Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Benefit of Conversion Therapy in Patients with Unresectable Hepatocellular Carcinoma: A Propensity Score-Matched Study
Authors Han R , Gan L , Sun L, Lang M, Tian X, Zhu K, Chen L , Li G, Song T
Received 23 July 2024
Accepted for publication 24 September 2024
Published 28 September 2024 Volume 2024:11 Pages 1835—1844
DOI https://doi.org/10.2147/JHC.S482803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Ruyu Han,1,* Leijuan Gan,1,* Liyu Sun,1 Mengran Lang,2 Xindi Tian,1 Kangwei Zhu,1 Lu Chen,1 Guangtao Li,1 Tianqiang Song1
1Department of Hepatobiliary Cancer, Liver Cancer Center, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin Key Laboratory of Digestive Cancer, Tianjin, 300060, People’s Republic of China; 2Hepatobiliary Surgery Department, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianqiang Song, Department of Hepatobiliary Cancer, Liver Cancer Center, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin Key Laboratory of Digestive Cancer, Tianjin, 300060, People’s Republic of China, Tel +86-22-23340123, Fax +86-22-23537796, Email [email protected]
Purpose: This study aimed to investigate the benefit of conversion therapy for patients with unresectable hepatocellular carcinoma (HCC).
Patients and Methods: A retrospective cohort study was conducted involving 40 patients initially deemed unresectable HCC (uHCC). They received surgery following successful conversion therapy involving lenvatinib. The patients were matched in a 1:1 ratio to with a control group who underwent direct surgery, based on pre-treatment clinical data.
Results: The median recurrence-free survival (RFS) duration for the conversion therapy cohort was notably longer than that of the direct surgery cohort (25 months vs 11 months). Furthermore, the 1- and 2-year RFS rates were significantly higher in the conversion therapy group compared to the direct surgery group (1 year: 70.5% vs 40.1%; 2 years: 49.0% vs 19.1%). The survival curves indicated a statistically significantly longer RFS in the conversion therapy cohort compared to the direct surgery cohort (P = 0.007). While patients achieving good remission based on both RECIST 1.1 and mRECIST criteria showed superior median RFS, no significant disparity was observed in the survival curves. The subgroup analysis revealed significantly improved prognosis among patients in the conversion therapy group who were male, older, had a history of alcohol consumption, were non-smokers, had liver cirrhosis, possessed Child-Pugh A liver function, had a tumor diameter exceeding 5 cm, and had an AFP ≥ 400 ng/mL. Among the cohort of 40 patients, only 8 individuals encountered severe adverse reactions, which were managed through dose reduction. None of the patients experienced multiple severe adverse reactions concurrently.
Conclusion: For patients with unresectable hepatocellular carcinoma, conversion therapy offers a significantly better prognosis than direct surgery for uHCC patients.
Keywords: hepatocellular carcinoma, conversion therapy, recurrence-free survival, hepatic resection
Introduction
Hepatocellular carcinoma (HCC) presents a formidable challenge to public health, characterized by high incidence and mortality rates compared to other malignancies.1 The primary radical interventions for HCC encompass hepatic resection (HR), liver transplantation (LT), and radiofrequency ablation (RFA).2 These modalities exclusively recommended for patients in the early stages.3 Unfortunately, the majority of HCC cases are diagnosed at intermediate to advanced stages, precluding the feasibility of surgical interventions.4,5 Historically, therapeutic options for these patients were constrained due to the absence of effective pharmaceutical interventions.6 However, the advent of targeted therapies and immunotherapies has substantially broadened the therapeutic armamentarium for HCC, yielding increasingly efficacious treatments.7–9 Consequently, the landscape of therapeutic options for patients with intermediate and advanced HCC has progressively expanded.
In recent years, conversion therapy has emerged as a key component in the diagnostic and therapeutic strategy for intermediate and advanced HCC.10,11 Conversion therapy aims to offer localized or systemic treatment to patients initially deemed unresectable, potentially enabling them to undergo radical treatment.5 Such patients can be categorized into two distinct groups. One category comprises those who are unresectable due to systemic conditions that render them unable to tolerate surgical trauma, have compromised liver function, or possess insufficient remaining liver volume. The second group includes patients whose HCC is technically resectable, yet the outcomes of resection are no better than those of non-surgical treatments, a condition referred to as oncologically or biologically unresectable While the definition of surgically unresectable HCC is widely accepted, the definition of oncologically unresectable HCC is dynamic and more controversial. Our previous study found that triple therapy of lenvatinib plus sintilimab plus arterially directed therapy transformed unresectable hepatocellular carcinoma into resectable disease.12 The debate over whether conversion therapy can provide a survival benefit compared to direct surgery remains unresolved. Therefore, this study aimed to compare patients who underwent surgery after successful conversion with those who underwent surgery directly, seeking to understand the benefit of conversion therapy relative to direct surgery for oncology patients.
Materials and Methods
Patients
We conducted a retrospective analysis of patients with initially unresectable HCC (uHCC) who received lenvatinib between May 2019 and December 2021 at the Cancer Research Institute and Hospital of Tianjin Medical University, followed by surgical intervention. Patients meeting the following criteria were included: (A) age ≥18 years; (B) definitive postoperative pathological diagnosis of HCC, and clinical diagnosis based on the latest clinical guidelines at the time; (C) initial diagnosis of unresectable disease; (D) receiving at least two cycles of lenvatinib initially; (E) undergoing subsequent partial hepatectomy or RFA; (F) liver function Child-Pugh Grade A or B and (G) presented with at least one measurable intrahepatic lesion based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 or modified RECIST (mRECIST).13,14 Exclusion criteria comprised contraindications to therapy (absolute neutrophil count <1.2 × 109/L, platelet count <60 × 109/L, albumin concentration <30 g/L, total bilirubin concentration ≥30 μmol/L, aspartate and glutamic transaminases ≥5 × upper limit of normal range (ULN), creatinine clearance ≥1.5 × ULN, left ventricular ejection fraction <45%, a history of other malignancies, incomplete clinical data, and death or loss to follow-up within 1 month of surgery). In addition, clinical data were collected from HCC patients who received surgical treatment from January 2014 to December 2021 at the same hospital. Propensity score matching (PSM) was conducted in a 1:1 ratio based on the clinicopathological data before initial treatment. Due to the limited number of Barcelona Clinic Liver Cancer (BCLC) stage C patients in the direct surgery group, the two groups were categorized into stages A, B, and C according to BCLC staging and matched separately.15
Treatments
Lenvatinib-based combination therapy was used as conversion therapy. In the conversion group, lenvatinib (Lenvima®, Eisai, Tokyo, Japan) was taken orally. The first dose of anti-PD-1 therapy was administered within 7 days of the start of lenvatinib treatment. The specific dosing regimen, including the combination with immunotherapy or interventional therapy, was determined by two or more experienced clinicians. Drug doses were administered in accordance with guidelines and adjusted based on the patient’s performance status, liver function, and treatment tolerance. The specific interventional regimen, such as TACE, HAIC, or a combination thereof, was decided upon by an experienced interventionalist.
Data Collection and Imaging Evaluation
The study encompassed a comprehensive analysis of various laboratory and imaging parameters, including age, sex, hepatitis status, cirrhosis presence, tumor size, number of tumors, Child-Pugh classification, BCLC staging, Eastern Cooperative Oncology Group performance status (ECOG PS), presence of extrahepatic metastases, presence of vascular invasion, albumin-bilirubin (ALBI) classification,16 and alpha-fetoprotein (AFP) levels. All laboratory assessments were conducted within 3 days prior to initial treatment, while imaging evaluations, utilizing enhanced magnetic resonance imaging (MRI) or computed tomography (CT), were performed within 7 days before the initial treatment and subsequently every 2 to 3 months. Imaging interpretations were independently performed by two radiologists, with disagreements resolved by a more experienced radiologist. The feasibility and approach of surgical intervention were assessed by a panel of two or more experienced clinicians.
Study Endpoints
The primary study endpoint of this study was recurrence-free survival (RFS), defined as the duration from the initiation of radical surgery to the identification of disease recurrence. The secondary endpoint was treatment-related adverse events (TRAEs), assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. During the data collection process, researchers adhered to the 2013 revised Declaration of Helsinki by maintaining blinding to clinical outcomes. Approval for the study was obtained from the ethics committee of Cancer Research Institute and the Hospital of Tianjin Medical University. The study followed Good Clinical Practice Guidelines and relevant local regulations. Prior to analysis, data containing potentially identifying information were anonymized and de-identified. As the study was retrospective, did not intervene in the routine diagnosis and treatment of patients, and all information that could potentially reveal the identity of the patients was anonymized and de-identified, informed consent was waived with the agreement of the ethics committee.
Statistical Analysis
PSM was conducted to ensure comparability between the two patient groups based on their sex, age, liver cirrhosis, AFP, Child-Pugh classification, tumor diameter, and tumor number at baseline. The mean ± standard deviation (SD) was used to describe the normally distributed continuous variables, while the non-normal distributed variables were described using median and quartiles. Student’s t-test or Wilcoxon rank-sum test was used for continuous variables depending on their distribution. Categorical data were presented as numbers and percentages and analyzed using Pearson’s chi-square test or Fisher’s exact test. Survival analyses were performed using the Kaplan–Meier method, and the Log rank test was used to compare survival curves. COX regression was used to calculate the variables affecting RFS. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. A two-sided P-value <0.05 was considered statistically significant. Statistical analyses were conducted using SPSS 26.0 software and R 4.2.3.
Results
Patients
A total of 40 patients undergoing conversion therapy were included in the study. The flow chart for patient inclusion is shown in Figure 1. There were 12 patients classified as BCLC stage B, and 17 patients as stage C. Additionally, 11 patients belonged to stage A with tumor rupture or insufficient remaining functional liver volume. All patients did not meet the criteria for surgery in the BCLC and NCCN guidelines. Among them, 11 patients received a combination of camrelizumab, 1 patient received pembrolizumab, 4 patients received toripalimab, 2 patients received tislelizumab and 22 patients received sintilimab. Furthermore, 28 out of the 40 patients underwent a combination of interventional therapies. Of these, 5 patients received 1 cycle, 6 patients received 2 cycles, 16 patients received 3 cycles, and 1 patient received 5 cycles of interventional therapies. A conversion therapy group (n = 40) and a direct surgery group (n = 40) were established. The clinical baseline data after matching are shown in Table 1. No significant differences were shown between the 2 matched groups.
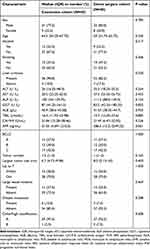 |
Table 1 Clinicopathologic Characteristics of Patients in Two Cohorts |
 |
Figure 1 Flowchart of patient selection. |
Survival
The median follow-up period for patients undergoing conversion therapy was 20 months (IQRs: 16–33 months). The median RFS for conversion cohort was significantly longer compared to the direct surgery cohort (25 months vs 11 months). The median overall survival (OS) for the conversion therapy cohort was significantly longer than that of direct surgery cohort (NA vs 56 months). The 1-year RFS rate for conversion cohort was notably higher than that of direct surgery cohort (70.5% vs 40.1%), with a similar trend observed in the 2-year RFS (49.0% vs 19.1%). Moreover, both the 1-year and 2-year OS rates were significantly higher in the conversion therapy cohort compared to the direct surgery cohort (1-year: 94.4% vs 87.0%; 2-year: 83.7% vs 74.5%).
Using RFS as the outcome variable, both groups underwent survival analysis, revealing that the RFS of the conversion cohort was superior to than that of the direct surgery cohort (P = 0.007) (Figure 2A). While OS was improved in the conversion cohort compared to the direct surgery cohort, no statistical difference was observed between the two groups (P = 0.11) (Figure 2B). Using the optimal degree of tumor remission before surgery as a variable for survival analysis, the median RFS according to the RECIST 1.1 criteria was 23 months for stable disease (SD) patients, with less than half of the partial response (PR) patients experiencing recurrence up to the end of the follow-up period. Conversely, according to mRECIST criteria, the median RFS was 4 months for SD patients, 25 months for PR patients, and less than half of complete response (CR) patients experienced a recurrence by the end of the follow-up period. Median RFS was more favorable in patients with good remission according to both RECIST 1.1 and mRECIST criteria, though no significant difference was found in survival curves (Figure 3A and B).
 |
Figure 2 Kaplan–Meier Curves Between Conversion and Direct Surgery Cohorts (A: RFS; B: OS). |
 |
Figure 3 Kaplan–Meier curves for RFS based on tumor response (A: mRECIST; B: RECIST 1.1). |
Subgroup Analysis
The subgroup analysis illustrates the impact of various factors on RFS in the two groups (Figure 4). The analysis revealed that patients in the conversion group experienced a notably improved prognosis if they were male, older, had a history of alcohol consumption, did not smoke, had liver cirrhosis, possessed Child-Pugh A liver function, had a tumor diameter exceeding 5 cm, and had an AFP level ≥400 ng/mL.
 |
Figure 4 Forest plot for RFS of the matched cohorts of patients. |
Safety Analysis
The median dosing cycle for patients in the conversion cohort was 3 (IQRs: 2–6). No fatalities attributed to treatment were observed in the study. Out of 40 patients, 27 experienced TRAEs. Table 2 presents TRAEs, highlighting hand and foot skin reactions (20%) and liver function abnormalities (20%) being the most prevalent. Conversely, hypertension (7.5%) emerged as the most frequent serious AE (SAE). Only hypertension, rash, muscle pain and abnormal liver function emerged with serious AEs, while all other AEs were graded 1–2. Among the cohort, only 8 individuals (20%) encountered SAEs, which were managed through dose reduction. Notably, none of the patients experienced multiple SAEs concurrently.
 |
Table 2 Treatment-Related Adverse Events |
Discussion
HCC ranks as the sixth most prevalent cancer globally and stands as the third leading cause of death.1 In China, nearly 70% of HCC patients receive a diagnosis at an intermediate to advanced stage, thereby missing the opportunity for curative treatment.17 The median OS for patients with intermediate to advanced disease is only 4–27 months.18 The notion of conversion therapy has increasingly permeated the diagnostic and treatment landscape of HCC. This approach involves administering local or systemic treatments to initially unresectable patients, offering them a chance for surgical intervention.10
However, there are limited studies comparing the prognosis between direct surgery and conversion surgery due to the low number of advanced patients undergoing surgery. In this study, we conducted a retrospective review of patients who received HR or RFA over the past 8 years. We categorized them into three groups based on preoperative staging and matched them with propensity scores for individuals who underwent conversion therapy. Subsequently, we compared the difference in survival between the two groups, aiming to elucidate the benefits of conversion therapy compared to direct surgery.
Lenvatinib, an oral small-molecule inhibitor targeting multi-receptor tyrosine kinases, is approved as a first-line agent for uHCC patients in several regions, including the United States, European Union, Japan, and China.3,19,20 In an open Phase 3 multicenter clinical trial, lenvatinib demonstrated non-inferiority to sorafenib in terms of OS in untreated advanced HCC. Based on mRECIST criteria, lenvatinib significantly enhances objective response rate (ORR), median time to progression and median progression-free survival compared to sorafenib.9 It is endorsed as a first-line systemic therapy option for advanced HCC by guidelines such as NCCN, ESMO, AASLD and EASL.3,19,21,22 Lenvatinib exerts its effects by targeting the VEGF pathway, thereby inhibiting tumor angiogenesis and mitigating the elevated metastasis and invasiveness associated with VEGF overexpression.23 Furthermore, lenvatinib enhances T lymphocyte infiltration within an immunosuppressive microenvironment.24 Given that immune checkpoint inhibitors (ICIs) require T lymphocyte infiltration to function effectively, lenvatinib facilitates an optimal immunotherapeutic milieu for anti-PD-1 therapy.25 Concurrently, ICIs synergistically contribute to the restoration of an immune-supportive environment and promote vascular normalization.26 Therefore, there is a synergistic effect between lenvatinib and ICIs in antitumor. A phase 1b clinical trial revealed a 46% ORR when combining lenvatinib with pembrolizumab for uHCC patients.27 Shindoh et al found that conversion surgery after lenvatinib treatment could confer a significant survival benefit in some patients with advanced HCC.28 Hence, conversion therapy stands as a viable treatment modality for uHCC patients.
After matching the propensity scores, no significant differences were observed between the two groups in terms of baseline characteristics. Survival curves revealed that the conversion cohort exhibited a significantly better prognosis compared to the direct surgery cohort (P = 0.007). The risk of recurrence was notably lower in the conversion therapy group compared to direct surgery. Moreover, the RFS in the conversion cohort was calculated from the time of surgical treatment to the detection of recurrence, without accounting for the time between conversion therapy and surgery. Therefore, the actual prognosis of the conversion cohort is likely significantly better. Survival analysis incorporating the optimal degree of tumor remission before surgery as a variable indicated that patients achieving CR or PR had a significantly better prognosis than those with SD, regardless of RECIST 1.1 or mRECIST criteria. Although the survival analysis did not show a significant difference, this observation may be attributed to the small sample size.
Through subgroup analysis of both groups, we found that the prognosis was significantly favored in the conversion cohort among patients who were male, older, had a history of alcohol consumption, did not have a history of smoking, had liver cirrhosis, had Child-Pugh class A, had a tumor diameter of more than 5 cm and had an AFP ≥400 ng/mL. No segment of the population exhibited a better prognosis in the direct surgery cohort than compared to the conversion cohort. This finding may be attributed to the notably superior prognosis observed in the conversion cohort compared to the direct surgery cohort.
Upon tallying the TRAEs among patients in the conversion cohort. It was noted that out of 40 patients, only 8 individuals (20%) encountered SAEs, which were alleviated and managed through dose reduction. Only hypertension, rash, muscle pain and abnormal liver function emerged as SAEs, while all other AEs were graded 1–2. None of the patients experienced multiple SAEs concurrently and there were no treatment-related deaths. This suggests that the safety profile of conversion therapy is acceptable When compared with direct surgery, conversion therapy yielded a significantly better prognosis and acceptable AEs. Therefore, for non-surgical patients, receiving combination therapy including lenvatinib may serve as an optional and effective treatment modality.
There are some limitations of this study. First, to ensure an adequate follow-up time, data collection was restricted to patients up to December 2021, resulting in a relatively small sample size in the conversion cohort. Second, to attain a sufficient sample size, lenvatinib was predominantly utilized as the foundation of conversion therapy, complemented by immunotherapy and interventional therapy based on the patient’s condition. Although various conversion therapy regimens were administered to different patients, the primary focus of this study was to evaluate the significance of conversion therapy overall, rather than evaluating specific conversion regimens. Nonetheless, the study’s results underscored the benefits of conversion therapy compared to direct surgery. Third, among the 40 patients in the conversion cohort, more than half (n = 21) received lenvatinib as postoperative adjuvant therapy, with 12 of them undergoing combined immunotherapy, and an additional 4 patients receiving adjuvant PD-1 antibody monotherapy. Incomplete data on patients’ postoperative adjuvant therapy precluded its analysis as a study variable, potentially impacting patients’ RFS and consequently influencing the study results. Finally, as a single-center study, the generalizability of our findings may be limited, highlighting the necessity for large-scale multicenter studies to validate our results.
Conclusion
For patients with uHCC, opting for conversion therapy, which involves initial treatment with lenvatinib followed by surgery, yields a markedly improved long-term prognosis compared to undergoing direct surgery. Additionally, conversion therapy demonstrates an acceptable safety profile.
Acknowledgments
This work is supported by the grant from the National Natural Science Foundation of China (82173317), the Scientific research projects of Tianjin Education Commission (2022KJ227) and the Doctoral Start-up Fund of Tianjin Medical University Cancer Institute & Hospital (B2208).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
3. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
4. Song T, Lang M, Ren S, Gan L, Lu W. The past, present and future of conversion therapy for liver cancer. Am J Cancer Res. 2021;11(10):4711–4724.
5. Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11(2):227–252. doi:10.21037/hbsn-21-328
6. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
7. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
8. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
9. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
10. Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15(3):155–160. doi:10.5582/bst.2021.01091
11. Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240(2):299–305. doi:10.1097/01.sla.0000133123.11932.19
12. Gan L, Lang M, Tian X, et al. A retrospective analysis of conversion therapy with lenvatinib, sintilimab, and arterially-directed therapy in patients with initially unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2023;10:673–686. doi:10.2147/JHC.S404675
13. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
14. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
15. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
16. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol: off J Am Soc Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
17. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
18. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int: off J Int Assoc Study Liver. 2015;35(9):2155–2166. doi:10.1111/liv.12818
19. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
20. Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6(1):18. doi:10.1186/2045-824X-6-18
21. Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Nat Comprehensive Cancer Network. 2021;19(5):541–565. doi:10.6004/jnccn.2021.0022
22. Vogel A, Martinelli E, Vogel A. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol: off J European Soc Med Oncol. 2021;32(6):801–805. doi:10.1016/j.annonc.2021.02.014
23. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616. doi:10.1038/s41571-018-0073-4
24. Hutchinson L. Targeted therapies: lenvatinib SELECTs survival benefit. Nat Rev Endocrinol. 2017;13(9):500. doi:10.1038/nrendo.2017.96
25. Reig M, Bruix J. Lenvatinib: can a non-inferiority trial change clinical practice? Lancet. 2018;391(10126):1123–1124. doi:10.1016/S0140-6736(18)30208-3
26. Wang Y, Jiang M, Zhu J, et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed Pharmacothe. 2020;132:110797. doi:10.1016/j.biopha.2020.110797
27. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol: off J Am Soc Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
28. Shindoh J, Kawamura Y, Kobayashi Y, et al. Prognostic impact of surgical intervention after lenvatinib treatment for advanced hepatocellular carcinoma. Ann Surg Oncol. 2021;28(12):7663–7672. doi:10.1245/s10434-021-09974-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

