Back to Journals » Journal of Pain Research » Volume 18
Balanced Opioid-Free Anesthesia on Chronic Postsurgical Pain After Video-Assisted Thoracoscopic Surgery: A Randomized Controlled Trial Protocol
Authors Huo WW, Qian J , Zhao HX , Dou W , Chen SM, Ji FH , Peng K
Received 23 January 2025
Accepted for publication 3 May 2025
Published 15 May 2025 Volume 2025:18 Pages 2459—2466
DOI https://doi.org/10.2147/JPR.S519022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Karina Gritsenko
Wen-wen Huo,1,2,* Jia-yu Qian,1,3,* Han-xue Zhao,1,2,* Wei Dou,1,2 Shao-mu Chen,4 Fu-hai Ji,1,2 Ke Peng1,2
1Department of Anesthesiology, First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China; 2Institute of Anesthesiology, Soochow University, Suzhou, Jiangsu, People’s Republic of China; 3Department of Anesthesiology, Zhangjiagang First People’s Hospital, Zhangjiagang, Jiangsu, People’s Republic of China; 4Department of Thoracic Surgery, First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ke Peng, Department of Anesthesiology, First Affiliated Hospital of Soochow University, 899 Pinghai Road, Suzhou, Jiangsu, 215006, People’s Republic of China, Tel +86-15962155989, Email [email protected]
Background: Opioids are widely used for anesthesia and postoperative analgesia; however, their use is related to increased risks of untoward effects including hyperalgesia and chronic postsurgical pain (CPSP). We aim to compare opioid-free anesthesia (OFA) with opioid-based anesthesia (OBA) on the incidence of CPSP after video-assisted thoracoscopic surgery (VATS).
Methods: This randomized controlled clinical trial was approved by the Medical Ethics Committee of the First Affiliated Hospital of Soochow University, Suzhou, China. A total of 180 adult patients undergoing VATS lung resection will be randomized to receive one of two balanced anesthesia regimens: OFA (dexmedetomidine, esketamine, and sevoflurane) or OBA (sufentanil and sevoflurane). A standardized multimodal analgesia comprises erector spinae plane block, intravenous flurbiprofen axetil, and patient-controlled sufentanil analgesia. The primary outcome is the incidence of CPSP at 3 months after surgery. Secondary outcomes include acute postoperative pain at rest and while coughing (at discharge from post-anesthesia care unit and 6, 24 and 48 hours after surgery), the incidences of postoperative pain at 1 month and 6 months, postoperative 24- and 48-hour sufentanil consumption, adverse events (postoperative nausea and vomiting, headache, dizziness, hallucination, and nightmare), length of post-anesthesia care unit and hospital stay, and the 15-item quality of recovery scores at 48 hours after surgery.
Discussion: We hypothesize that the OFA strategy would decrease the incidence of CPSP, reduce postoperative adverse events, and enhance quality of recovery following VATS procedures.
Registration: Chinese Clinical Trial Registry (ChiCTR2400081099).
Keywords: chronic postsurgical pain, multimodal analgesia, opioid free, quality of recovery, video-assisted thoracoscopic surgery
Introduction
Video-assisted thoracoscopic surgery (VATS) has been increasingly performed for lung lesion resection. Although less invasiveness compared to thoracotomy, these procedures still result in significant pain.1–4 Approximately 15–60% of patients undergoing thoracic surgery develop chronic postsurgical pain (CPSP).5–7 CPSP is pain that develops or increases in intensity after surgical procedures or tissue injury and persists beyond the healing process, that is, at least 3 months after the initiating event.8–10 Opioid medications (such as morphine, fentanyl, sufentanil, and oxycodone) are commonly used in clinical anesthesia and postoperative analgesia. While opioids exert strong antinociceptive and analgesic effects, their use can lead to adverse events including postoperative nausea and vomiting (PONV), pruritus, dizziness, respiratory depression, gastrointestinal paralysis, hyperalgesia, CPSP, and opioid abuse.11–13 Multimodal analgesia is recommended for optimizing pain management and reducing opioid-related side effects.
Balanced anesthesia refers to the simultaneous use of different medications to achieve an anesthetic state while reducing undesirable side effects.14–16 Over the recent years, the use of OFA has been in the spotlight of clinical research.17,18 Our recent work demonstrated the feasibility of providing general anesthesia without using opioids for surgical patients, demonstrating the benefits of OFA in reducing PONV compared to conventional opioid-based anesthesia (OBA).19–21 A previous study also suggested that OFA was associated with reduced acute postoperative pain and analgesia-related adverse events after lung surgery.22 Whether OFA affects the incidence of CPSP in patients undergoing VATS remains unknown. Herein, we design this clinical trial to hypothesize that the OFA strategy, compared to OBA, would reduce the rate of CPSP, mitigate opioid-related adverse effects, and enhance recovery in patients undergoing VATS procedures.
Methods
Ethics and Registration
The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Soochow University (Approval No. 2024–027) on January 31, 2024. We registered it at the Chinese Clinical Trial Registry (registration number: ChiCTR2400081099) on February 22, 2024. All participants will sign the informed consent. We will strictly follow the Declaration of Helsinki. We report this study protocol in accordance to the guidelines of Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement. (Table S1).23
Study Design and Status
This is a single-center, prospective, randomized, patient-blind and assessor-blind, controlled clinical trial with two parallel groups and superiority design. A total of 180 patients will be enrolled at the First Affiliated Hospital of Soochow University in Suzhou, China. The enrollment of patients is ongoing by the time of this protocol submission. The study flow diagram is presented in Figure 1.
 |
Figure 1 Flow diagram of this trial. Abbreviations: OFA, opioid-free anesthesia; OBA, opioid-based anesthesia. |
Inclusion Criteria
1. Age ≥18 years old;
2. American Society of Anesthesiologists grade I–III;
3. Undergoing elective VATS lung resection under general anesthesia and being transferred to surgical wards postoperatively;
Exclusion Criteria
1. Body mass index ≥35 kg/m2;
2. Allergy or contraindication to the medications in this study;
3. Coronary heart disease, myocardial infarction, sick sinus syndrome or severe bradycardia (heart rate [HR] <50 beats/min), atrioventricular block (second-degree or greater), or ejection fraction <40%;
4. Liver dysfunction (Child-Pugh class C) or renal failure (renal replacement therapy);
5. Parkinson’s disease, Alzheimer’s disease, seizures, epilepsy;
6. History of chronic pain (defined as pain with numeric rating scale [NRS] score ≥1 that lasts or recurs for more than 3 months,) or preoperative use of any sedative or analgesic;
7. Inability to participate in this study (eg, cognitive deficiencies) or refusal for participation.
Randomization and Blinding
A research coordinator who will not participate in the subsequent study process performs the online randomization (1:1 and block sizes of 4). The random codes will be kept in sealed opaque envelopes. Shortly before anesthesia, a research nurse blinded to the randomization opens the envelope and assigns patients to either the OBA or OFA group. The anesthesia team is aware of the study groups due to the nature of anesthetic treatment (dexmedetomidine infusion during anesthesia induction in the OFA group but not in the OBA group); however, the study hypothesis will not be disclosed. Patients, other perioperative care stuff, and outcome assessors will be fully blinded to the group assignment.
Study Interventions
Figure 2 illustrates the study interventions in the two groups. Patients in the OFA group will receive dexmedetomidine infusion (0.6 µg/kg over 10 min), esketamine 0.3 mg/kg, and propofol 1.5–2.0 mg/kg for anesthesia induction, while patients in the OBA group will receive sufentanil 0.3 µg/kg and propofol 1.5–2.0 mg/kg. During surgery, general anesthesia will be maintained with inhalation of 1–3% sevoflurane, and dexmedetomidine infusion at a rate of 0.2–1.0 µg/kg/h plus boluses of esketamine 0.1 mg/kg as needed (in the OFA group) or boluses of sufentanil 0.1 µg/kg (in the OBA group). This OFA regimen has been successfully applied for patients undergoing thoracoscopic surgery in our recent study.19
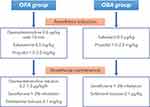 |
Figure 2 Study interventions in the two groups. Abbreviations: OFA, opioid-free anesthesia; OBA, opioid-based anesthesia. |
Anesthesia and Multimodal Analgesia
Patients will be monitored with electrocardiogram, non-invasive blood pressure, pulse oximetry, end-tidal carbon dioxide, nasopharyngeal temperature, bispectral index (BIS) and invasive blood pressure via a radial artery catheter. During anesthesia induction, rocuronium 0.6 mg/kg will be administered for intubation with a double-lumen endotracheal tube. The correct position will be confirmed by auscultation and flexible fiberoptic bronchoscopy. One-lung ventilation will be performed with tidal volume 6–8 mL/kg and respiratory rate 12–15 times/min. Depth of anesthesia will be maintained at BIS values 40–60. All patients will receive intravenous (i.v). dexamethasone 5 mg during anesthesia induction and palonosetron 0.25 mg before the end of surgery, as prophylaxis of PONV. Intraoperative fluid infusion and hemodynamic events including hypotension (mean blood pressure reduction >30% of baseline), hypertension (mean blood pressure increase >30% of baseline), bradycardia (HR < 45 beats/min), and tachycardia (HR > 100 beats/min) will be managed at the discretion of the anesthesiologists. Patients will be extubated after surgery and transferred to the post-anesthesia care unit (PACU).
Patients in both groups will receive a standardized multimodal analgesia, including erector spinae plane block (ESPB), i.v. flurbiprofen axetil, and patient-controlled intravenous analgesia (PCIA). After anesthesia induction and intubation, patients will be placed in a lateral position. The ESPB procedures will be performed by a skilled anesthesiologist before the start of surgery. A 22-gauge block needle will be advanced to reach the T5 transverse process craniocaudally under the guidance of ultrasound using the in-plane technique. After an aspiration test, 0.375% ropivacaine 20 mL will be injected, and the linear spread of the anesthetics between the transverse processes and erector spinae muscle indicates the successful block. Flurbiprofen axetil 50 mg will be administered intraoperatively and twice daily during postoperative day 1 and 2. Patients will also receive PCIA (sufentanil 100 μg diluted with normal saline to a final volume of 100 mL) during the first two postoperative days. The device is programmed to deliver a bolus dose of sufentanil 1 μg with a lockout time of 5 minutes. Patients will be instructed to press the button for pain relief.
Primary Outcome
The primary outcome is the incidence of CPSP at 3 months after surgery, defined as NRS pain scores ≥1, which is in line with the recent studies.24–26 The outcome measurement with the NRS will be performed by an independent researcher blinded to group assignment via telephone follow-up. The NRS scores range from 0 to 10, where 0 denotes no pain and 10 denotes the worst pain imaginable. As pain cutoffs of NRS ≥1, ≥3, or ≥4 have been used to define CPSP,27 we will also assess pain with NRS scores ≥4 in this study.
Secondary Outcomes
Secondary outcomes include: (1) the NRS pain scores at rest and while coughing at PACU discharge, and 6, 24 and 48 hours postoperatively, (2) the incidences of pain at 1 month and 6 months postoperatively; (3) the total amount of sufentanil consumption until 24 and 48 hours postoperatively; (4) the incidences of PONV, headache, dizziness, hallucination, and nightmare during 0–48 hours postoperatively; (5) length of PACU stay, (6) length of postoperative hospital stay, and (7) quality of recovery at 48 hours after surgery, assessed using the 15-item quality of recovery (QoR-15) scale.
Data Collection and Monitoring
Table 1 illustrates the schedule of patient enrollment, study interventions, and outcome assessment according to the SPIRIT guideline. A day before surgery, an independent research staff who is unaware of the randomization will screen for eligible patients and collect demographic and baseline data. Intraoperative data (use of anesthetics and analgesics, fluid infusion, hemodynamic events and interventions, etc.) and surgical characteristics will be extracted from the Anesthesia Information System (MedicalSystem DoCare v5.0, Suzhou, China). Postoperative outcomes will be assessed by an independent researcher blinded to the group allocation. If patients are discharged from the hospital, follow-up will be completed by telephone. Data will be documented in the designated case report forms. An electronic database will be established based on the collected data. There is a Data Monitoring Committee (DMC) at the First Affiliated Hospital of Soochow University independent of the research team. The implementation of this trial and all relevant data will be monitored by the DMC.
 |
Table 1 Schedule of Patient Enrollment, Study Interventions, and Outcome Assessment |
Sample Size Calculation
According to the literature, the incidence of chronic pain after VATS is approximately 15–60%.5–7 We hypothesize that 20% of patients in the OFA group would have CPSP at 3 months after surgery compared to 40% in the OBA group. Based on this assumption at a two-sided significance level of 0.05 and power of 80%, we calculate that 80 patients are needed in each group using the Z-test (PASS 15.0 software). After considering an attrition rate of ~10%, we determine a required sample size of 180 (n = 90 in each group).
Statistical Analysis
The distribution of continuous data will be assessed with the Shapiro–Wilk test. Data in normal distribution will be presented as mean (standard deviation) and compared using the independent t test, while data in non-normal distribution will be shown as median (interquartile range) and compared with the Mann–Whitney rank-sum test. Categorical data will be presented as number (percentage) and compared with the Chi-square test or Fisher’s exact test.
For the primary outcome of the incidence of CPSP, the treatment effect of OFA vs OBA will be assessed using the relative risk (RR) with the 95% confidence interval (CI). A two-sided P < 0.05 will be applied to determine the statistical significance. A prespecified subgroup analysis for the primary outcome will be performed according to sex, age, acute postoperative pain, and postoperative sufentanil consumption. The secondary outcomes will be assessed using the RR or difference in means or medians with the corresponding 95% CI. A false discovery rate q < 0.05 will be applied using the Benjamin–Hochberg method to correct for multiple comparisons of the secondary outcomes. Data will be analyzed on the intention-to-treat basis. Missing data will not be imputed. Statistical analyses will be done using the SPSS (v.25.0, IBM SPSS, Chicago, IL, USA), based on a dataset without the details of group allocation and patient personal information.
Discussion
In this randomized controlled trial, we plan to enroll 180 adult patients to compare the use of balanced OFA with OBA in patients who undergo VATS lung resection. Our primary hypothesis is that OFA could decrease the incidence of CPSP 3 months postoperatively. Additionally, we will assess acute postoperative pain, total amount of PICA sufentanil, adverse events (PONV, headache, dizziness, hallucination, and nightmare), length of PACU and hospital stay, and the QoR-15 scores at 48 h after surgery. This trial will be conducted and results will be reported on the basis of the Consolidated Standards of Reporting Trials guideline.28
OFA refers to the avoidance of opioids during surgery while inhibiting nociception by using alternative medications and techniques. The common components of OFA include dexmedetomidine, N-methyl-D-aspartate (NMDA) antagonists (ketamine or esketamine), lidocaine, and regional anesthesia.29–32 Dexmedetomidine is a highly selective α-2 adrenergic agonist with analgesic properties and has been used as an opioid substitute in various surgical procedures.33,34 A high dose of dexmedetomidine may induce hemodynamic adverse events such as bradycardia and hypotension.35 Esketamine is an NMDA antagonist with a stronger analgesic effect and a faster orientation recovery time compared to ketamine. Esketamine has been used in OFA to reduce opioid-related side effects and postoperative pain.36–38 A high dose of esketamine may lead to mental side effects such as headache, dizziness, hallucination, nightmare. The doses of dexmedetomidine and esketamine in the current study are within the clinical normal ranges and are unlikely to induce those adverse effects, which has been demonstrated in our recent study.19
Ultrasound-guided ESPB will be applied in this study, which deposits local analgesics into the interfascial plane between the transverse spinal processes and the erector spinae muscle.39 ESPB can block the dorsal and ventral branches of the thoracic spinal nerves, with a sympathetic nerve block to some extent. ESPB has been reported to provide effective analgesia in many types of surgeries including VATS procedures, and the use of ESPB is associated with a lower incidence of complications compared with systemic analgesia.40,41
As far as we know, this is the first randomized controlled trial powered to assess the effects of OFA on CPSP after VATS lung surgery. The primary outcome of this trial—the incidence of CPSP at 3 months postoperatively—is defined as NRS ≥ 1. This threshold was selected based on the recent studies.24–27 We aim to determine whether OFA reduces the risk of any persistent pain, including mild pain, which is often underreported but clinically relevant. Even mild pain may impair quality of life or progress to more severe chronic pain states. In addition, we will assess pain with NRS ≥ 4 in this study. Nonetheless, there are some limitations. First, perioperative pain control affects the development of postoperative chronic pain. The ESPB-based multimodal analgesia will be employed in our patients, which may not be the routine clinical practice during thoracic surgery in other medical centers. Second, the anesthesia team cannot be blinded to the study groups due to the nature of drug administration. Next, chronic pain may be more prevalent after thoracotomy than after thoracoscopic surgery. Future studies are needed to assess whether applying OFA would mitigate acute and chronic pain following thoracotomy.
In conclusion, this prospective randomized controlled study will elucidate the effects of balanced OFA vs OBA on the incidence of CPSP after VATS lung surgery. We expect that our results would strengthen the clinical evidence to optimize pain management for patients undergoing these surgical procedures.
Funding
This work will be supported by National Natural Science Foundation of China (82301387 and 82471290), Suzhou Basic Research Pilot Project (SSD2024082), Suzhou Medical Health Science and Technology Innovation Project (SKY2022136), Clinical Diagnosis and Treatment Technology Innovation Project of the First Affiliated Hospital of Soochow University (CXZL-Q-240714), and Suzhou Key Laboratory of Anesthesiology (SZS2023013). The funders have no role in the study design, data collection, data analysis, interpretation, or writing of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi:10.1016/S1470-2045(16)00173-X
2. Schug SA, Bruce J. Risk stratification for the development of chronic postsurgical pain. Pain Rep. 2017;2(6):e627. doi:10.1097/PR9.0000000000000627
3. Tawfic Q, Kumar K, Pirani Z, Armstrong K. Prevention of chronic post-surgical pain: the importance of early identification of risk factors. J Anesth. 2017;31(3):424–431. doi:10.1007/s00540-017-2339-x
4. Tong Y, Wei P, Wang S, et al. Characteristics of postoperative pain after VATS and pain-related factors: the experience in national cancer center of China. J Pain Res. 2020;13:1861–1867. doi:10.2147/JPR.S249134
5. Wei S, Zhang G, Ma J, et al. Randomized controlled trial of an alternative drainage strategy vs routine chest tube insertion for postoperative pain after thoracoscopic wedge resection. BMC Anesthesiol. 2022;22(1):27. doi:10.1186/s12871-022-01569-w
6. Feray S, Lemoine A, Aveline C, Quesnel C. Pain management after thoracic surgery or chest trauma. Minerva Anestesiol. 2023;89(11):1022–1033. doi:10.23736/S0375-9393.23.17291-9
7. Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ. A prospective study of chronic pain after thoracic surgery. Anesthesiology. 2017;126(5):938–951. doi:10.1097/ALN.0000000000001576
8. Pogatzki-Zahn EM, Forget P. ICD-11: a major step forward towards the prediction and prevention of chronic postsurgical pain. Eur J Anaesthesiol. 2024;41(6):399–401. doi:10.1097/EJA.0000000000001996
9. Stamer UM, P L, Hofer DM, Barke A, Korwisi B. Chronic postsurgical pain in the ICD-11: implications for anaesthesiology and pain medicine. Br J Anaesth. 2025. doi:10.1016/j.bja.2025.02.005
10. Moka E, Aguirre JA, Sauter AR, P L, European Society of Regional A, Pain T. Chronic postsurgical pain and transitional pain services: a narrative review highlighting European perspectives. Reg Anesth Pain Med. 2025;50(2):205–212. doi:10.1136/rapm-2024-105614
11. Fletcher D, Martinez V. How can we prevent opioid induced hyperalgesia in surgical patients? Br J Anaesth. 2016;116(4):447–449. doi:10.1093/bja/aew050
12. Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393(10180):1558–1568. doi:10.1016/S0140-6736(19)30430-1
13. Kim DD, Ramirez MF, Cata JP. Opioid use, misuse, and abuse: a narrative review about interventions to reduce opioid consumption and related adverse events in the perioperative setting. Minerva Anestesiol. 2022;88(4):300–307. doi:10.23736/S0375-9393.21.15937-1
14. Hu Y, Zhang QY, Qin GC, et al. Balanced opioid-free anesthesia with lidocaine and esketamine versus balanced anesthesia with sufentanil for gynecological endoscopic surgery: a randomized controlled trial. Sci Rep. 2024;14(1):11759. doi:10.1038/s41598-024-62824-3
15. Coppens M, Steenhout A, De Baerdemaeker L. Adjuvants for balanced anesthesia in ambulatory surgery. Best Pract Res Clin Anaesthesiol. 2023;37(3):409–420. doi:10.1016/j.bpa.2022.12.003
16. Helene Beloeil MG, Lebuffe G, Gerbaud A, et al. Balanced opioid-free anesthesia with dexmedetomidine__versus balanced anesthesia with remifentanil for major__or intermediate noncardiac surgery. Anesthesiology. 2021;134(4):541–551. doi:10.1097/ALN.0000000000003725
17. Feenstra ML, Jansen S, Eshuis WJ, van Berge Henegouwen MI, Hollmann MW, Hermanides J. Opioid-free anesthesia: a systematic review and meta-analysis. J Clin Anesth. 2023;90:111215. doi:10.1016/j.jclinane.2023.111215
18. Léger M, Perrault T, Pessiot-Royer S, et al. Opioid-free anesthesia protocol on the early quality of recovery after major surgery (SOFA Trial): a randomized clinical trial. Anesthesiology. 2024;140(4):679–689. doi:10.1097/ALN.0000000000004840
19. Feng CD, Xu Y, Chen S, et al. Opioid-free anaesthesia reduces postoperative nausea and vomiting after thoracoscopic lung resection: a randomised controlled trial. Br J Anaesth. 2024;132(2):267–276. doi:10.1016/j.bja.2023.11.008
20. Wang D, Ji FH, Peng K. Comparison of opioid-free and opioid-inclusive propofol anaesthesia for thyroid and parathyroid surgery: a reply. Anaesthesia. 2025;80(2):213–214. doi:10.1111/anae.16438
21. Wang D, Sun Y, Zhu YJ, et al. Comparison of opioid-free and opioid-inclusive propofol anaesthesia for thyroid and parathyroid surgery: a randomised controlled trial. Anaesthesia. 2024;79(10):1072–1080. doi:10.1111/anae.16382
22. Wang S, Li Y, Liang C, Han X, Wang J, Miao C. Opioid-free anesthesia reduces the severity of acute postoperative motion-induced pain and patient-controlled epidural analgesia-related adverse events in lung surgery: randomized clinical trial. Front Med. 2023. doi:10.3389/fmed.2023.1243311
23. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e7586
24. Wu XD, Zeng FF, Yu XX, et al. Development and validation of a prediction model for chronic post-surgical pain after thoracic surgery in elderly patients: a retrospective cohort study. J Pain Res. 2022;15:3079–3091. doi:10.2147/JPR.S368295
25. Hu JH, Shi HJ, Han ZY, Liu H, Ji FH, Peng K. Protocol for development and validation of multivariable prediction models for chronic postsurgical pain following video-assisted thoracic surgery. J Pain Res. 2023;16:2251–2256. doi:10.2147/JPR.S416450
26. Wang HT, Liu W, Luo AL, Ma C, Huang YG. Prevalence and risk factors of chronic post-thoracotomy pain in Chinese patients from Peking Union Medical College Hospital. Chin Med J. 2012;125(17):3033–3038.
27. Hofer DM, Lehmann T, Zaslansky R, et al. Rethinking the definition of chronic postsurgical pain: composites of patient-reported pain-related outcomes vs pain intensities alone. Pain. 2022;163(12):2457–2465. doi:10.1097/j.pain.0000000000002653
28. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi:10.1136/bmj.c869
29. Berlier J, Carabalona J-F, Tête H, et al. Effects of opioid-free anesthesia on postoperative morphine consumption after bariatric surgery. J Clin Anesth. 2022;81:110906. doi:10.1016/j.jclinane.2022.110906
30. Matthes K, Gromski MA, Schneider BE, Spiegel JE. Opioid-free single-incision laparoscopic (SIL) cholecystectomy using bilateral TAP blocks. J Clin Anesth. 2012;24(1):65–67. doi:10.1016/j.jclinane.2011.04.014
31. Chen L, He W, Liu X, Lv F, Li Y. Application of opioid-free general anesthesia for gynecological laparoscopic surgery under ERAS protocol: a non-inferiority randomized controlled trial. BMC Anesthesiol. 2023;23(1). doi:10.1186/s12871-023-01994-5
32. Selim J, Jarlier X, Clavier T, et al. Impact of opioid-free anesthesia after video-assisted thoracic surgery: a propensity score study. Ann Thorac Surg. 2022;114(1):218–224. doi:10.1016/j.athoracsur.2021.09.014
33. Gaszynski T, Gaszynska E, Szewczyk T. Dexmedetomidine for awake intubation and an opioid-free general anesthesia in a superobese patient with suspected difficult intubation. Drug Des Devel Ther. 2014;8:909–912. doi:10.2147/DDDT.S64587
34. Li Y, Wang B, Zhang LL, et al. Dexmedetomidine combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique. Anesth Analg. 2016;122(4):1202–1210. doi:10.1213/ANE.0000000000001165
35. Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36(6):926–939. doi:10.1007/s00134-010-1877-6
36. Yan H, Chen W, Chen Y, et al. Opioid-free versus opioid-based anesthesia on postoperative pain after thoracoscopic surgery: the use of intravenous and epidural esketamine. Anesthesia Analg. 2023;137(2):399–408. doi:10.1213/ANE.0000000000006547
37. Massoth C, Schwellenbach J, Saadat-Gilani K, et al. Impact of opioid-free anaesthesia on postoperative nausea, vomiting and pain after gynaecological laparoscopy - A randomised controlled trial. J Clin Anesth. 2021;75:110437. doi:10.1016/j.jclinane.2021.110437
38. Chen HY, Meng XY, Gao H, et al. Esketamine-based opioid-free anaesthesia alleviates postoperative nausea and vomiting in patients who underwent laparoscopic surgery: study protocol for a randomized, double-blinded, multicentre trial. Trials. 2023;24(1):13. doi:10.1186/s13063-022-07003-3
39. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med Sep-Oct. 2016;41(5):621–627. doi:10.1097/AAP.0000000000000451
40. Finnerty DT, McMahon A, McNamara JR, Hartigan SD, Griffin M, Buggy DJ. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br J Anaesth. 2020;125(5):802–810. doi:10.1016/j.bja.2020.06.020
41. Yao Y, Fu S, Dai S, et al. Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: a prospective, randomized, controlled trial. J Clin Anesth. 2020;63:109783. doi:10.1016/j.jclinane.2020.109783
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

