Back to Journals » Nature and Science of Sleep » Volume 16
Association of Short Sleep Duration and Obstructive Sleep Apnea with Central Obesity: A Retrospective Study Utilizing Anthropometric Measures
Authors Li Y , Lu Y, Zhao Y, Lyu Z
Received 10 July 2024
Accepted for publication 25 September 2024
Published 2 October 2024 Volume 2024:16 Pages 1545—1556
DOI https://doi.org/10.2147/NSS.S483984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Sarah L Appleton
Yi Li,1– 3,* Yixuan Lu,1,2,* Youdan Zhao,1 Zhi Lyu1,2
1Department of Senior Cadres Ward, Zhongshan Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, People’s Republic of China; 2The School of Clinical Medicine, Fujian Medical University, Fuzhou, People’s Republic of China; 3Department of General Medicine, Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhi Lyu, Department of Senior Cadres Ward, Zhongshan Hospital of Xiamen University, No. 201-209 hubin South Road, Siming District, Xiamen, 361000, People’s Republic of China, Tel +86-13779950606, Email [email protected]
Background: Central obesity, as measured by examination instruments, has been shown to be associated with both OSA and short sleep duration. However, objective measurement tools like CT, MRI, and DXA are expensive, cause radiation exposure, and have limited availability, especially in resource-limited settings. Thus, this study aimed to demonstrate the relevance of Body Mass Index (BMI) and Waist-to-Height Ratio (WHtR) as surrogate indicators of visceral obesity in the assessment of OSA and short sleep duration. We also intend to evaluate whether WHtR, in combination with BMI, can be a suitable surrogate marker for visceral adiposity.
Methods: We recruited 333 adults with complete polysomnographic (PSG) records retrospectively. Logistic regression helped to assess the association of BMI and WHtR as surrogates for central adiposity with OSA and short sleep duration. Moreover, ROC curve analysis was conducted to evaluate the predictive ability of BMI and WHtR.
Results: Following the relevant adjustments, logistic regression analysis results showed that the combination of WHtR and BMI acting as central obesity surrogates was significantly associated with OSA and short sleep duration (p< 0.05). According to univariate regression analysis, sleep latency and wake after sleep onset were independent predictors of the risk of central obesity in patients with short sleep duration and OSA. Additionally, ROC curve analysis demonstrated that the combination of BMI and WHtR provided a better assessment of central adiposity in patients with OSA and short sleep duration, compared to each measure alone.
Conclusion: BMI and WHtR are significantly associated with OSA and short sleep duration, and might serve as a potential surrogate marker for central obesity. Sleep latency and wake after sleep onset can independently predict the risk of central obesity in patients with short sleep time and OSA. Thus, larger prospective studies are needed to verify our findings.
Keywords: body mass index, central obesity, obstructive sleep apnea, short sleep duration, waist-to-height ratio
Introduction
Adequate sleep is necessary for good health. Recent recommendations from the American Academy of Sleep Medicine (AASM) state that adults require a minimum of seven hours of sleep per night.1 However, the Centers for Disease Control and Prevention (CDC) and the Maternal and Child Health Bureau (MCHB) data show that 32.5% of American adults do not follow this recommendation.1 Moreover, sleeping <6 hours per night can hamper human health.2
Obstructive Sleep Apnea (OSA) is characterized by the partial or complete collapse of the upper airway during sleep.3 Its main symptoms encompass difficulty in breathing, snoring, and excessive daytime sleepiness.4 Additionally, OSA is now recognized as a neurological disorder characterized by structural changes in the cerebral cortex.5 Studies utilizing electroencephalograms (EEGs) for OSAs have revealed that it manifests as slowed electrical activity at night.6 Approximately half of the global population is affected by OSA.7 Thus, the higher prevalence and mortality rates associated with OSA are now emerging as significant public health challenges.
Various lifestyle changes have significantly increased obesity rates.8 Correspondingly, the prevalence of OSA and short sleep duration have increased simultaneously.9 Currently, OSA is the most common sleep-related breathing disorder.10 Central obesity is a key factor for understanding the relationship between body fat distribution and metabolic dysfunction.11 Furthermore, OSA is closely associated with both central and general obesity.12 Similarly, sleep duration has also been linked to central and general obesity.13,14 Since OSA and short sleep duration display common pathophysiological effects, they may exacerbate visceral obesity by the metabolic risk.15 However, limited evidence is available on the combined effects of OSA and short sleep duration on central obesity. The potential mechanisms connecting OSA, short sleep duration, and central obesity encompass hormonal changes, increased sympathetic nerve excitability, oxidative stress, and inflammation.9 One study involving a large Chinese population demonstrated that OSA combined with short sleep duration had an additive effect on insulin resistance, which is closely linked to central obesity.16 A Korean prospective study suggested a correlation between OSA, short sleep duration, and visceral obesity; however, the visceral obesity severity was influenced by the interaction of sleep deprivation and OSA. Although visceral fat is commonly measured by CT,9 dual X-ray absorptiometry (DXA) is a well-established technology for assessing visceral fat in clinical and scientific settings. However, both these modalities are expensive, require specialized technicians, cause radiation exposure, and are often unavailable in poor rural areas and developing countries.17 Additionally, obesity causes local obstruction in the pharyngeal region, involving tissues like the tongue,18 upper airway fat,19 and parapharyngeal fat.20 Previous studies have used MRI to evaluate these areas with promising results.18,19 However, the use of MRI is limited like other advanced assessment tools. Therefore, it is crucial to identify alternative indicators for better outcomes.
Although waist circumference is the most common indicator of central obesity, it is easily influenced by factors like height, gender, and race. Conversely, the Waist-to-Height Ratio (WHtR) is minimally affected by height and gender. International standards typically use a WHtR >0.5 as the diagnostic criterion for central obesity.21 One study found that waist circumference and body mass index (BMI) are the most accurate and accessible surrogate markers of central obesity in adults.22 Furthermore, the WHtR is an optimal predictor for obesity and metabolic syndrome in Chinese adults.23 However, WHtR alone is insufficient for predicting the risk of obesity-related diseases. BMI is the most common indicator for assessing obesity. However, using BMI alone cannot accurately determine the relationship between obesity and related diseases.24
None of the studies have examined WHtR as an indicator of central obesity concerning OSA and short sleep duration to date. Few of the studies have only described the association between obesity indicators, like BMI and WHtR, as well as OSA or short sleep duration.25–30 The association between OSA and short sleep time was not evaluated and short sleep time was not considered as an independent factor. Additionally, a surrogate visceral obesity marker, visceral adiposity index (VAI), does not apply to other diseases and requires biochemical testing, thereby limiting its use.22
Currently, there is a lack of studies evaluating the association between central obesity and OSA and short sleep time through simple body measurement measures that can replace instruments such as CT. In other words, can body measurement methods replicate the effectiveness of instrument measurements in proving the association between central obesity and OSA, short sleep duration?
This study aimed to evaluate the combination of WHtR and BMI as a surrogate marker for central obesity and its relationship with OSA and short sleep duration. We also sought to determine whether WHtR plus BMI can be an effective measure for assessing OSA and short sleep duration.
Materials and Methods
Study Participants
We enrolled subjects who were registered at the Sleep Monitoring Center, Department of Respiratory and Critical Care Medicine, Zhongshan Hospital of Xiamen University from January 1, 2019, to December 31, 2021. Located on Xiamen Island, Xiamen University of Zhongshan Hospital is a large tertiary-level hospital that serves both Siming and Huli Districts. Therefore, the hospital’s patient population can be considered representative of the general population of Xiamen Island. We included consecutive adults who were suspected of having sleep-disordered breathing and underwent polysomnography (PSG) due to snoring or daytime sleepiness. The study maintained strict confidentiality of the patients’ data and was conducted in strict compliance with the Declaration of Helsinki and was approved by the Ethics Committee of the Zhongshan Hospital of Xiamen University (xmzsyyky2023-124). Informed consent was waived off due to the study’s retrospective nature. The inclusion criteria were: adults with complete nocturnal PSG data; those with fully independent behavioral as well as cognitive functioning, and patients having electronic medical history of relevant personal comorbidities. The exclusion criteria were: OSA patients who were receiving continuous positive airway pressure (CPAP) treatment; individuals in the acute phase of cardiovascular or pulmonary diseases; those with congestive heart failure, chronic renal failure, and chronic obstructive pulmonary disease (COPD), and patients with other sleep disorders like central sleep apnea or narcolepsy. The study’s flow chart is shown in Figure 1.
Sleep Monitoring Data
We followed the standard protocols recommended by the American Academy of Sleep Medicine for conducting overnight PSG. PSG data processing was done using ProFusion PSG 3 software (Beijing Zhonghe Beide Trading Co., Ltd., Beijing, China) and analyzed by certified PSG experts. Before 9:00 PM, PSG sensors were placed on all patients after their arrival. They chose their own lights-out time and were awakened at 7:00 AM. Patients were advised to go to bed earlier, and no additional activities were permitted until morning once the lights were off.
Using thermocouple readings, apneas were defined as a reduction in airflow of >90% from the baseline for >10 seconds. Hypopneas were defined as a reduction in nasal pressure airflow signal of >30% from the baseline for >10 seconds. We diagnosed OSA using the International Classification of Diseases, Tenth Revision (ICD-10: G47.3), and Ninth Revision (ICD-9: 347.2A). This diagnosis was based on the patient’s subjective symptoms as well as clinical examination and sleep study results, with an apnea-hypopnea index (AHI) of ≥5 events per hour. Subsequently, OSA severity was categorized as mild (5.0–14.9 events/hour), moderate (15.0–29.9 events/hour), or severe (≥30.0 events/hour) grades, respectively. Several patient characteristics were also collected, like the total sleep time (TST), the minimum and average oxygen saturation, sleep efficiency, sleep latency, wake after sleep onset, rapid eye movement (REM), non-rapid eye movement (NREM), the N1-N3 stages and the Epworth Sleepiness Scale (ESS).
Currently, there are no precise diagnostic criteria for determining short sleep duration. According to the American National Sleep Foundation consensus, sleep duration <6 hours was detrimental to health, and short sleep duration was defined as TST <6 hours.2
Clinical Variables and Risk Factors for Central Obesity
We collected patients’baseline demographic details from the hospital’s Jiahe Electronic Medical Record System. All clinical variables and central obesity-associated risk factors were retrospectively collected. These included demographic characteristics and lifestyle factors like age, sex, BMI, smoking history, and alcohol consumption. After reviewing medical histories, we diagnosed comorbidities like hypertension, diabetes, coronary heart disease, and hyperlipidemia. Having mean systolic and diastolic blood pressures of ≥140 mm Hg and ≥90 mm Hg, or self-reported use of antihypertensive medication indicated hypertension.31 Diabetes was diagnosed using the following criteria: fasting blood glucose level ≥7.0 mmol/L (126 mg/dL); oral glucose tolerance test showing blood glucose level ≥11.1 mmol/L (200 mg/dL), two hours post-meal, and random blood glucose level ≥11.1 mmol/L (200 mg/dL) as well as classic diabetes symptoms like polyuria, polydipsia, and unexplained weight loss. Thus, diabetes was diagnosed if any of these criteria were met.32 Coronary heart disease was considered if cardiovascular disease was caused by reduced myocardial blood flow, ie, due to coronary artery stenosis or occlusion. A serum cholesterol level of ≥240 mg/dL, a triglyceride level of ≥200 mg/dL, and a low-density lipoprotein level of ≥160 mg/dL, or when the patient was on lipid-lowering medication indicated hyperlipidemia.33 However, patients with cardiovascular disease history and those taking lipid-lowering medications to prevent cardiovascular disease recurrence were not considered hyperlipidemic.
Waist-to-Height Ratio
The Sleep Monitoring Center’s trained staff measured patients’ waist circumferences and height. These measurements were taken in the evening post-PSG. During these measurements, individuals wore light clothing, were shoeless, and stood upright with shoulders in a relaxed position. Measured in centimeters (cm), waist circumference was measured at the midpoint between the lower rib margin and the anterior superior iliac spine. Patients stood barefoot, and their height was measured in cm. Both height and waist circumference measurements were recorded with one decimal place precision. The WHtR was calculated by: WHtR = waist circumference(cm)/height(cm).
Grouping
Since all study patients were Chinese, the Chinese BMI obesity categories were employed for classification.34 The international WHtR cutoff value for diagnosing central obesity is 0.5. Based on the previous literature,35 the WHtR cutoff value was determined as 0.5. Patients were categorized into four groups based on their BMI and WHtR values: non-obese individuals (BMI<28kg/m2, WHtR<0.5), non-obese individuals with visceral fat (BMI<28kg/m2, WHtR≥0.5), non-centrally obese individuals (BMI≥28kg/m2, WHtR<0.5), and centrally obese individuals (BMI≥28kg/m2, WHtR≥0.5).
Statistical Analysis
All statistical analyses were performed by SPSS software 27.0 version (IBM, New York, USA). The normal continuous and categorical data were presented as mean ± standard deviation (SD) and percentages, respectively. Group differences in mean were assessed using a one-way analysis of variance. The control group included those non-OSA individuals who did not have short sleep duration. Multivariate logistic regression analysis helped to explore the relationships between the three BMI/WHtR-defined groups as well as OSA and short sleep duration. Moreover, REM and N3 sleep stages were considered as short-sleep factors in OSA patients,36 while wake after sleep onset and sleep efficiency were associated with sleep duration.37 Additionally, average blood oxygen levels were considered important indicators for diagnosing the severity of OSA-related hypoxia.38 Therefore, the REM stage, N3 stage, sleep efficiency, and wake after sleep onset were considered confounding variables in PSG. We included numerous variables like age, gender, height, current smoking and drinking status, diabetes, hypertension, coronary heart disease, hyperlipidemia, sleep efficiency, average blood oxygen level, wake after sleep onset, N3 stage, and REM period to account for potential confounding factors. Moreover, the association between sleep duration and OSA with central obesity, defined by WHtR and BMI, was examined by integrating TST recorded in PSG, considering OSA status, and using non-OSA individuals with normal sleep duration as the reference. Two-tailed p-values <0.05 denoted statistical significance.
Results
Table 1 shows the patients’ characteristics, grouped as per their obesity status. We included 333 patients, comprising 39 non-obese, 175 non-obese with visceral fat, 20 non-central obese, and 99 central obese individuals, respectively. The study population’s obesity and non-obesity rates were 26.7% and 73.3%, respectively. Moreover, 51.3% of patients were diagnosed with OSA, while 61.5% had short sleep duration in the non-obese cohort. Among non-obese individuals with visceral fat, 78.3% were OSA-positive, while 60.6% of them experienced short sleep duration. In the non-central obese group, 85.0% and 45.0% of patients had OSA and short sleep duration, respectively. Among the centrally obese individuals, 92.9% were diagnosed with OSA, whereas 39.4% of them had short sleep duration. Significant differences were observed in age, AHI, minimum oxygen saturation levels, and average oxygen saturation among the four patient groups (p<0.05). Even if the groups’ mean sleep durations were not significant when categorizing patients into short and normal sleepers, a correlation between short sleep duration and central fat distribution was observed (p<0.05).
 |
Table 1 The Clinical Data, Physical Measurement Data and PSG Characteristics of the Crowd |
A multivariate logistic regression model helped assess the relationship between the four BMI/WHtR-defined groups and the presence of OSA as well as short sleep duration alone. The confounding factors were: age, gender, height, current smoking and drinking status, diabetes, hypertension, coronary heart disease, hyperlipidemia, sleep efficiency, average blood oxygen level, wake after sleep onset, N3 stage, and REM period. After confounding factor adjustment, the odds ratio (OR) for short sleep duration and OSA in the centrally obese group were 4.125 (p=0.033) and 4.02 (p=0.033), compared to the non-obese group (Table 2).
 |
Table 2 Multivariate Logistic Analysis of OSA and Short Sleep Duration in Group 4 (BMI≥28kg/m2, WHtR≥0.5, Central Obesity) |
Lastly, we investigated the combined effects of sleep duration and OSA in the four BMI/WHtR groups. The individuals without OSA and normal sleep duration served as the reference patients. After adjusting for the aforementioned confounding variables, In the central obesity group, a synergistic effect of OSA and short sleep duration on the risk of central obesity was observed (Table 3, Figure 2). However, the combination of OSA and short sleep duration was significantly associated with central obesity in this group.
 |
Table 3 Multivariate Logistic Analysis of OSA and Short Sleep Duration Combinations in Group 4 (BMI≥28kg/m2, WHtR≥0.5, Central Obesity) |
Additionally, we used univariate logistic regression analysis to identify risk factors for central obesity in short sleepers plus OSA in PSG. While central obesity (BMI≥28 kg/m², WHtR≥0.5) was designated as the dependent variable, sleep latency, REM, NREM, N1-N3 stages, wake after sleep onset frequency, sleep efficiency, average as well as lowest blood oxygen levels, and ESS score were used as independent variables. Moreover, univariate logistic regression analysis revealed that sleep latency and wake after sleep onset times were independent predictors of the risk of central obesity in short sleepers combined with OSA (p<0.05, Table 4).
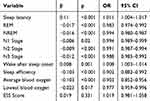 |
Table 4 Univariate Logistic Regression Results Used to Explore Independent Predictors of Central Obesity Risk in People with OSA and Short Sleep Duration |
We used the receiver operating characteristic (ROC) curve to evaluate the predictive power of BMI plus WHtR as a central obesity predictor in individuals with OSA and short sleep duration. The area under the curve (AUC) values were 0.627, 0.584, and 0.626 for BMI+WHtR, WHtR, and BMI groups, respectively. Thus, combining BMI and WHtR can provide a more reliable assessment of central obesity in patients with OSA and short sleep duration, compared to using BMI or WHtR alone (Figure 3).
 |
Figure 3 The AUCs of BMI+WHtR, WHtR and BMI. Abbreviations: BMI, Body Mass Index; WHtR, Waist-to-height ratio. |
The F-test (ANOVA method) was used to calculate the minimum sample size. With an alpha level and a power of 0.05 and 0.8, the minimum sample size was 91. Since our actual sample size was 333, we suggest that the sample was sufficient to draw reliable conclusions.
Discussion
This study is the first to explore the relationship between central obesity and OSA, as well as short sleep duration, using a comprehensive evaluation technique combining WHtR with BMI. By conducting this hospital-based cross-sectional analysis comprising 333 snoring patients, we examined the association between central obesity, defined by WHtR in conjunction with BMI, and OSA as well as short sleep duration. Our findings revealed that central obesity assessed by simple measurement measures was associated with comorbid OSA and short sleep duration, even after confounder adjustments; these findings were comparable with instrumental evaluation outcomes.
We divided our patients according to different BMI and WHtR levels to investigate the effects of fat mass and distribution on the incidence of OSA and short sleep duration. The four categories included patients with central obesity, those without central obesity, those with central fat but not obesity, and those without obesity, based on their obesity profiles. Our findings indicate a combined effect of OSA and short sleep duration on central obesity as well as align with the results of Kim et al.9 Although their study utilized CT for assessment, it is costly and similar to other body measurement indicators.39 A large-scale Chinese study showed that BMI alone cannot assess central obesity.40 In line with recommendations from World Health Organization (WHO) experts, using other body measurement indicators with BMI might offer a more comprehensive evaluation for the affected individuals.41 Our results revealed that the combination of BMI and WHtR, as a central obesity indicator, is significantly associated with OSA and short sleep duration. Therefore, clinicians can utilize these simple and accessible body measurements when advanced measurement instruments are not accessible. This offers a practical approach to identifying high-risk individuals, ensuring timely OSA treatment and sleep interventions, and reducing metabolic risk.
The relationship between OSA and central obesity is well-documented. Hence, OSA was associated with central obesity even after confounding variable adjustment in our study. Many previous studies have yielded inconsistent results after investigating the relationship between central obesity and sleep duration. A study on 9059 Chinese adults suggested that reduced sleep duration was associated with an enhanced risk of central obesity.42 Similarly, another Chinese study with 21,958 participants revealed an increased risk of central obesity in short sleep duration individuals.43 However, a prospective UK study involving 5021 individuals and a Spanish study44 found no such associations.44,45 These discrepancies might be because of variations in race, participant characteristics, gender ratios, sample sizes, or insufficient confounding factor adjustments. Moreover, we found that the combination of OSA and short sleep duration was associated with central obesity, though these effects might have been amplified by our single-center, retrospective design, and a smaller sample size.
In our study, the central obesity risk increased by 19-fold after combining OSA and short sleep duration. Central obesity is closely associated with metabolic syndrome,46 while another study showed that WHtR can effectively predict the occurrence of metabolic syndrome.44 The majority of our patients were males. One study suggested that WHtR can effectively assess obesity in men, especially when visceral fat is considered.47 Moreover, there are no studies that have used anthropometric measures to assess OSA combined with central obesity. Additionally, we also used the ROC curve to evaluate the predictive ability of combining WHtR and BMI. However, we could not establish it as a reliable alternative indicator due to our limited sample size and the study’s retrospective design. Hence, larger, prospective cohort studies are needed to confirm our results.
In PSG, the sleep latency and the wake after sleep onset frequency were determined as risk factors for central obesity in short sleepers along with OSA. However, previous studies have not examined the risk factors for central obesity in short sleep duration and OSA in PSG. Our study is the first one to provide risk factors for central obesity in short sleepers along with OSA. The arousal index is considered for measuring sleep fragmentation. In a sleep health study involving 2835 men and 2888 women, the number of wake after sleep onset was closely related to obesity.48 Although the mechanism linking sleep fragmentation and obesity is ambiguous, neuronal activity dysregulation is considered a potential mechanism.48 In case of sleep fragmentation, the body might regulate appetite by increasing the neuronal activity expressing orexin, an excitatory peptide that promotes wakefulness and feeding.49 In a multi-site cohort study, shorter sleep latency was associated with reduced risk of obesity.50 Although sleep latency increases the risk of obesity by affecting sleep efficiency,51 the pathophysiology is unclear. In one study, sleep latency increased insulin resistance by affecting sleep efficiency.52
Recent laboratory and clinical findings suggest common pathophysiological traits between OSA, short sleep duration, and obesity. Moreover, the synergistic mechanisms underlying obesity induced by OSA and short sleep duration have not yet been extensively explored. The association among OSA, short sleep duration, and obesity may stem from alterations in metabolic hormone levels, leading to diminished energy expenditure and heightened appetite.53,54 This might be because shorter sleep duration increases wakefulness and provides more opportunities for eating. Additionally, OSA patients experience hormonal changes due to fragmented sleep, and the increased work of breathing at night further elevates appetite, compounding the effect.55 Moreover, patients with both OSA and short sleep duration exhibit abnormal sympathetic nervous system activation.56,57 Hormones like leptin, implicated in both OSA and short sleep duration, might cause sympathetic nervous system activation.58 Moreover, systemic inflammation and oxidative stress are common pathophysiological features in individuals with OSA and short sleep duration.59,60 Consequently, the co-occurrence of OSA and short sleep duration exacerbates these pathophysiological changes, amplifying the onset and progression of obesity.
Interestingly, OSA might promote obesity through potential factors, like mood disorders. Additionally, OSA is associated with several mood disorders, including schizophrenia, anxiety, and depression.61–63 The association between OSA and anxiety has been verified in two recent Mendelian randomization studies.64,65 Another prospective study showed that patients undergoing OSA treatment experienced reduced anxiety.66 Moreover, anxiety, depression, and stress can greatly contribute to the development of obesity.67 Therefore, we speculate that OSA may indirectly promote the development of obesity through its association with mood disorders.
Considering the close relationship between obesity and OSA, they might share a common molecular basis. A genome-wide association study suggested that the POMC might be a common susceptibility gene in both conditions.68 Additionally, two genes, CD40LG and GZMB, may also be involved in the immune infiltration of adipocytes and the endocrine dysregulation processes observed in OSA.69 Thus, these findings offer new insights into the underlying mechanisms connecting obesity and OSA.
Due to the rising prevalence of obesity, more patients are being hospitalized and evaluated for obesity-related respiratory diseases. This phenomenon is raising concerns about the future medical burden.70 However, artificial intelligence is now playing an important role in identifying obesity-related diseases, like OSA. A recent study developed an algorithm to predict the OSA severity by incorporating clinical characteristics like age, gender, BMI, and diabetes.71,72 By using heart rate and blood oxygen data as the main features, an XGBoost algorithm-based machine learning diagnostic model was developed to accurately identify children with OSA of varying severity.73 This advancement can also lessen the future medical burden. However, as the two-way relationship between obesity and sleep disorders is gradually recognized, the medical community’s future objectives should also include timely identification of sleep disorders-associated obesity risks and minimizing the metabolic risks caused by them.55
Our study had several limitations. Since we had a retrospective cross-sectional design, establishing a clear causal relationship between short sleep duration, OSA, and WHtR was challenging. Additionally, larger confidence intervals were seen due to small sample sizes in certain subgroups when sleep duration and OSA were combined. Since the study was conducted in a hospital-based respiratory sleep monitoring center, this might have introduced the first night effect and environmental adaptation biases. Thus, future investigations might benefit from dynamic PSG conducted over several nights or the use of wearable technologies or home sleep monitoring devices for data collection. Given the bidirectional causality between OSA and obesity, as well as the possibility of reverse causality and bidirectional relationships with short sleep duration, these factors should be carefully considered.15,30 Additionally, consistent WHtR measurements across different healthcare providers with standardized training are crucial. Lastly, we did not conduct gender-based subgroup analyses, despite the predominance of males in OSA cases. Thus, additional exploration into the interplay between gender, OSA, and short sleep duration is necessary for such patients.
Conclusion
BMI combined with WHtR as a surrogate marker for central obesity is associated with OSA and short sleep duration, showing potential as a predictive marker. Sleep latency and wake after sleep onset can independently predict the risk of central obesity in patients with short sleep duration and OSA. However, due to the limitations of our study design, our results should be interpreted with caution. Hence, larger studies are needed to confirm the benefits of combining BMI with WHtR.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The author would like to thank doctors in Department of Respiratory and Critical Care Medicine, Zhongshan Hospital of Xiamen University in data collection. At the same time, the authors thank Dr. Xiaobin Zhang from the Department of Respiratory and Critical Care Medicine, Zhongshan Hospital of Xiamen University, for his guidance during the revision process of the article.
Funding
This research did not receive any specific grant from funding agencies.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ramar K, Malhotra RK, Carden KA, et al. Sleep is essential to health: an American academy of sleep medicine position statement. J Clin Sleep Med. 2021;17(10):2115–2119. doi:10.5664/jcsm.9476
2. Hirshkowitz M, Whiton K, Albert SM, et al. National sleep foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi:10.1016/j.sleh.2015.10.004
3. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi:10.1056/NEJM199304293281704
4. Su L, Chen J, Qu H, Luo C, Wu J, Jiao Y. Association between snoring frequency and male serum testosterone: findings from the 2015-2016 national health and nutrition examination survey. Sleep Med. 2022;100:1–5.
5. Feng Y, Wu J, Yuan M, Xu T, Li J, Hou D. Causal association between brain structure and obstructive sleep apnea: a Mendelian randomization study. Sleep Med. 2024;122:14–19. doi:10.1016/j.sleep.2024.07.032
6. Puskás S, Kozák N, Sulina D, Csiba L, Magyar MT. Quantitative EEG in obstructive sleep apnea syndrome: a review of the literature. Rev Neurosci. 2017;28(3):265–270. doi:10.1515/revneuro-2016-0064
7. de Araujo Dantas AB, Gonçalves FM, Martins AA, et al. Worldwide prevalence and associated risk factors of obstructive sleep apnea: a meta-analysis and meta-regression. Sleep Breath. 2023;27(6):2083–2109. doi:10.1007/s11325-023-02810-7
8. Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35(6):561–566. doi:10.4093/dmj.2011.35.6.561
9. Kim NH, Lee SK, Eun CR, et al. Short sleep duration combined with obstructive sleep apnea is associated with visceral obesity in Korean adults. Sleep. 2013;36(5):723–729. doi:10.5665/sleep.2636
10. Molnár V, Molnár A, Lakner Z, et al. Examination of the diaphragm in obstructive sleep apnea using ultrasound imaging. Sleep Breath. 2022;26(3):1333–1339. doi:10.1007/s11325-021-02472-3
11. Zhong P, Tan S, Zhu Z, et al. Normal-weight central obesity and risk of cardiovascular and microvascular events in adults with prediabetes or diabetes: Chinese and British cohorts. Diabetes Metab Res Rev. 2023;39(8):e3707. doi:10.1002/dmrr.3707
12. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. doi:10.1378/chest.09-0360
13. Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA sleep study. Am J Epidemiol. 2009;170(7):805–813. doi:10.1093/aje/kwp230
14. Loredo JS, Weng J, Ramos AR, et al. Sleep patterns and obesity: Hispanic community health study/study of latinos sueño ancillar study. Chest. 2019;156(2):348–356. doi:10.1016/j.chest.2018.12.004
15. Muscogiuri G, Barrea L, Annunziata G, et al. Obesity and sleep disturbance: the chicken or the egg? Crit. Rev. Food Sci. Nutr. 2019;59(13):2158–2165. doi:10.1080/10408398.2018.1506979
16. Xu H, Liang C, Zou J, et al. Interaction between obstructive sleep apnea and short sleep duration on insulin resistance: a large-scale study: OSA, short sleep duration and insulin resistance. Respir Res. 2020;21(1):151. doi:10.1186/s12931-020-01416-x
17. Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. doi:10.1259/bjr/38447238
18. Molnár V, Lakner Z, Molnár A, et al. Ultrasound and magnetic resonance imaging of the tongue in obstructive sleep apnoea. Appl Sci. 2022;12(19):9583. doi:10.3390/app12199583
19. Molnár V, Lakner Z, Molnár A, et al. The predictive role of the upper-airway adipose tissue in the pathogenesis of obstructive sleep apnoea. Life. 2022;12(10):1543.
20. Molnár V, Molnár A, Lakner Z, et al. The prognostic role of ultrasound and magnetic resonance imaging in obstructive sleep apnoea based on lateral oropharyngeal wall obstruction. Sleep Breath. 2023;27(1):319–328. doi:10.1007/s11325-022-02597-z
21. Liu X, He M, Li Y. Adult obesity diagnostic tool: a narrative review. Medicine (Baltimore). 2024;103(17):e37946. doi:10.1097/MD.0000000000037946
22. Borruel S, Moltó JF, Alpañés M, et al. Surrogate markers of visceral adiposity in young adults: waist circumference and body mass index are more accurate than waist hip ratio, model of adipose distribution and visceral adiposity index. PLoS One. 2014;9(12):e114112. doi:10.1371/journal.pone.0114112
23. Shao J, Yu L, Shen X, Li D, Wang K. Waist-to-height ratio, an optimal predictor for obesity and metabolic syndrome in Chinese adults. J Nutr Health Aging. 2010;14(9):782–785. doi:10.1007/s12603-010-0106-x
24. Garrow J. Body composition for the investigation of obesity. Basic Life Sci. 1990;55:183–190. doi:10.1007/978-1-4613-1473-8_26
25. Banhiran W, Junlapan A, Assanasen P, Chongkolwatana C. Physical predictors for moderate to severe obstructive sleep apnea in snoring patients. Sleep Breath. 2014;18(1):151–158. doi:10.1007/s11325-013-0863-y
26. Unal Y, Ozturk DA, Tosun K, Kutlu G. Association between obstructive sleep apnea syndrome and waist-to-height ratio. Sleep Breath. 2019;23(2):523–529. doi:10.1007/s11325-018-1725-4
27. Yazdanpanah MH, Farjam M, Naghizadeh MM, Jedi F, Mohebi K, Homayounfar R. Sleep duration and anthropometric indices in an Iranian population: the fasa PERSIAN cohort study. Sci Rep. 2021;11(1):16249. doi:10.1038/s41598-021-95796-9
28. Gu M, Liu CC, Hsu CC, et al. Associations of sleep duration with physical fitness performance and self-perception of health: a cross-sectional study of Taiwanese adults aged 23-45. BMC Public Health. 2021;21(1):594. doi:10.1186/s12889-021-10636-9
29. Kim JH, Koo YC, Cho HJ, Kang JW. Relationship between various anthropometric measures and apnea-hypopnea index in Korean men. Auris Nasus Larynx. 2018;45(2):295–300. doi:10.1016/j.anl.2017.05.005
30. Koolhaas CM, Kocevska D, Te Lindert BHW, et al. Objectively measured sleep and body mass index: a prospective bidirectional study in middle-aged and older adults. Sleep Med. 2019;57:43–50. doi:10.1016/j.sleep.2019.01.034
31. Bakris G, Ali W, Parati G. ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J Am Coll Cardiol. 2019;73(23):3018–3026. doi:10.1016/j.jacc.2019.03.507
32. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–s27. doi:10.2337/dc18-S002
33. Joint Committee on the Revision of the Guidelines for the Prevention and Treatment of Dyslipidemia in Chinese Adults. Chinese guideline for the management of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44(10):833–853. doi:10.3760/cma.j.issn.0253-3758.2016.10.005
34. Wang Y, Mao L, Zhang X. Waist-hip ratio is an independent predictor of moderate-to-severe OSA in nonobese males: a cross-sectional study. BMC Pulm Med. 2022;22(1):151. doi:10.1186/s12890-022-01886-3
35. Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13(3):275–286. doi:10.1111/j.1467-789X.2011.00952.x
36. Nozawa S, Urushihata K, Machida R, Hanaoka M. Sleep architecture of short sleep time in patients with obstructive sleep apnea: a retrospective single-facility study. Sleep & Breathing = Schlaf & Atmung. 2022;26(4):1633–1640. doi:10.1007/s11325-021-02533-7
37. Harrison EI, Roth RH, Lobo JM, et al. Sleep time and efficiency in patients undergoing laboratory-based polysomnography. J Clin Sleep Med. 2021;17(8):1591–1598. doi:10.5664/jcsm.9252
38. Suen C, Ryan CM, Mubashir T, et al. Sleep study and oximetry parameters for predicting postoperative complications in patients with OSA. Chest. 2019;155(4):855–867. doi:10.1016/j.chest.2018.09.030
39. Lee YG, Lee YJ, Jeong DU. Differential effects of obesity on obstructive sleep apnea syndrome according to age. Psychiatry Invest. 2017;14(5):656–661. doi:10.4306/pi.2017.14.5.656
40. Zeng Q, Wang L, Dong S, et al. CT-derived abdominal adiposity: distributions and better predictive ability than BMI in a nationwide study of 59,429 adults in China. Metabolism. 2021;115:154456. doi:10.1016/j.metabol.2020.154456
41. Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO expert consultation on waist circumference and waist-hip ratio. Eur J Clin Nutr. 2010;64(1):2–5. doi:10.1038/ejcn.2009.139
42. Fan Y, Zhang L, Wang Y, et al. Gender differences in the association between sleep duration and body mass index, percentage of body fat and visceral fat area among Chinese adults: a cross-sectional study. BMC Endocr Disord. 2021;21(1):247. doi:10.1186/s12902-021-00913-4
43. Ning X, Lv J, Guo Y, et al. Association of sleep duration with weight gain and general and central obesity risk in Chinese adults: a prospective study. Obesity. 2020;28(2):468–474. doi:10.1002/oby.22713
44. Pouragha H, Amiri M, Saraei M, Pouryaghoub G, Mehrdad R. Body impedance analyzer and anthropometric indicators; predictors of metabolic syndrome. J Diabetes Metab Disord. 2021;20(2):1169–1178. doi:10.1007/s40200-021-00836-w
45. Stranges S, Cappuccio FP, Kandala NB, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II study. Am J Epidemiol. 2008;167(3):321–329. doi:10.1093/aje/kwm302
46. Chen GP, Qi JC, Wang BY, et al. Applicability of visceral adiposity index in predicting metabolic syndrome in adults with obstructive sleep apnea: a cross-sectional study. BMC Pulm Med. 2016;16(1):37. doi:10.1186/s12890-016-0198-0
47. Moltrer M, Pala L, Cosentino C, Mannucci E, Rotella CM, Cresci B. Body mass index (BMI), waist circumference (WC), waist-to-height ratio (WHtR) e waist body mass index (wBMI): which is better? Endocrine. 2022;76(3):578–583. doi:10.1007/s12020-022-03030-x
48. Zhao B, Sun S, He X, Yang J, Ma X, Yan B. Sleep fragmentation and the risk of obesity: the sleep heart health study. Obesity. 2021;29(8):1387–1393. doi:10.1002/oby.23193
49. Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252(2):125–141. doi:10.1530/JOE-21-0155
50. Hawkins MS, Pokutnaya DY, Duan D, et al. Associations between sleep health and obesity and weight change in adults: the daily24 multisite cohort study. Sleep Health. 2023;9(5):767–773. doi:10.1016/j.sleh.2023.03.006
51. Kanagasabai T, Dhanoa R, Kuk JL, Ardern CI. Association between sleep habits and metabolically healthy obesity in adults: a cross-sectional study. J Obes. 2017;2017:5272984. doi:10.1155/2017/5272984
52. Hashemipour S, Ghorbani A, Khashayar A, Olfati H. Association of sleep quality with insulin resistance in obese or overweight subjects. Sleep Sci. 2021;14(Spec 1):75–78. doi:10.5935/1984-0063.20200084
53. Sanner BM, Kollhosser P, Buechner N, Zidek W, Tepel M. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur Respir J. 2004;23(4):601–604. doi:10.1183/09031936.04.00067804
54. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi:10.1371/journal.pmed.0010062
55. Ong CW, O’Driscoll DM, Truby H, Naughton MT, Hamilton GS. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med Rev. 2013;17(2):123–131. doi:10.1016/j.smrv.2012.05.002
56. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi:10.1172/JCI118235
57. Spiegel K, Leproult R, L’Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–5771. doi:10.1210/jc.2004-1003
58. Simonds SE, Pryor JT, Ravussin E, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159(6):1404–1416. doi:10.1016/j.cell.2014.10.058
59. Ferrie JE, Kivimäki M, Akbaraly TN, et al. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II study. Am J Epidemiol. 2013;178(6):956–961. doi:10.1093/aje/kwt072
60. Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121(8):1014–1021. doi:10.1161/CIRCULATIONAHA.109.900357
61. Wu YY, Chang ET, Yang YC, Chen SF, Hsu CY, Shen YC. Risk of obstructive sleep apnea in patients with schizophrenia: a nationwide population-based cohort study. Soc Psychiatry Psychiatr Epidemiol. 2020;55(12):1671–1677. doi:10.1007/s00127-020-01870-4
62. Pan ML, Tsao HM, Hsu CC, et al. Bidirectional association between obstructive sleep apnea and depression: a population-based longitudinal study. Medicine. 2016;95(37):e4833. doi:10.1097/MD.0000000000004833
63. Diaz SV, Brown LK. Relationships between obstructive sleep apnea and anxiety. Curr Opin Pulm Med. 2016;22(6):563–569. doi:10.1097/MCP.0000000000000326
64. Wang X, Song S, Dong N, et al. The causal relationship between depression and obstructive sleep apnea: a bidirectional Mendelian randomization study. J Psychosom Res. 2024;179:111620. doi:10.1016/j.jpsychores.2024.111620
65. Liu H, Wang X, Feng H, et al. Obstructive sleep apnea and mental disorders: a bidirectional Mendelian randomization study. BMC Psychiatry. 2024;24(1):304. doi:10.1186/s12888-024-05754-8
66. Maniaci A, Ferlito S, Lechien JR, et al. Anxiety, depression and sleepiness in OSA patients treated with barbed reposition pharyngoplasty: a prospective study. Eur Arch Otorhinolaryngol. 2022;279(8):4189–4198. doi:10.1007/s00405-022-07369-9
67. Wurtman J, Wurtman R. The Trajectory from Mood to Obesity. Curr Obes Rep. 2018;7(1):1–5. doi:10.1007/s13679-017-0291-6
68. Patel SR. Shared genetic risk factors for obstructive sleep apnea and obesity. J Appl Physiol (1985). 2005;99(4):1600–1606. doi:10.1152/japplphysiol.00501.2005
69. Ming X, Cai W, Li Z, et al. CD40LG and GZMB were correlated with adipose tissue macrophage infiltration and involved in obstructive sleep apnea related metabolic dysregulation: evidence from bioinformatics analysis. Front Genet. 2023;14:1128139. doi:10.3389/fgene.2023.1128139
70. Meurling IJ, Shea DO, Garvey JF. Obesity and sleep: a growing concern. Curr Opin Pulm Med. 2019;25(6):602–608. doi:10.1097/MCP.0000000000000627
71. Maniaci A, Riela PM, Iannella G, et al. Machine learning identification of obstructive sleep apnea severity through the patient clinical features: a retrospective study. Life. 2023;13(3):702. doi:10.3390/life13030702
72. Molnár V, Kunos L, Tamás L, Lakner Z. Evaluation of the applicability of artificial intelligence for the prediction of obstructive sleep apnoea. Appl Sci. 2023;13(7):4231. doi:10.3390/app13074231
73. Ye P, Qin H, Zhan X, et al. Diagnosis of obstructive sleep apnea in children based on the XGBoost algorithm using nocturnal heart rate and blood oxygen feature. Am J Otolaryngol. 2023;44(2):103714. doi:10.1016/j.amjoto.2022.103714
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



