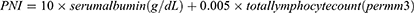Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Association of Prognostic Nutritional Index with Post-Discharge Bleeding After Percutaneous Coronary Intervention in ACS Patients on DAPT
Authors Elkenany NM, Sabah ZU , Agiba NA, Elmahdy HK, Elsherbiny EAY , Said SRA , Nassef EM, Alhawy AME , Ahmed MSM , Elsharkawy AMS, Hussein AMM , Elmalah AA
Received 21 October 2024
Accepted for publication 15 March 2025
Published 10 April 2025 Volume 2025:21 Pages 455—466
DOI https://doi.org/10.2147/TCRM.S496656
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Nasima Mohamed Elkenany,1 Zia Ul Sabah,2 Nadia Ahmed Agiba,1 Hanaa Kamel Elmahdy,1 Eman Aziz Yousef Elsherbiny,1 Salwa Rashad Aly Said,1 Eman Mostafa Nassef,1 Ahmed Mohamed Ewis Alhawy,3 Marwan Sayed Mohamed Ahmed,3 Ashraf Mohammed Said Elsharkawy,3 Amr Mahmoud Mohamed Hussein,3 Abeer Ahmed Elmalah1
1Faculty of Medicine (for Girls), Al Azhar University, Cairo, Egypt; 2Department of Medicine, College of Medicine, King Khalid University; Prince Faisal Bin Khalid Cardiac Centre, Abha, Aseer, Saudi Arabia; 3Faculty of Medicine (for Boys), Al Azhar University, Cairo, Egypt
Correspondence: Zia Ul Sabah, Department of Medicine, College of Medicine, King Khalid University, Abha, Saudi Arabia, Tel +966536130888, Email [email protected]
Purpose: Malnutrition increases bleeding risk by reducing thrombogenicity, impairing platelet aggregation, prolonging bleeding time, and promoting systemic inflammation, which affects vascular permeability and angiogenesis. The Prognostic Nutritional Index (PNI), calculated from serum albumin and lymphocyte count, reflects both nutritional and inflammatory status. This study aimed to assess PNI’s association with bleeding risk in acute coronary syndrome (ACS) patients on dual antiplatelet therapy (DAPT).
Patients and Methods: This prospective, single-center observational cohort study enrolled 1843 patients presenting with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI). ROC analysis determined 42.7 as the optimal PNI cut-off value for risk stratification. Participants were stratified into distinct groups based on Prognostic Nutritional Index (PNI) cut-off values, a composite marker derived from serum albumin levels and peripheral lymphocyte counts, reflecting both nutritional and inflammatory status. Patients were prospectively followed for 12 months post-discharge to assess the occurrence of actionable bleeding events, with the aim of evaluating the association between PNI and post-PCI bleeding risk.
Results: The study cohort had a mean age of 66.4, with 65.16% male. After PCI, 98.04% were on DAPT. Patients were divided into Group I (PNI ≥ 42.7, n = 1290) and Group II (PNI < 42.7, n = 553). During follow-up, 5.58% of patients experienced actionable bleeding, with 3.5% in Group I and 10.3% in Group II (p < 0.0001). Multivariable Cox regression analysis revealed that PNI < 42.7 was a significant independent predictor of bleeding (HR: 1.7; 95% CI: 1.1– 2.5; p < 0.003).
Conclusion: Baseline PNI is an independent predictor of post-discharge bleeding in ACS patients on DAPT after PCI, suggesting it could be a valuable tool for risk stratification of bleeding in these patients.
Keywords: prognostic nutritional index, acute coronary syndrome, dual antiplatelet therapy
Graphical Abstract:

Introduction
The use of dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor has resulted in marked decrease of ischemic events and led to improved outcomes in acute coronary syndrome (ACS).1–3 Following treatment with percutaneous coronary intervention (PCI), antithrombotic treatment with DAPT typically continues for up to 12 months post-discharge to downscale the risks of stent thrombosis and accompanying ischemic events.4 However, the benefits of DAPT come with significantly increased risks of bleeding which severely tax and limit the clinical benefits of DAPT.5,6 Bleeding associated with the use of DAPT has been shown to be a significant contributing factor for both the mortality and morbidity.7–9 Previously it has been demonstrated that risk of mortality posed by bleeding is comparable to that of myocardial infarction (MI).9 It has also been shown that unlike ischemic events, there is a significant and proportionate correlation between major bleeding events and risk of mortality.10 Various studies, both randomised controlled trials (RCT’s) and observational studies have reported a 1.3–5.6% incidence of major bleeding in patients with ACS within 1 year of discharge 11–13 and a 33.33% of patients on DAPT encountering a bleeding event within 1-year post-discharge.11 An earlier study showed that the risk for re-hospitalization due to bleeding was higher in conservatively treated patients compared to invasively treated patients.14 Earlier variables, age, female gender, lower weight, low hemoglobin, diabetes, hypertension, renal failure, atrial fibrillation, prior bleeding, and hemorrhagic or ischemic stroke have demonstrated as significant predictors of bleeding.15–19
Current risk stratification tools for post-discharge bleeding after PCI, such as the PRECISE-DAPT and PARIS risk scores, rely on clinical variables like age, renal function, hemoglobin levels, and prior bleeding history. While these tools are widely used, studies have highlighted their modest predictive accuracy. For instance, the PRECISE-DAPT score, though validated in multiple cohorts, has shown only moderate discrimination (C-statistic ~0.60–0.65) for predicting bleeding events, particularly in diverse populations.20,21 Similarly, the PARIS bleeding risk score has demonstrated limited predictive performance (C-statistic ~0.64), as reported in its derivation and validation studies.22,23 These limitations underscore the need for more robust risk assessment tools.
Furthermore, previous investigations have reported that malnutrition is significantly associated with low-thrombogenicity hence bleeding risk,24 increased bleeding risk in patients on Vitamin K antagonists,25 significant decrease in mean platelet aggregation (MPA),26 prolongation of bleeding time and purpura,27,28 vascular dysfunction and remodelling,29,30 decreased Th1 cytokines (IL-2 and IFN-g), proinflammatory (TNF, IL-6, IL-1a, and IL-1b) cytokines and hyperactivation of Th2 cytokines (IL-4, IL-5, and IL-13),31–33 thus, promoting generalized systemic inflammation which negatively affects vascular permeability,34,35 and angiogenesis,36 thereby significantly increasing odds of bleeding.
The prognostic nutritional index (PNI) propounded by Buzby et al,37 is an easy to calculate mathematical index from serum albumin concentration and peripheral blood lymphocytes, and it is capable of comprehensively reflecting the nutritional and inflammatory status of an individual.38 Previously, PNI has been utilized to assess various cancers,39–41 postoperative acute kidney injury (AKI),42 prognosis of cardiovascular diseases,43–45 and evaluating long-term outcomes in elderly patients with an ischemic stroke.46 However, PNI has mostly been used for survival and mortality analysis in various disease conditions and not as a predictive factor for diseases or events of interest. Additionally, the trade-off between the utility and safety of DAPT is challenging, more so in case of patients at higher risk of bleeding,5,16,47,48 therefore it becomes imperative to stratify patients based on the probability of risk of bleeding before putting them on DAPT.
Hence, we hypothesized that PNI may have a significant association with bleeding risk in ACS patients on DAPT. The aim of the study was to test this hypothesis and evaluate the predictive value of PNI in determining the risk of bleeding in ACS patients on DAPT.
Patients and Methods
Study Design
This prospective, single-center observational cohort study was conducted at Prince Faisal bin Khalid Cardiac Center, Abha, Saudi Arabia. Data pertaining to all patients who presented with acute cardiac syndrome (ACS) and underwent percutaneous coronary intervention (PCI) from March 2021 to March 2023 were recorded. The definition of ACS considered was unstable angina (UA) with accompanying chest pain and ischemic changes on electrocardiogram, or type 1 acute myocardial infarction (MI) conforming to the universal definition of myocardial infarction.49
Inclusion Criteria
Patients were only considered eligible for inclusion if they presented with ACS and had at least one lesion in a native coronary artery having a reference diameter of 2.25–4.25 mm and a stenosis ≥ 70%, as well as, suitable for PCI with stent placement.
Exclusion Criteria
Patients were excluded if they have had a major surgical procedure, or a major pathological bleeding event within past 3 months, history of diathesis for bleeding or coagulopathy, or a life expectancy of not more than 2 years. Patients were also excluded if they had known hypersensitivity or contraindication for aspirin, ticagrelor, clopidogrel, heparin, zotarolimus, everolimus, biolimus, or contrast agents.
Procedural Details
PCI was performed conforming to established standard practices. For anticoagulation either low-molecular weight or unfractionated heparin was used. Using glycoprotein IIb/IIIa inhibitors, predilation or postdilation, thrombus aspiration, and use of intravascular imaging or fractional flow reserve (FFR) / instantaneous wave-free ratio (iFR) was left at the operators’ discretion. Oral administration of 300 mg aspirin and a 300 mg or 600 mg clopidogrel loading dose was ensured at least 12 hours before PCI. In case the administration of loading dose was not achieved 12 hours before PCI, a loading dose of 600 mg clopidogrel was given as early as possible before PCI.
After successful PCI all patients were put on optimal pharmacological therapy, including DAPT, statins, beta blockers or if indicated on angiotensin-receptor blockers (ARBs)/ angiotensin-converting enzyme inhibitors (ACE-inhibitors)/direct renin inhibitors in conformity with ACC/AHA50 and ESC4 guidelines.
End Points, Data Recorded and Definitions
The primary endpoint of this study was post-discharge actionable bleeding occurring at least 7 days after PCI. Fixing the 7-day lower-limit was based on the upper-limit of current hospitalization time frames for ACS, as well as to account for any in-hospital events attributable to invasive procedures.51
Data regarding baseline clinical, laboratory, procedural, and medications at discharge were prospectively recorded. Bleeding Academic Research Consortium (BARC) criteria were used to classify bleeding.52 During the follow-up period actionable bleeding was defined as BARC 2 or 3 bleed. Chronic Kidney Disease Epidemiology Collaboration equation (the CKD-EPI) was utilized to calculate estimated glomerular filtration rate (eGFR).53
Additionally, baseline prognostic nutritional index (PNI) was calculated as,54
Follow-Up
The clinical follow-up of all patients was done at 1, 3, 6, and 12 months from index PCI. At each follow-up point, data on clinical status, endpoint events if any, any other adverse events, and adherence to DAPT were recorded.
Ethical Declaration and Approval
This study was carried out in conformity with the 2013 revision of the principles of Declaration of Helsinki55 and approved by the institutional ethics committee of King Khalid University, Abha, Saudi Arabia vide number: ECM #2024-3328. The study protocol was approved by the institutional review board and all the study subjects provided written informed consent.
Statistical Analysis
With a 95% confidence level (Z = 1.96), maximum variability (p = 0.5), and a 0.05 margin of error (e), Cochran’s sample size equation56 determined a minimum required sample size of 385. Our study population (N = 1843) significantly exceeded this threshold, ensuring robust statistical power for analysis. Continuous baseline data was presented as means ± standard deviations or median (Quartile1, Quartile3), and intergroup comparisons of such data was done using students t-test or Mann-Whitney U-test, as found appropriate. The categorical data was presented as numbers and percentages. Pearson Chi-square test was used to compare categorical variables. PNI was treated as a binary variable, after identifying the optimum cut-off value using receiver-operating characteristic curve (ROC) analysis. Univariable and Multivariable Cox proportional hazards regression models were employed to identify independent predictors of actionable bleeding, as unlike logistic regression they account for time-to-event data and provide time-dependent hazard ratios, offering superior insights into bleeding risk over the 12-month follow-up. Only those variables which assumed significance in univariable model p(<0.05), were included in the multivariable Cox proportional regression model. Statistical analyses were conducted using R (R Core Team, 2023), RStudio (RStudio Team, 2023). Our study design is depicted in Figure 1.
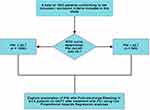 |
Figure 1 Study Design. |
Results
Baseline Characteristics of Study Cohort
Between March 2021 to March 2023, a total of 1843 ACS patients undergoing PCI and conforming to the set inclusion/exclusion criteria were enrolled. Data including demographics, clinical details, co-morbidities, presentation time diagnosis, laboratory parameters, procedural details, medications at discharge were recorded. Furthermore, data on study endpoint events (actionable bleeding) were recorded for each enrolled patient for a follow-up period of 1-year. Since the last patient was enrolled in March 2023, the follow-up period extended to March 2024. Baseline features of the study population are presented in Table 1. The mean age of the study cohort was 66.4 ± 9.80, with 65.16% being male. The most common presentation time event in the study population was STEMI (56.75%), followed by NSTEMI (32.17%) and UA (10.85%) respectively. Post-PCI, 98.04% of the cohort were put on DAPT. PNI, in the whole cohort ranged from 31.46 to 51.8, with a median of 44.6. ROC analysis identified 42.7 as the optimal cut-off value for PNI, as depicted in Figure 2.
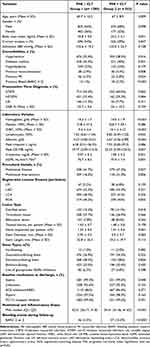 |
Table 1 Baseline Characteristics of Acute Coronary Syndrome Study Population |
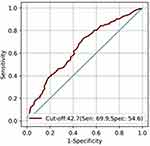 |
Figure 2 Receiver operating characteristic curve of PNI. |
To study the correlations between PNI and other clinical characteristics of the study population, subjects were stratified into two groups with respect to PNI, Group I (PNI ≥ 42.7, n =1290) and Group II (PNI < 42.7, n = 553), as presented in Table 1. Out of the total 1843 study subjects, 103 (5.58%) patients reported at least one event of actionable bleeding during the follow-up period. The projection across the two groups revealed that 3.5% (46/1290) patients from Group I reported the endpoint of actionable bleeding as against 10.3% (57/553) patients from Group II: p < 0.0001. The study subjects in Group II compared to Group I, were older, had higher prevalence of comorbid conditions, lower baseline mean Hb value (P < 0.0001), higher mean WBC count (p < 0.0001), lower mean albumin value (P<0.0001), lower lymphocyte count (P = 0.032), and a lower mean Egfr (P = 0.001). However, the two groups did not differ in presentation time event types, interventional findings, and medications at discharge including DAPT. No patients were lost to follow-up and no mortality was reported.
Association of PNI with Actionable Bleeding During Follow-up
To explore the association between PNI and actionable bleeding during follow-up, univariable Cox regression analysis was done, with actionable bleeding as dependent variable. For this analysis PNI was treated as a categorical variable (PNI ≥ 42.7 or PNI < 42.7). Results of the univariate Cox regression analysis are presented in Supplementary Table 1. To test the predictive significance of PNI, statistically significant variates in univariable Cox regression were included in the multivariable Cox regression analysis. The multivariable Cox regression analysis identified PNI (< 42.7) as a significant independent predictor of actionable bleeding (HR: 1.7; 95% CI: 1.1–2.5; p < 0.003), as presented in Table 2.
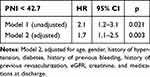 |
Table 2 Association of PNI with Post-Discharge Bleeding in ACS Patients on DAPT After Treatment With PCI |
In addition to PNI, the other independent predictors of actionable bleeding during follow-up as identified by multivariable Cox regression are presented in Table 3. Additionally, Kaplan-Meier analysis was performed to assess the actionable bleeding event analysis over a 12-month follow-up period. The median time to first bleeding could not be estimated for either group, as the bleeding-free probability remained above 50% at the end of the study period. At 12 months, the bleeding-free probability was 96.4% (95% CI: 94.8–97.2) in Group 1 and 83.3% (95% CI: 73.4–86.8) in Group 2. The Log rank test indicated statistically significant difference between the groups (p < 0.0001). These results suggest a low overall risk of bleeding in Group 1 (PNI ≥ 42.7) compared to Group 2 (PNI < 42.7) during the study period (Figure 3).
 |
Table 3 Predictors of Post-Discharge Bleeding in ACS Patients on DAPT After Treatment with PCI |
 |
Figure 3 Kaplan-Meier Curve Analysis. |
Discussion
The present study involved 1843 ACS patients who were treated with PCI and discharged on DAPT. The central finding of the study was that the nutritional and inflammatory status of a patient as indicated by PNI is a significant predictor (HR: 1.7;95% CI:1.1–2.5;p=0.003) of post-discharge bleeding in ACS patients on DAPT after treatment with PCI (Table 2). The study confirmed previously identified predictors of bleeding (age, history of hypertension, and history of bleeding).15,57 Additionally, the present study also found that female gender and increase in Hb at discharge were associated with lower risk of bleeding, in with PLATO trial and an earlier study respectively.12,58
PNI as a tool for prognostication was initially proposed by Buzby in 1980 to predict outcomes of patients undergoing gastrointestinal surgery.37 He calculated the nutritional status of patients by fitting a linear regression model on serum albumin, serum transferrin, delayed hypersensitivity, and triceps skinfold. Later, in 1984, Onodera modified the formula of PNI calculation by only retaining albumin from the earlier model and adding lymphocyte count in a mathematical equation to represent a subject’s nutritional and inflammatory status.38 Previously, investigators have explored the applications of PNI in the prognosis of various cancers,39–42 cardiovascular diseases,43–45 and postoperative acute kidney injury (AKI).42 In a 2016 study a significant association between lower PNI and unfavourable outcomes in elderly STEMI patients treated with primary PCI was proposed,59 which was later on confirmed by two independent investigations held in 2017.60,61 Both of the investigations reported that PNI was a significant independent prognostic factor in all STEMI patients treated with PCI. PNI is calculated from serum albumin values and lymphocyte counts and is a biological marker of chronic inflammatory and nutritional status.38 Low albumin levels have previously been linked to increased risk of bleeding in ACS patients undergoing PCI.62 Moreover, malnutrition can induce chronic inflammation,63 which can reduce total lymphocyte and CD4 counts,64 which in turn leads to endothelial dysfunction,65 and a 2023 study reported endothelial dysfunction as an independent predictor of major bleeding in patients with acute coronary syndrome (ACS).66
To our information this is the first study to investigate the association of PNI and post-discharge bleeding in ACS patients treated with PCI. In this study the optimal PNI cut-off value was 42.7, which is in line with previous studies reporting an ideal PNI threshold between 40 and 50.67 However, studies have reported non-uniform PNI thresholds for different diseases. This can be attributed to the fact that diseases have varied characteristics and pathogenesis, so, setting a uniform cut-off value for PNI may not be clinically wise. Therefore, cut-off values for PNI should be set separately for different disease conditions. Additionally, other malnutrition screening indices, such as, universal screening tool for malnutrition are not suitable to use in practical setting as the procedure of calculation is complex and also requires professional assistance.68 PNI is well suited for use in clinical settings given its easy calculation from routine parameters, albumin and lymphocyte count.
Current risk stratification tools for post-discharge bleeding after PCI, including PRECISE-DAPT,21 PARIS,23 and ARC-HBR,69 have demonstrated moderate discriminative performance (PRECISE-DAPT and PARIS: C-statistics ~0.60–0.65; ARC-HBR: C-statistics ~0.65–0.75) in predicting bleeding events across multiple cohorts. While these tools are validated and widely utilized, their reliance on clinical variables such as age, renal function, and prior bleeding history limits their predictive accuracy, particularly in capturing systemic physiological vulnerabilities.
In contrast, PNI, derived from serum albumin and lymphocyte counts, offers a novel, biomarker-driven approach to risk stratification. PNI reflects both nutritional status and systemic inflammation, which are intrinsically linked to impaired thrombogenicity, prolonged bleeding time, and increased vascular fragility, which are key pathophysiological contributors to bleeding risk in patients on dual antiplatelet therapy (DAPT). Unlike existing tools, PNI provides a holistic assessment of physiological resilience, potentially enhancing risk prediction beyond traditional clinical variables. By integrating PNI into risk stratification protocols, clinicians may better identify high-risk patients, enabling more individualized DAPT strategies and optimizing post-PCI outcomes. Thus, PNI represents a significant advancement over current methods, addressing critical gaps in bleeding risk assessment.
Therefore, we believe that utilizing PNI as a predictive index along with other risk scores such as PRECISE-DAPT (Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy) score,20 PARIS,22 or ARC-HBR69 criteria can go a long way in risk stratification of post-discharge bleeding events in ACS patients put on DAPT after treatment with PCI, thus allow clinicians to take informed decisions and direct targeted treatments accordingly.
Limitations
Single centre study, demographic component largely drawn from a particular geography (Aseer region, Kingdom of Saudi Arabia), might limit the generalization of the results. Moreover, changes in PNI post-discharge were not accounted, therefore, the effects of changes in PNI on outcomes could not be determined. All these constitute the main limitations of this study. We acknowledge that variations in sociodemographic factors, clinical characteristics, and practice patterns, such as PCI techniques and DAPT regimens, may introduce potential biases, thereby influencing outcomes and limiting external validity. While our findings provide valuable insights into the role of PNI in post-PCI bleeding risk stratification, future multicentre studies are warranted to validate these results in diverse populations and settings.
Conclusion
The baseline PNI is a significant independent predictor of post-discharge bleeding in ACS patients put on DAPT after treatment with PCI. The integration of PNI into risk stratification protocols holds significant clinical implications, particularly for optimizing post-PCI bleeding risk assessment in patients on DAPT. By incorporating PNI values, clinicians can leverage a biomarker-driven approach that reflects both nutritional deficiency and systemic inflammation, which are key pathophysiological contributors to bleeding risk. This holistic assessment could enhance the identification of high-risk patients, enabling more tailored DAPT strategies, such as de-escalation or shorter durations, thereby balancing ischemic and bleeding risks. However, to ensure broader applicability beyond the regional population studied, multicentre validation across diverse sociodemographic and clinical settings is essential. Such efforts would confirm PNI’s utility in global clinical practice, potentially transforming risk stratification paradigms and improving patient outcomes.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
This study was approved by the Research Ethics Committee at King Khalid University (HAPO-06-B-001) vide approval No: ECM #2024-3328 and written informed consent was obtained from all the participants.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for encouragement and funding this work.
Author Contributions
All authors have significantly and equally contributed to the conception, study design, execution of the work as well as to data acquisition, analysis, and interpretation. Each author actively participated in drafting, revising, and critically reviewing the manuscript. All authors approved this version for publishing and also agreed on the submission to this journal. Furthermore, all authors commit to being accountable for the integrity and accuracy of the work.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia for funding this work through Large Group Project under grant number: RPG 2/571/45.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wiviott SD, Braunwald E, McCabe CH. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi:10.1056/NEJMoa0706482
2. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi:10.1056/NEJMoa0904327
3. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation [published correction appears in]. N Engl J Med. 2001;345(7):494–502. doi:10.1056/NEJMoa010746
4. Valgimigli M, Bueno H, Byrne RA, et al. ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–260. doi:10.1093/eurheartj/ehx419
5. Baber U, Dangas G, Chandrasekhar J, et al. Time-dependent associations between actionable bleeding, coronary thrombotic events, and mortality following percutaneous coronary intervention: results from the Paris registry. JACC: Cardiovasc Interv. 2016;9(13):1349–1357. doi:10.1016/j.jcin.2016.04.009
6. Pocock SJ, Mehran R, Clayton TC, et al. Prognostic modeling of individual patient risk and mortality impact of ischemic and hemorrhagic complications. Circulation. 2010;121(1):43–51. doi:10.1161/CIRCULATIONAHA.109.878017
7. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114(8):774–782. doi:10.1161/CIRCULATIONAHA.106.612812
8. Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96(9):1200–1206. doi:10.1016/j.amjcard.2005.06.056
9. Ducrocq G, Schulte PJ, Budaj A, et al. Balancing the risk of spontaneous ischemic and major bleeding events in acute coronary syndromes. Am Heart J. 2017;186:91–99. doi:10.1016/j.ahj.2017.01.010
10. Mehran R, Pocock SJ, Stone GW, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J. 2009;30(12):1457–1466. doi:10.1093/eurheartj/ehp110
11. Ismail N, Jordan KP, Rao S, et al. Incidence and prognostic impact of post discharge bleeding post acute coronary syndrome within an outpatient setting: a systematic review. BMJ Open. 2019;9(2):e023337. doi:10.1136/bmjopen-2018-023337
12. Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient outcomes (PLATO) trial [published correction appears in]. Eur Heart J. 2011;32(23):2933–2944. doi:10.1093/eurheartj/ehr422
13. Garay A, Formiga F, Raposeiras-Roubín S, et al. Prediction of post-discharge bleeding in elderly patients with acute coronary syndromes: insights from the BleeMACS registry. Tromb Haemost. 2018;118:929–938. doi:10.1055/s-0038-1635259
14. Brinkert M, Southern DA, James MT, Knudtson ML, Anderson TJ, Charbonneau F. Incidence and prognostic implications of late bleeding after myocardial infarction or unstable angina according to treatment strategy. Cana J Cardiol. 2017;33(8):998–1005. doi:10.1016/j.cjca.2017.05.001
15. Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the global registry of acute coronary events (GRACE). Eur Heart J. 2003;24(20):1815–1823. doi:10.1016/s0195-668x(03)00485-8
16. Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55(23):2556–2566. doi:10.1016/j.jacc.2009.09.076
17. Simonsson M, Winell H, Olsson H, et al. Development and validation of a novel risk score for in-hospital major bleeding in acute myocardial infarction:-the swedeheart score. J Am Heart Assoc. 2019;8(5):e012157. doi:10.1161/JAHA.119.012157
18. Alfredsson J, Neely B, Neely ML, et al. Predicting the risk of bleeding during dual antiplatelet therapy after acute coronary syndromes. Heart. 2017;103(15):1168–1176. doi:10.1136/heartjnl-2016-310090
19. Kikkert WJ, Hassell MECJ, Delewi R, et al. Predictors and prognostic consequence of gastrointestinal bleeding in patients with ST-segment elevation myocardial infarction. Int J Cardiol. 2015;184:128–134. doi:10.1016/j.ijcard.2015.01.041
20. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389(10073):1025–1034. doi:10.1016/S0140-6736(17)30397-5
21. Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the academic research consortium for high bleeding risk. Eur Heart J. 2019;40(31):2632–2653. doi:10.1093/eurheartj/ehz372
22. Baber U, Mehran R, Giustino G, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS0. J Am Coll Cardiol. 2015;65(21):2224–2234. doi:10.1016/j.jacc.2015.03.552
23. Chichareon P, Modolo R, Kogame N, et al. Validation of the Paris bleeding score in patients undergoing percutaneous coronary intervention: insights from the GLOBAL LEADERS trial. Eur Heart J. 2019;40(23):1848–1858. doi:10.1093/eurheartj/ehz169
24. Nakanishi N, Kaikita K, Ishii M, et al. Malnutrition-associated high bleeding risk with low thrombogenicity in patients undergoing percutaneous coronary intervention. Nutr Metab Cardiovasc Dis. 2022;32(5):1227–1235. doi:10.1016/j.numecd.2022.01.016
25. Moustafa F, Dopeux L, Mulliez A, et al. Severe undernutrition increases bleeding risk on vitamin-K antagonists. Clin Nutr. 2021;40(4):2237–2243. doi:10.1016/j.clnu.2020.10.002
26. Uner A, Calişkan U, Oner AF, Koç H, Kasap AF. Platelet functions in patients with protein-energy malnutrition. Clin Appl Thromb Hemost. 2001;7(4):286–288. doi:10.1177/107602960100700406
27. Dorantes S, Barron I, Arias N, Vázquez J, Soto R. Pathogenesis of purpura in the children with severe malnutrition. J Pediatr. 1964;65(3):438. doi:10.1016/S0022-3476(64)80409-1
28. Tyagi A, Chandra J, Narayan S, Sharma D. Platelet function tests in protein energy malnutrition. Indian Pediatr. 1992;29(2):228.
29. Maia AR, Batista TM, Victorio JA, et al. Taurine supplementation reduces blood pressure and prevents endothelial dysfunction and oxidative stress in post-weaning protein-restricted rats. PLoS One. 2014;9(8):e105851. doi:10.1371/journal.pone.0105851
30. Torrens C, Kelsall CJ, Hopkins LA, Anthony FW, Curzen NP, Hanson MA. Atorvastatin restores endothelial function in offspring of protein-restricted rats in a cholesterol-independent manner. Hypertension. 2009;53(4):661–667. doi:10.1161/HYPERTENSIONAHA.108.122820
31. Sinha P, Davis J, Saag L, et al. Undernutrition and tuberculosis: public health implications. J Infect Dis. 2019;219(9):1356–1363. doi:10.1093/infdis/jiy675
32. Chandrasekaran P, Saravanan N, Bethunaickan R, Tripathy SM. Modulator of immune responses in tuberculosis. Front Immunol. 2017;8:1316. doi:10.3389/fimmu.2017.01316
33. Anuradha R, Munisankar S, Bhootra Y, et al. Coexistent malnutrition is associated with perturbations in systemic and antigen-specific cytokine responses in latent tuberculosis infection. Clin Vaccine Immunol. 2016;23(4):339–345. doi:10.1128/CVI.00009-16
34. Ferrero-Miliani L, Nielsen O, Andersen P, Girardin S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147(2):227–235. doi:10.1111/j.1365-2249.2006.03261.x
35. Chertov O, Yang D, Howard O, Oppenheim JJ. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol Rev. 2000;177(1):68–78. doi:10.1034/j.1600-065X.2000.17702.x
36. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204–7218. doi:10.18632/oncotarget.23208
37. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi:10.1016/0002-9610(80)90246-9
38. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005.
39. Lee JY, Kim HI, Kim YN, et al. Clinical significance of the prognostic nutritional index for predicting short- and long-term surgical outcomes after gastrectomy: a retrospective analysis of 7781 gastric cancer patients. Medicine. 2016;95(18):e3539. doi:10.1097/MD.0000000000003539
40. Yang Y, Gao P, Song Y, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol. 2016;42(8):1176–1182. doi:10.1016/j.ejso.2016.05.029
41. Okada S, Shimada J, Kato D, Tsunezuka H, Teramukai S, Inoue M. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg. 2017;104(1):296–302. doi:10.1016/j.athoracsur.2017.01.085
42. Sim JH, Jun IG, Moon YJ, et al. Association of preoperative prognostic nutritional index and postoperative acute kidney injury in patients who underwent hepatectomy for hepatocellular carcinoma. J Pers Med. 2021;11(5):428. doi:10.3390/jpm11050428
43. Cheng YL, Sung SH, Cheng HM, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6(6):e004876. doi:10.1161/JAHA.116.004876
44. Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. 2020;76(7):828–840. doi:10.1016/j.jacc.2020.06.058
45. Hayashi J, Uchida T, Ri S, et al. Clinical significance of the prognostic nutritional index in patients undergoing cardiovascular surgery. Gen Thorac Cardiovasc Surg. 2020;68(8):774–779. doi:10.1007/s11748-020-01300-x
46. Yuan K, Zhu S, Wang H, et al. Association between malnutrition and long-term mortality in older adults with ischemic stroke. Clin Nutr. 2021;40(5):2535–2542. doi:10.1016/j.clnu.2021.04.018
47. Faggioni M, Baber U, Afshar AE, et al. Effects of body mass index on clinical outcomes in female patients undergoing percutaneous coronary intervention with drug-eluting stents: results from a patient-level pooled analysis of randomized controlled trials. JACC: Cardiovasc Interv. 2018;11(1):68–76. doi:10.1016/j.jcin.2017.06.060
48. Sorrentino S, Baber U, Claessen BE, et al. Determinants of significant out-of-hospital bleeding in patients undergoing percutaneous coronary intervention. Thromb Haemost. 2018;118(11):1997–2005. doi:10.1055/s-0038-1673687
49. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi:10.1161/CIRCULATIONAHA.107.187397
50. Levine GN, Bates ER, Bittl JA, et al. ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2016;68(10):1082–1115. doi:10.1016/j.jacc.2016.03.513
51. Tickoo S, Bhardwaj A, Fonarow GC, Liang L, Bhatt DL, Cannon CP. Relation between hospital length of stay and quality of care in patients with acute coronary syndromes (from the American Heart Association’s get with the guidelines—coronary artery disease data set). Am J Cardiol. 2016;117(2):201–205. doi:10.1016/j.amjcard.2015.10.027
52. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736–2747. doi:10.1161/CIRCULATIONAHA.110.009449
53. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate [published correction appears in]. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
54. Jeon HG, Choi DK, Sung HH, et al. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol. 2016;23(1):321–327. doi:10.1245/s10434-015-4614-0
55. Nicogossian A, Kloiber O, Stabile B. The revised World Medical Association’s declaration of Helsinki 2013: enhancing the protection of human research Subjects and empowering ethics review committees. World Med Health Policy. 2014;6(1):1–3. doi:10.1002/wmh3.79
56. Cochran WG. Sampling Techniques.
57. Ismail N, Jordan KP, Kadam UT, Edwards JJ, Kinnaird T, Mamas MA. Bleeding after hospital discharge following acute coronary syndrome: incidence, types, timing, and predictors. J Am Heart Assoc. 2019;8(21):e013679. doi:10.1161/JAHA.119.013679
58. Bassand JP, Afzal R, Eikelboom J, et al. Relationship between baseline haemoglobin and major bleeding complications in acute coronary syndromes. Eur Heart J. 2009;31(1):50–58. doi:10.1093/eurheartj/ehp401
59. Basta G, Chatzianagnostou K, Paradossi U, et al. The prognostic impact of objective nutritional indices in elderly patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Int J Cardiol. 2016;221:987–992. doi:10.1016/j.ijcard.2016.07.039
60. Chen QJ, Qu HJ, Li DZ, et al. Prognostic nutritional index predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Sci Rep. 2017;7(1):3285. doi:10.1038/s41598-017-03364-x
61. Keskin M, Hayıroğlu MI, Keskin T, et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutr Metab Cardiovasc Dis. 2017;27(5):438–446. doi:10.1016/j.numecd.2017.01.005
62. Yoshioka G, Natsuaki M, Goriki Y, et al. Serum albumin and bleeding events after percutaneous coronary intervention in patients with acute Myocardial infarction (from the HAGAKURE-ACS registry). Am J Cardiol. 2022;165:19–26. doi:10.1016/j.amjcard.2021.10.043
63. Fatyga P, Pac A, Fedyk-łukasik M, Grodzicki T, Skalska A. The relationship between malnutrition risk and inflammatory biomarkers in outpatient geriatric population. Eur Geriatr Med. 2020;11(3):383–391. doi:10.1007/s41999-020-00303-4
64. Moysidou E, Lioulios G, Xochelli A, et al. Different types of chronic inflammation engender distinctive immunosenescent profiles in affected patients. Int J mol Sci. 2022;23(23):14688. doi:10.3390/ijms232314688
65. Wang J, Li X, Hou WJ, Dong LX, Cao J. Endothelial function and T-lymphocyte subsets in patients with overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea. Chin Med J. 2019;132(14):1654–1659. doi:10.1097/CM9.0000000000000312
66. Yoshii T, Matsuzawa Y, Kato S, et al. Endothelial dysfunction predicts bleeding and cardiovascular death in acute coronary syndrome. Int J Cardiol. 2023;376:11–17. doi:10.1016/j.ijcard.2023.01.079
67. Ma S, Zhang B, Lu T, et al. Value of the prognostic nutritional index (PNI) in patients with newly diagnosed, CD5-positive diffuse large B-cell lymphoma: a multicenter retrospective study of the Huaihai lymphoma working group. Cancer. 2022;128(19):3487–3494. doi:10.1002/cncr.34405
68. Kang MK, Kim TJ, Kim Y, et al. Geriatric nutritional risk index predicts poor outcomes in patients with acute ischemic stroke - automated undernutrition screen tool. PLoS One. 2020;15(2):e0228738. doi:10.1371/journal.pone.0228738
69. Ueki Y, Bär S, Losdat S, et al. Validation of the academic research consortium for high bleeding risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention. 2020;16(5):371–379. doi:10.4244/EIJ-D-20-00052
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Impact of Patient Adherence to Dual Antiplatelet Medication Following Percutaneous Coronary Intervention on the Occurrence of Adverse Cardiovascular Events
Mansurova JA, Orekhov A, Zhunuspekova AS, Kassymova AA, Karazhanova LK
Patient Preference and Adherence 2024, 18:425-434
Published Date: 17 February 2024