Back to Journals » Cancer Management and Research » Volume 17
Association of PD-L1 Expression with Lymph Node Metastasis and Clinical Stage in Ampulla of Vater Cancer: An Observational Study
Authors Andrianto A, Rudiman R , Ruchimat T, Lukman K , Sulthana BAAS, Purnama A, Wijaya A, Primastari E , Nugraha P
Received 10 January 2025
Accepted for publication 2 April 2025
Published 13 May 2025 Volume 2025:17 Pages 965—974
DOI https://doi.org/10.2147/CMAR.S513961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Andrianto Andrianto,1 Reno Rudiman,1 Tommy Ruchimat,2 Kiki Lukman,1 Bambang Am Am Setya Sulthana,2 Andriana Purnama,2 Alma Wijaya,2 Etis Primastari,3 Prapanca Nugraha1
1Department of Surgery, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia; 2Department of Surgery, Dr. Hasan Sadikin General Hospital, Bandung, Indonesia; 3Department of Pathological Anatomy, Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Andrianto Andrianto, Email [email protected] Prapanca Nugraha, Email [email protected]
Background: Ampulla of Vater cancer is a subtype of periampullary cancer originating from pancreatic ducts and the bile ducts. Immune checkpoint proteins, particularly Programmed Death-Ligand 1 (PD-L1), show a crucial function in influencing cancer progression, tumor microenvironment, and immune evasion. This study investigates the association between PD-L1 expression and clinical characteristics in patients with ampulla of Vater cancer.
Methods: A retrospective observational study was carried out at a general hospital in West Java, Indonesia, from July 2019 to June 2024. Forty-four patients diagnosed with ampulla of Vater cancer were included. PD-L1 expression was evaluated using immunohistochemistry, and clinicopathological data were analyzed using chi-square, Mann–Whitney, and independent t-tests.
Results: There were 44 research subject. The PD-L1 expression was positive in 59.1% of patients and negatively associated with carcinoembryonic antigen (CEA) levels (p = 0.010). There was a significant association between PD-L1 positivity and lymph node involvement (p = 0.042) and clinical stage (p = 0.017). No significant association was found between PD-L1 expression and age, sex, histopathological grade, or distant metastasis.
Conclusion: PD-L1 expression in ampulla of Vater cancer is significantly associated with higher lymph node metastasis and advanced clinical stage but not with age, sex, or tumor differentiation. These findings suggest PD-L1 as a potential prognostic marker and therapeutic target.
Plain Language Summary: Ampulla of Vater cancer is a rare type of gastrointestinal cancer that develops where the bile duct and pancreatic duct meet. This cancer can evade the immune system by using proteins like PD-L1, which help cancer cells avoid being attacked by immune cells. This study looked at how PD-L1 expression relates to various clinical and pathological features in patients with this cancer. Researchers studied medical records from 44 patients diagnosed with ampulla of Vater cancer in a hospital in West Java, Indonesia, between 2019 and 2024. They examined tissue samples for PD-L1 expression and analyzed patient characteristics such as age, sex, cancer stage, and levels of specific tumor markers. The PD-L1 expression were positive on 59.1% of the patients. Patients with positive PD-L1 were more likely to have cancer spread to lymph nodes and more advanced cancer stages. Higher levels of the tumor marker CEA were associated with lower PD-L1 expression. The PD-L1 expression was not related to patient age, sex, tumor grade, or distant metastasis. The PD-L1 expression in ampulla of Vater cancer is associated with more advanced disease and lymph node involvement, making it a potential marker for predicting cancer progression and a possible target for immunotherapy. However, more research with larger patient groups is needed to confirm these findings.
Keywords: Ampulla of Vater cancer, immune checkpoint inhibitors, PD-L1 expression, lymphatic metastasis, periampullary cancer
Introduction
Ampulla of Vater cancer is one of the cancers that is grouped in periampullary cancer. Periampullary cancer itself is a general term used to grouping cancer originating from the proximal part of the pancreas, distal part of the bile duct, and the cancer of second portion of duodenum. Ampulla of Vater cancer differs from periampullary cancer as a tumor that is topographically centred in the ampulla of Vater area, which can originating from three different components: the pancreatic duct, bile duct, and ampulla or common duct. Hence, this tumor can show morphology cell from pancreas, biliary, and intestinal.1–4
Periampullary cancers include four distinct types of cancer: duodenal adenocarcinoma (DA), distal cholangiocarcinoma (DC), pancreatic ductal adenocarcinoma (PDAC), and ampulla of Vater cancer (AC), and These four types account for 5% of all gastrointestinal malignancies.1,5 Periampullary cancers cause gastric outlet obstruction by compromising the duodenal lumen. In the United States, periampullary malignancies collectively cause over 30 thousand cancer-related deaths annually. The liver is the primary organ of metastasis, followed by lymph nodes, peritoneum, lung, bone, kidney, and rarely skin. Cranial metastases with skin involvement have been reported infrequently.6,7
The development, progression, and metastasis of periampullary carcinoma are significantly influenced by the tumor microenvironment (TME). Immunohistochemical components include CD3, CD4, CD8, FoxP3, and PD-L1.
The PD-1/PD-L1 pathway plays a key role in regulating immune tolerance within the tumor microenvironment.8 The activity of PD-L1 or PD-L2 contributes to the activation, proliferation, and function of cytotoxic T cells, ultimately weakening the anti-tumor immune response. By binding to its receptor, PD-L1 promotes tumor growth by activating signalling pathways that enhance cancer cell proliferation and survival. Moreover, PD-L1 is implicated in further tumor progression and has been found to exert non-immune proliferative effects on various types of cancer cells.9–11
Programmed Death-Ligand 1 (PD-L1) protein is involved in the process of immunosuppression. Under normal conditions, PD-L1 binds to the inhibitory receptor programmed cell death protein 1 (PD-1) on T lymphocytes, suppressing their activation. As a transmembrane molecule, PD-L1 functions through the PD-1/PD-L1 signalling pathway to hinder T cell differentiation and proliferation. In a healthy immune system, PD-L1 helps maintain a balance between protective immunity and immune tolerance. However, in the tumor microenvironment, various mechanisms lead to increased PD-L1 expression, resulting in abnormal activation of the PD-L1/PD-1 signalling pathway. This suppresses T cell proliferation and differentiation, induces T cell apoptosis, and inhibits cytokine signalling and secretion. Ultimately, these effects contribute to cancer cell invasion and metastasis.12,13
Immune checkpoint inhibitors (ICIs), such as CTLA-4 and PD-L1, have recently become promising therapeutic targets for cancer treatment. These therapeutic antibodies aim to deactivate immune checkpoint proteins, shifting the balance from immune suppression to immune activation. Immune checkpoints encompass various regulatory pathways that suppress the immune system, playing a crucial role in maintaining immune tolerance and preventing potential damage caused by excessive immune responses.14,15
The discovery of immune checkpoint proteins such as PD-1/PD-L1 and CTLA-4 represents a significant breakthrough in cancer immunotherapy. Humanized monoclonal antibodies targeting these proteins have demonstrated effectiveness in treating cancer from head and neck, kidney cancer, lung cancer, and melanoma. The United States Food and Drug Administration (FDA) has approved three types of immune checkpoint inhibitors (ICIs): PD-1 inhibitors (Nivolumab, Pembrolizumab, Cemiplimab), PD-L1 inhibitors (Atezolizumab, Durvalumab, Avelumab), and CTLA-4 inhibitors (Ipilimumab). Despite their success, not all patients respond equally to these treatments, highlighting the importance of biomarkers such as tumor mutation burden (TMB), PD-L1 expression, the microbiome, hypoxia, interferon-γ, and extracellular matrix (ECM) in predicting the effectiveness of ICI therapies. Research underscores that disrupting the PD-1/PD-L1 interaction could enhance immune responses against tumors, making it a pivotal approach in cancer immunotherapy. Evaluating PD-L1 expression could assist clinicians in selecting the most suitable ICI therapy for individual patients.16
PD-1 is a key suppressive immune checkpoint predominantly expressed on macrophages, B lymphocytes, dendritic cells (DCs), monocytes, tumor-specific activated T cells, myeloid cells, and natural killer (NK) cells, particularly under conditions of chronic antigen exposure. PD-L1, one of the primary ligands of PD-1, has been identified as a valuable biomarker for predicting prognosis and sensitivity to PD-1/PD-L1 inhibitors.12,17 This study aims to explore and analyze the relationship between PD-L1 expression and the clinicopathological characteristics of patients with ampulla of Vater carcinoma.
Materials and Methods
Study Setting
This study employs a retrospective observational design conducted in the West Java, Indonesia population, following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.18,19 It evaluates the relationship between PD-L1 expression and clinicopathological features in patients with ampulla of Vater carcinoma.
Inclusion and Exclusion Criteria
The subjects included patients who visited a digestive care center in a tertiary hospital in West Java, Indonesia, from July 2019 to June 2024. Inclusion criteria required patients to have a histopathological diagnosis of ampulla of Vater carcinoma through endoscopic biopsy or open surgery. Exclusion criteria eliminated patients whose surgeries were performed at other hospitals and lacked pathological anatomy paraffin blocks.
Immunohistochemistry Staining
PD-L1 expression was assessed using immunohistochemistry, quantifying the percentage of tumor cells (TC) exhibiting membrane staining of any intensity with brown cytoplasmic and/or membrane staining. Formalin-fixed paraffin-embedded tissue blocks were sectioned into 3-μm-thick slices and incubated overnight at 38°C. The specimens were then deparaffinized using xylene and gradually rehydrated with ethanol solutions of decreasing concentration. To suppress nonspecific endogenous peroxidase activity, the samples were treated with a peroxidase solution in methanol, incubated for 10–15 minutes, and then rinsed with running water. Epitope retrieval was carried out using an antigen retrieval process with ethylenediamine tetraacetic acid (EDTA) solution in a decloaking chamber at 100°C for 20 minutes. Immunohistochemical staining was performed using a labeled streptavidin-biotin immunoperoxidase complex. The primary antibodies used were anti-PD-L1 rabbit monoclonal (SP142, Abcam, Inc., Cambridge, USA) at a 1:300 dilution and anti-CD8 rabbit monoclonal (clone SP16, Abcam, Inc., Cambridge, USA) at a 1:200 dilution. Following primary antibody application, the samples were incubated and rinsed twice with phosphate-buffered saline (PBS) for five minutes each. The secondary antibody used was the Star Trek Universal HRP Detection STUHRP700L10-KIT (Biocare). After secondary antibody application, counterstaining was performed using Mayer’s hematoxylin, followed by a 10-minute incubation. The slides were then rinsed with distilled water, and a bluing agent containing 0.25% lithium carbonate was applied for 10–20 seconds.
The immunohistochemical expression of CD8+ tumor-infiltrating lymphocytes (TILs) was assessed semi-quantitatively based on the distribution of staining. Lymphocytes showing CD8 positivity were identified by brown staining on the cell membrane and partially within the cytoplasm. Each slide was examined under a microscope at 400× magnification, with five non-necrotic regions selected for evaluation, excluding adjacent normal tissue or fibrosis. The expression of CD8+ T lymphocytes in the stromal area surrounding tumor cells was assessed using a density scoring system based on the percentage of positive cells: <20% (score 0), 20%–50% (score 1), and >50% (score 2), with scores categorized as negative (0), low (1), or high (2). Similarly, PD-L1 immunohistochemical expression was evaluated semi-quantitatively by analyzing the proportion of tumor cells (TC) with membrane staining of any intensity. Tumor cells expressing PD-L1 exhibited brown staining on the membrane and/or cytoplasm. Each slide was analyzed under 400× magnification, selecting five regions without significant inflammation or necrosis. Adjacent normal tissue or fibrosis was excluded. The tumor cell score was determined as the percentage of positive cells relative to the total tumor population. As Marletta et al and Azriyantha et al the percentage thresholds were categorized as follows: negative (TC0: <1%), TC1 (1–4%), TC2 (5–49%), and TC3 (≥50%).20,21
Clinicopathological Data Collection
Clinicopathological data, including age, sex, histopathological grade, stage, carcinoembryonic antigen (CEA), and carbohydrate antigen 19–9 (CA 19–9), were collected from medical records.
Data Analysis
Data analysis was conducted using SPSS version 26. Patient characteristics were presented as proportions. Differences were analyzed using the following statistical tests: chi-square test (a) for categorical variables, Mann–Whitney test (b) for non-normally distributed continuous variables, and independent t-test (c) for normally distributed continuous variables. A p-value <0.05 was considered statistically significant.
Result
There were 44 subjects included in this research. Table 1 shows the research subject characteristics that include age, sex, grade of histopathological features, stage, CEA, CA-19-9, and PD-L1 expression. Most of the subjects were >50 years old with an average age of 54.7±11.5. The gender distribution showed that 45.5% of the subjects were male and 54.5% were female. In terms of tumor differentiation grade, the majority of the subjects had well-differentiated tumors (86.4%). Based on T stage, most of the subjects were in stage T4 (86.4%). For the N stage, the majority of the subjects were in stage N2 (90.9%). Metastasis was found in 50% of the subjects, with liver metastasis (36.4%) being the most common, followed by lung metastasis (9.1%) and omentum (4.5%). In terms of disease stage, 4.5% were in stage 2, 45.5% in stage 3, and 50% in stage 4. There were no strong positive expressions found in this study; from the immunohistochemistry, there were only three categories found: negative as seen in Figure 1; moderate positive expression as seen in Figure 2; and strong positive expression as seen in Figure 3. There was no very strong positive PD-L1 expression. The negative expression of PD-L1 in this study was seen in 18 subjects (40.9%), moderate positive in 12 subjects (27.3%), and strong positive in 14 subjects (31.8%). Table 2 shows the association between PD-L1 expression and clinicopathological features of research subjects. Significant relationships were found between PD-L1 and N stage, stage of the disease, and CEA level (p < 0.05).
 |
Table 1 Characteristics of Research Subjects |
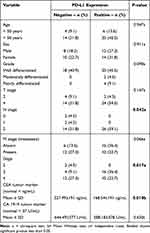 |
Table 2 The Expression of PD-L1 and Clinicopathological Characteristic of Ampulla of Vater Cancer Patients |
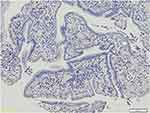 |
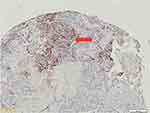
Figure 1 Negative expression of PD-L1 showed the proportion of PD-L1 colored tumor cells among visible tumor cells less than 1%. |
Discussion
Based on the demographic data, the majority of the subjects in this study were over 50 years old, with an average age of 54.7 ± 11.5 years. The gender distribution showed that 45.5% of subjects were male and 54.5% were female. These findings align with the study by Miyazaki et al, which indicated that individuals over 60 years old are at an increased risk for ampullary cancer.22 The increasing incidence of periampullary cancer with age suggests that aging plays a role in cancer susceptibility, possibly due to cumulative genetic mutations and prolonged exposure to carcinogenic factors.
This study did not find a significant association between PD-L1 expression and distant metastasis in Ampulla of Vater cancer patients. PD-L1 expression was detected in 16 patients (36.4%) without metastasis and in 10 patients (22.7%) with metastasis, with a p-value > 0.05, indicating no statistically significant relationship. This contrasts with several studies suggesting that PD-L1 expression is linked to poor prognosis and disease progression. For instance, Saraggi et al reported that PD-L1 expression on tumor cells is associated with worse clinical outcomes.23 In periampullary cancers, particularly pancreatic cancer, 80% of cases exhibit PD-L1 expression, with 20% showing significantly higher levels than normal pancreatic tissue, associated with poor prognosis. However, our findings indicate that while PD-L1 may play a role in disease progression, it may not directly influence distant metastasis in Ampulla of Vater cancer. These differences could be due to variations in sample size, tumor biology, and microenvironmental factors affecting PD-L1 expression in different cancer subtypes.24–26
Despite the lack of a significant association with distant metastasis, our study found a strong relationship between PD-L1 expression and both N classification and clinical stage. This aligns with research by Matsumoto et al, who examined 45 patients with Ampulla of Vater carcinoma and found that high PD-L1 expression associated with larger tumor size, advanced disease stage, and poorer overall survival.27 Similarly, Thakur et al analyzed data from 115 periampullary cancer patients, including 32 with Ampulla of Vater carcinoma, and concluded that PD-L1 overexpression was linked to improved overall survival (OS) and disease-free survival (DFS).24 Their findings suggest that while PD-L1 expression may be associated with tumor progression, its impact on prognosis may vary depending on other clinical factors such as tumor location, size, and lymphatic involvement.
A possible explanation for the discrepancy between N stage and M stage could be the distinction between lymphatic metastasis (regional spread) and distant metastasis (systemic spread).28 While our study found a significant association between PD-L1 expression and lymph node involvement (N classification), it did not establish a statistically significant link with distant metastasis (M classification). This suggests that PD-L1 may play a more prominent role in early tumor progression and regional spread rather than distant dissemination, reinforcing its potential as a prognostic biomarker and a target for immunotherapy.
Additionally, a significant relationship was observed between PD-L1 expression and CEA tumor marker levels. Patients with negative PD-L1 expression had a mean CEA value of 327.99 ± 191 ng/mL, while those with positive PD-L1 expression had a significantly lower mean value of 168.54 ± 191 ng/mL (p = 0.010). This finding suggests a potential link between PD-L1 and tumor marker dynamics, consistent with research by Chen et al, which explored the interaction between PD-L1 and CEA in pancreatic cancer. Their study demonstrated that inhibiting the PD-1/PD-L1 pathway enhanced the function of CEA691-specific cytotoxic T cells, suggesting that PD-L1 inhibition could be a promising therapeutic strategy for tumors expressing high levels of CEA. This underscores the complex role of PD-L1 in immune evasion and highlights its potential as a therapeutic target in periampullary malignancies.26 Elevated levels of CEA are often associated with advanced disease, poor prognosis, and increased tumor burden in Ampulla of Vater cancer. The observed difference in mean CEA values between PD-L1-positive and PD-L1-negative patients suggests a possible interaction between tumor immunogenicity and biomarker expression, which may influence tumor progression, response to therapy, or immune evasion mechanisms. The inverse relationship between PD-L1 expression and CEA levels may suggest a distinct tumor biology in Ampulla of Vater cancer. One possible explanation is that tumors with lower PD-L1 expression might exhibit more aggressive behavior, leading to increased CEA secretion, whereas PD-L1-positive tumors might rely more on immune evasion strategies rather than excessive tumor marker production. This finding does not necessarily contradict previous research but highlights the complex interplay between tumor markers and immune checkpoint molecules, which may differ between cancer types.
Emerging evidence supports the role of PD-L1 as a prognostic and predictive marker in periampullary cancers. Studies indicate that inhibiting PD-L1 can suppress pancreatic cancer progression in mouse models by increasing interferon-gamma (IFN-γ) levels and reducing interleukin-10 (IL-10), thereby modulating the tumor microenvironment to favor immune response. PD-L1-positive tumors have been shown to contain higher levels of tumor-infiltrating regulatory T cells (Tregs), which contribute to immune evasion. Furthermore, PD-L1 expression on peripheral CD8+ T cells has been found to be significantly higher in pancreatic ductal adenocarcinoma (PDAC) patients compared to those with intraductal mucinous papillary neoplasms or healthy individuals, highlighting the diagnostic and therapeutic relevance of PD-1/PD-L1 signaling in pancreatic malignancies. These findings reinforce the importance of continued research into the role of PD-L1 in immune regulation and its potential as a therapeutic target in Ampulla of Vater cancer.12–15
Although our study provides valuable insights into the relationship between PD-L1 expression and tumor progression, it has several limitations, primarily the small sample size. The low prevalence of Ampulla of Vater cancer at our institution restricted the number of cases available for analysis, potentially impacting the statistical power of our findings. Additionally, we did not assess the influence of tumor size and histological subtype, both of which are critical in understanding the association between CEA levels and PD-L1 expression. Future research incorporating these variables would provide a more comprehensive analysis of their potential impact. To strengthen our findings, larger, multi-center studies with expanded sample sizes and multiple immune checkpoint markers (such as CTLA-4) are essential to validate the prognostic and therapeutic implications of immune markers expression in Ampulla of Vater cancer. Further research in this area could enhance our understanding of PD-L1’s role in tumor metastasis and support the development of targeted immunotherapeutic strategies for this rare malignancy.
Conclusion
This study shows that there is no significant relationship between PD-L1 expression in ampulla of Vater cancer patients with age, sex, and pathological differentiation. However, positive expression of PD-L1 is associated with higher lymphatic metastasis and a late stage of the disease.
Data Sharing Statement
Analyzed data and tables presented within the paper. Raw data and immunohistochemistry pictures are available upon request to the corresponding author.
Ethical Declaration
The ethics committee of Dr. Hasan Sadikin General Hospital gave permission for data collection with No. Ethical Approval DP.04.03/D.XIV.6.5/509/2024. The study collected data from pathological data and electronic medical records in the hospital; there was no need for a signed informed consent form. The Ethics Committee granted a waiver of informed consent for this study as it was a retrospective analysis using anonymized patient data, posing minimal risk to participants and making individual consent impractical. To ensure ethical compliance, all patient data were de-identified before analysis, with strict confidentiality maintained in accordance with the Declaration of Helsinki. No personally identifiable information was disclosed, and robust data security measures were implemented to prevent unauthorized access, ensuring compliance with ethical standards and patient privacy protections.
Acknowledgments
The authors want to thank and appreciate all the residents of general surgery, anatomical pathology residents, and digestive surgery trainees for their support in this study.
Funding
The author declared that there was no funding received in the preparation of this study.
Disclosure
There is no conflict of interest in the writing of this paper.
References
1. Hu W, Duan Z, Zhang Y. et al. Remission from the 5-Fu-based chemotherapy to gemcitabine-based chemotherapy-based on the pathological classification of periampullary carcinoma: a case report and literature review. Onco Targets Ther. 2022;15:891. doi:10.2147/OTT.S372053
2. Hester CA, Dogeas E, Augustine MM, et al. Incidence and comparative outcomes of periampullary cancer: a population‐based analysis demonstrating improved outcomes and increased use of adjuvant therapy from 2004 to 2012. J Surg Oncol. 2019;119(3):303–317. doi:10.1002/jso.25336
3. Hugenschmidt H, Labori KJ, Brunborg C, et al. Circulating tumor cells are an independent predictor of shorter survival in patients undergoing resection for pancreatic and periampullary adenocarcinoma. Ann Surg. 2020;271(3):549–558. doi:10.1097/SLA.0000000000003035
4. Duan Z, Zhang Y, Tang Y, Gao R, Bao J, Liang B. Adjuvant therapy for periampullary carcinoma and the significance of histopathological typing: a systematic review. Transl Oncol. 2022;20:101414. doi:10.1016/j.tranon.2022.101414
5. Romiti A, Barucca V, Zullo A, et al. Tumors of ampulla of Vater: a case series and review of chemotherapy options. World J Gastrointestinal Oncol. 2012;4(3):60. doi:10.4251/wjgo.v4.i3.60
6. Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educat Book. 2014;34(1):112–115. doi:10.14694/EdBook_AM.2014.34.112
7. Zackria R, Botejue M, Hwang AW. Primary duodenal periampullary adenocarcinoma: an uncommon presentation. Cureus. 2021;13(4). doi:10.7759/cureus.14323
8. Wang Q, Shao X, Zhang Y, et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023;12(10):11149–11165. doi:10.1002/cam4.5698
9. Broggi G, Angelico G, Farina J, et al. Tumor-associated microenvironment, PD-L1 expression and their relationship with immunotherapy in glioblastoma, IDH-wild type: a comprehensive review with emphasis on the implications for neuropathologists. Pathol Res Pract. 2024;254:155144. doi:10.1016/j.prp.2024.155144
10. Liu B, Arakawa Y, Yokogawa R, et al. PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3+ and CD8+ infiltrating lymphocytes. PLoS One. 2018;13(4):e0194594. doi:10.1371/journal.pone.0194594
11. Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 expression in cytotoxic CD8+ tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res. 2018;24(2):407–419. doi:10.1158/1078-0432.CCR-17-2156
12. Han Y, Liu D, Li L.PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727.
13. Gutic B, Bozanovic T, Mandic A, et al. Programmed cell death-1 and its ligands: current knowledge and possibilities in immunotherapy. Clinics. 2023;78:100177. doi:10.1016/j.clinsp.2023.100177
14. Wojtukiewicz MZ, Rek MM, Karpowicz K, et al. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021;40(3):949–982. doi:10.1007/s10555-021-09976-0
15. Kong X, Zhang J, Chen S, et al. Immune checkpoint inhibitors: breakthroughs in cancer treatment. Cancer Biol Med. 2024;00(55):2095–3941. doi:10.20892/j.issn.2095-3941.2024.0055
16. Shiravand Y, Khodadadi F, Kashani SM, et al. Immune checkpoint inhibitors in cancer therapy. Current Oncol. 2022;29(5):3044–3060. doi:10.3390/curroncol29050247
17. Tang Q, Chen Y, Li X, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442. doi:10.3389/fimmu.2022.964442
18. Vandenbroucke JP, Elm EV, Altman DG, et al. Strobe initiative. strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Internal Med. 2007;147(8):W–163. doi:10.7326/0003-4819-147-8-200710160-00010-w1
19. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31. doi:10.4103/sja.SJA_543_18
20. Marletta S, Fusco N, Munari E, et al. Atlas of PD-L1 for pathologists: indications, scores, diagnostic platforms and reporting systems. J Personaliz Med. 2022;12(7):1073. doi:10.3390/jpm12071073
21. Azriyantha MR, Lukman K, Sulthana BA, et al. Association between tumor-infiltrating lymphocyte-T CD8+ and programmed death-ligand 1 protein with the occurrence of metastasis in colorectal cancer patients: an observational study. Trends Immunotherap. 2024;8(2). doi:10.24294/ti.v8.i2.6221
22. Miyazaki M, Yoshitomi H, Miyakawa S, et al. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepato-Biliary-Pancreat Sci. 2015;22(4):249–273. doi:10.1002/jhbp.233
23. Saraggi D, Galuppini F, Remo A, et al. PD‐L1 overexpression in ampulla of Vater carcinoma and its pre‐invasive lesions. Histopathology. 2017;71(3):470–474. doi:10.1111/his.13254
24. Thakur N, Paik KY, Hwang G, Chong Y. High expression of PD-L1 is associated with better survival in pancreatic/periampullary cancers and correlates with epithelial to mesenchymal transition. Diagnostics. 2021;11(4):597. doi:10.3390/diagnostics11040597
25. Zhang Z, Xiong Q, Xu Y, Cai X, Zhang L, Zhu Q. The pd-L1 expression and tumor-infiltrating immune cells predict an unfavorable prognosis in pancreatic ductal adenocarcinoma and adenosquamous carcinoma. J Clin Med. 2023;12(4):1398. doi:10.3390/jcm12041398
26. Chen Y, Xue SA, Behboudi S, Mohammad GH, Pereira SP, Morris EC. Ex vivo PD-L1/PD-1 pathway blockade reverses dysfunction of circulating CEA-specific T cells in pancreatic cancer patients. Clin Cancer Res. 2017;23(20):6178–6189. doi:10.1158/1078-0432.CCR-17-1185
27. Matsumoto K, Ohara T, Fujisawa M, et al. Diagnostic utility of the PD-L1 immunostaining in biopsy specimens of patients with biliary tract neoplasms. J Gastrointestinal Surg. 2022;26(6):1213–1223. doi:10.1007/s11605-021-05197-6
28. Ji H, Hu C, Yang X, et al. Lymph node metastasis in cancer progression: molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduction and Targeted Therapy. 2023;8(1):367. doi:10.1038/s41392-023-01576-4
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.