Back to Journals » Infection and Drug Resistance » Volume 18
Bacteremia in the Gulf Cooperation Council Region: A Review of the Literature 2013–2023
Authors Al-Musawi T , Al-Agha R, Al-Khiami S, Al-Shamari H, Baghdadi M, Bosaeed M , Abdel Hadi H, Mady A, Sabra N
Received 27 September 2024
Accepted for publication 31 March 2025
Published 7 May 2025 Volume 2025:18 Pages 2329—2355
DOI https://doi.org/10.2147/IDR.S497241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Tariq Al-Musawi,1,2 Rawan Al-Agha,3 Safaa Al-Khiami,4 Hussain Al-Shamari,5 Malak Baghdadi,6 Mohammad Bosaeed,7– 9 Hamad Abdel Hadi,10,11 Ahmed Mady,12,13 Nisrine Sabra14
1Department of Critical Care Medicine, Dallah Hospital, Al-Khobar, Saudi Arabia; 2Department of Medicine, Royal College of Surgeons in Ireland-Medical University of Bahrain, Manama, Bahrain; 3Internal Medicine Department, Salmaniya Medical Complex-Governmental Hospitals, Manama, Kingdom of Bahrain; 4Infectious Disease Department, Ibrahim bin Hamad Obaidullah Hospital, Ras Al-Khaimah, United Arab Emirates; 5Microbiology Unit, Sabah Al-Ahmad Urology Center, Kuwait City, Kuwait; 6Medical Affairs, Pfizer, Jeddah, Saudi Arabia; 7Department of Medicine, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia; 8Department of Medicine, College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia; 9Department of Infectious Diseases Research, King Abdullah International Medical Research Center, Riyadh, Saudi Arabia; 10Division of Infectious Diseases, Communicable Diseases Centre, Hamad Medical Corporation, Doha, Qatar; 11College of Medicine, Qatar University, Doha, Qatar; 12Critical Care Department, King Saud Medical City, Riyadh, Saudi Arabia; 13Department of Anesthesiology and ICU, Tanta University Hospitals, Tanta, Egypt; 14Medical Affairs, Pfizer, Dubai Media City, Dubai, United Arab Emirates
Correspondence: Tariq Al-Musawi, Dallah Hospital, Al-Khobar, Saudi Arabia, Email [email protected]
Abstract: Bloodstream infections (BSIs) are amongst the leading healthcare-associated infections (HCAIs), and their comprehensive evaluation and management are of global and regional importance. This narrative review examines and reports data on BSIs from the Gulf Cooperation Council (GCC) region covering the period between 2013 and 2023. The reviewed literature demonstrated that BSIs were frequently associated with critical care settings such as the Intensive Care Unit (ICU) and were often associated with invasive lines and devices [such as central-line associated BSI (CLABSI)]. Fever was the main presenting symptom, while diabetes mellitus and hypertension were the common associated comorbidities. High mortality rates were reported for BSIs, particularly when caused by multidrug-resistant (MDR) Gram-negative pathogens. There was a wide range of antimicrobial resistance rates reported across the region; however, carbapenem-resistance rates exceeding 30% were reported for Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae. Few publications included molecular mechanisms of carbapenem resistance; however, when mechanisms were reported they were dominated by OXA-48. In conclusion, the lack of structured surveillance programs and networks to monitor microbiological phenotypic and genotypic patterns as well as clinical outcomes across the region means there is paucity of uniform data on BSIs across the GCC region. To bridge this gap, we recommend timely surveillance programs for the monitoring of resistance and outcomes.
Keywords: bacteremia, bloodstream infection, Gulf, review, antimicrobial
Introduction
Bacteremia is defined as the presence of viable bacteria in the bloodstream acquired from a variety of sites, such as the urinary tract, respiratory system, or through the breach of gastrointestinal mucosa or skin and soft tissue barriers.1 The resulting bacteremia might be transient and asymptomatic, where the body clears the bacterial burden without interventions with no subsequent harm, or may lead to an established bloodstream infection (BSI).1 BSI is facilitated through specific bacterial virulence factors that allow the transmission of pathogens to the bloodstream, evading multiple defense barriers and subsequent survival to manifest as clinical disease.2 Globally, BSIs are considered a significant healthcare burden with an estimation of 575,000–677,000 episodes annually in North America and greater than 1,200,000 in Europe, as well as being ranked amongst the top seven causes of mortality in North America and Europe.3 The clinical presentation of BSIs is variable since it can present as either primary bacteremia with direct invasion into the bloodstream, or secondary, where the infection is related to other primary infection sites such as urinary or respiratory tracts.1,4,5 One of the important features of BSIs is the ability of the bacteria to infect distant organs through hematogenous spread, seeding to remote sites such as cardiac valves, bone tissues and brain parenchyma, causing infections such as infective endocarditis, osteomyelitis and brain abscesses.6,7 Central lines are also associated with BSI and for the correct evaluation of central-line associated infections (CLABSIs), the Centers for Disease Control and Prevention (CDC) guidance advocates establishing appropriate eligibility criteria: presence of a central line for more than two consecutive days and the isolation of pathogens consistent with CLABSI.4
The hallmark for the initial diagnosis of a BSI is a positive blood culture, used to confirm the presence of bacteremia; therefore, the evaluation can be confounded by the spurious contamination by skin commensals such as coagulase-negative staphylococci and Corynebacterium spp.4,8 Such possible contamination should be carefully evaluated before the initiation of appropriate management. Major causes of Gram-positive bacteremia are Staphylococcus aureus, coagulase-negative staphylococci as well as Streptococcus and Enterococcus species.9 Bacteremia caused by Gram-negative bacteria are dominated by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii. In contrast to Gram-positive bacteremia, the appropriate management of Gram-negative bacteremia can be difficult due to the escalating challenges of antimicrobial resistance (AMR).10 Multidrug-resistant (MDR) pathogens are a prominent challenge especially in healthcare-associated infections (HCAIs) and amongst patients with multiple comorbidities and in critical care settings.5,11 In general, BSIs are associated with significant morbidity and mortality,12 which is amplified in the presence of MDR particularly if associated with HCAIs.
Risk factors associated with a BSI include comorbidities such as diabetes mellitus and an immunocompromised state such as patients with organ transplants, recipients of chemotherapy or people living with HIV.13–16 In addition, patients with organ dysfunction such as heart failure, chronic respiratory and end stage renal disease (particularly those on assisted supportive devices or receiving dialysis) are at increased risk.15,16 Old age and being resident in a long-term facility are independent risks for developing a BSI with directly increased mortality.15,16 Although BSIs can be insidious, typical symptoms include fever, chills and rigors accompanied by raised inflammatory markers and are associated with tachypnoea and hypotension.
As the effective treatment of bacterial BSI is of global and regional importance, focus has been directed towards improving surveillance and monitoring. In the Gulf Cooperation Council (GCC) region, expert working groups have been deliberating to consolidate appropriate therapeutic recommendations and specific guidance considering regional challenges such as variability of local infrastructure and resistance patterns, the availability of novel antimicrobials, and expertise.17 This narrative study will review data on non-transient bacteremia (BSI) from the GCC region published between 2013 and 2023. The alphabetically arranged region includes Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates.
Methods
Search Strategy
A literature search was conducted for English-language articles published between May 2013 and May 2023 using medical search engines PubMed (Medline®), PubMed Central and Embase®. Search protocol terms included “bacteraemia”, “bacteremia”, “septicaemia”, “septicemia”, “bloodstream infection”, “blood-stream infection” in combination with “Bahrain”, “Kuwait”, “Oman”, “Qatar”, “Saudi Arabia”, “KSA”, “United Arab Emirates”, “UAE”, “Gulf Cooperation Council” and “GCC”. No patient age restrictions were applied to the searches. Article types included in the searches were full articles, reviews, randomized clinical trials, brief communication, regional and national reports as well as case series. Letters, editorials, notes and conference abstracts were excluded.
Literature from the search results was collated into an Excel database, which catalogued, by country, publication title, authors, journal title and volume, publication year and abstract. The publication titles and abstracts in the database were then screened to identify suitable literature for inclusion. From the identified publications, the full text was collected and reviewed to assess eligibility for inclusion in the review, and a final list of publications was drawn up. Articles were excluded if they reported on sepsis (defined as organ dysfunction caused by a dysregulated host response to infection)18 without reporting BSIs, or were from neighboring regions or countries without specifically focusing on the GCC countries. Articles were similarly excluded if they were a case series reporting less than 10 cases.
Outcomes
Outcomes of interest included the prevalence, diagnosis and epidemiology of BSI in the region, risk factors associated with BSIs, clinical outcomes and mortality, causal pathogens, antimicrobial susceptibility and treatment. Antimicrobial susceptibility data were only included if data were presented on at least 10 isolates.
A summary of the literature captured for this review, by country and outcome, is presented in Supplemental Table 1.
Prevalence of BSI in the GCC Region
Literature from the region shows that BSIs are prominent HCAIs particularly in intensive care settings such as ICUs19–21 and are often device-associated.19–23 In Kuwait, between 2018 and 2019 the most frequently occurring HCAI reported in the ICU were BSI (42.3%), followed by pneumonia (28.8%), UTI (15.3%) and skin and soft tissue infections (SSTI) (9.6%).19 Abulhasan et al24 reported a rate of HCAI of 11.9/100 patients (22.1/1000 ICU days) among neurocritical care patients in a study from a tertiary care hospital in Kuwait between 2015 and 2017. Among these HCAIs, the most common infection type was UTI (36.7% [40/109]), followed by BSI (27.5% [30/109]) and pneumonia (14.7% [16/109]); of the 30 patients with BSI in the study, 66.7% (20/30) were CLABSIs. Similarly, in a point prevalence survey in Saudi Arabia (from September to December 2016), among 184 HCAIs, surgical site infections were the most common (32.6%), followed by BSI (19.5%).25 In a second point prevalence study, from 2017, the most common infection types among 114 HCAIs were identified as pneumonia (27.2%), UTIs (20.2%) and BSI (10.5%);21 the same study also identified that 19.2% of HCAI were device-associated. Gupta et al23 reported the most frequent HCAIs in critical care as device-associated. Furthermore, Saleem et al26 have reported that, among culture positive HCAI, CLABSIs were the third most common infection in the ICU, after ventilator-associated pneumonia and catheter-associated urinary tract infections.
Two studies focused on infections caused by A. baumannii. Saleem et al27 found CLABSI was the third most common A. baumannii infection in 2019 (11.4%, 4/35) but accounted for 4.3% (2/47) of A. baumannii infections in 2020. In the second study, of 321 patients, the most common infection types were respiratory (58.6%) and SSTI (29.3%), followed by BSI (8.6%) and UTI (2.1%).28 In the case of carbapenem-resistant Enterobacterales infections, BSI was more prevalent with 40.7% (77/189) reported as BSI, followed by pneumonia (23.8%, 45/189) and complicated UTI (23.8%, 45/189).29 Conversely, in a retrospective matched case-controlled study among children (≤17 years old) with carbapenem-resistant Enterobacterales infections in Saudi Arabia in 2016–2017, only 10.5% (2/19) of patients had BSI; the majority of infections were related to bodily fluids (eg, pleural, pericardial, cerebrospinal, etc) and wound discharge (9/19, 47.4%) or urinary tract (8/19, 42.1%).30 Among patients in a children’s hospital with infections due to Stenotrophomonas maltophilia, the main infection types were pneumonia (45.8%, 22/48) and BSI (29.2%, 14/48).31
Two studies from Saudi Arabia reported SSTI as the most common infection associated with Gram-positive organisms. In a study of MRSA, the majority of infections were SSTIs, followed by BSIs and lower respiratory tract infections,32 and among patients with infections caused by Streptococcus anginosus group organisms, the most common infection types were SSTIs (55.2%, 58/105) followed by IAIs (23.8%, 25/105) and BSIs (14.3%, 15/105).33 In their retrospective cohort study of adult patients with BSIs caused by enterococci between 2009 and 2018 from Qatar, Ali et al20 determined that the most common sources of BSIs were central-line infections (18.3% 48/263); however, in 119 cases (45.2%) there was no identified source of infection.
A number of studies looked at immunocompromised patients. Among adult febrile neutropenic cancer patients in Saudi Arabia, the most common infection was BSI (33.3%, 46/138) followed by UTIs (29.7%, 41/138) and wounds (19.6%, 27/138).34 Al-Mulla et al35 reported on the rates of bacteremia among pediatric oncology patients between 2004 and 2011 in Qatar and found that the rates among patients with acute lymphoblastic and acute myelocytic leukemia (33/66, 50.0% and 5/9, 55.6%, respectively) were significantly higher than among patients with lymphoma, solid and brain tumors (12/39, 30.8%; 14/56, 25.0%; 6/15, 40.0%, respectively) (P = 0.038).
Similarly, among febrile patients from Saudi Arabia aged <12 years with sickle cell disease who had up-to-date vaccination and were in receipt of penicillin prophylaxis, the overall rate of serious bacterial infection was reported as 3.6% (30/833) with 11 (1.3%) cases of bacteremia.36
Central Line–Associated Bloodstream Infections (CLABSI)
Rates of CLABSIs reported in the region are shown in Table 1. In their study of CLABSIs across Saudi Arabia, Bahrain and Oman, Balkhy et al37 reported a rate of 3.1 per 1000 central-line days (95% CI, 2.8–3.3) across age groups and ward types (Table 1).
 |
Table 1 Rates of Central-Line Associated Bloodstream Infections (CLABSI) Reported in the GCC Region, by Age Group |
Use of Care Bundles
A number of studies in the region have reported on efforts made to reduce CLABSIs through the use of care bundles.60 A care bundle is a set of evidence-based interventions performed collectively to improve patient outcomes and, in the case of CLABSIs, includes hand hygiene, skin antisepsis, barrier precautions (eg, sterile gloves and gowns) and timely removal of the central line.48 A number of studies across the region have reported a successful reduction in rates of CLABSIs through the use of care bundles (Table 1),39,41–45,47–49,54,56,57 with Khalid et al56 reporting a 15-month period with no CLABSIs following the introduction of care bundles. Alrebish et al59 attributed their low rate of CLABSI (2.6/1000 central-line days) to the 100% compliance with the CLABSI bundle, and Yaseen et al42 reported a change from 2.0/1000 central-line days with 37% bundle compliance in 2008 to 0.0/1000 central-line days with 98.0–100% bundle compliance in 2014–2015. In a study in ICUs across the United Arab Emirates (UAE), the introduction of a number of interventions resulted in a reduction in CLABSIs from 2.56/1000 catheter-days to 1.79/1000 (Table 1).39 Interventions in the study by Latif et al39 included a focus on collaboration, training, a checklist of evidence-based practices, safety program and performance feedback as described in the Keystone ICU Project.61 Hand hygiene interventions have also been shown to be effective in reducing the incidence of CLABSI (Table 1).38,58
The importance of infection control procedures was shown in a study of an outbreak of bacteremia in Saudi Arabia caused by Burkholderia cepacia-contaminated ultrasound probe gel.62 Also, rates of CLABSI were reported to have increased in Saudi Arabia during the COVID-19 pandemic (from 2.16/1000 central-line days in 2019 [pre-pandemic] to 2.50/1000 central-line days in 2020–2021 [during the pandemic], P = 0.01), which the authors suggested was due to the impact of the pandemic on infection control practices and surveillance.52
Pediatric Patients
Al-Tawfiq et al50 reported a higher rate of CLABSI in pediatric and neonatal ICUs than seen in adult ICUs (3.38/1000 central-line days, 2.28/1000 central-line days and 1.21–2.13/1000 central-line days, respectively, Table 1). A retrospective, observational study from Qatar (January 2019 – June 2020) looked at the impact of introducing closed intravenous administration sets in the neonatal ICU on the incidence of CLABSIs.55 The study reported the rate of CLABSIs dropped from 2.87/1000 central-line days pre-intervention to a post-intervention rate of 0.22/1000 central-line days and concluded that compliance with infection control bundles and the use of pre-assembled closed intravenous administration sets may have a positive impact on CLABSI rates. Among children receiving parenteral nutrition, Al Lawati et al53 reported a CLABSI rate of 14/1000 central-line days (Table 1) and a mortality rate of 25% among patients with a CLABSI. They concluded that the introduction of care bundles, which were not widely utilized at their hospital, would be a useful tool to reduce the rates of CLABSIs.
As part of efforts to reduce CLABSI, Bayoumi et al63 carried out a retrospective observational study to look at the impact of antimicrobial-impregnated peripherally inserted central catheters (PICCs) versus conventional PICCs in a neonatal ICU in Qatar between 2017 and 2020. When comparing the two PICCs, they found no difference in the rates of CLABSI; however, they did observe a higher rate of elective removal after completion of therapy when the antimicrobial-impregnated PICC was used.
Central Line Location
There is debate on whether the access location of a central venous catheter (CVC) impacts the chance of developing a BSI. Alhazmi et al64 reported on adult hemodialysis patients in Saudi Arabia, among whom 90 had arteriovenous fistulas for vascular access and 69 had a CVC. In the arteriovenous fistula group, three (3/90, 3.3%) patients developed a BSI and in the CVC group 18 (18/69, 26.1%) patients developed a BSI, and the authors concluded that the type of vascular access was a risk factor for developing BSIs in hemodialysis patients.64 In an analysis comparing femoral lines with internal jugular in pediatric patients, Al-Sofyani et al65 found that femoral lines were 4.67 times more likely to be associated with a CLABSI, although the difference was not statistically significant. Al-Khawaja et al47 reported that the most common site among adult patients who developed a CLABSI was the femoral vein, followed by the jugular vein.
Secondary BSIs
Although central lines are a common source of BSI, other infection sites can also lead to a BSI, including genitourinary, SSTI, gastrointestinal tract, bone and joint, reproductive tract and the central nervous system.20 While endovascular infections were the most common source of infection among adults with MRSA bacteremia in a tertiary care center in Saudi Arabia (30.4% of cases), 19.6% of patients had pneumonia and 19.0% had SSTIs.66 Arabi et al67 reported that among renal transplant patients who developed a first UTI within the first 6 months after transplant, the UTIs were complicated with bacteremia in 6.2% (6/97) of cases. In a study assessing the impact of the timing of ureteric stent removal in renal transplant recipients on UTI rates, UTI recurrence and hospitalization, the occurrence of UTIs while stents were still in place was associated with bacteremia and hospitalization.68
Diagnosis of BSIs
Signs and Symptoms
Fever is the symptom most commonly reported for patients with BSIs although a great deal of the reported evidence in the region is for BSI caused by Gram-positive bacteria.69–71 In a retrospective study of pediatric and adult patients with Group A streptococcal bacteremia in Saudi Arabia between 2007 and 2015, common symptoms of bacteremia among the 33 patients included fever (69.7%), cough (48.5%), vomiting (33.3%) and tachycardia (30.3%).69 El-Kady et al70 assessed symptoms of BSIs according to the causative organism among hemodialysis patients, concluding that fever was more common with S. aureus (20.9%) but rigors were more common with coagulase-negative staphylococci (19.4%). They also found that, for BSIs caused by Gram-negative bacilli, the percentage of patients who presented with fever, rigors, vomiting or hypotension was similar (6.5%, 7.2%, 8.6%, and 7.9%, respectively).70
For bacteremia due to the Gram-negative S. maltophilia among pediatric patients in Saudi Arabia, the most common signs and symptoms were fever (67.6% of patients) and respiratory symptoms (38.2%).72 Of note, the study included polymicrobial infections with the other organisms identified as Enterobacter spp., Acinetobacter spp., Pseudomonas spp., Klebsiella spp., Enterococcus spp., coagulase-negative staphylococci and streptococci.
Microbiology Laboratory methods
Accurate microbiological identification is essential as sample contamination can complicate the diagnosis of bacteremia. Skin commensals, in particular coagulase-negative staphylococci, can be identified in blood culture samples but occur because of the contamination during sample collection, not because they are true pathogens.8 The US National Healthcare Safety Network has defined false-positive blood infections as the culture of commensal skin bacteria such as coagulase-negative staphylococci, Aerococcus spp, Micrococcus spp., Propionibacterium spp., some species of Bacillus spp. (excluding Bacillus anthracis), Corynebacterium spp. or viridans group streptococci from a single blood culture (only one bottle in a series).4 Studies in the GCC region have described steps taken to identify true BSIs. Al-Otaibi et al73 defined bacteremia as isolates of the same bacterial species from at least one set of blood cultures (ie, from two bottles taken at the same time). Nasef et al74 described a contaminant as a single positive coagulase-negative staphylococci culture from two simultaneous blood samples. The same study also concluded that the use of multiplex PCR-based blood culture identification reduced the amount of time to organism identification and time to appropriate antimicrobial therapy; however, there was no significant change in mortality rate, recurrence of bacteremia, length of stay, ICU admission or cost when compared with conventional culture methods.74
The Clinical and Laboratory Standards Institute (CLSI) guidance states that healthcare facilities should aim for ≤1% blood culture contamination rate.75
Risk Factors for Acquiring a BSI
Adults
Significant risk factors for the development of a CLABSI in the adult ICU include heart failure, infection (eg, respiratory or urinary tract), pressure ulcers and tracheostomy.51 In a retrospective study of adult patients (defined in the study as >14 years) in a single tertiary center in Saudi Arabia, the predictors of bacteremia were determined as length of hospitalization (OR, 1.30; 95% CI, 1.14–1.48), presence of a central line (OR, 1.37; 95% CI 1.21–1.55) and a lactic acid concentration of ≥2 mmol/L (OR, 1.31; 95% CI 1.13–1.52).76 Risk factors for acquiring an MDR Pseudomonas aeruginosa infection include the presence of an invasive device, healthcare contact, antimicrobial exposure within 90 days of developing the infection and previous isolation of P. aeruginosa.77 In the case of MDR A. baumannii BSIs, risk factors among patients in the ICU were the presence of an intravascular device or a higher Sequential Organ Failure Assessment (SOFA) score.78 Al-Dorzi et al79 reported that most patients in their ICU study of BSIs due to Acinetobacter spp. had a CVC (98.3%) and received mechanical ventilation (59.3%). Among cancer patients with bacteremia caused by Gram-negative bacteria in Saudi Arabia, Al-Otaibi et al73 reported that 54% were hematological and 46% were solid tumors, and the risk factors for developing bacteremia included admission to the ICU and the presence of a central line. Most patients in the study were >18 years (49/56).
Regarding Gram-positive organisms, factors that were independently associated with bacteremia due to coagulase-negative staphylococci in Saudi Arabia included the presence of a CVC (P ≤ 0.0001), prior antibiotic therapy (P ≤ 0.0001), more than one positive blood culture (P ≤ 0.0001) and admission to the ICU (P = 0.007).8
Age has also been shown to be a factor, with Bandy et al80 reporting that the majority of BSIs in their study were in patients ≥60 years of age (132/222, 59.5%), with 47/222 (21.2%) coming from patients 41–59 years, 29/222 (13.1%) from patients 21–40 years of age and 14/222 (6.3%) from patients ≤20 years.
Neonates and Pediatric Patients
Using a multivariate logistic regression analysis, Bayoumi et al63 found that gestational age at birth, insertion technique and number of insertion attempts were associated with more CLABSIs among neonates.
Comorbid Conditions
Diabetes mellitus has been reported as a common underlying condition for both Gram-positive and Gram-negative BSIs,66,69,71,77,81,82 as well as CLABSI among dialysis patients,83 and hemodialysis-related bacteremia.84 Hypertension has also been reported to be common.66,81,82,84 Immunosuppression was reported as an underlying condition for patients in Saudi Arabia with Group A streptococcal bacteremia (18.2% of 33 patients);69 other conditions included cardiac disease (33.3%), malignancy (27.3%), recent trauma (18.2%) and recent influenza infection (18.2%). In a case series with Elizabethkingia meningoseptica bacteremia, all 12 patients had at least one underlying condition or intervention, and these included chronic illness and surgery.85
Similar underlying conditions are reported for patients with bacteremia due to MDR organisms. Studies of bacteremia due to MDR P. aeruginosa and carbapenem-resistant Enterobacterales in Saudi Arabia reported diabetes mellitus, hypertension and chronic kidney failure as common underlying conditions,81,82 in addition to the presence of malignancy.
Clinical Outcomes and Mortality Associated with BSI
Across the studies there was a large range of mortality rates associated with BSI. Crude mortality among adult and pediatric ICU patients from Kuwait with CLABSI was reported as 27.3% by Al-Mousa et al40 which increased to 38.9% for patients from neonatal ICUs (Table 2). Among adults, rates ranged from 10% to ≥67%,86,87 with the highest rates attributed to infections caused by antimicrobial-resistant pathogens (Table 2). In their retrospective analysis of carbapenem-resistant bacteremia between 2007 and 2016, Balkhair et al88 found that patients with A. baumannii infections were significantly younger (41.2 years) than patients with infections due to P. aeruginosa or K. pneumoniae (51.3 and 52.1 years, respectively) (P = 0.049). Also, of the 775 patients with bacteremia in the study, 252 (32.5%) died within 30 days of bacteremia onset.88 Rates of 30-day all-cause mortality were 40.2% for A. baumannii, 31.5% for K. pneumoniae and 28.6% for P. aeruginosa bacteremia (Table 2). In a later study by Balkhair et al87 reporting results from 2017 to 2020, mortality was higher among patients with BSIs caused by carbapenem-resistant isolates than those with carbapenem-susceptible infections, and 30-day all-cause mortality was similar for infections caused by all four carbapenem-resistant species studied (68.8%, P. aeruginosa; 67.8%, K. pneumoniae; 67.6%, A. baumannii; 71.4%, E. coli, Table 2). In patients >14 years of age, carbapenem-resistant bacteremia has also been shown to be an independent predictor for mortality in a study from Saudi Arabia and in Oman, the use of broad-spectrum antibiotics (eg, carbapenems and piperacillin-tazobactam), and mechanical ventilation were associated with mortality among patients with carbapenem-resistant Enterobacterales bacteremia.29,89
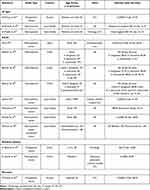 |
Table 2 Rates of Mortality From Bloodstream Infections (BSIs) in the GCC Region, by Age Group |
In a study of infections caused by A. baumannii carried out in Oman, all-cause mortality rates were higher among adult patients with pneumonia when compared with other infections (P = 0.000, OR 2.85); however, patients with bacteremia died earlier (28-day mortality) (P = 0.042, OR 2.83).28 Among patients with neutropenia in Saudi Arabia, mortality was associated with the presence of bacteremia (P = 0.016).92 For S. maltophilia bacteremia among pediatric patients, the mortality rate was reported as 33.8% within 7 days of diagnosis.72
Al-Otaibi et al73 reported a high mortality rate (32.1%) among cancer patients with bacteremia at a hospital in Saudi Arabia (Table 2); they also reported a high rate of MDR isolates (43.5%). Comparing outcomes for patients inside and outside the ICU, Alotaibi et al90 reported that adult patients with CLABSI in the ICU and those admitted to the ICU after infection had higher mortality than those outside the ICU (P < 0.0001), although CLABSI-related sequelae were not associated with increased mortality (P < 0.595).
Length of Stay and Hospital Costs
The time taken to diagnose a BSI and prescribe an appropriate antimicrobial is critical for effective treatment. Mahrous et al86 analyzed the impact of rapid diagnostic testing coupled with a pharmacist-guided antimicrobial stewardship program on clinical outcomes in the treatment of adults with BSIs. The intervention reduced the time to culture identification from 96 hours to 22 hours (P < 0.0001) and the median time to targeted antimicrobials from 22 hours to 2 hours (P < 0.0001). They concluded that the length of stay was significantly shorter after the practice was introduced (6.5 vs 8 days, P = 0.03).86 In addition, the intervention reduced mortality (from 18% to 10%, Table 2) and 30-day re-admission.
Retrospective studies of adults and pediatric patients have shown that the presence of a CLABSI is associated with a longer length of stay.93,94 Alotaibi et al93 reported that the presence of CLABSI in adults increased the length of stay by 13.13 ± 9.53 days. The length of stay was also significantly longer when central-line removal was delayed (P < 0.001). In a pediatric study, the rate of CLABSI was 16.6% among patients admitted for more than 30 days and 0.6% among patients admitted for ≤30 days.94
In Bahrain, adult patients with a CLABSI had a longer median length of stay in the ICU before insertion of the central line (7.6 days compared with 2.8 days for the control group, P < 0.05).47 Al-Khawaja et al45 calculated, following the care bundle-related reduction in CLABSI (from 2.3 patients per month to 1 patient per month), that 367 hospital days were saved, which corresponded with a reduction in hospital costs of 1,100,553 USD.
Complications
Al-Khadidi et al69 reported the most frequent complications of Group A beta-hemolytic bacteremia as shock (10/33, 30.3%), acute respiratory distress syndrome (7/33, 21.2%), renal impairment (7/33, 21.2%) and pneumonia (5/33, 15.2%). Abazid et al95 reported that septicemia among patients in a critical care unit was a predictor of developing delirium. Among 139 adult hemodialysis patients with CLABSI, El-Kady et al70 reported that 43.2% developed complications with hospital admission and endocarditis being the most common (15.8% and 11.5%, respectively). Also, 10.1% required ICU admission and 5.8% developed septic embolism.70
Causal Pathogens of BSIs
Adults
Gram-negative organisms predominate as the causative pathogens of BSI among adults in the region. In the UAE, among adults with a confirmed BSI, Gram-negative isolates were reported to be more common that Gram-positive isolates [71.8% (148/206) and 24.3% (50/206), respectively], with the most common organism reported to be E. coli (31.1%, 64/206).74 In their study of HCAI among ICU patients in Kuwait, Alfouzan et al19 reported Gram-negative species as more commonly occurring than Gram-positives among BSI, with A. baumannii and K. pneumoniae the most frequent (both 21.5% of isolates); 9.2% of isolates were S. aureus. In Saudi Arabia, Gram-negative species and MDR isolates have also been more frequently identified than Gram-positive species among adult patients with bacteremia in a number of studies.64,80,96 Al-Khawaja et al47 determined that, among patients with a confirmed CLABSI, Gram-negative bacteria were more common (56%) than Gram-positive (41%) pathogens; however, at a species level, the most frequently isolated species were coagulase-negative staphylococci (18%) followed by Acinetobacter spp. (15%) and Enterococcus spp. (15%). In a 1-year (2019) prospective study, Saleem et al26 reported that K. pneumoniae was the most common pathogen among CLABSI cases in an ICU in Saudi Arabia. Using direct DNA sequencing-based analysis, Alzahrani et al97 found Gram-negative species to be more common among adults with leukemia and lymphoma. In a study of Gram-negative BSI among cancer patients in Saudi Arabia, the most common organisms were E. coli (29.5%, 18/61), A. baumannii (18.0%, 11/61), Pseudomonas spp. (16.4%, 10/61) and K. pneumoniae (13.1%, 8/61).73 Among hemodialysis patients in Saudi Arabia with CLABSI, Gram-negative organisms have also been reported to be more common than Gram-positive organisms (61.4%, 129/210; 38.6%, 81/210, respectively), with E. cloacae being the most common Gram-negative species.83
Conversely, a retrospective analysis by a microbiology laboratory in Oman between 2016 and 2017 reported that, among isolates from blood samples, 59.2% (837/1415) were Gram-positive and 40.1% (568/1415) were Gram-negative.98 Among adult patients with febrile neutropenia in Saudi Arabia, an equal number of Gram-positive and Gram-negative isolates were isolates from blood cultures (n = 23).34 Among adult hemodialysis patients in Saudi Arabia, CLABSIs were more commonly due to Gram-positive bacteria (97/139, 69.8%) than Gram-negative (42/139, 30.2%).70 Abdelfattah et al99 also reported that Gram-positive bacteria were a more common cause of BSI among dialysis patients, with 52% of all BSI caused by S. aureus.
Badawy et al100 in their study of dialysis patients in Kuwait in 2012 determined that common skin commensals (including coagulase-negative staphylococci and diphtheroids) were the most frequently isolated species followed by Gram-negative rods (40.2% and 31.8%, respectively); 12.1% of all blood culture isolates were S. aureus. The study authors stated that they could not determine if the skin commensals were causative pathogens or specimen contamination.100 Similarly, in Oman, Staphylococcus epidermidis has been reported as the most frequent microorganism in CLABSI.51 Care must be taken when attributing BSIs to common skin commensals. Asaad et al8 reported that of the coagulase-negative staphylococci that grew from 208 positive blood cultures, 75 (36.1%) were causative pathogens and 133 (63.9%) were contaminants. Further to this, the emergency department was identified as the most common area for contamination (81.3%, 26/32 culture-positive samples) while the ICU was the least common (11.8%, 2/17 of samples).8 Al-Tawfiq reported that, of 67 patients with blood cultures positive for coagulase-negative Staphylococcus, antimicrobial therapy was stopped in 65% of cases because there was no evidence of infection.101
Neonates and Pediatric Patients
In contrast to many of the adult studies, those presenting data on neonates and pediatric patients reported that Gram-positive organisms were more common than Gram-negatives as a cause of BSIs. In their study of pediatric oncology patients in Qatar, Al-Mulla et al35 found that Gram-positive pathogens were more common than Gram-negative pathogens (64/116, 55.2% and 52/116, 44.8%, respectively); however, the percentages changed over time, with a higher percentage of Gram-positive isolates in 2004–2005 (17/29, 58.6%) than in 2010–2011 (15/28, 53.6%). K. pneumoniae was the most common Gram-negative species and S. epidermidis the most common Gram-positive. Gram-positive organisms were also the most common pathogen among neonates in an ICU in Oman.102 In Saudi Arabia, between May 2016 and December 2017, among non-neutropenic children with cancer and a CLABSI, Gram-positive isolates were more common than Gram-negatives (8/13, 61.5% and 5/13, 38.5%, respectively), although it should be noted that the number of isolates was small.103 A similar result was reported among pediatric patients in receipt of stem cell transplants who developed bacteremia (55.6%, 10/18 Gram-positive; 44.4%, 8/18 Gram-negative).104 Also, among neonates, Gram-positive BSIs have been shown to be more common than Gram-negative BSIs (64.2% and 32.5%, respectively), with S. aureus and K. pneumoniae being the most common species from the two groups, respectively.105 Group B streptococci have also been reported to be a common cause of BSIs among neonates.106
As seen among the studies of adults, not all studies reported the same pattern. In pediatric (≤13 years) oncology patients in Oman, Staphylococcus was the most common Gram-positive organism (68.8%, 22/32) and Klebsiella the most common Gram-negative (21.4%, 9/42); however, Gram-negative organisms were more common overall with 42 isolates (56.8%) compared with 32 Gram-positive isolates (43.3%).91 In a study of neonates at a COVID-19 segregation center in Saudi Arabia in 2020, Gram-negative organisms were reported to be more common than Gram-positives (68.2% and 31.8%, respectively), and among patients with sickle cell disease between 2005 and 2015 in Saudi Arabia, 7.8% (25/320) reported a bacterial infection, three of which were from the bloodstream and all caused by Salmonella spp.107,108
Rarely Reported Pathogens
Rarely reported causes of bacteremia include Sphingomonas paucimobilis (Bahrain, hemodialysis patients);84 non-typhoidal Salmonella (Kuwait);109 Actinomyces odontolyticus (Qatar);110 Streptococcus gallolyticus subsp gallolyticus (Qatar);111 Bacteroides fragilis (Kuwait);112 and Chryseobacterium/Elizabethkingia spp (Saudi Arabia).85,113
Antimicrobial Susceptibility Among Gram-Positive Pathogens
Antimicrobial susceptibility (or resistance) among Gram-positive pathogens causing BSIs, as reported across the GCC countries, is presented in Table 3. Antimicrobials for which results were frequently reported included oxacillin, gentamicin, tetracycline, trimethoprim-sulfamethoxazole, ciprofloxacin, clindamycin and vancomycin. Among S. epidermidis and Staphylococcus haemolyticus isolates, susceptibility was high to daptomycin and vancomycin. There was little data available on S. aureus; however, Alhunaif et al66 reported on 184 MRSA isolates from adults with BSIs in Saudi Arabia between January 2013 and June 2017 and ≥98% were susceptible to vancomycin, linezolid and tigecycline. All 196 isolates of Group B streptococci from BSIs isolated in Qatar between January 2015 and March 2019 were susceptible to penicillin, ceftriaxone and vancomycin and 71.4% were susceptible to clindamycin (Table 3);71 however, the study also reported an increasing trend in infections, from 1.48 per 100,000 people to 2.09 per 100,000. In an analysis of 263 enterococci from BSI by Ali et al20 in Qatar 2009–2018, the majority of isolates were susceptible to vancomycin, ampicillin, linezolid, daptomycin and gentamicin (Table 3); however, the study did not distinguish between enterococcal infections and polymicrobial infections that included enterococci.20
 |
Table 3 Antimicrobial Susceptibility and/or Resistance Among Gram-Positive Pathogens Causing Bloodstream Infections (BSIs) in GCC Countries |
Multidrug Resistance
Among pediatric oncology patients in Oman (January 2015 – October 2017), 38% (n = 12) of Gram-positive organisms were resistant to ≥4 antimicrobials; however, no isolates were resistant to vancomycin and among neonates in Saudi Arabia, 54.5% of S. aureus isolates were resistant to ≥3 antimicrobials.91,105
Antimicrobial Susceptibility Among Gram-Negative Pathogens
Rates of antimicrobial susceptibility (or resistance) among Gram-negative pathogens causing BSIs, as reported across the GCC countries, are presented in Table 4. Studies reported variable rates of susceptibility or resistance; however, high rates of susceptibility (≥80%) were reported by a number of studies among P. aeruginosa to cefepime, gentamicin, amikacin and meropenem (Table 4). Low rates of susceptibility among A. baumannii isolates were reported to a range of antimicrobials; however, Bandy et al80 reported 100% colistin susceptibility. Among K. pneumoniae, susceptibility to meropenem and ciprofloxacin was >80% and to cephalosporins ≥70% across a number of studies (Table 4).
 |
Table 4 Antimicrobial Susceptibility and/or Resistance Among Gram-Negative Pathogens Causing Bloodstream Infections (BSIs) in GCC Countries |
Carbapenem Resistance
Carbapenem-resistant pathogens are an increasing problem worldwide, and carbapenemase-positive bacteria have been identified as causative pathogens in BSIs in the GCC region. In Oman, K. pneumoniae from BSI were susceptible to carbapenems prior to 2010;88 however, resistance increased to 40% in 2014. The same study reported that of 66 carbapenem-resistant K. pneumoniae isolates tested, 21.2% were also resistant to colistin. In a retrospective study of Gram-negative infections between 2017 and 2020, also in Oman, 46.4% (84/181) of K. pneumoniae were carbapenem-resistant.87 In addition, 29.9% (32/107) of P. aeruginosa, 80.4% (37/46) of A. baumannii and 2.9% (7/244) of E. coli in the same study were carbapenem-resistant and, in total, 13.4% (16/119) of the carbapenem-resistant isolates were resistant to both carbapenems and colistin (15 of 16 were K. pneumoniae).87 In Saudi Arabia, a cross-section retrospective study at a single center identified bacteremia as the most common infection among patients with carbapenem-resistant Enterobacterales.116
Multidrug Resistance
MDR Gram-negative infections are increasingly problematic to treat. A study of MDR bacteria in Oman in 2012 reported that BSIs and pneumonia were the infections most frequently associated with multidrug resistance and, although the study did not specify the bacterial species isolated from blood, across the infection sources the most common bacteria were A. baumannii and E. coli.117 Among pediatric oncology patients in Oman (January 2015–October 2017), 33.3% (14/42) of Gram-negative bacteria from blood cultures were resistant to ≥4 antimicrobials, and the highest rates of resistance were reported for ampicillin and ceftriaxone (both, n=15/42, 35.7%), piperacillin-tazobactam (n = 13, 31.0%) and cefepime (n = 12, 28.6%).91
Among oncology patients in Saudi Arabia, Al-Otaibi et al73 reported low rates of susceptibility among A. baumannii between 2013 and 2015 (Table 4); the same study also found that the MDR rate among P. aeruginosa and A. baumannii was 43.5% and that the rate of ESBL producers was 34.6% among isolates of E. coli and K. pneumoniae. For isolates collected in Saudi Arabia in 2019, multidrug resistance among E. coli and K. pneumoniae was reported to be 78.3% (18/23) and 49.2% (31/63), respectively, and 4.3% (1/23) of E. coli and 46.0% (29/63) of K. pneumoniae were carbapenemase producers.80 In addition, a retrospective study of 60 ICU patients (98.3% with central lines) with Acinetobacter BSIs in Saudi Arabia between 2005 and 2010 reported that all cases were MDR, 90% were resistant to carbapenems and one isolate was resistant to colistin.79 The same study concluded that the mortality risk for Acinetobacter BSI was lower among patients who received appropriate antimicrobial therapy.
In Qatar, between October 2014 and September 2017, <5% (16/362) of P. aeruginosa from BSIs were MDR;77 against these MDR isolates 12.5% were susceptible to meropenem, 50% were susceptible to ceftazidime-avibactam and ceftolozane-tazobactam, and 100% were susceptible to colistin. MDR A. baumannii, E. cloacae, K. pneumoniae and P. aeruginosa were identified among the Gram-negative organisms isolated from CLABSI in pediatric/neonatal ICUs in Kuwait in 2015;54 however, following infection control interventions, along with a decrease in the number of CLABSIs, no MDR isolates were collected in 2016 (January–March). Among neonates in Saudi Arabia 45% (9/20) of K. pneumoniae isolates were determined to be resistant to ≥3 antimicrobials.105
Among isolates from hemodialysis patients in Saudi Arabia, 46.7% of K. pneumoniae were ESBL-producers and 13.3% were MDR;70 however, only 15 isolates were included in the study.
Resistant Genotypes
Among carbapenem-resistant Enterobacterales, particularly K. pneumoniae, isolated from bacteremia patients in Saudi Arabia and Oman, OXA-48 was the most common genotype.81,87 NDM-positive isolates were also reported.81,87 In the UAE, four ertapenem-non-susceptible Enterobacterales collected between 2011 and 2012 from patients with healthcare-associated bacteremia were positive for blaOXA-48 and all four isolates were susceptible to tigecycline and colistin and with MICs for meropenem of between 0.25 and 0.5 mg/L.118 Also, in the UAE, between January 2016 and October 2017, patients with bacteremia caused by Gram-negative pathogens that were positive for AmpC β-lactamases were reported.74 The carbapenemase VIM-4 has been reported among K. pneumoniae and E. coli isolates in Kuwait.119
A range of resistance genes were identified among MDR P. aeruginosa collected between 2014 and 2017, including CTX-M-15, VIM, OXA, PDC and genes encoding aminoglycoside modifying enzymes.77
Antimicrobial Management & Clinical Outcomes
The selection of the appropriate antimicrobial treatment for bacteremia should be guided by the most likely source of the infection, microbiological data and knowledge of the local antibiogram. Bacteremia was reported to be the second most common reason for prescribing antimicrobials in a study in Qatar, with teicoplanin being a commonly prescribed antimicrobial for primary bacteremia.120,121 Also, in Qatar, between October 2014 and September 2017, the most commonly used antimicrobial agents against MDR P. aeruginosa BSI were meropenem (75.0%, 12/16 cases) and colistin (62.5%, 10/16 cases), with clinical response achieved in 50% of patients (8/16) and the 30-day all-cause mortality was 31.3% (5/16).77 A retrospective study by Hakeam et al82 in Saudi Arabia compared the treatment of MDR P. aeruginosa bacteremia with ceftolozane-tazobactam and colistin, concluding that the risk of mortality was similar between the two groups, although clinical success was higher in the ceftolozane-tazobactam group (76.5% and 41.4%, respectively). The study also noted that colistin was always used in combination, most frequently with a carbapenem (22/29 cases, 75.9%), whereas ceftolozane-tazobactam was used in combination with an antipseudomonal antimicrobial in 64.7% of cases (11/17).82
A retrospective, multi-center cohort study of adults in Saudi Arabia compared outcomes for patients with carbapenem-resistant Enterobacterales bacteremia who received ceftazidime-avibactam and those who received colistin (32 and 29 patients, respectively).81 In comparison with the colistin group, the adjusted risk for 14-day mortality was lower in the ceftazidime-avibactam group (hazard ratio 0.32; 95% confidence interval [CI] 0.10–0.99; P = 0.049); however, the crude 14-day mortality, the adjusted 30-day mortality and bacterial eradication were similar between the two groups.81 Alraddadi et al29 reported a protective effect when ceftazidime-avibactam was part of the treatment regimen for carbapenem-resistant Enterobacterales bacteremia; however, when age, Charlson comorbidity index and Pitts Bacteremia Score (PBS) were controlled for, the association between 30-day all-cause mortality and treatment with ceftazidime-avibactam was no longer significant (P = 0.06). Colistin is considered a last option in the treatment of BSI, often in cases caused by carbapenem-resistant organisms. However, the use of colistin is complicated by difficulties in determining the appropriate dose and possible toxicity.122 Colistin-resistant Enterobacterales have been reported in the region (Saudi Arabia).123
Another study of infections caused by carbapenem-resistant Enterobacterales in Saudi Arabia compared treatment of adults with ceftazidime-avibactam to other antimicrobials.124 The study included a range of infections and the rate of bacteremia was 70% (7/10) in the group that received ceftazidime-avibactam and 53.6% (15/28) in the group that received a comparator.124 In both groups, the time to clearance of bacteremia was similar (median values: 4 days and 5 days, respectively).124 A retrospective observational study from the UAE looked at the microbiological cure of bacteremia and pneumonia among patients using renal replacement therapy and receiving ceftazidime-avibactam. A total of 22 patients had bacteremia, and microbiological cure was achieved in 20 patients (90.9%) and through the use of multivariate logistic regression, the study determined that Enterobacterales, daily drug dose and bacteremia were associated with microbiological cure.125
Among hemodialysis patients in Saudi Arabia, vancomycin was used to treat BSIs in 55.2% of cases, ceftazidime in 48.3% of cases and gentamicin in 13.8%.64 Alshukairi et al96 reported the most commonly used antimicrobials in the treatment of bacteremia among febrile neutropenic adults to be carbapenems, vancomycin and aminoglycosides; however, following an imipenem de-escalation study, piperacillin-tazobactam use increased (P = 0.018) and meropenem use decreased significantly (P = 0.009).
Combination Therapy
Combination therapy with piperacillin-tazobactam and amikacin was reported to be a common combination in Oman in the treatment of pediatric oncology patients with BSIs.91 Other combinations reported in the study included cefepime and amikacin; meropenem, piperacillin-tazobactam or cefepime in combination in vancomycin; and ceftazidime in combination with amikacin.
Antimicrobial Stewardship
Antimicrobial stewardship programs are vital for supporting the appropriate use of antimicrobials. In a study across 6 hospitals in Saudi Arabia, the overall appropriateness of antimicrobial therapy was found to be lowest in BSIs.126 Al-Omari et al127 demonstrated that following the introduction of a stewardship program, HCAIs decreased, with the rate of CLABSIs reducing by 94.1%.
Gaps & Recommendations
While a great deal of research has been published on the impact of CLABSI in the region, more information on the clinical impact of other types of BSIs is needed, particularly in the case of secondary infections, as they may benefit from different clinical management. In addition, the majority of available information in the region is related to healthcare-acquired, and in particular ICU-acquired, BSIs. More information is needed on the risk factors and pathogens associated with community-acquired BSIs. There is also a lack of published data on important pathogens such as Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae and anaerobes.
With the population in the region ageing, more analysis on the impact and appropriate treatment of BSIs among older age groups is also important. More local information on antimicrobial susceptibility profiles and associated resistance mechanisms of organisms causing BSI would also support the appropriate selection of antimicrobial agents, although the region is known to have a high prevalence of OXA-48.128 Accurate data on colistin susceptibility in the region, using reliable reference methods, would also be beneficial.
There is a lack of national BSI surveillance data, including yearly antibiograms, from most GCC countries, such data would enable the monitoring of epidemiology and resistance trends. One recommendation would be to develop a surveillance network across the GCC region for the BSIs, causative organisms and their antibiograms. Valuable data are also lacking on the costs associated with BSI in the region.
Limitations
This review has comprehensively covered 10 years of data regarding an important aspect of invasive bacterial disease; however, it has some limitations. One of the prime limitations of this review was the fact that not all studies included an undisputed description of their definition of a BSI. As discussed in this review, positive blood cultures may not mean that the patient had a BSI. Also, some of the studies included were from a single center, potentially resulting in giving data undue weight and, as single centers, the reported data may not be reflective of the wider area or country. Additionally, a large number of the studies were from centers in Saudi Arabia resulting in the underrepresentation of other GCC countries. However, these limitations highlight the fact that there is a lack of systematic surveillance of BSI in GCC countries, meaning that data must be aggregated from studies, often from a single center, across the region, with sporadic representation of patients, locations and the use of variable definitions of BSI.
Also, there was a focus on CLABSI in the literature, this is a commonly occurring type of BSI so the focus is not unexpected; however, more studies on other types of BSIs would strengthen the evidence base. In addition, infective endocarditis and osteomyelitis, as hematogenous infections, were perhaps underrepresented in our literature search because they were not included as specific terms.
Finally, because sepsis was excluded from the search terms, no information was collected from the literature about the rates of patients with BSIs who developed sepsis.
Discussion and Conclusions
BSIs are common in the GCC region. However, rates of BSI in the studies included in this review varied by country, setting and population and a number of studies reported that BSI were frequently device-associated.21,23,26 In Saudi Arabia, Bahrain and Oman, the reported rate of CLABSIs was 146% higher than a study of US hospitals but 33% lower than an international hospital study.37 These differences might be related to healthcare expenditures in the different countries/regions. Another international study also reported a correlation between infection risk and the socio-economic level of a country.129 Device associated infections are recognized as an important infection control target and a number of GCC region studies reported on the effective use of infection control procedures and care bundles to reduce rates of CLABSI.39,41–45,47–49,54,56,57
Across the region, a range of Gram-positive and Gram-negative species were reported to cause BSI, with most publications focusing on CLABSI. Species reported included S. aureus and MRSA, S. epidermidis, Enterococcus faecalis, K. pneumoniae, A. baumannii, P. aeruginosa, E. coli, S. maltophilia, Serratia marcescens, Enterobacter aerogenes, Enterobacter cloacae and Citrobacter koseri.20,26,27,31,32,41,43,44,54,64,66,72,76,79,83,87,99,130 Gram-negative organisms were more commonly reported among adults; however, among neonates and pediatric patients, the majority of studies across the region reported that Gram-positive organisms were more common. The likely causative organisms of an infection will vary due to a number of factors, including patient location (eg, ICU, general ward, and community), comorbidities, and geographic location.
High rates of mortality were associated with antimicrobial-resistant pathogens, particularly carbapenem-resistant Gram-negatives such as carbapenem-resistant Enterobacterales, P. aeruginosa and A. baumannii.87,131–133 Patient location was also reported to be associated with mortality, with patients in the ICU with a CLABSI having a higher mortality rate than those outside the ICU.90
Carbapenem resistance (imipenem and/or meropenem) was reported to be ≥30% against P. aeruginosa, A. baumannii and K. pneumoniae in a number of studies included in this review.70,73,80,115 Among E. coli, rates of carbapenem resistance were lower. Antimicrobial resistance is of global concern with many countries reporting increasing rates of resistance, particularly for classes such as the carbapenems.10 There was a lack of susceptibility data for newer agents such as ceftazidime-avibactam and meropenem-vaborbactam in the region and in vitro and clinical studies involving BSI for these agents would be beneficial.
This review has revealed a number of gaps in the literature from the GCC region. While a great deal of the literature focuses on CLABSI, more information on other BSI, and particularly secondary infections would be beneficial, as would information on pathogens such as Streptococcus pneumoniae, Haemophilus influenzae and anaerobes. The interpretation of antimicrobial susceptibility data is limited because of the number of studies that were single centers and included specific patient groups. The GCC region would benefit from surveillance networks to monitor BSIs, causative organisms and antibiograms.
In conclusion, BSIs present significant morbidity and mortality in the GCC region with device associated infections making up an important proportion of such infections. Infection control, and the use of care bundles, has been shown to reduce the burden of device-associated infections. Antimicrobial resistance is a global problem, from which the GCC region is not immune, and it impacts the appropriate selection of antimicrobials and the effective treatment of BSI. The development of national BSI surveillance networks to inform clinicians would aid treatment and decision-making.
Funding
This work is funded by Pfizer and personnel at Pfizer were involved in the decision to publish this article. Medical writing support was provided by Wendy Hartley, PhD of Micron Research Ltd. (Ely, UK), and was funded by Pfizer.
Disclosure
Hussain Al-Shamari has received speaking fees from Pfizer and MSD. Hamad Abdel Hadi has received honoraria from Pfizer, MSD and AstraZeneca for educational activities. Malak Baghdadi and Nisrine Sabra are employees of Pfizer. Malak Baghdadi reports non-financial support from Micron Research, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Seifert H. The clinical importance of microbiological findings in the diagnosis and management of bloodstream infections. Clin Infect Dis. 2009;48(Suppl 4):S238–S245. doi:10.1086/598188
2. Holmes CL, Anderson MT, Mobley HLT, Bachman MA. Pathogenesis of Gram-negative bacteremia. Clin Microbiol Rev. 2021;34(2):e00234–20. doi:10.1128/CMR.00234-20
3. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501–509. doi:10.1111/1469-0691.12195
4. National Healthcare Safety Network. Atlanta, Georgia: bloodstream infection event (central line-associated bloodstream infection and non-central line associated bloodstream infection). 2023. Available from:: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf.
5. Timsit J-F, Ruppé E, Barbier F, Tabah A, Bassetti M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266–284. doi:10.1007/s00134-020-05950-6
6. Saginur R, Suh KN. Staphylococcus aureus bacteraemia of unknown primary source: where do we stand? Int J Antimicrob Agents. 2008;32(Suppl 1):S21–25. doi:10.1016/j.ijantimicag.2008.06.008
7. Autore G, Bernardi L, Esposito S. Update on acute bone and joint infections in paediatrics: a narrative review on the most recent evidence-based recommendations and appropriate antinfective therapy. Antibiotics. 2020;9(8):486. doi:10.3390/antibiotics9080486
8. Asaad AM, Qureshi MA, Hasan SM. Clinical significance of coagulase-negative staphylococci isolates from nosocomial bloodstream infections. Infect Dis. 2016;48(5):356–360. doi:10.3109/23744235.2015.1122833
9. Shelburne S, Musher DM. Management of Gram-positive bacterial disease: staphylococcus aureus, streptococcal, pneumococcal and enterococcal infections. In: Safdar A, editor. Principles and Practice of Cancer Infectious Diseases. New York: Springer; 2011:409–420. doi:10.1007/978-1-60761-644-3_35
10. World Health Organization. Geneva: global antimicrobial resistance and use surveillance system (GLASS) report. 2022. Available from: https://www.who.int/publications/i/item/9789240062702.
11. Pace MC, Corrente A, Passavanti MB, et al. Burden of severe infections due to carbapenem-resistant pathogens in intensive care unit. World J Clin Cases. 2023;11(13):2874–2889. doi:10.12998/wjcc.v11.i13.2874
12. McNamara JF, Righi E, Wright H, Hartel GF, Harris PNA, Paterson DL. Long-term morbidity and mortality following bloodstream infection: a systematic literature review. J Infect. 2018;77(1):1–8. doi:10.1016/j.jinf.2018.03.005
13. Cabrero EL, Robledo RT, Cuñado AC, et al. Risk factors of catheter- associated bloodstream infection: systematic review and meta-analysis. PLoS One. 2023;18(3):e0282290. doi:10.1371/journal.pone.0282290
14. Stoeckle M, Kaech C, Trampuz A, Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med Wkly. 2008;138(35–36):512–519. doi:10.4414/smw.2008.12228
15. Laupland KB, Pasquill K, Dagasso G, Parfitt EC, Steele L, Schonheyder HC. Population-based risk factors for community-onset bloodstream infections. Eur J Clin Microbiol Infect Dis. 2020;39(4):753–758. doi:10.1007/s10096-019-03777-8
16. Laupland KB, Pasquill K, Steele L, Parfitt EC. Burden of bloodstream infection in older persons: a population-based study. BMC Geriatr. 2021;21(1):31. doi:10.1186/s12877-020-01984-z
17. Al Salman J, Al Dabal L, Bassetti M, et al. Management of infections caused by WHO critical priority gram-negative pathogens in Arab countries of the Middle East: a consensus paper. Int J Antimicrob Agents. 2020;56(4):106104. doi:10.1016/j.ijantimicag.2020.106104
18. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
19. Alfouzan W, Dhar R, Abdo NM, Alali WQ, Rabaan AA. Epidemiology and microbiological profile of common healthcare associated infections among patients in the intensive care unit of a general hospital in Kuwait: a retrospective observational study. J Epidemiol Glob Health. 2021;11(3):302–309. doi:10.2991/jegh.k.210524.001
20. Ali GA, Goravey W, Najim MS, et al. Epidemiology, microbiological and clinical characteristics of Enterococcus species bloodstream infections: a 10-year retrospective cohort study from Qatar. Ann Med Surg Lond. 2022;80:104258. doi:10.1016/j.amsu.2022.104258
21. Alshamrani MM, El-Saed A, Alsaedi A, et al. Burden of healthcare-associated infections at six tertiary-care hospitals in Saudi Arabia: a point prevalence survey. Infect Control Hosp Epidemiol. 2019;40(3):355–357. doi:10.1017/ice.2018.338
22. Al-Tawfiq JA, Amalraj A, Memish ZA. Reduction and surveillance of device-associated infections in adult intensive care units at a Saudi Arabian hospital, 2004–2011. Int J Infect Dis. 2013;17(12):e1207–e1211. doi:10.1016/j.ijid.2013.06.015
23. Gupta SK, Al Khaleefah FK, Al Harbi IS, et al. An intervention study for the prevention and control of health care-associated infection in the critical cares area of a tertiary care hospital in Saudi Arabia. Indian J Crit Care Med. 2018;22(12):858–861. doi:10.4103/ijccm.IJCCM_270_18
24. Abulhasan YB, Abdullah AA, Shetty SA, Ramadan MA, Yousef W, Mokaddas EM. Health care-associated infections in a neurocritical care unit of a developing country. Neurocrit Care. 2020;32(3):836–846. doi:10.1007/s12028-019-00856-8
25. Alamer A, Alharbi F, Aldhilan A, et al. Healthcare-associated infections (HAIs): challenges and measures taken by the radiology department to control infection transmission. Vaccines. 2022;10(12):2060. doi:10.3390/vaccines10122060
26. Saleem M, Khaja ASS, Hossain A, et al. Pathogen burden among ICU patients in a tertiary care hospital in Hail Saudi Arabia with particular reference to β-lactamases profile. Infect Drug Resist. 2023;16:769–778. doi:10.2147/IDR.S394777
27. Saleem M, Khaja ASS, Hossain A, et al. Molecular characterization and antibiogram of Acinetobacter baumannii clinical isolates recovered from the patients with ventilator-associated pneumonia. Healthcare. 2022;10(11):2210. doi:10.3390/healthcare10112210
28. Alrahmany D, Omar AF, Alreesi A, Harb G, Ghazi IM. Acinetobacter baumannii infection-related mortality in hospitalized patients: risk factors and potential targets for clinical and antimicrobial stewardship interventions. Antibiotics. 2022;11(8):1086. doi:10.3390/antibiotics11081086
29. Alraddadi BM, Heaphy ELG, Aljishi Y, et al. Molecular epidemiology and outcome of carbapenem-resistant Enterobacterales in Saudi Arabia. BMC Infect Dis. 2022;22(1):542. doi:10.1186/s12879-022-07507-y
30. Alzomor OA, Alfawaz TS, Abu-Shaheen A, Alshehri MA, Al Shahrani D. A matched case-control study to assess the carbapenem-resistant Enterobacteriaceae infections among hospitalized children at King Fahad Medical City, Riyadh, Saudi Arabia. Saudi Med J. 2019;40(11):1105–1110. doi:10.15537/smj.2019.11.24586
31. Alqahtani JM. Emergence of Stenotrophomonas maltophilia nosocomial isolates in a Saudi children’s hospital. Risk factors and clinical characteristics. Saudi Med J. 2017;38(5):521–527. doi:10.15537/smj.2017.5.16375
32. Al-Hamad AM, Alfaraj AA, Altowaileb J, et al. Incidence and antibiotic susceptibility of MRSA infections in a Saudi Arabian Hospital: a 10-year surveillance study. J Infect Dev Ctries. 2018;12(6):454–461. doi:10.3855/jidc.9778
33. Al Majid F, Aldrees A, Barry M, Binkhamis K, Allam A, A Almohaya A. Streptococcus anginosus group infections: management and outcome at a tertiary care hospital. J Infect Public Health. 2020;13(11):1749–1754. doi:10.1016/j.jiph.2020.07.017
34. Sirkhazi M, Sarriff A, Aziz NA, Almana F, Arafat O, Shorman M. Bacterial spectrum, isolation sites and susceptibility patterns of pathogens in adult febrile neutropenic cancer patients at a specialist hospital in Saudi Arabia. World J Oncol. 2014;5(5–6):196–203. doi:10.14740/wjon850w
35. Al-Mulla NA, Taj-Aldeen SJ, El Shafie S, Janahi M, Al-Nasser AA, Chandra P. Bacterial bloodstream infections and antimicrobial susceptibility pattern in pediatric hematology/oncology patients after anticancer chemotherapy. Infect Drug Resist. 2014;7:289–299. doi:10.2147/IDR.S70486
36. Alzomor O, Aljobair F, Al Kasim F, et al. Frequency of serious bacterial infection among febrile sickle cell disease children in the era of the conjugate vaccine: a retrospective study. Int J Pediatr Adolesc Med. 2022;9(3):165–170. doi:10.1016/j.ijpam.2022.05.002
37. Balkhy HH, El-Saed A, Al-Abri SS, et al. Rates of central line-associated bloodstream infection in tertiary care hospitals in 3 Arabian gulf countries: 6-year surveillance study. Am J Infect Control. 2017;45(5):e49–e51. doi:10.1016/j.ajic.2017.01.027
38. Al-Tawfiq JA, Abed MS, Al-Yami N, Birrer RB. Promoting and sustaining a hospital-wide, multifaceted hand hygiene program resulted in significant reduction in health care-associated infections. Am J Infect Control. 2013;41(6):482–486. doi:10.1016/j.ajic.2012.08.009
39. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi health services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816–822. doi:10.1017/ice.2015.70
40. Al-Mousa HH, Omar AA, Rosenthal VD, et al. Device-associated infection rates, bacterial resistance, length of stay, and mortality in Kuwait: international Nosocomial Infection Consortium findings. Am J Infect Control. 2016;44(4):444–449. doi:10.1016/j.ajic.2015.10.031
41. Al-Abdely HM, Alshehri AD, Rosenthal VD, et al. Prospective multicentre study in intensive care units in five cities from the Kingdom of Saudi Arabia: impact of the International nosocomial infection control consortium (INICC) multidimensional approach on rates of central line-associated bloodstream infection. J Infect Prev. 2017;18(1):25–34. doi:10.1177/1757177416669424
42. Yaseen M, Al-Hameed F, Osman K, et al. A project to reduce the rate of central line associated bloodstream infection in ICU patients to a target of zero. BMJ Qual Improv Rep. 2016;5(1):
43. Salama MF, Jamal W, Al Mousa H, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34–41. doi:10.1016/j.jiph.2015.05.001
44. Bukhari S, Banjar A, Baghdadi S, Baltow B, Ashshi A, Hussain W. Central line associated blood stream infection rate after intervention and comparing outcome with national healthcare safety network and international nosocomial infection control consortium data. Ann Med Health Sci Res. 2014;4(5):682–686. doi:10.4103/2141-9248.141499
45. Al-Khawaja S, Saeed NK, Rosenthal VD, et al. Impact of international nosocomial infection control consortium’s multidimensional approach on central line-associated bloodstream infection rates in Bahrain. J Vasc Access. 2020;21(4):481–489. doi:10.1177/1129729819888426
46. Gaid E, Assiri A, McNabb S, Banjar W. Device-associated nosocomial infection in general hospitals, Kingdom of Saudi Arabia, 2013–2016. J Epidemiol Glob Health. 2018;7(Suppl 1):S35–S40. doi:10.1016/j.jegh.2017.10.008
47. Al-Khawaja S, Saeed NK, Al-Khawaja S, Azzam N, Al-Biltagi M. Trends of central line-associated bloodstream infections in the intensive care unit in the Kingdom of Bahrain: four years’ experience. World J Crit Care Med. 2021;10(5):220–231. doi:10.5492/wjccm.v10.i5.220
48. Gupta P, Thomas M, Patel A, et al. Bundle approach used to achieve zero central line-associated bloodstream infections in an adult coronary intensive care unit. BMJ Open Qual. 2021;10(1):e001200. doi:10.1136/bmjoq-2020-001200
49. Mazi WA, Abdulwahab MH, Alashqar MA, et al. Sustained low incidence rates of central line-associated blood stream infections in the intensive care unit. Infect Drug Resist. 2021;14:889–894. doi:10.2147/IDR.S290791
50. Al-Tawfiq JA, Abdrabalnabi R, Taher A, et al. Surveillance of device associated infections in intensive care units at a Saudi Arabian Hospital, 2017–2020. J Infect Public Health. 2023;16(6):917–921. doi:10.1016/j.jiph.2023.04.007
51. Al-Shukri RH, Al-Rawajfah OM, Al-Daken L, Al-Busaidi M. ICU-acquired central line-associated bloodstream infection and its associated factors in Oman. Am J Infect Control. 2022;50(9):1026–1031. doi:10.1016/j.ajic.2021.12.024
52. Alsaffar MJ, Alsheddi FM, Humayun T, et al. Impact of COVID-19 pandemic on the rates of central line-associated bloodstream infection and catheter-associated urinary tract infection in an intensive care setting: national experience. Am J Infect Control. 2023;51(10):1108–1113. doi:10.1016/j.ajic.2023.03.016
53. Al Lawati TT, Al Jamie A, Al Mufarraji N. Central line associated sepsis in children receiving parenteral nutrition in Oman. J Infect Public Health. 2017;10(5):829–832. doi:10.1016/j.jiph.2017.01.022
54. Hamza WS, Hamed AS-TM, Alfadhli MA, Ramadan -MA-M. A multidisciplinary intervention to reduce central line-associated bloodstream infection in pediatrics and neonatal intensive care units. Pediatr Neonatol. 2022;63(1):71–77. doi:10.1016/j.pedneo.2021.08.010
55. van Rens MFPT, Hugill K, Francia ALV, et al. Closed intravenous systems for central vascular access: a difference maker for CLABSI rates in neonates? J Vasc Access. 2023;24:1390–1397. doi:10.1177/11297298221085480
56. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209–1213. doi:10.1016/j.ajic.2013.05.028
57. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of society for healthcare epidemiology of America/infectious diseases society of America practice guidelines. Am J Infect Control. 2014;42(8):865–867. doi:10.1016/j.ajic.2014.05.005
58. Al-Tawfiq JA, Treble M, Abdrabalnabi R, Okeahialam C, Khazindar S, Myers S. Using targeted solution tools as an initiative to improve hand hygiene: challenges and lessons learned. Epidemiol Infect. 2018;146(2):276–282. doi:10.1017/S0950268817002758
59. Alrebish SA, Yusufoglu HS, Alotibi RF, Abdulkhalik NS, Ahmed NJ, Khan AH. Epidemiology of healthcare-associated infections and adherence to the HAI prevention strategies. Healthcare. 2022;11(1):63. doi:10.3390/healthcare11010063
60. Institute for Healthcare Improvement (IHI). Cambridge, MA: how-to guide: prevent central line-associated bloodstream infections. 2012. Available from: www.ihi.org.
61. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. doi:10.1056/NEJMoa061115
62. Abdelfattah R, Al-Jumaah S, Al-Qahtani A, Al-Thawadi S, Barron I, Al-Mofada S. Outbreak of Burkholderia cepacia bacteraemia in a tertiary care centre due to contaminated ultrasound probe gel. J Hosp Infect. 2018;98(3):289–294. doi:10.1016/j.jhin.2017.09.010
63. Bayoumi MAA, van Rens MFPT, Chandra P, et al. Does the antimicrobial-impregnated peripherally inserted central catheter decrease the CLABSI rate in neonates? Results from a retrospective cohort study. Front Pediatr. 2022;10:1012800. doi:10.3389/fped.2022.1012800
64. Alhazmi SH, Noor SO, Alshamrani MM, Farahat FM. Bloodstream infection at hemodialysis facilities in Jeddah: a medical record review. Ann Saudi Med. 2019;39(4):258–264. doi:10.5144/0256-4947.2019.258
65. Al-Sofyani KA, Uddin MS. Can inverse probability treatment weighting (IPTW) be used to assess differences of CRBSI rates between non-tunneled femoral and jugular CVCs in PICU patients? BMC Infect Dis. 2022;22(1):598. doi:10.1186/s12879-022-07571-4
66. Alhunaif SA, Almansour S, Almutairi R, et al. Methicillin-resistant Staphylococcus aureus bacteremia: epidemiology, clinical characteristics, risk factors, and outcomes in a tertiary care center in Riyadh, Saudi Arabia. Cureus. 2021;13(5):e14934. doi:10.7759/cureus.14934
67. Arabi Z, Al Thiab K, Altheaby A, et al. Urinary tract infections in the first 6 months after renal transplantation. Int J Nephrol. 2021;2021:3033276. doi:10.1155/2021/3033276
68. Arabi Z, Al Thiab K, Altheaby A, et al. The impact of timing of stent removal on the incidence of UTI, recurrence, symptomatology, resistance, and hospitalization in renal transplant recipients. J Transplant. 2021;2021:3428260. doi:10.1155/2021/3428260
69. Al-Khadidi FJ, AlSheheri MA, AlFawaz TS, Enani MA, AlAqeel AA, AlShahrani DA. Group A streptococcal bacteraemia: experience at King Fahad Medical City in Riyadh, Saudi Arabia. Saudi Med J. 2017;38(10):1034–1037. doi:10.15537/smj.2017.10.20966
70. El-Kady RAE-H, Waggas D, AkL A. Microbial repercussion on hemodialysis catheter-related bloodstream infection outcome: a 2-year retrospective study. Infect Drug Resist. 2021;14:4067–4075. doi:10.2147/IDR.S333438
71. Ali M, Alamin MA, Ali GA, et al. Microbiological and clinical characteristics of invasive Group B Streptococcal blood stream infections in children and adults from Qatar. BMC Infect Dis. 2022;22(1):881. doi:10.1186/s12879-022-07801-9
72. Alsuhaibani M, Aljarbou A, Althawadi S, Alsweed A, Al-Hajjar S. Stenotrophomonas maltophilia bacteremia in children: risk factors and mortality rate. Antimicrob Resist Infect Control. 2021;10(1):19. doi:10.1186/s13756-021-00888-w
73. Al-Otaibi FE, Bukhari EE, Badr M, Alrabiaa AA. Prevalence and risk factors of Gram-negative bacilli causing blood stream infection in patients with malignancy. Saudi Med J. 2016;37(9):979–984. doi:10.15537/smj.2016.9.14211
74. Nasef R, El Lababidi R, Alatoom A, Krishnaprasad S, Bonilla F. The impact of integrating rapid PCR-based blood culture identification panel to an established antimicrobial stewardship program in the United Arab of Emirates. Int J Infect Dis. 2020;91:124–128. doi:10.1016/j.ijid.2019.11.028
75. Clinical Laboratory and Standards Institute. Principles and procedures for blood cultures. 2nd ed. CLSI guideline M47. 2022.
76. Mahmoud E, Al Dhoayan M, Bosaeed M, Al Johani S, Arabi YM. Developing machine-learning prediction algorithm for bacteremia in admitted patients. Infect Drug Resist. 2021;14:757–765. doi:10.2147/IDR.S293496
77. Ahmed MAS, Hamid JM, Husain AA, et al. Clinical outcomes, molecular epidemiology and resistance mechanisms of multidrug-resistant Pseudomonas aeruginosa isolated from bloodstream infections from Qatar. Ann Med. 2021;53(1):2345–2353. doi:10.1080/07853890.2021.2012588
78. Alenazi TA, Bin Shaman MS, Suliman DM, et al. The impact of multidrug-resistant Acinetobacter baumannii infection in critically ill patients with or without COVID-19 infection. Healthcare. 2023;11(4):487. doi:10.3390/healthcare11040487
79. Al-Dorzi HM, Asiri AM, Shimemri A, et al. Impact of empirical antimicrobial therapy on the outcome of critically ill patients with Acinetobacter bacteremia. Ann Thorac Med. 2015;10(4):256–262. doi:10.4103/1817-1737.164302
80. Bandy A, Almaeen AH. Pathogenic spectrum of blood stream infections and resistance pattern in Gram-negative bacteria from Aljouf region of Saudi Arabia. PLoS One. 2020;15(6):e0233704. doi:10.1371/journal.pone.0233704
81. Hakeam HA, Alsahli H, Albabtain L, Alassaf S, Al Duhailib Z, Sahar Althawadi S. Effectiveness of ceftazidime-avibactam versus colistin in treating carbapenem-resistant Enterobacteriaceae bacteremia. Int J Infect Dis. 2021;109:1–7. doi:10.1016/j.ijid.2021.05.079
82. Hakeam HA, Askar G, Al Sulaiman K, et al. Treatment of multidrug-resistant Pseudomonas aeruginosa bacteremia using ceftolozane-tazobactam-based or colistin-based antibiotic regimens: a multicenter retrospective study. J Infect Public Health. 2022;15(10):1081–1088. doi:10.1016/j.jiph.2022.08.020
83. Mohsin B. Pattern of causative micro-organisms in catheter related blood stream infections in dialysis patients: experience from Saudi Arabia. J Ayub Med Coll Abbottabad. 2017;29(4):635–640.
84. Al-Said J, Pagaduan A, Murdeshwar S. Successful elimination of hemodialysis-related bacteremia and vascular access infection. Saudi J Kidney Dis Transpl. 2013;24(6):1228–1232. doi:10.4103/1319-2442.121313
85. Aldoghaim FS, Kaabia N, Alyami AM, et al. Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) bacteraemia: a series of 12 cases at Prince Sultan Military Medical City KSA. New Microbes New Infect. 2019;32:100617. doi:10.1016/j.nmni.2019.100617
86. Mahrous AJ, Thabit AK, Elarabi S, Fleisher J. Clinical impact of pharmacist-directed antimicrobial stewardship guidance following blood culture rapid diagnostic testing. J Hosp Infect. 2020;106(3):436–446. doi:10.1016/j.jhin.2020.09.010
87. Balkhair A, Al Saadi K, Al Adawi B. Epidemiology and mortality outcome of carbapenem- and colistin-resistant Klebsiella pneumoniae, Escherichia coli, Acinetobacter baumannii, and Pseudomonas aeruginosa bloodstream infections. IJID Reg. 2023;7:1–5. doi:10.1016/j.ijregi.2023.01.002
88. Balkhair A, Al-Muharrmi Z, Al’Adawi B, et al. Prevalence and 30-day all-cause mortality of carbapenem-and colistin-resistant bacteraemia caused by Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae: description of a decade-long trend. Int J Infect Dis. 2019;85:10–15. doi:10.1016/j.ijid.2019.05.004
89. Khamis F, Al-Zakwani I, Molai M, et al. Demographic, clinical, and outcome characteristics of carbapenem-resistant Enterobacteriaceae over a 10-year period (2010–2020) in Oman. IJID Reg. 2022;4:165–170. doi:10.1016/j.ijregi.2022.08.001
90. Alotaibi NH, Barri AM, Somily AM. The attributable factors that increase the likelihood of central line associated blood stream infection related in-hospital 30-day mortality. Cureus. 2023;15(3):e35898. doi:10.7759/cureus.35898
91. Al Battashi A, Al Harrassi B, Al maskari N, Al Hashami H, Al Awaidy S. Alarming antibiotic resistance in pediatric oncology patients: a three-year prospective cohort study from Oman. Infect Drug Resist. 2022;15:3939–3947. doi:10.2147/IDR.S369909
92. Al-Tawfiq JA, Hinedi K, Khairallah H, et al. Epidemiology and source of infection in patients with febrile neutropenia: a ten-year longitudinal study. J Infect Public Health. 2019;12(3):364–366. doi:10.1016/j.jiph.2018.12.006
93. Alotaibi NH, Barri A, Elahi MA. Length of stay in patients with central line-associated bloodstream infection at a tertiary hospital in the Kingdom of Saudi Arabia. Cureus. 2020;12(10):e10820. doi:10.7759/cureus.10820
94. Alshaikh R, AlKhalifah A, Fayed A, AlYousef S. Factors influencing the length of stay among patients admitted to a tertiary pediatric intensive care unit in Saudi Arabia. Front Pediatr. 2022;10:1093160. doi:10.3389/fped.2022.1093160
95. Abazid RM, Al-Harbi SA, Allihimy AS, et al. Incidence of delirium in the critical care unit and risk factors in the Central Region, Saudi Arabia. Saudi Med J. 2021;42(4):445–448. doi:10.15537/smj.2021.42.4.20200754
96. Alshukairi A, Alserehi H, El-Saed A, et al. A de-escalation protocol for febrile neutropenia cases and its impact on carbapenem resistance: a retrospective, quasi-experimental single-center study. J Infect Public Health. 2016;9(4):443–451. doi:10.1016/j.jiph.2015.11.004
97. Alzahrani FM, Al-Amri A, Shaikh SS, et al. Direct DNA sequencing-based analysis of microbiota associated with hematological malignancies in the Eastern Province of Saudi Arabia. Biomed Res Int. 2021;2021(1):4202019. doi:10.1155/2021/4202019
98. Al Rahmany D, Albeloushi A, Alreesi I, et al. Exploring bacterial resistance in Northern Oman, a foundation for implementing evidence-based antimicrobial stewardship program. Int J Infect Dis. 2019;83:77–82. doi:10.1016/j.ijid.2019.04.004
99. Abdelfattah RR, Al-Jumaah S, Al-Korbi L, Al-Qahtani T. Three years’ experience of dialysis event surveillance. Am J Infect Control. 2019;47(7):793–797. doi:10.1016/j.ajic.2018.12.011
100. Badawy DA, Mowafi HS, Al-Mousa HH. Surveillance of dialysis events: 12-month experience at five outpatient adult hemodialysis centers in Kuwait. J Infect Public Health. 2014;7(5):386–391. doi:10.1016/j.jiph.2014.04.008
101. Al-Tawfiq JA. The pattern and impact of infectious diseases consultation on antimicrobial prescription. J Glob Infect Dis. 2013;5(2):45–48. doi:10.4103/0974-777X.112266
102. Abdellatif M, Al-Khabori M, Rahman AU, Khan AA, Al-Farsi A, Ali K. Outcome of late-onset neonatal sepsis at a tertiary hospital in Oman. Oman Med J. 2019;34(4):302–307. doi:10.5001/omj.2019.60
103. AlAzmi A, Jastaniah W, AlDabbagh M, Elimam N. A clinical approach to non-neutropenic fever in children with cancer. J Oncol Pharm Pract. 2021;27(3):560–569. doi:10.1177/1078155220925161
104. Alrugaib T, Alsultan A, Elbashir E, Albdah B, Alharbi M, Essa MF. Antimicrobial prophylaxis and the rate of blood stream infections and Clostridioides difficile in pediatric stem cell transplantation: a single-center retrospective study. Pediatr Transplant. 2023;27(1):e14375. doi:10.1111/petr.14375
105. Alarjani KM, Almutairi AM, AlQahtany FS, Soundharrajan I. Methicillin and multidrug resistant pathogenic Staphylococcus aureus associated sepsis in hospitalized neonatal infections and antibiotic susceptibility. J Infect Public Health. 2021;14(11):1630–1634. doi:10.1016/j.jiph.2021.08.031
106. Al Luhidan L, Madani A, Albanyan EA, et al. Neonatal group B streptococcal infection in a tertiary care hospital in Saudi Arabia: a 13-year experience. Pediatr Infect Dis J. 2019;38(7):731–734. doi:10.1097/INF.0000000000002269
107. Mahmoud AM, Alpakistany TA, Ismail KA, et al. Effect of COVID-19 pandemic on neonatal sepsis. Clin Lab. 2022;68(5). doi:10.7754/Clin.Lab.2021.210711
108. Alsaif MA, Abdulbaqi M, Al Noaim K, Aghbari M, Alabdulqader M, Robinson JL. Prevalence of serious bacterial infections in children with sickle cell disease at King Abdulaziz Hospital, Al Ahsa. Mediterr J Hematol Infect Dis. 2021;13(1):e2021002. doi:10.4084/MJHID.2021.002
109. Albert MJ, Bulach D, Alfouzan W, et al. Non-typhoidal Salmonella blood stream infection in Kuwait: clinical and microbiological characteristics. PLoS Negl Trop Dis. 2019;13(4):e0007293. doi:10.1371/journal.pntd.0007293
110. Ali M, Razok A, Hisham Ziglam H. A 5-year retrospective study of Actinomyces odontolyticus bacteremia in the state of Qatar, case series. Ann Med Surg Lond. 2022;76:103583. doi:10.1016/j.amsu.2022.103583
111. Sasi S, Abid FB, Wilson GJ, Zaqout A, Nair AP, Chitrambika P. Intrauterine infection and postpartum bacteremia due to Streptococcus gallolyticus subsp gallolyticus: an emerging concern. IDCases. 2022;29:e01562. doi:10.1016/j.idcr.2022.e01562
112. Jamal W, Khodakhast FB, AlAzmi A, Sόki J, AlHashem G, Rotimi VO. Prevalence and antimicrobial susceptibility of enterotoxigenic extra-intestinal Bacteroides fragilis among 13-year collection of isolates in Kuwait. BMC Microbiol. 2020;20(1):14. doi:10.1186/s12866-020-1703-4
113. Alyami AM, Kaabia NM, AlQasim MA, et al. Chryseobacterium/Elizabethkingia species infections in Saudi Arabia. Saudi Med J. 2020;41(3):309–313. doi:10.15537/smj.2020.3.24985
114. Al-Matary A, Heena H, AlSarheed AS, et al. Characteristics of neonatal sepsis at a tertiary care hospital in Saudi Arabia. J Infect Public Health. 2019;12(5):666–672. doi:10.1016/j.jiph.2019.03.007
115. Aloraifi RI, Alharthi AF, Almefleh AA, et al. Prevalence of carbapenem non-susceptible gram-negative bacteria at tertiary care hospitals in Saudi Arabia. Cureus. 2023;15(1):e33767. doi:10.7759/cureus.33767
116. Al Khamis M, AlMusa Z, Hashhoush M, Alsaif N, Salam A, Atta M. Carbapenem-resistant Enterobacteriaceae: a retrospective review of presentation, treatment, and clinical outcomes in a tertiary care referral hospital. Cureus. 2022;14(7):e27094. doi:10.7759/cureus.27094
117. Balkhair A, Al-Farsi YM, Al-Muharrmi Z, et al. Epidemiology of multi-drug resistant organisms in a teaching hospital in Oman: a one-year hospital-based study. Sci World J. 2014;2014:157102. doi:10.1155/2014/157102
118. Ahn C, Butt AA, Rivera JI, et al. OXA-48-producing Enterobacteriaceae causing bacteremia, United Arab Emirates. Int J Infect Dis. 2015;30:36–37. doi:10.1016/j.ijid.2014.11.008
119. Jamal W, Rotimi VO, Albert MJ, Khodakhast F, Nordmann P, Poirel L. High prevalence of VIM-4 and NDM-1 metallo-β-lactamase among carbapenem-resistant Enterobacteriaceae. J Med Microbiol. 2013;62(8):1239–1244. doi:10.1099/jmm.0.059915-0
120. Nasr Z, Babiker A, Elbasheer M, Osman A, Elazzazy S, Wilby KJ. Practice implications of an antimicrobial stewardship intervention in a tertiary care teaching hospital, Qatar. East Mediterr Health J. 2019;25(3):172–180. doi:10.26719/emhj.18.026
121. Elajez R, Abdallah I, Bakdach D, et al. Thrombocytopenia associated with teicoplanin use: a retrospective observational study. Ann Pharmacother. 2022:10600280221078123. 10.1177/10600280221078123
122. Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. doi:10.1002/phar.2209
123. Garbati MA, Bin Abdulhak A, Baba K, Sakkijha H. Infection due to colistin-resistant Enterobacteriaceae in critically-ill patients. J Infect Dev Ctries. 2013;7(10):713–719. doi:10.3855/jidc.2851
124. Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, Wali G. Efficacy of ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. BMC Infect Dis. 2019;19(1):772. doi:10.1186/s12879-019-4409-1
125. El Nekidy WS, Al Ali M, Abidi E, et al. Microbiologic outcomes of ceftazidime-avibactam dosing in patients with sepsis utilizing renal replacement therapies. Hemodial Int. 2023;27(3):289–295. doi:10.1111/hdi.13090
126. Alsaedi AA, El-Saed A, Althaqafi A, Bhutta MJ, Abukhzam B, Alshamrani M. Antimicrobial therapy, resistance, and appropriateness in healthcare-associated and community-associated infections; a point prevalence survey. J Infect Chemother. 2022;28(10):1358–1363. doi:10.1016/j.jiac.2022.06.003
127. Al-Omari A, Al Mutair A, Alhumaid S, et al. The impact of antimicrobial stewardship program implementation at four tertiary private hospitals: results of a five-years pre-post analysis. Antimicrob Resist Infect Control. 2020;9(1):95. doi:10.1186/s13756-020-00751-4
128. Boyd SE, Holmes A, Peck R, Livermore DM, Hope W. OXA-48-Like β-Lactamases: global epidemiology, treatment options, and development pipeline. Antimicrob Agents Chemother. 2022;66(8):e0021622. doi:10.1128/aac.00216-22
129. Rosenthal VD, Jarvis WR, Jamulitrat S, et al. Socioeconomic impact on device-associated infections in pediatric intensive care units of 16 limited-resource countries: international nosocomial infection control consortium findings. Pediatr Crit Care Med. 2012;13(4):399–406. doi:10.1097/PCC.0b013e318238b260
130. Kabrah AM, Kabrah SM, Bahwerth FS, Naof F Alredaini NF. Antibiotic resistance profile of common bacteria isolated from blood stream, lower respiratory tract and urinary infections in intensive care unit in Saudi Arabia: a retrospective study. Ethiop J Health Sci. 2021;31(6):1231–1240. doi:10.4314/ejhs.v31i6.19
131. Li D, Huang X, Rao H, et al. Klebsiella pneumoniae bacteremia mortality: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2023;13:1157010. doi:10.3389/fcimb.2023.1157010
132. Liu Y-C, Lu C-Y, Yen T-Y, et al. Clinical characteristics and outcomes of carbapenem-resistant Enterobacterales bacteremia in pediatric patients. J Microbiol Immunol Infect. 2023;56(1):84–92. doi:10.1016/j.jmii.2022.09.010
133. Vivo A, Fitzpatrick MA, Suda KJ, et al. Epidemiology and outcomes associated with carbapenem-resistant Acinetobacter baumannii and carbapenem-resistant Pseudomonas aeruginosa: a retrospective cohort study. BMC Infect Dis. 2022;22(1):491. doi:10.1186/s12879-022-07436-w
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

