connection between MDZ and 1-OH-MDZ. Both MDZ and 1-OH-MDZ were most accurately characterized by a one-compartment model. IIV was characterized utilizing an exponential model for IIV, whereas for MDZ and 1-OH-MDZ, intra-individual variability was characterized using a combination model and an additive model, respectively. The covariate screening process is provided in Supplementary Table 2. The specifications of the primary model are displayed in Table 2.
 |
Table 2 The Specifications of the Primary Model |
 |
Figure 2 The structural model for MDZ and 1-OH-MDZ. |
Final Model Evaluation
The GOF plots of the final model exhibited effective data characterization, as seen in Figure 3.The model’s ability to estimate individual concentrations was demonstrated by the uniformly distributed individual predicted values for MDZ and its metabolite 1-OH-MDZ when plotted against observed concentrations, aligning closely with the line of unity. Nevertheless, the population estimate of MDZ, and its metabolites failed to accurately account for elevated concentrations. It is probable that the limited number of patient cases with varying dosing and inconsistent sampling times contributed to this outcome. Supplementary Figure 1 provides additional GOF plots. The evaluation of VPC predictive performance showed high levels of accuracy. Figure 4 displays the median concentrations and 95% confidence intervals for MDZ and 1-OH-MDZ as shown by VPC.
Simulations
The simulation results indicate that MDZ plasma concentrations are affected by the AST levels of patients and the NR1I2 rs2461817 genotype. As patients’ AST levels increase, so do MDZ plasma concentrations. As an illustration, the increase in AST levels from 22 IU/L to 60 IU/L leads to a decrease in MDZ CL from 27.8 L/h to 18.7 L/h (Figure 5). Furthermore, various genotypes of NR1I2 rs2461817 have an impact on MDZ plasma concentrations. Patients with the rs2461817 mutant homozygous genotype had higher MDZ plasma concentrations compared to those with wild-type homozygous or mutant heterozygous genotypes. In addition, the CL value decreases from 22.6 L/h to 13.4 L/h in these patients (Figure 6).
Moreover, Figure 7 demonstrates the influence of BW on 1-OH-MDZ plasma levels. The simulation results demonstrate that plasma concentrations of 1-OH-MDZ exhibit variability in relation to BW. As BW increases from 54 kg to 65 kg, the CL of 1-OH-MDZ increases from 53.9 L/h to 72.3 L/h.
Discussion
The first prospective PopPK investigation of MDZ in Chinese ICU patients receiving MV is presented in this work. We looked at PK and pharmacogenetic data from 61 MV patients on continuous MDZ therapy. By combining the AST levels and the NR1I2 rs2461817 polymorphism, our covariate analysis explained 11.3% of the IIV in MDZ CL. Incorporating BW as a covariate for the CL of 1-OH-MDZ led to a decrease in IIV from 62.0% to 57.7%, explaining 4.5% of the IIV in 1-OH-MDZ CL.
Our data indicate that one-compartment models accurately reflect the PK characteristics of MDZ and 1-OH-MDZ. This is consistent with the PK analyses published for MDZ. However, some studies have suggested two-compartment models for MDZ.18 It is important to note that there is limited research on the PK of continuous MDZ infusion in adult patients. These studies primarily focused on patients with acute renal failure (ARF) and acute respiratory distress syndrome (ARDS) related to COVID-19.22,25 To date, no analysis has been conducted on Chinese ICU patients using PopPK. The population mean values of MDZ CL in our study showed significant variations compared to those reported by Swart and Smeets, with our values being 22.6 L/h, 5.47 L/h, and 6.7 L/h, respectively.22,24 Although our study included patients with various disease types, most participants were postoperative patients in relatively good health. This may explain why the estimated MDZ CL was similar to that of healthy individuals.34–36
Although we did not observe a significant correlation between MDZ CL and APACHE II scores in our study, further research on the relationship between APACHE II scores and drug CL or Vd in critically ill patients suggests a potential link. For instance, in the MDZ PopPK model by Swart et al, patients with an APACHE II score ≥26 had a significantly increased Vd.37 Additionally, a prospective observational study in ICU patients treated for tuberculosis found that subtherapeutic rifampin concentrations were often associated with higher APACHE II scores (P=0.03).38 In a propofol study, patients with an APACHE II score ≤20 had an average CL of 92 L/h (95% CI, 81–104), while those with an APACHE II score >20 had an average CL of 117 L/h (95% CI, 89–144).37 These findings suggest that APACHE II scores may influence PK characteristics, particularly CL and Vd, in critically ill patients. This could help explain the relatively higher MDZ CL observed in our study, potentially due to the lower APACHE II scores and the relatively mild conditions of our cohort.
Furthermore, the population mean value of 1-OH-MDZ CL in our study closely matched that of Swart et al (67.1 L/h vs 62 L/h), but it differed significantly from the value reported by Smeets et al (132 L/h).22,24 The MDZ Vd estimate in our study was 15.80 L, which aligns with the lower range of Vd values observed for MDZ in healthy subjects (0.4–2.0 L/kg).22,24,39 The calculated Vd for MDZ, excluding compartmentalization, is closer to the central compartment volume (27.2 L) reported by Seng et al.23 Variations in patient disease types, study duration, and ALB levels may explain the significant differences in Vd observed between our study and previous research. Swart and Smeets included patients with COVID-19-related ARDS and ARF, with observation periods of 11 to 611 hours and 24 to 715 hours, respectively.22,25 In contrast, our study was conducted over a shorter duration (0–24 hours) and included ICU patients with diverse conditions. Vree et al demonstrated that a decrease in serum ALB levels (<25 g/L) is associated with increased Vd and slower MDZ elimination.40 However, the majority of patients in our study had ALB levels within the normal range. Additionally, previous studies suggest that fat mass can significantly impact PK parameters.41 Although most of our patients had a body mass index (BMI) within the normal range, we did not measure fat mass, which may have contributed to variability in Vd. Future PK studies should extend the trial duration and explore the effects of fat mass, patient stratification, and other variables on Vd.
The identifiability of the model structure is essential in constructing a PopPK model. This guarantees the calculation of all PK parameters based on specified input, system, and output circumstances. Studies examining parent pharmaceuticals and their metabolites have discovered that it is not possible to directly compute the fraction of the parent drug metabolized to its metabolite (FMET) and the Vd of the metabolite. However, it is possible to determine their ratio.42,43 In order to make the model less complex and easier to understand, it is sometimes customary to set either the FMET or the Vd of the metabolite.44,45 When examining studies on the PK of MDZ and 1-OH-MDZ, the majority have established a constant value for FMET. According to the study conducted by Seng et al, the FMET value indicates that 60% of MDZ is transformed to 1-OH-MDZ.23
This study revealed a notable correlation between the NR1I2 rs2461817 SNP and MDZ CL. Specifically, individuals with the homozygous mutation of rs2461817 exhibited a 41% decrease in MDZ CL compared to those with the wild-type and heterozygous variants. This discovery suggests that genetic differences in the NR1I2 gene can influence the production and activity of CYP3A, thereby affecting MDZ metabolism. Our study highlights this noteworthy genetic relationship.
We investigated the factors contributing to the limited influence of the CYP3A4 and CYP3A5 genotypes on MDZ CL in our study. We propose that MDZ metabolism in ICU patients is influenced by multiple factors. Beyond genetic factors, drug-drug interactions (DDIs), inflammatory cytokines (such as TNF-α),and other clinical factors may inhibit or diminish the regulatory effects of PXR on CYP3A4 and CYP3A5.6,46 Therefore, while PXR gene regulation does play a role, clinical factors may obscure or attenuate the genetic influence on MDZ metabolism, which may explain the minimal effects of CYP3A4 and CYP3A5 genotypes on MDZ CL in our study. In conclusion, while genetic factors related to CYP3A4 and CYP3A5 may have some impact, further research is needed to better understand how these factors interact with clinical variables to optimize drug metabolism and pharmacotherapy.
Our study also found a negative correlation between AST levels and MDZ CL. Simulation results indicated that when AST levels increased from 22 IU/L to 60 IU/L, MDZ CL decreased by approximately 33%. This is consistent with the known role of CYP3A4 and CYP3A5 in MDZ metabolism. Similar findings were reported by Seng et al, who showed a reduction in MDZ CL as TBIL levels increased.23 Both AST and TBIL are biomarkers of hepatic function, with AST reflecting hepatocellular injury.47–49 Elevated AST levels typically indicate liver damage, particularly under acute stress conditions common in critically ill patients. While ALT is more sensitive for detecting liver injury in chronic liver diseases, AST may be more relevant in acute conditions, such as in ICU patients undergoing metabolic stress. Notably, AST is also a marker of mitochondrial damage, with approximately 80% of AST located in mitochondria, which play a critical role in liver function and drug metabolism.50 This finding aligns with a PopPK study of linezolid in critically ill pediatric patients, where AST was similarly identified as a key covariate for drug CL.51 This may explain the closer association between AST levels, MDZ metabolism, and liver function in ICU patients, where acute liver damage is more pronounced.
BW was found to be a significant covariate for the CL of 1-OH-MDZ, consistent with the results reported by Seng et al.23 In the ICU setting, drug CL is often closely associated with liver and renal function, and the workload of these organs may be more directly related to the patient’s total body weight rather than just fat mass.52 ICU patients’ weight can fluctuate significantly due to factors such as nutritional status, fluid resuscitation, and disease severity.53 Since BW is a dynamic indicator, it is better able to capture these fluctuations and may more accurately reflect the overall factors affecting drug PK. For this reason, BW appears to have a more direct relationship with 1-OH-MDZ CL compared to BMI. While BMI reflects the ratio of fat to lean mass, BW encompasses all body components, including muscle and fluids, which may have a greater impact on metabolic capacity. Moreover, the effect of BW on 1-OH-MDZ Vd might be harder to capture given the substantial weight fluctuations in ICU patients. Thus, BW’s influence on 1-OH-MDZ CL may be more pronounced in our study due to its more direct relationship with metabolic capacity and drug CL.
The primary pathway for 1-OH-MDZ CL involves the process of glucuronidation by uridine diphosphate glucuronosyltransferases (UGT) 1A4 and 2B4/7, resulting in the formation of hydroxy midazolam glucuronide.54 Prior research has demonstrated that obesity can enhance UGT activity in both mice and humans.47,55 Xu et al discovered that in obese mice, fatty liver caused an increase in hepatic UGT expression. This was accompanied by elevated mRNA levels and binding activity of many nuclear receptors, including PXR.55 These findings suggest that higher BW may affect the expression and function of UGT by stimulating the production of nuclear factors such as PXR. This, in turn, may speed up the metabolism of 1-OH-MDZ. Although our study did not assess UGT genes, which could help clarify this connection, further research should consider incorporating UGT genes and other body composition factors, such as fat mass, to enhance our understanding of MDZ metabolism in ICU patients.
Clinicians are also concerned about DDIs involving MDZ. When MDZ is administered alongside CYP3A enzyme inducers, inhibitors, or competitive inhibitors, these drugs can affect the metabolism of MDZ, resulting in alterations in its plasma concentration. Some commonly co-administered drugs include carbamazepine (an antiepileptic), olanzapine (an antipsychotic), itraconazole, fluconazole, voriconazole (azole antifungals), propofol, and fentanyl (an opioid analgesic). Interactions with anesthetics can lead to an increase in MDZ concentrations. Propofol-induced reduction in MDZ CL may be attributed to both hemodynamic suppression and enzyme inhibition caused by propofol.56 It is important to mention that the hemodynamic effects of propofol may also impact the distribution of MDZ to peripheral tissues, thus influencing its central volume of distribution.56 However, in a small subset of cases (36.1%), the co-administration of propofol did not have a significant effect on the Vd of MDZ as a clinical factor. In addition, the co-administration of fentanyl and MDZ may competitively inhibit CYP3A4, resulting in a reduction of MDZ CL.57 Regrettably, our study did not observe a noteworthy influence of fentanyl on MDZ plasma concentrations. This outcome may be attributed to the limited size of our cohort. In addition, the use of methylprednisolone was infrequent, and the connection between corticosteroids and MDZ PK parameters is still uncertain.58,59 Additional PK studies involving larger groups of participants are necessary to gain a comprehensive understanding of the clinical importance of these factors. This will allow for a more comprehensive understanding of the diverse characteristics of ICU patients.
Many restrictions apply to this study. Firstly, the reported Vd and CL of 1-OH-MDZ are “apparent” values because we could not determine the proportion of MDZ metabolized to 1-OH-MDZ. More samples collected after injection should be the goal of future research to better comprehend this conversion ratio. Secondly, this study did not develop a population pharmacokinetic/ pharmacodynamic (PK/PD) model connecting MDZ plasma concentration with sedation scores (RASS scores) due to the inadequate data and its subjectivity. Future research will involve a larger cohort and objective PD indicators, such as EEG, to establish a robust PK/PD model, thereby optimizing MDZ dosing and enhancing clinical outcomes. Thirdly, we did not quantify 1-hydroxymidazolam glucuronide, a Phase II metabolite. 1-OH-MDZ reacts with glucuronic acid to produce 1-OH-MG, which is a less potent form and is eliminated by the kidneys. However, in ICU patients with renal failure, there is a risk of 1-OH-MG buildup, which could result in minor sedative effects.18,60 To improve the applicability of the PopPK model, future studies should include 1-OH-MG and perform external validation.
Furthermore, the absence of known plasma concentration ranges for MDZ and 1-OH-MDZ complicates the assessment of existing dosing regimens, as it hinders the ability to attain targeted sedation levels. There is no recommended range for 1-OH-MDZ. However, a Ramsay score of 2–4 corresponds to a median MDZ plasma concentration of 200–300 ng/mL.60 Since the Ramsay and RASS scales correlate highly in ICU settings,61 200–300 ng/mL may be a good target dosage for mild to moderate sedation. Our MCS data suggest that patients with the NR1I2 rs2461817 wild-type homozygous or heterozygous genotype and AST ≤37 IU/L may not be appropriate candidates for the usual dosage regimen. Improving efficacy and lowering side effects will be possible with the use of model-based MCS and the establishment of pharmacodynamic markers.
Conclusions
In this diverse ICU population, we successfully developed a PopPK model for MDZ. Our findings confirm the significant impact of the NR1I2 rs2461817 genotype on MDZ CL. Additionally, we identified several important factors influencing the CL of MDZ and 1-OH-MDZ, including AST levels and BW. These findings highlight the importance of incorporating genetic and clinical factors into MDZ dosing strategy. Specifically, for a patient with NR1I2 rs2461817 genotype homozygous variant, lower dose should be considered when MDZ is used for sedation in ICU patients. Future work should focus on refining these PK models and integrating PD factors to improve dose prediction and optimize therapeutic outcomes.
Data Sharing Statement
The relevant author can be contacted with any request for any of the data used in this work.
Ethics Approval
The ethical committee of Fujian Medical University Union Hospital gave its approval to the study, which was carried out in compliance with the Declaration of Helsinki (No. 2021YF003-01).
Informed Consent Statement
All participants to the study gave their informed permission.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Startup Fund for Scientific Research, Fujian Medical University (Grant number: 2020QH1085), the Fujian Province Youth and Middle-aged Teacher Education Scientific Research Project (Grant number: JAT200159), the Fujian Provincial Natural Science Foundation of China (Grant number: 2024J01633), and the Fifth Batch of Key Discipline Construction Funds from the hospital (Grant number: 07220003).
Disclosure
The authors assert that they have no conflicts of interest.
References
1. Tanaka LMS, Azevedo LCP, Park M, et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Crit Care. 2014;18(4):R156. doi:10.1186/cc13995
2. Zhou Y, Yang J, Wang B, et al. Sequential use of midazolam and dexmedetomidine for long-term sedation may reduce weaning time in selected critically ill, mechanically ventilated patients: a randomized controlled study. Crit Care. 2022;26(1):122. doi:10.1186/s13054-022-03967-5
3. Jackson DL, Proudfoot CW, Cann KF, Walsh TS. The incidence of sub-optimal sedation in the ICU: a systematic review. Crit Care. 2009;13(6):R204. doi:10.1186/cc8212
4. Eikermann M, Sarge TW. Postoperative interruption of sedation on arrival in the surgical ICU: a new standard of care. Lancet Respir Med. 2017;5(10):764–765. doi:10.1016/s2213-2600(17)30350-8
5. Swart EL, van Schijndel RJ, van Loenen AC, Thijs LG. Continuous infusion of lorazepam versus midazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med. 1999;27(8):1461–1465. doi:10.1097/00003246-199908000-00009
6. Peter JU, Dieudonné P, Zolk O. Pharmacokinetics, pharmacodynamics, and side effects of midazolam: a review and case example. Pharmaceuticals. 2024;17(4). doi:10.3390/ph17040473
7. Oldenhof H, de Jong M, Steenhoek A, Janknegt R. Clinical pharmacokinetics of midazolam in intensive care patients, a wide interpatient variability? Clin Pharmacol Ther. 1988;43(3):263–269. doi:10.1038/clpt.1988.31
8. Shafer A, Doze VA, White PF. Pharmacokinetic variability of midazolam infusions in critically ill patients. Crit Care Med. 1990;18(9):1039–1041. doi:10.1097/00003246-199009000-00024
9. Pentikäinen PJ, Välisalmi L, Himberg JJ, Crevoisier C. Pharmacokinetics of midazolam following intravenous and oral administration in patients with chronic liver disease and in healthy subjects. J Clin Pharmacol. 1989;29(3):272–277. doi:10.1002/j.1552-4604.1989.tb03327.x
10. Shelly MP, Sultan MA, Bodenham A, Park GR. Midazolam infusions in critically ill patients. Eur J Anaesthesiol. 1991;8(1):21–27.
11. van Rongen A, Vaughns JD, Moorthy GS, Barrett JS, Knibbe CA, van den Anker JN. Population pharmacokinetics of midazolam and its metabolites in overweight and obese adolescents. Br J Clin Pharmacol. 2015;80(5):1185–1196. doi:10.1111/bcp.12693
12. Xie YL, Jin X, Yan SS, et al. Population pharmacokinetics of intravenous colistin sulfate and dosage optimization in critically ill patients. Front Pharmacol. 2022;13:967412. doi:10.3389/fphar.2022.967412
13. Chen CY, Xie M, Gong J, et al. Population pharmacokinetic analysis and dosing regimen optimization of teicoplanin in critically ill patients with sepsis. Front Pharmacol. 2023;14:1132367. doi:10.3389/fphar.2023.1132367
14. Lin XB, Li ZW, Yan M, et al. Population pharmacokinetics of voriconazole and CYP2C19 polymorphisms for optimizing dosing regimens in renal transplant recipients. Br J Clin Pharmacol. 2018;84(7):1587–1597. doi:10.1111/bcp.13595
15. Zuppa AF, Conrado DJ, Zane NR, et al. Midazolam dose optimization in critically ill pediatric patients with acute respiratory failure: a population pharmacokinetic-pharmacogenomic study. Crit Care Med. 2019;47(4):e301–e309. doi:10.1097/ccm.0000000000003638
16. Yalcin N, Sürmelioğlu N, Allegaert K. Population pharmacokinetics in critically ill neonates and infants undergoing extracorporeal membrane oxygenation: a literature review. BMJ Paediatr Open. 2022;6(1). doi:10.1136/bmjpo-2022-001512
17. Neupane B, Pandya H, Pandya T, et al. Inflammation and cardiovascular status impact midazolam pharmacokinetics in critically ill children: an observational, prospective, controlled study. Pharmacol Res Perspect. 2022;10(5):e01004. doi:10.1002/prp2.1004
18. Franken LG, Masman AD, de Winter BCM, et al. Hypoalbuminaemia and decreased midazolam clearance in terminally ill adult patients, an inflammatory effect? Br J Clin Pharmacol. 2017;83(8):1701–1712. doi:10.1111/bcp.13259
19. Vet NJ, Brussee JM, de Hoog M, et al. Inflammation and organ failure severely affect midazolam clearance in critically ill children. Am J Respir Crit Care Med. 2016;194(1):58–66. doi:10.1164/rccm.201510-2114OC
20. Kos MK, Miksić M, Jovanović M, Roškar R, Grosek Š, Grabnar I. Maturation of midazolam clearance in critically ill children with severe bronchiolitis: a population pharmacokinetic analysis. Eur J Pharm Sci. 2020;141:105095. doi:10.1016/j.ejps.2019.105095
21. Bienert A, Bartkowska-Sniatkowska A, Wiczling P, et al. Assessing circadian rhythms during prolonged midazolam infusion in the pediatric intensive care unit (PICU) children. Pharmacol Rep. 2013;65(1):107–121. doi:10.1016/s1734-1140(13)70969-1
22. Smeets TJL, Valkenburg AJ, van der Jagt M, et al. Hyperinflammation reduces midazolam metabolism in critically ill adults with COVID-19. Clin Pharmacokinet. 2022;61(7):973–983. doi:10.1007/s40262-022-01122-5
23. Seng KY, Hee KH, Soon GH, et al. CYP3A5*3 and bilirubin predict midazolam population pharmacokinetics in Asian cancer patients. J Clin Pharmacol. 2014;54(2):215–224. doi:10.1002/jcph.230
24. Swart EL, de Jongh J, Zuideveld KP, Danhof M, Thijs LG, Strack van Schijndel RJ. Population pharmacokinetics of lorazepam and midazolam and their metabolites in intensive care patients on continuous venovenous hemofiltration. Am J Kidney Dis. 2005;45(2):360–371. doi:10.1053/j.ajkd.2004.09.004
25. Swart EL, Zuideveld KP, de Jongh J, Danhof M, Thijs LG, Strack van Schijndel RM. Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients. Br J Clin Pharmacol. 2004;57(2):135–145. doi:10.1046/j.1365-2125.2003.01957.x
26. Lee SJ, Lee SS, Jeong HE, et al. The CYP3A4*18 allele, the most frequent coding variant in asian populations, does not significantly affect the midazolam disposition in heterozygous individuals. Drug Metab Dispos. 2007;35(11):2095–2101. doi:10.1124/dmd.107.016733
27. Flores-Pérez C, Castillejos-López MJ, Chávez-Pacheco JL, et al. The rs776746 variant of CYP3A5 is associated with intravenous midazolam plasma levels and higher clearance in critically ill Mexican paediatric patients. J Clin Pharm Ther. 2021;46(3):633–639. doi:10.1111/jcpt.13388
28. Eap CB, Buclin T, Hustert E, et al. Pharmacokinetics of midazolam in CYP3A4- and CYP3A5-genotyped subjects. Eur J Clin Pharmacol. 2004;60(4):231–236. doi:10.1007/s00228-004-0767-7
29. Staudinger JL. Clinical applications of small molecule inhibitors of Pregnane X receptor. Mol Cell Endocrinol. 2019;485:61–71. doi:10.1016/j.mce.2019.02.002
30. Schipani A, Siccardi M, D’Avolio A, et al. Population pharmacokinetic modeling of the association between 63396C->T pregnane X receptor polymorphism and unboosted atazanavir clearance. Antimicrob Agents Chemother. 2010;54(12):5242–5250. doi:10.1128/aac.00781-10
31. Li D, Zhu H, Luo X, Ge W. PXR haplotype clusters will affect the pharmacokinetics of ciclosporin in Chinese renal transplant recipients. J Pharm Pharmacol. 2020;72(2):271–278. doi:10.1111/jphp.13206
32. Irby DJ, Ibrahim ME, Dauki AM, et al. Approaches to handling missing or “problematic” pharmacology data: pharmacokinetics. CPT Pharmacometrics Syst Pharmacol. 2021;10(4):291–308. doi:10.1002/psp4.12611
33. Li X, Shao Y, Wang Z, Zhu J. Risk prediction and treatment assessment in glioma patients using SEER database: a prospective observational study. BMJ Open. 2023;13(12):e079341. doi:10.1136/bmjopen-2023-079341
34. van Rongen A, Kervezee L, Brill M, et al. Population pharmacokinetic model characterizing 24-hour variation in the pharmacokinetics of oral and intravenous midazolam in healthy volunteers. CPT Pharmacometrics Syst Pharmacol. 2015;4(8):454–464. doi:10.1002/psp4.12007
35. Wiebe ST, Meid AD, Mikus G. Composite midazolam and 1ʹ-OH midazolam population pharmacokinetic model for constitutive, inhibited and induced CYP3A activity. J Pharmacokinet Pharmacodyn. 2020;47(6):527–542. doi:10.1007/s10928-020-09704-1
36. Lammers LA, Achterbergh R, Romijn JA, Mathôt RAA. Nutritional Status Differentially Alters Cytochrome P450 3A4 (CYP3A4) and Uridine 5ʹ-Diphospho-Glucuronosyltransferase (UGT) mediated drug metabolism: effect of short-term fasting and high fat diet on midazolam metabolism. Eur J Drug Metab Pharmacokinet. 2018;43(6):751–767. doi:10.1007/s13318-018-0487-5
37. Tse AHW, Ling L, Lee A, Joynt GM. Altered pharmacokinetics in prolonged infusions of sedatives and analgesics among adult critically ill patients: a systematic review. Clin Ther. 2018;40(9):1598–1615.e1592. doi:10.1016/j.clinthera.2018.07.021
38. Koegelenberg CF, Nortje A, Lalla U, et al. The pharmacokinetics of enteral antituberculosis drugs in patients requiring intensive care. S Afr Med J. 2013;103(6):394–398. doi:10.7196/samj.6344
39. Garzone PD, Kroboth PD. Pharmacokinetics of the newer benzodiazepines. Clin Pharmacokinet. 1989;16(6):337–364. doi:10.2165/00003088-198916060-00002
40. Vree TB, Shimoda M, Driessen JJ, et al. Decreased plasma albumin concentration results in increased volume of distribution and decreased elimination of midazolam in intensive care patients. Clin Pharmacol Ther. 1989;46(5):537–544. doi:10.1038/clpt.1989.182
41. Wasmann RE, Svensson EM, Schalkwijk SJ, Brüggemann RJ, Ter Heine R. Normal fat mass cannot be reliably estimated in typical pharmacokinetic studies. Eur J Clin Pharmacol. 2021;77(5):727–733. doi:10.1007/s00228-020-03042-4
42. Saruwatari J, Ogusu N, Shimomasuda M, et al. Effects of CYP2C19 and P450 oxidoreductase polymorphisms on the population pharmacokinetics of clobazam and N-desmethylclobazam in Japanese patients with epilepsy. Ther Drug Monit. 2014;36(3):302–309. doi:10.1097/ftd.0000000000000015
43. Evans ND, Godfrey KR, Chapman MJ, Chappell MJ, Aarons L, Duffull SB. An identifiability analysis of a parent-metabolite pharmacokinetic model for ivabradine. J Pharmacokinet Pharmacodyn. 2001;28(1):93–105. doi:10.1023/a:1011521819898
44. Ahlers SJ, Välitalo PA, Peeters MY, et al. Morphine glucuronidation and elimination in intensive care patients: a comparison with healthy volunteers. Anesth Analg. 2015;121(5):1261–1273. doi:10.1213/ane.0000000000000936
45. Franken LG, Masman AD, de Winter BC, et al. Pharmacokinetics of morphine, Morphine-3-Glucuronide and Morphine-6-Glucuronide in terminally ill adult patients. Clin Pharmacokinet. 2016;55(6):697–709. doi:10.1007/s40262-015-0345-4
46. Gu X, Ke S, Liu D, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281(26):17882–17889. doi:10.1074/jbc.M601302200
47. Brill MJ, Diepstraten J, van Rongen A, Van kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. doi:10.2165/11599410-000000000-00000
48. Zhang P, Wang CY, Li YX, Pan Y, Niu JQ, He SM. Determination of the upper cut-off values of serum alanine aminotransferase and aspartate aminotransferase in Chinese. World J Gastroenterol. 2015;21(8):2419–2424. doi:10.3748/wjg.v21.i8.2419
49. Zuo L, Ling J, Hu N, Chen R. Establishment and validation of a population pharmacokinetic model for apatinib in patients with tumors. BMC Cancer. 2024;24(1):1346. doi:10.1186/s12885-024-13118-4
50. Gong X, Gao Y, Guo G, et al. Effect of matrine on primary human hepatocytes in vitro. Cytotechnology. 2015;67(2):255–265. doi:10.1007/s10616-013-9680-1
51. Yang M, Zhao L, Wang X, et al. Population pharmacokinetics and dosage optimization of linezolid in critically ill pediatric patients. Antimicrob Agents Chemother. 2023;95(5). doi:10.1128/aac.02504-20
52. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. doi:10.2165/11318100-000000000-00000
53. You JW, Lee SJ, Kim YE, et al. Association between weight change and clinical outcomes in critically ill patients. J Crit Care. 2013;28(6):923–927. doi:10.1016/j.jcrc.2013.07.055
54. Ahsman MJ, Hanekamp M, Wildschut ED, Tibboel D, Mathot RA. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2010;49(6):407–419. doi:10.2165/11319970-000000000-00000
55. Xu J, Kulkarni SR, Li L, Slitt AL. UDP-glucuronosyltransferase expression in mouse liver is increased in obesity- and fasting-induced steatosis. Drug Metab Dispos. 2012;40(2):259–266. doi:10.1124/dmd.111.039925
56. Lichtenbelt BJ, Olofsen E, Dahan A, Van Kleef JW, Struys MM, Vuyk J. Propofol reduces the distribution and clearance of midazolam. Anesth Analg. 2010;110(6):1597–1606. doi:10.1213/ANE.0b013e3181da91bb
57. Hase I, Oda Y, Tanaka K, Mizutani K, Nakamoto T, Asada A. I.v. fentanyl decreases the clearance of midazolam. Br J Anaesth. 1997;79(6):740–743. doi:10.1093/bja/79.6.740
58. Higashikawa F, Murakami T, Kaneda T, Takano M. In-vivo and in-vitro metabolic clearance of midazolam, a cytochrome P450 3A substrate, by the liver under normal and increased enzyme activity in rats. J Pharm Pharmacol. 1999;51(4):405–410. doi:10.1211/0022357991772600
59. Nakajima M, Suzuki T, Sasaki T, et al. Effects of chronic administration of glucocorticoid on midazolam pharmacokinetics in humans. Ther Drug Monit. 1999;21(5):507–513. doi:10.1097/00007691-199910000-00003
60. Spina SP, Ensom MH. Clinical pharmacokinetic monitoring of midazolam in critically ill patients. Pharmacotherapy. 2007;27(3):389–398. doi:10.1592/phco.27.3.389
61. Namigar T, Serap K, Esra AT, et al. [The correlation among the Ramsay sedation scale, Richmond agitation sedation scale and Riker sedation agitation scale during midazolam-remifentanil sedation]. Rev Bras Anestesiol. 2017;67(4):347–354. doi:10.1016/j.bjan.2017.03.006
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.








