Back to Journals » Drug Design, Development and Therapy » Volume 19
Bibliometric and Visualized Analysis of Artemisinin and Its Derivatives in Cancer
Authors Feng J , He Y, Bai T, You Q, Zhu H, Jia C, Liu B, Li S
Received 7 January 2025
Accepted for publication 21 May 2025
Published 30 June 2025 Volume 2025:19 Pages 5517—5538
DOI https://doi.org/10.2147/DDDT.S514219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Solomon Tadesse Zeleke
Jiaming Feng,1,* Yuqing He,1,* Ting Bai,1,* Qianhui You,1,* Huici Zhu,1 Chengyao Jia,2 Baonian Liu,1 Shaoling Li3
1Department of Anatomy, School of Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China; 2Guanghua School of Stomatology, Sun Yat-Sen University, Guangzhou, 510080, People’s Republic of China; 3Department of Pathology, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai, 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Baonian Liu, Department of Anatomy, School of Integrative Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China, Email [email protected] Shaoling Li, Department of Pathology, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai, 200433, People’s Republic of China, Email [email protected]
Background: Artemisinin, found in the traditional Chinese medicine (TCM) Artemisia annua, has demonstrated remarkable efficacy in therapeutics and holds significant potential as a pharmaceutical drug in cancer. Until now, there have been no systematic scientometrics studies to analyze the research trend of artemisinin and its derivatives in cancer.
Aim of the Study: We conducted this bibliometric study with the aim of providing researchers in the field with an all-around summary of artemisinin and its derivatives in cancer, visualizing current research advances, identifying hotspots, and ultimately outlining cutting-edge trends for future development.
Methods: A total of 927 relevant publications from 1990 to 2023 were accessed from the WOSCC database and analyzed bibliometrically utilizing software including CiteSpace VOSviewer and Microsoft Excel.
Results: Global publications in artemisinin and tumor therapy have steadily increased. China is considered the leading country in terms of publication numbers, and Chinese scholars have been influential in the field of artemisinin and its derivatives in the prevention and treatment of cancer. Cluster analysis of co-cited references depicted artemisinin-type drugs, bax-mediated intrinsic pathways, and infected erythrocytes as the most noteworthy topics. In contrast, the keywords analysis revealed a strong emphasis on the issues of cancer, in-vitro, cycle arrest, anticancer activity, and plasmodium-falciparum.
Conclusion: This study provides a comprehensive overview of relevant research trends by explicitly analyzing the published literature associated with artemisinin and its derivatives in cancer. The results of this multifaceted analysis can provide valuable data that offers ample insights for the field and for researchers wishing to venture into the field.
Keywords: artemisinin, cancer, traditional Chinese medicine, therapeutic agent, bibliometric analysis
Introduction
Cancer constitutes a substantial global public health burden1 and stands as the leading cause of mortality among non-communicable diseases (NCDs), as well as the foremost obstacle to enhancing life expectancy worldwide.2 Even with technological advances, the incidence of some cancers continues to rise.3 However, due to its complex nature and the resilience of malignant cells to current treatments, cancer continues to be a formidable challenge in the realm of medical research.4,5 It is gratifying that traditional Chinese medicine has a more significant role in preventing and treating cancer,6–8 with artemisinin-based drugs having a preventive and curative effect on a wide range of diseases.
Artemisinin, discovered in the traditional Chinese medicine (TCM) Artemisia annua,9,10 is a compound identified as a sesquiterpene lactone that features an endoperoxide structure.11 Artemisia annua has been recognized for its extensive application in traditional Chinese medicine, specifically for alleviating fevers and inflammatory conditions, with its utilization for malaria treatment tracing back over two millennia.12 Artemisinin was discovered through research on traditional herbal medicine and is now an important drug for malaria treatment.13 These plant-derived peroxides uniquely kill plasmodium in the pre-erythrocytic stage, blocking their advancement into mature periods with growing pathogenicity.14,15 Beyond malaria, artemisinin and its derivatives, such as artesunate, exhibit broad therapeutic potential against parasitic diseases like schistosomiasis and clonorchiasis,16 as well as bacterial infections,17 inflammatory conditions (eg, SM934 tested in lupus patients),18 and fibrosis19 These compounds also demonstrate anti-inflammatory effects,15,20 inhibit angiogenesis,21 and suppress tumor cell growth by exerting antiproliferative and cytotoxic effects,22 inhibiting cell migration and invasion, modulating gene expression and signaling pathways, and inducing cell cycle arrest and apoptosis.13,23 Notably, research on fibrosis reveals mechanistic parallels with tumor stroma, offering cross-disciplinary insights for cancer therapy, which has garnered significant attention for their potential antitumor applications.24
Numerous studies have been released on using artemisinin and its derivatives in cancer treatment. Bibliometrics is a methodological framework that uses quantitative analysis to examine a large corpus of literature within a specific research domain.25,26 It employs mathematical and statistical methodologies by utilizing visualization techniques to demonstrate various dimensions and trends characterizing the development of a scientific topic.27–29 CiteSpace is a visual analysis software designed to visualize and analyze structural and temporal patterns in scientific literature.30 It facilitates systematic scientometric reviews by providing an outline of the fundamental domain of knowledge, assisting in the recognition of primary subjects and patterns within research fields.31 VOSviewer is designed to construct and visualize bibliometric networks of scientific publications, such as co-authorship networks and keyword co-occurrence networks, helping to reveal the structure and trends of a research field through graphical representations.32 Pajek facilitated the adjustment and enhancement of the layout of the cluster map.33,34
Although crucial contributions have been made in researching artemisinin application and its derivatives in the field of malignancy, many challenges remain.35,36 The selective study of artemisinin and its derivatives is not sufficiently in-depth. Although these compounds have shown cytotoxicity towards certain cancer cells, their lack of specificity may cause harm to healthy tissues.14 Additionally, while their pharmacological effects have been tentatively elucidated,37,38 the specific mechanisms of action remain unclear,39 which limits the precise application of artemisinin and its derivatives in treating malignant neoplasm. Furthermore, despite the potential of artemisinin and its derivatives in anticancer therapy demonstrated by in vitro and animal tests, clinical research remains limited, with only a few trials and case reports demonstrating their efficacy as adjuvants or primary agents in cancer treatment.40–43 Therefore, further research should focus on these areas. This research enhanced the understanding of artemisinin and its derivatives through bibliometric analysis. It improves current research and elevates anticancer investigations of the drug to a more sophisticated level of scientific understanding and practical implementation, thereby enabling its novel application within the realm of contemporary medicine.
Methods
Searching Strategy and Data Collection
The process of sourcing published materials was carried out by utilizing the Web of Science database (https://www.webofscience.com/wos/) on 4 January 2024. All publications were output within text-only files using the “Full Record and Cited References” format. The applied search formula was TS = (artemisinin*) AND TS = (cancer* OR anticancer* OR tumor* OR tumor* OR oncology OR neoplasm* OR carcinoma* OR lymphoma* OR sarcoma* OR leukemia*) AND DT = (Article OR Review) AND LA = (English) AND DOP = (1990–01-01/2023-12-31). Two investigators (Ting Bai and Yuqing He) regained and screened the papers. Any divergent viewpoints were resolved through constructive dialogue with the respective authors until a unanimous agreement had been reached. Figure 1A illustrates the flowchart for data collection and analysis.
 |
Figure 1 (A) Flowchart of bibliometric analysis. (B) The number of articles about Artemisinin and its Derivatives in cancer per year from 1993 to 2023. |
Data Standardization
Nonstandardized keywords were screened in accordance with established standards to eliminate redundant repetition in the keyword co-occurrence graph. This is because inconsistencies in lexical categories and the forms of words in both singular and plural can lead to such repetition. Additionally, countries/regions were standardized, such as the unification of England, Scotland, Northern Ireland, and Wales to form the United Kingdom and the incorporation of Taiwan into China.
Visualized Analysis
The software utilized in our investigation was as outlined below: CiteSpace (version 6.2. R7, Chaomei Chen, Drexel University, Philadelphia, PA, United States),44 VOSviewer (version 1.6.20, Leiden University, Leiden, Netherlands),32 Pajek (version 5.17, University of Ljubljana, Ljubljana, Slovenia).33 The data was analyzed using these applications, and the results were transferred to multiple tables that condensed bibliometric parameters such as publication numbers and years, titles, countries and institutions, authors, journals, total and average citations, keywords, and references. The network maps, the time view, and the density view of the map were generated using VOSviewer. The VOSviewer map depicted that the nodes corresponding to weighting were the links indicating collaborations or co-occurring presences within a single piece of literature. The thickness of the link represented positive correlations with link strength. In the module visualizing networks, clusters were differentiated using colors. In the overlay visualization module, White symbolized the previous period of research, while purple denoted the current phase, and the colors of nodes were indicative of the average publication year. CiteSpace was utilized to generate burst maps, a dual map overlay of journals, and to identify co-citation references. The dark nodes represent older publications, while the bright nodes represent subsequent articles. Additionally, in the burst module, keywords and references were arranged based on the start of the year of the burst.
Results
Annual Publications and Citations
A total of 927 papers were involved, and the annual number of publications and citations were calculated. Figure 1B illustrates a consistent increase in publications and citations on artemisinin and tumor therapy from 1993 to 2023, with an accelerated growth rate after 2018. Specifically, annual publications increased from 62 in 2018 to 99 in 2020, reflecting heightened research interest, possibly due to emerging evidence of artemisinin’s anticancer potential and increased funding for traditional Chinese medicine research. Citations peaked at 4493 in 2021, indicating growing academic impact. After screening up to the data collection cutoff date, the 2023 literature (n=80) did not set a new record in quantity, but still demonstrated sustained momentum.
Analysis of Cooperation Status
Countries/Regions
A total of 69 countries have contributed to the study of artemisinin and tumors. The most productive country/region was China (n = 536, 57.82%), in addition to the United States (n = 129, 13.92%), Germany (n = 90, 9.71%), India (n = 48, 5.18%), and South Korea (n = 33, 3.56%) (Figure 2A). The top 10 countries, such as one North American country, three European countries, five Asian countries, and one African country, have published 950 papers, constituting 80.85% of all published papers. Figure 2B displays the substantial collaboration among the top thirty countries/regions with the highest productivity. In general, among nations with a large number of publications and enhanced collaborative efforts, China is the most significant.
Institutions
Approximately 1000 institutions worldwide have conducted studies on the application of artemisinin and its derivatives in tumor research. Table 1 shows the top 10 institutions by the number of literature outputs and citations. Johannes Gutenberg University Mainz had the most important publications (n = 46, 4.96%), followed by the Chinese Academy of Sciences (n = 41, 4.42%). The main institutions and their collaborative relationships in the field are shown in Figure 2C. And the investigation of co-occurrent institutions found that close cooperation existed among institutions with many publications, and domestic collaboration exhibited greater frequency than international cooperation.
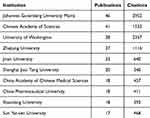 |
Table 1 The Top 10 Productive Institutions |
Authors
Over 4000 researchers engaged in research on artemisinin and tumors. Efferth, Thomas (n = 59) from Germany was the author with the most publications, followed by Tsogoeva, Svetlana B. (n = 17), and Sasaki, Tomikazu (n = 13) (Table 2). The analysis of author co-occurrence revealed multiple research groups and collaborative efforts between researchers in the field (Figure 2D). The nodes’ size was positively associated with the publication number of a certain author, while the thickness of the lines between nodes indicated cooperation frequency.
 |
Table 2 The Top 10 Productive Authors |
 |
Table 3 The Top 10 Journals of Publications on Artemisinin and Its Derivatives in Cancer (Sorted by Total Citations) |
Analysis of Journals
As of December 2023, 376 SCI journals have published articles on artemisinin and tumors. Table 3 provides a summary of the top 10 journals with the largest publication quantity arranged by total, accounting for 34.31% of the total citations. ANTICANCER RESEARCH was the most published journal (n = 17,4.52%), and CANCER LETTERS was the most cited (citations = 1141). JOURNAL OF MEDICINAL CHEMISTRY was the second most cited journal, with 1045 citations. The cited journal is on the left, and the cited journal is on the right of the map, which shows the topic distribution of journals by means of the dual map overlay of journals, as shown in Figure 3. The dual map overlay presents that almost all the articles about artemisinin and tumors were published in one discipline (“molecular biology immunology”). The primary sources of knowledge in this field are predominantly the two disciplines located on the right side of the map. (“molecular biology genetics” and “chemistry materials physics”).
 |
Figure 3 The dual map overlay of journals. |
Analysis of Co-Cited References
Co-cited references indicate that they have been cited by multiple studies included in this study. In order to investigate the knowledge base and background of artemisinin and tumors, we conducted an analysis of reference co-citations through CiteSpace. In Figure 4A, the arrangement of co-cited references in the distribution network from 1993 to 2023 was depicted, including references cited more than 30 times on the map. Additionally, as indicated by the color bar, the more faded the color of the node, the later it was referenced. The top 10 co-cited references are listed in Table 4. Among them, the paper entitled the antimalarial artesunate is also active against cancer “has garnered the highest number of co-citations (n = 218).45 The paper entitled” Qinghaosu (Artemisinin): an Antimalarial Drug from China ranked second (n = 182). The first was published in the International Journal of Oncology, and the second was published in SCIENCE. All of the top 10 co-cited references were published in 1985, with around two-thirds published in the last two decades.
 |
Table 4 The Top 10 Co-Cited References |
 |
Figure 4 The map of co-citation references (A) and the largest 10 clusters (B). |
A total of 18 clusters were identified, as stated in the log-likelihood ratio algorithm of CiteSpace. The 10 largest clusters among them are shown in Figure 4B. In the timeline view, the cluster labels are at the far end of the line, and different colored nodes on the same line represent references from different years within a cluster, with the nodes closer to the right representing the latest references. Modularity Q (0.851) is above the threshold of 0.3, and Mean Silhouette (0.9306) values surpass 0.7, indicating that the clusters are compelling and hold structural significance. The 10 largest clusters were identified as “artemisinin-type drug” (cluster #0), “bax-mediated intrinsic pathway” (cluster #1), “infected erythrocyte” (cluster #2), “potential synergism” (cluster #3), “biological action” (cluster #4), “inhibits hypoxia” (cluster #5), “tumor cell” (cluster #6), “kaposis sarcoma xenograft tumor” (cluster #7), “botanical compound” (cluster #8) and “artemisinin-tagged iron-carrying compound” (cluster #9), respectively. The largest and most recent cluster, Cluster #0, comprises 245 references.
The citation burst in a reference is when those references are cited significantly more often than usual over time, and this type of study can facilitate the exploration of how research hotspots have evolved over the years. The top 50 with the most robust citation burst are shown in Figure 5, where the red bar indicates a high frequency of citations, and the blue bar indicates a low citation frequency.
 |
Figure 5 Visual analysis of reference bursts. |
The reference with the most robust burst strength (strength = 35.07, burst period = 2018–2023) is “From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy”,49 published by Efferth T. Table 5 shows the references that remain in a state of the burst.
 |
Table 5 References Currently in a State of Burst |
Analysis of Keywords
Keywords serve as the fundamental components of articles. Utilizing co-occurrence analysis of keywords can effectively pinpoint active research areas in the field. Among 3440 keywords in 927 papers, the keywords with a frequency of occurrence greater than 15 times were extracted and clustered, and 109 keywords were obtained (Figure 6A). The average publishing year was between 2009 and 2021. The nodes’ different colors represent the five clusters found, including 36, 23, 18, 18, and 14 keywords, respectively. Cluster 1 (red) is mainly correlated with drug application. Keywords in this cluster include “cancer”, “in-vitro”, “cycle arrest”, “anticancer activity”, and “plasmodium-falciparum”. Cluster 2 (green) contains keywords associated with the pharmacological mechanism of artemisinin in the treatment of tumors, such as “artemisinin”, “dihydroartemisinin”, “mechanism”, “cell” and “ferroptosis”. Cluster 3 (blue), Cluster 4 (yellow), and Cluster 5 (purple) emphasize artemisinin drugs, expression, and outcome, respectively. In the density view (Figure 6B), “artemisinin”, “apoptosis”, “artesunate”, “dihydroartemisinin” and “in-vitro” exhibited a high density on the map, demonstrating the importance of these topics and the possible interests of research in the field of artemisinin and tumors.
We employed the keyword burst and time view tools to investigate the progression of research trends, anticipate emerging topics, and discover potential hotspots. Keywords in Figure 6C were color-coded based on their average publishing year. Keywords that appear early are displayed in white, while recent keywords are in dark purple. The latest keywords involved “hybrid molecule”, “cell death”, “oral artesunate”, “colorectal cancer”, “phase”, “ferroptosis”, and “autophagy”. Figure 6D shows the keyword burst in artemisinin and tumors over the last three decades. The keywords that continued to exhibit a state of burst are “nanoparticles”, “therapy”, “oral artesunate”, “cycle arrest”, “metabolism”, “mechanism”, “autophagy”, “cell death”, “combination”, “ferroptosis”, “hybrid molecule”, “colorectal cancer”, “lung cancer”, indicating the potential research hotspots in the future.
Discussion
Our study conducts a bibliometric analysis focusing on the recent advances, research hotspots, and future trends of artemisinin and its derivatives in cancer treatment. We conducted a systematic literature review of articles published in the WOSCC database from 1990 to 2023 associated with the application of artemisinin and its derivatives in cancer. Our comprehensive bibliometric evaluation included 927 papers from 376 journals from 69 countries/regions. By analyzing tables, visualized results, and statistics on countries, regions, authors, institutions, and keywords in the field, we offered valuable perspectives on emerging hotspots and trends in this area of research.
Bibliometric Information
As illustrated in Figure 1B, the number of publications generally exhibits an annual upward trend. The publication curve with dots peaked in 2020. Additionally, the figure illustrates that the rate of increase in citations exceeds that of publications and exhibits a similar trend to the publication volume.
Artemisinin was discovered in the 1970s, and Tu Youyou and her team successfully extracted it from the Artemisia annua plant in 1972. This discovery provided a novel effective drug for malaria treatment.64 For over 40 years, this drug class has continued attracting widespread attention from researchers as a novel therapeutic agent.65 Artemisinins are a novel class of compounds that demonstrated potent therapeutic effects in inflammation,15 immune diseases,66 shock,67,68 cancer,7,55,69 and other conditions.70 In recent years, with the global epidemic of cancer, the repurposing of “old” drugs for new indications has gradually gained popularity. The emerging research field of using artemisinin and its derivatives for cancer treatment has also developed rapidly. Between 1993 and 2000, the research on artemisinin-based drugs in cancer therapy did not attract much attention. Since 2001, this field has attracted growing interest, and the number of related research reports has rapidly increased. The clinical application of artemisinin and its derivatives in cancer is a current research direction, which will provide insightful perspectives for the future development of safe and reliable anticancer drugs.
Efferth, Thomas, who received the Willmar Schwabe Award in 2006, is the most productive author (59 papers). He and his team mainly focus on applying artemisinin-based drugs in cancer treatment, including the anti-multidrug resistance capability of artesunate and its mechanism of action.59,71,72 Chinese scholars have published the most research, four times that of the second-ranked United States. Eight out of the top ten most productive institutions are in China, which may be related to China’s large population base. Additionally, China is one of the countries with a high incidence and mortality rate of cancer, which can also contribute to this outcome. Hence, it is unsurprising that Chinese researchers are the most active in cancer research. Notably, among the top 18 most-cited articles, 10 were written by Chinese scholars. This indicates, to some extent, the influence of Chinese scholars on artemisinin and its derivatives in cancer prevention and treatment.
Among the top 10 most cited publications (Table 6), Efferth et al were the first to propose the anticancer effects of artemisinin-based drugs. They suggested that artesunate could be a potential candidate for cancer chemotherapeutics.45 In 2008, Hou et al investigated the in vivo and in vitro anticancer effects of Artesunate and Dihydroartemisinin and their impact on cell proliferation and apoptosis-related gene protein expression. They concluded that their use alone or in combination with other therapies has a promising therapeutic effect on human liver cancer.9 In 2010, Ghantous et al explored the application of sesquiterpene lactones in clinical cancer trials and considered them hopeful candidates in cancer drug discovery.73 As the anticancer effects of artemisinin-based drugs are gradually uncovered, these drugs are increasingly favored in cancer prevention and treatment.
 |
Table 6 The Top 10 Most Cited Articles |
Furthermore, as shown in Table 1, although most institutions publishing research on artemisinin and its derivatives are based in China, the most co-cited articles originate from leading journals such as International Journal of Oncology and Science. These highly cited papers, such as “The antimalarial artesunate is also active against cancer” (co-cited 218 times) and “Qinghaosu (Artemisinin): an Antimalarial Drug from China” (co-cited 182 times), are frequently referenced due to their pioneering contributions to the field. For instance, the former, published by Efferth et al, was among the first to systematically demonstrate artesunate’s anticancer potential across multiple cancer cell lines, establishing a foundational framework for subsequent studies. Similarly, the latter, published in Science, provided a comprehensive overview of artemisinin’s discovery and its pharmacological properties, serving as a seminal reference for both malaria and cancer research. These papers are highly cited because they introduced novel concepts, provided robust experimental evidence, and were published in high-impact journals, enhancing their visibility and influence. This suggests that the global impact of research in this field is driven by studies that combine innovative insights with rigorous methodologies, regardless of the authors’ geographical origins.
Overall, the bibliometric results indicate that studies on artemisinin and its derivatives in cancer prevention and treatment are still thriving. Researchers from diverse nations are driving forward this field from different perspectives, collectively contributing to a rapid academic revolution that will continue from the present into the future.
Detection of Research Hotspots and Future Directions
Specific Mechanisms of Action of Artemisinin and Its Derivatives Against Cancer
The classification of specific anticancer mechanisms covers multifaceted interventions targeting cancer biological processes and molecular mechanisms, of which the regulation of signaling pathways is central. These mechanisms include intervening in the cell cycle to block tumor cell proliferation, activating apoptosis to induce tumor cell death, inhibiting key signaling pathways to limit tumor growth and division, adopting anti-angiogenic measures to reduce the blood supply to tumors, utilizing immune modulation to enhance the body’s attack on tumors, influencing gene expression in tumor cells through genetic and epigenetic regulation, and depriving metabolic reprogramming of energy required by cancer cells.24,80
Artemisinin and its derivatives have important roles in the regulation of anticancer signaling pathways, the main ones of which are suppression of PI3K/AKT/mTOR pathway, which affects the cell cycle, cell dormancy, and proliferation;81,82 activation of AMPK signaling pathway, which regulates cell growth and metabolic reprogramming;83 inhibition of STAT3 signaling pathway, which affects cancer cell growth, survival, and immune escape;84–87 activation of JNK signaling pathway, which promotes cell apoptosis and autophagy;88,89 inhibition of NF-κB activity, which reduces tumor cell sensitivity to apoptosis and promotes tumor growth. Pathway promotes apoptosis and autophagy.90–93
The PI3K/AKT/mTOR pathway is a significant cell survival and proliferation signaling pathway, and artemisinin and its derivatives can reduce the viability and proliferation rate of cancer cells by inhibiting this pathway. This signaling pathway is also intimately connected with the progression of the cell cycle. By blocking the PI3K/AKT/mTOR pathway, artemisinins and their derivatives can lead to cell cycle arrest and prevent the proliferation of cancer cells.94 In some cases, artemisinin and its derivatives possess the capacity to trigger autophagy and cancer cell apoptosis by inhibiting mTOR activity,95–97 an important mechanism of their anticancer effects. The PI3K/AKT/mTOR pathway has also been related to drug resistance in cancers, and artemisinin and its derivatives, by inhibiting this pathway, may help overcome the resistance of certain cancers to chemotherapeutic drugs.98,99 The cancers it affects include breast cancers,100,101 prostate cancers,102,103 ovarian cancers,104,105 pancreatic cancers,106,107 lung cancers,108,109 stomach cancers110,111 and liver cancers.112
Artemisinin and its derivatives may directly inhibit the function of PI3K, AKT, or mTOR kinases, thereby blocking signaling. They may also interfere with interactions between critical molecules in the signaling pathway, such as preventing AKT’s phosphorylation or inhibiting the mTOR complex’s assembly. Artemisinin and its derivatives may also affect the PI3K/AKT/mTOR pathway by modulating factors upstream or downstream of the pathway, such as inhibiting the activity of growth factor receptors or modulating the expression of downstream effector molecules. By inhibiting the PI3K/AKT/mTOR pathway through these mechanisms, artemisinin and its derivatives hinder the proliferation and survival of cancer cells at various levels, providing an important basis for their action as potential anticancer drugs.
Artemisinin and its derivatives belong to the class of sesquiterpene lactones, and in the most cited article, “What made sesquiterpene lactones reach cancer clinical trials?” Ghantous et al summarized the specific mechanism of action of sesquiterpene lactones, including artemisinin, on tumors and cancer stem cells and pointed out that in clinical trials for cancer, sesquiterpene lactones (SLs) can diffuse through the cell membrane and selectively target specific signaling pathways and mechanisms in cancer cells. These include the NF-kB pathway, sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) pump, high intracellular iron content, cell surface transferrin receptors, and epigenetic regulation mechanisms. Glutathione can also diffuse through the cell membrane and interact with the SERCA pump through lipophilic interactions, inhibiting its function of transporting Ca2+ from the cytoplasm to the endoplasmic reticulum. Numerous cancer cells exhibit elevated levels of intracellular iron and transferrin receptors. Intracellular iron can bind to the endoperoxide bridge of artemisinin, leading to its activation and the production of toxic reactive oxygen species. Additionally, artemisinin can inhibit the activity of NF-kB. NF-kB is a vital transcription factor within cells that regulates various cellular processes, including inflammation, immune responses, cell growth, and death. In the development of cancer, the activity of NF-kB is abnormally elevated and closely associated with tumor formation, advancement, metastasis, and resistance to anticancer treatments. Therefore, inhibiting the activity of NF-kB is considered a potential anticancer strategy. The specific effects of inhibiting NF-kB include suppressing tumor cell proliferation and survival, reducing tumor growth promoted by inflammatory responses, blocking tumor metastasis and invasion, and enhancing the effectiveness of anticancer therapies.
STAT3 serves as a downstream mediator of various cytokines, such as IL-6 and IL-4, and growth factors, including EGF and hypoxia. It has become a significant target due to its role in enhancing the stemness of tumor cells, triggering abnormal proliferation and malignant transformation, and evading apoptosis metastasis and immune responses.113 Phosphorylated STAT3 dimers in cancer cells stimulate the gene transcription related to the regulation of cell cycle, adhesion, angiogenesis, and metastasis. Sustained STAT3 activation is an adverse prognostic factor in certain cancers. In immune cells, IL-6 triggers STAT-3 phosphorylation to facilitate IL-10 secretion, which leads to both maintenance of a robust immunosuppressive microenvironment and autocrine activation of immune STAT-3. ARTs can polarize monocytes to adopt a tumoricidal phenotype by inhibiting STAT3 in human primary monocytes. Additionally, suppressing Fas downregulation mediated by STAT3 or decreasing phosphorylated STAT3 can hinder tumor proliferation and metastasis while inducing cell apoptosis.114
Artemisinin and its derivatives exert their effects on the MAPK pathway through various mechanisms. MAPKs, which include c-Jun N-terminal kinase (JNK), ERKs, and p38 MAPK, play a significant role in multiple cellular processes.115,116 VEGF’s downstream signaling pathways can activate ERKs, promoting cell survival and proliferation. By interacting with endothelial cells (ECs) receptors phosphorylating Raf and activating MEK1/2 and ERKs, VEGF can enhance EC survival and proliferation.115 JNK and p38 MAPK are involved in both cytotoxic and cytoprotective activities.116–118 JNK, a protein enhancing angiogenesis, responds to stressors, phosphorylating c-jun of AP-1, leading to nuclear translocation and upregulating pro-angiogenic stimuli. p38 MAPK responds similarly to stress stimuli.117–123 Additionally, both JNK and p38 MAPK also mediate apoptosis.124–126 Impeding ERK-related cytoprotective activities can inhibit angiogenesis.
Artemisinin compounds exhibit both cytotoxic and cytostatic effects on cancer cells and cause cell cycle arrest in multiple cell types. Inhibitors such as the Cyclin-dependent kinase-associated protein (CIP) and kinase-inhibitory protein (KIP), including p57, p21, and p27,127 participate in regulating cell division. Artemisinin and its derivatives disrupt cell division by interfering with different phases of the cell cycle. Artemisinin has been found to typically cause growth arrest during the G0/G1 to S transition. Nevertheless, growth arrest during all stages of the cell cycle has been frequently observed. ARTs also trigger apoptotic cell death in several cell types, where the mitochondrial-mediated apoptotic signaling pathways exert a crucial role.123,128
Directions of Artemisinin and Its Derivatives in Tumor Therapy
Artemisinin and its derivatives, such as dihydroartemisinin (DHA), artesunate (ARS), and artemether, have emerged as promising candidates in cancer therapy due to their multifaceted antitumor effects, including induction of apoptosis, ferroptosis, cell cycle arrest, and inhibition of angiogenesis. Preclinical studies have demonstrated efficacy across a broad spectrum of cancers, including breast, colorectal, lung, liver, and cervical cancers. However, significant challenges remain in translating these findings into clinical practice, including suboptimal bioavailability,129 and limited clinical trial data. To overcome these hurdles and maximize the therapeutic potential of artemisinin-based compounds, future research should prioritize the following specific development directions, which aim to enhance efficacy, specificity, and clinical applicability.
Optimization of Drug Delivery Systems: one of the primary limitations of artemisinin and its derivatives is their poor pharmacokinetic profile, characterized by low solubility, rapid metabolism, and short half-life in vivo.130 These factors reduce the drugs’ ability to reach therapeutic concentrations at tumor sites while increasing the risk of systemic toxicity. Future research should focus on developing advanced drug delivery systems to address these challenges. Nanomedicine offers a promising avenue, with approaches such as nanoparticles, liposomes, micelles, and dendrimers showing potential to enhance tumor-specific delivery. For example, transferrin-eight-arm-polyethylene glycol–dihydroartemisinin nanoparticles (TF-8armPEG–DHA NPs) have demonstrated improved tumor accumulation and reduced off-target effects in preclinical models of non-small cell lung cancer (NSCLC).131 Expanding on such models, researchers could explore stimuli-responsive nanoparticles that release artemisinin in response to tumor-specific cues, such as pH, hypoxia, or enzyme activity, thereby improving precision and efficacy. Additionally, organelle-targeted delivery systems hold significant promise. Artemisinin’s mechanism of action, particularly its induction of reactive oxygen species (ROS) via interaction with intracellular iron, suggests that targeting mitochondria or endoplasmic reticulum could amplify its cytotoxic effects.14 Conjugates like triphenylphosphonium (TPP)-linked artemisinin have shown enhanced mitochondrial localization and cytotoxicity in cancer cells.132 Future studies should investigate novel conjugates and nanoparticle designs to optimize subcellular targeting, potentially increasing potency while minimizing damage to healthy tissues. These advancements could pave the way for clinical trials by providing formulations that achieve higher therapeutic indices.
Combination Therapies to Overcome Resistance: cancer’s heterogeneity and propensity for developing resistance to monotherapies underscore the need for combination strategies. Artemisinin and its derivatives are well-suited for this approach due to their ability to modulate multiple signaling pathways, including PI3K/AKT/mTOR,82 NF-κB,133 STAT3,84 and MAPK,134 which are often implicated in tumor progression and drug resistance. Future research should systematically evaluate artemisinin-based combinations with conventional chemotherapeutics, targeted therapies, and immunotherapies to identify synergistic effects. For instance, preclinical studies have shown that combining artesunate with cisplatin enhances cytotoxicity in ovarian cancer cells.135 Similarly, DHA has been reported to sensitize pancreatic cancer cells to gemcitabine, suggesting potential for combination regimens in cancers with poor prognosis.136 Beyond chemotherapeutics, integrating artemisinin with immunotherapies, such as immune checkpoint inhibitors (eg, anti-PD-1/PD-L1 antibodies), could exploit its immunomodulatory properties.82 Artemisinin’s ability to suppress regulatory T cells and myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment (TME) suggests it could enhance T-cell-mediated antitumor immunity.137 Clinical trials exploring these combinations, particularly in immunologically “cold” tumors like pancreatic or liver cancer, could establish artemisinin as a versatile adjuvant. To accelerate progress, high-throughput screening platforms, as demonstrated in recent studies could be employed to identify optimal drug pairings and dosing schedules, minimizing trial-and-error in clinical settings.138
Elucidation of Molecular Mechanisms: while artemisinin’s antitumor effects—such as induction of apoptosis, ferroptosis, autophagy, and cell cycle arrest—are well-documented, the precise molecular interactions driving these outcomes remain incompletely understood. This knowledge gap hinders the rational design of more potent and selective derivatives. Future research should leverage advanced omics technologies, including genomics, transcriptomics, proteomics, and metabolomics, to comprehensively map artemisinin’s interactome in cancer cells. Moreover, understanding the role of tumor-specific factors, such as iron metabolism or oxidative stress, could guide the development of derivatives tailored to particular cancer types. Computational modeling and machine learning approaches could further predict artemisinin’s efficacy across diverse genetic backgrounds, enabling personalized treatment strategies. By elucidating these mechanisms, researchers can design analogs with enhanced specificity, reducing off-target effects and paving the way for precision oncology applications.
740Clinical Trial Expansion and Biomarker Development: The scarcity of clinical trials remains a critical bottleneck in artemisinin’s development as a cancer therapeutic. While preclinical data are robust, only a handful of studies have evaluated artemisinin or its derivatives in human patients, often as adjuvants rather than primary agents. Future research should prioritize designing and conducting phase I/II clinical trials to assess safety, pharmacokinetics, and preliminary efficacy in specific cancer types with strong preclinical evidence. These trials should incorporate rigorous endpoints to establish clinical utility. Equally important is the identification of biomarkers to predict treatment response and guide patient selection. Artemisinin’s efficacy is closely tied to factors like intracellular iron levels, antioxidant enzyme activity and expression of signaling molecules like VEGF or EGFR. Developing assays to measure these biomarkers in patient samples could enable stratification, ensuring that artemisinin is administered to those most likely to benefit. Collaborative efforts between academia and industry will be essential to fund and execute these trials, bringing artemisinin closer to regulatory approval.
Targeting the Tumor Microenvironment: The tumor microenvironment plays a pivotal role in cancer progression, immune evasion, and therapy resistance. Artemisinin’s ability to modulate the TME, particularly through inhibition of immunosuppressive cells like Tregs and MDSCs, offers a unique opportunity to enhance antitumor immunity. Future research should explore how artemisinin derivatives can reshape the TME to favor immune activation. Additionally, artemisinin’s anti-angiogenic effects, mediated through pathways like NF-κB and VEGF, suggest it could disrupt the vascular niche that supports tumor growth. Research should focus on combining artemisinin with anti-angiogenic agents, such as bevacizumab, to starve tumors of nutrients and oxygen. Furthermore, the role of cancer-associated fibroblasts (CAFs) and extracellular matrix remodeling in the TME remains underexplored in the context of artemisinin therapy. Investigating these interactions could uncover novel therapeutic targets, broadening artemisinin’s applicability across solid tumors.
Development of Novel Derivatives: To address limitations like low potency and non-specific cytotoxicity, future research should focus on synthesizing novel artemisinin derivatives with improved pharmacological properties. Structure-activity relationship (SAR) studies could guide the design of analogs that retain the critical endoperoxide bridge—essential for ROS generation—while enhancing lipophilicity, stability, or receptor affinity.
By pursuing these six development directions—optimized delivery, combination therapies, mechanistic elucidation, clinical trials, TME modulation, and novel derivatives—artemisinin and its derivatives can transition from promising preclinical agents to cornerstone therapies in oncology. These efforts will require interdisciplinary collaboration, integrating expertise in pharmacology, bioengineering, immunology, and clinical research.
Limitations
Our study retrieved literature from the WOSCC database through reliable retrieval strategies, but the limitations still need to be considered: All the data in our article were obtained from a single database, which may lead to some incomplete initial data. The algorithm used to generate the results inevitably has its flaws. Due to the limited time frame for retrieving relevant research, some recently published articles may not appear in the search results we provide. Therefore, further in-depth exploration is needed to elucidate the hotspots and scientific trends in the research of artemisinin and its derivatives in cancer. Nevertheless, this bibliometric analysis still delves deeply into the existing data and provides valuable insights into the origin and advancement of applicating artemisinin and its derivatives in cancer, thus enhancing the lucidity of potential hotspots and guiding directions for future study.
Conclusions
We conducted a bibliometric analysis to assess the outcomes and impacts of relevant research. Our study summarized the most recent advancements in artemisinin and its derivatives in the field of cancer, identified research hotspots, and explored the prospects for development in this area. Artemisinin and its derivatives have undoubtedly become a significant research direction in cancer prevention and treatment. Currently, the drug repurposing of artemisinin for various types of cancer is a popular research topic, and studies on various clinical settings and combination therapies are crucial. Moreover, delving into the mechanisms of activity of artemisinin and its derivatives on multiple cancers is a current research hotspot. In cancer treatment research, artemisinin and its derivatives, serving as safe, reliable, and cost-effective drugs, have already achieved encouraging results in preclinical studies. Future research in this field should focus on combination therapeutics involving artemisinin and its derivatives, drug resistance in cancer, and other key issues, utilizing multidisciplinary approaches and omics technologies to foster active collaboration among different research groups.
Abbreviations
NCD, non-communicable disease; TCM, traditional Chinese medicine; WOSCC, Web of Science Core Collection; PI3K, Phosphoinositide 3-kinase; AMPK, Adenosine Monophosphate-activated Protein Kinase; STAT3, Signal Transducer and Activator of Transcription 3; JNK, c-Jun N-terminal kinase; NF-κB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; mTOR, mammalian Target of Rapamycin; AKT, Protein Kinase B; MAPK, Mitogen-Activated Protein Kinase; VEGF, Vascular Endothelial Growth Factor; ERK, Extracellular Signal-Regulated Kinase; CIP, Cyclin-dependent kinase-associated protein; KIP, Kinase-Inhibitory Protein; SERCA, Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase; IL-6, Interleukin-6; IL-4, Interleukin-4; EGF, Epidermal Growth Factor; IL-10, Interleukin-10; ARTs, Artemisinin and its derivatives; ECs, Endothelial Cells; AP-1, Activator Protein 1; DHA, Dihydroartemisinin; ARS, Artesunate; ROS, Reactive Oxygen Species; NSCLC, Non-Small Cell Lung Cancer; TPP, Triphenylphosphonium; MDR, Multidrug Resistance; MDSCs, Myeloid-Derived Suppressor Cells; TME, Tumor Microenvironment; CAFs, Cancer-Associated Fibroblasts; SAR, Structure-Activity Relationship.
Data Sharing Statement
All data generated or analyzed during this study are included in this article.
Acknowledgments
We thank VOSviewer and Pajek for free access by researchers.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Shanghai Municipal Health Commission (Grant no. 20224Y0128), the National Natural Science Foundation of China (82204831), the Shanghai Sailing Program (No. 22YF1448800), and the China Postdoctoral Science Foundation (No. 2021M692153).
Disclosure
The authors declare that they have no competing interests.
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi:10.3322/caac.20006
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
4. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi:10.1016/s0092-8674(00)81683-9
5. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
6. Liu J, Wang S, Zhang Y, Fan HT, Lin HS. Traditional Chinese medicine and cancer: history, present situation, and development. Thorac Cancer. 2015;6(5):561–569. doi:10.1111/1759-7714.12270
7. Crichton EP. Infusion fluids as culture media. Am J Clin Pathol. 1973;59(2):199–202. doi:10.1093/ajcp/59.2.199
8. Lu CL, Li X, Zhou HM, et al. Traditional Chinese medicine in cancer care: an overview of 5834 randomized controlled trials published in Chinese. Integr Cancer Ther. 2021;20:15347354211031650. doi:10.1177/15347354211031650
9. Hou J, Wang D, Zhang R, Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res. 2008;14(17):5519–5530. doi:10.1158/1078-0432.Ccr-08-0197
10. Wang J, Xu C, Wong YK, et al. Artemisinin, the magic drug discovered from traditional Chinese medicine. Engineering. 2019;5(1):32–39. doi:10.1016/j.eng.2018.11.011
11. Wen W, Yu R. Artemisinin biosynthesis and its regulatory enzymes: progress and perspective. Pharmacogn Rev. 2011;5(10):189–194. doi:10.4103/0973-7847.91118
12. Feng X, Cao S, Qiu F, Zhang B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol Ther. 2020;216:107650. doi:10.1016/j.pharmthera.2020.107650
13. Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7(12):864–874. doi:10.1038/nrmicro2239
14. Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32(13):1655–1660. doi:10.1016/s0020-7519(02)00194-7
15. Shi C, Li H, Yang Y, Hou L. Anti-inflammatory and immunoregulatory functions of artemisinin and its derivatives. Mediators Inflamm. 2015;2015(1):435713. doi:10.1155/2015/435713
16. Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228(4703):1049–1055. doi:10.1126/science.3887571
17. Chung IY, Jang HJ, Yoo YJ, et al. Artemisinin displays bactericidal activity via copper-mediated DNA damage. Virulence. 2022;13(1):149–159. doi:10.1080/21505594.2021.2021643
18. Lin Z, Liu Y, Chen L, et al. Artemisinin analogue SM934 protects against lupus-associated antiphospholipid syndrome via activation of Nrf2 and its targets. Sci China Life Sci. 2021;64(10):1702–1719. doi:10.1007/s11427-020-1840-1
19. Wang Y, You F, Xue J, Xue J. Novel use for old drugs: the emerging role of artemisinin and its derivatives in fibrosis. Pharmacol Res. 2020;157:104829. doi:10.1016/j.phrs.2020.104829
20. Kim WS, Choi WJ, Lee S, et al. Anti-inflammatory, antioxidant and antimicrobial effects of artemisinin extracts from Artemisia annua L. Korean J Physiol Pharmacol. 2015;19(1):21–27. doi:10.4196/kjpp.2015.19.1.21
21. Wei T, Liu J. Anti-angiogenic properties of artemisinin derivatives. Int J Mol Med. 2017;40(4):972–978. doi:10.3892/ijmm.2017.3085
22. Chen HH, Zhou HJ, Fang X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res. 2003;48(3):231–236. doi:10.1016/s1043-6618(03)00107-5
23. Zeng ZW, Chen D, Chen L, He B, Li Y. A comprehensive overview of artemisinin and its derivatives as anticancer agents. Eur J Med Chem. 2023;247:115000. doi:10.1016/j.ejmech.2022.115000
24. Augustin Y, Staines HM, Krishna S. Artemisinins as a novel anti-cancer therapy: targeting a global cancer pandemic through drug repurposing. Pharmacol Ther. 2020;216:107706. doi:10.1016/j.pharmthera.2020.107706
25. Rosenberg B. Understanding scientific literatures: a bibliometric approach. Information Storage and Retrieval. 1974;10(11–12):420–421. doi:10.1016/0020-0271(74)90051-5
26. Broadus RN. Toward a definition of “bibliometrics”. Scientometrics. 1987;12(5–6):373–379. doi:10.1007/bf02016680
27. Hood WW, Wilson CS. The literature of bibliometrics, scientometrics, and informetrics. Scientometrics. 2001;52(2):291–314. doi:10.1023/a:1017919924342
28. Cobo MJ, López-Herrera AG, Herrera-Viedma E, Herrera F. An approach for detecting, quantifying, and visualizing the evolution of a research field: a practical application to the fuzzy sets theory field. J Informetrics. 2011;5(1):146–166. doi:10.1016/j.joi.2010.10.002
29. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. Journal of Business Research. 2021;133:285–296. doi:10.1016/j.jbusres.2021.04.070
30. Chen C. Science mapping: a systematic review of the literature. J Data Inf Sci. 2017;2(2):1–40. doi:10.1515/jdis-2017-0006
31. Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. 2019;14(10):e0223994. doi:10.1371/journal.pone.0223994
32. van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3
33. Mrvar A, Batagelj V. Analysis and visualization of large networks with program package Pajek. Complex Adaptive Systems Modeling. 2016;4(1). doi:10.1186/s40294-016-0017-8
34. He X, Xu S, Tang L, Ling S, Wei X, Xu X. Insights into the history and tendency of liver transplantation for liver cancer: a bibliometric-based visual analysis. Int J Surg. 2024;110(1):406–418. doi:10.1097/JS9.0000000000000806
35. Wongsrichanalai C, Sibley CH. Fighting drug-resistant Plasmodium falciparum: the challenge of artemisinin resistance. Clin Microbiol Infect. 2013;19(10):908–916. doi:10.1111/1469-0691.12316
36. Xie D-Y, Ma D-M, Judd R, Jones AL. Artemisinin biosynthesis in Artemisia annua and metabolic engineering: questions, challenges, and perspectives. Phytochem Rev. 2016;15(6):1093–1114. doi:10.1007/s11101-016-9480-2
37. Zyad A, Tilaoui M, Jaafari A, Oukerrou MA, Mouse HA. More insights into the pharmacological effects of artemisinin. Phytother Res. 2018;32(2):216–229. doi:10.1002/ptr.5958
38. Nabi N, Singh S, Saffeullah P. An updated review on distribution, biosynthesis and pharmacological effects of artemisinin: a wonder drug. Phytochemistry. 2023;214:113798. doi:10.1016/j.phytochem.2023.113798
39. O’Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin--the debate continues. Molecules. 2010;15(3):1705–1721. doi:10.3390/molecules15031705
40. Zhang Z-Y, Yu S-Q, Miao L-Y, et al. Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue bao = Journal of Chinese Integrative Medicine. 2008;6(2):134–138. doi:10.3736/jcim20080206
41. Berger T, Dieckmann D, Efferth T, et al. Artesunate in the treatment of metastatic uveal melanoma - first experiences. Oncol Rep. 2005. doi:10.3892/or.14.6.1599
42. Singh N, Verma K. Case report of a laryngeal squamous cell carcinoma treated with artesunate. Arch Oncol. 2002;10(4):279–280. doi:10.2298/aoo0204279s
43. Kiani BH, Kayani WK, Khayam AU, Dilshad E, Ismail H, Mirza B. Artemisinin and its derivatives: a promising cancer therapy. Mol Biol Rep. 2020;47(8):6321–6336. doi:10.1007/s11033-020-05669-z
44. Chen C, Ibekwe‐SanJuan F, Hou J. The structure and dynamics of cocitation clusters: a multiple‐perspective cocitation analysis. J Am Soc Inf Sci Technol. 2010;61(7):1386–1409. doi:10.1002/asi.21309
45. Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18(4):767–773. doi:10.3892/ijo.18.4.767
46. Efferth T, Benakis A, Romero MR, et al. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med. 2004;37(7):998–1009. doi:10.1016/j.freeradbiomed.2004.06.023
47. Efferth T, Sauerbrey A, Olbrich A, et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003;64(2):382–394. doi:10.1124/mol.64.2.382
48. Singh NP, Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001;70(1):49–56. doi:10.1016/s0024-3205(01)01372-8
49. Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. 2017;46:65–83. doi:10.1016/j.semcancer.2017.02.009
50. Woerdenbag HJ, Moskal TA, Pras N, et al. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J Nat Prod. 1993;56(6):849–856. doi:10.1021/np50096a007
51. Lai H, Singh NP. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995;91(1):41–46. doi:10.1016/0304-3835(94)03716-v
52. Dell’Eva R, Pfeffer U, Vené R, et al. Inhibition of angiogenesis in vivo and growth of Kaposi’s sarcoma xenograft tumors by the anti-malarial artesunate. Biochem Pharmacol. 2004;68(12):2359–2366. doi:10.1016/j.bcp.2004.08.021
53. Wong YK, Xu C, Kalesh KA, et al. Artemisinin as an anticancer drug: recent advances in target profiling and mechanisms of action. Med Res Rev. 2017;37(6):1492–1517. doi:10.1002/med.21446
54. Bhaw-Luximon A, Jhurry D. Artemisinin and its derivatives in cancer therapy: status of progress, mechanism of action, and future perspectives. Cancer Chemother Pharmacol. 2017;79(3):451–466. doi:10.1007/s00280-017-3251-7
55. Efferth T. Cancer combination therapies with artemisinin-type drugs. Biochem Pharmacol. 2017;139:56–70. doi:10.1016/j.bcp.2017.03.019
56. Deeken JF, Wang H, Hartley M, et al. A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol. 2018;81(3):587–596. doi:10.1007/s00280-018-3533-8
57. Greenshields AL, Fernando W, Hoskin DW. The anti-malarial drug artesunate causes cell cycle arrest and apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3 breast cancer cells. Exp Mol Pathol. 2019;107:10–22. doi:10.1016/j.yexmp.2019.01.006
58. Våtsveen TK, Myhre MR, Steen CB, et al. Artesunate shows potent anti-tumor activity in B-cell lymphoma. J Hematol Oncol. 2018;11(1):23. doi:10.1186/s13045-018-0561-0
59. Sun X, Yan P, Zou C, et al. Targeting autophagy enhances the anticancer effect of artemisinin and its derivatives. Med Res Rev. 2019;39(6):2172–2193. doi:10.1002/med.21580
60. Cao Y, Feng YH, Gao LW, et al. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int Immunopharmacol. 2019;70:110–116. doi:10.1016/j.intimp.2019.01.041
61. Du J, Wang T, Li Y, et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356–369. doi:10.1016/j.freeradbiomed.2018.12.011
62. Chen GQ, Benthani FA, Wu J, Liang D, Bian ZX, Jiang X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020;27(1):242–254. doi:10.1038/s41418-019-0352-3
63. Gao F, Sun Z, Kong F, Xiao J. Artemisinin-derived hybrids and their anticancer activity. Eur J Med Chem. 2020;188:112044. doi:10.1016/j.ejmech.2020.112044
64. Krishna S, Bustamante L, Haynes RK, Staines HM. Artemisinins: their growing importance in medicine. Trends Pharmacol Sci. 2008;29(10):520–527. doi:10.1016/j.tips.2008.07.004
65. Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142(1):126–139. doi:10.1016/j.pharmthera.2013.12.001
66. Mu X, Wang C. Artemisinins-a promising new treatment for systemic lupus erythematosus: a descriptive review. Curr Rheumatol Rep. 2018;20(9):55. doi:10.1007/s11926-018-0764-y
67. Sordi R, Chiazza F, Johnson FL, et al. Inhibition of IkappaB kinase attenuates the organ injury and dysfunction associated with hemorrhagic shock. Mol Med. 2015;21(1):563–575. doi:10.2119/molmed.2015.00049
68. Sordi R, Nandra KK, Chiazza F, et al. Artesunate protects against the organ injury and dysfunction induced by severe hemorrhage and resuscitation. Ann Surg. 2017;265(2):408–417. doi:10.1097/SLA.0000000000001664
69. Krishna S, Ganapathi S, Ster IC, et al. A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine. 2015;2(1):82–90. doi:10.1016/j.ebiom.2014.11.010
70. Efferth T. Beyond malaria: the inhibition of viruses by artemisinin-type compounds. Biotechnol Adv. 2018;36(6):1730–1737. doi:10.1016/j.biotechadv.2018.01.001
71. Kelter G, Steinbach D, Konkimalla VB, et al. Role of transferrin receptor and the ABC transporters ABCB6 and ABCB7 for resistance and differentiation of tumor cells towards artesunate. PLoS One. 2007;2(8):e798. doi:10.1371/journal.pone.0000798
72. Bachmeier B, Fichtner I, Killian PH, Kronski E, Pfeffer U, Efferth T. Development of resistance towards artesunate in MDA-MB-231 human breast cancer cells. PLoS One. 2011;6(5):e20550. doi:10.1371/journal.pone.0020550
73. Ferreira JFS, Luthria DL, Sasaki T, Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15(5):3135–3170. doi:10.3390/molecules15053135
74. Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov Today. 2010;15(15–16):668–678. doi:10.1016/j.drudis.2010.06.002
75. Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi:10.1016/j.redox.2016.12.010
76. Chaturvedi D, Goswami A, Saikia PP, Barua NC, Rao PG. Artemisinin and its derivatives: a novel class of anti-malarial and anti-cancer agents. Chem Soc Rev. 2010;39(2):435–454. doi:10.1039/b816679j
77. Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol. 2012;2012:247597. doi:10.1155/2012/247597
78. Tan W, Lu J, Huang M, et al. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin Med. 2011;6(1):27. doi:10.1186/1749-8546-6-27
79. Ooko E, Saeed ME, Kadioglu O, et al. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22(11):1045–1054. doi:10.1016/j.phymed.2015.08.002
80. Ma Z, Woon CY, Liu CG, et al. Repurposing artemisinin and its derivatives as anticancer drugs: a chance or challenge? Front Pharmacol. 2021;12:828856. doi:10.3389/fphar.2021.828856
81. Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7(4):209–219. doi:10.1038/nrclinonc.2010.21
82. Zhang M, Wang L, Liu W, et al. Targeting inhibition of accumulation and function of myeloid-derived suppressor cells by artemisinin via PI3K/AKT, mTOR, and MAPK pathways enhances anti-PD-L1 immunotherapy in melanoma and liver tumors. J Immunol Res. 2022;2022:2253436. doi:10.1155/2022/2253436
83. Zhou X, Chen Y, Wang F, et al. Artesunate induces autophagy dependent apoptosis through upregulating ROS and activating AMPK-mTOR-ULK1 axis in human bladder cancer cells. Chem Biol Interact. 2020;331:109273. doi:10.1016/j.cbi.2020.109273
84. Yan X, Li P, Zhan Y, et al. Dihydroartemisinin suppresses STAT3 signaling and Mcl-1 and survivin expression to potentiate ABT-263-induced apoptosis in non-small cell lung cancer cells harboring EGFR or RAS mutation. Biochem Pharmacol. 2018;150:72–85. doi:10.1016/j.bcp.2018.01.031
85. Dang WZ, Li H, Jiang B, et al. Therapeutic effects of artesunate on lupus-prone MRL/lpr mice are dependent on T follicular helper cell differentiation and activation of JAK2-STAT3 signaling pathway. Phytomedicine. 2019;62:152965. doi:10.1016/j.phymed.2019.152965
86. Berkoz M, Ozkan-Yilmaz F, Ozluer-Hunt A, et al. Artesunate inhibits melanoma progression in vitro via suppressing STAT3 signaling pathway. Pharmacol Rep. 2021;73(2):650–663. doi:10.1007/s43440-021-00230-6
87. Chen Y, Wang F, Wu P, et al. Artesunate induces apoptosis, autophagy and ferroptosis in diffuse large B cell lymphoma cells by impairing STAT3 signaling. Cell Signal. 2021;88:110167. doi:10.1016/j.cellsig.2021.110167
88. Dong F, Han J, Jing G, et al. Dihydroartemisinin transiently activates the JNK/SAPK signaling pathway in endothelial cells. Oncol Lett. 2016;12(6):4699–4704. doi:10.3892/ol.2016.5223
89. Zhang S, Shi L, Ma H, et al. Dihydroartemisinin induces apoptosis in human gastric cancer cell line BGC-823 through activation of JNK1/2 and p38 MAPK signaling pathways. J Recept Signal Transduction Res. 2017;37(2):174–180. doi:10.1080/10799893.2016.1203942
90. Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW, Jeong HG. Suppression of PMA-induced tumor cell invasion by dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol. 2010;79(12):1714–1726. doi:10.1016/j.bcp.2010.02.003
91. Wang SJ, Gao Y, Chen H, et al. Dihydroartemisinin inactivates NF-kappaB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett. 2010;293(1):99–108. doi:10.1016/j.canlet.2010.01.001
92. Nunes JJ, Pandey SK, Yadav A, Goel S, Ateeq B. Targeting NF-kappa B signaling by artesunate restores sensitivity of castrate-resistant prostate cancer cells to antiandrogens. Neoplasia. 2017;19(4):333–345. doi:10.1016/j.neo.2017.02.002
93. Su T, Li F, Guan J, et al. Artemisinin and its derivatives prevent helicobacter pylori-induced gastric carcinogenesis via inhibition of NF-kappaB signaling. Phytomedicine. 2019;63:152968. doi:10.1016/j.phymed.2019.152968
94. Yin X, Liu Y, Qin J, et al. Artesunate suppresses the proliferation and development of estrogen receptor-alpha-positive endometrial cancer in HAND2-dependent pathway. Front Cell Dev Biol. 2020;8:606969. doi:10.3389/fcell.2020.606969
95. Zhang S, Feng R, Yuan F, et al. The therapeutic effects of dihydroartemisinin on cisplatin-resistant gastric cancer cells. Curr Pharm Biotechnol. 2022;23(2):276–286. doi:10.2174/1389201022666210217114825
96. Xu Z, Liu X, Zhuang D. Artesunate inhibits proliferation, migration, and invasion of thyroid cancer cells by regulating the PI3K/AKT/FKHR pathway. Biochem Cell Biol. 2022;100(1):85–92. doi:10.1139/bcb-2021-0275
97. Farhan M, Silva M, Xingan X, Zhou Z, Zheng W. Artemisinin inhibits the migration and invasion in uveal melanoma via inhibition of the PI3K/AKT/mTOR signaling pathway. Oxid Med Cell Longev. 2021;2021:9911537. doi:10.1155/2021/9911537
98. Jing W, Shuo L, Yingru X, et al. Artesunate promotes sensitivity to sorafenib in hepatocellular carcinoma. Biochem Biophys Res Commun. 2019;519(1):41–45. doi:10.1016/j.bbrc.2019.08.115
99. Wang Y, Yang Z, Zhu W, et al. Dihydroartemisinin inhibited stem cell-like properties and enhanced oxaliplatin sensitivity of colorectal cancer via AKT/mTOR signaling. Drug Dev Res. 2023;84(5):988–998. doi:10.1002/ddr.22067
100. Lee JJ, Loh K, Yap YS. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med. 2015;12(4):342–354. doi:10.7497/j.issn.2095-3941.2015.0089
101. Zhu K, Wu Y, He P, et al. PI3K/AKT/mTOR-targeted therapy for breast cancer. Cells. 2022;11(16):2508. doi:10.3390/cells11162508
102. Chang L, Graham PH, Ni J, et al. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol. 2015;96(3):507–517. doi:10.1016/j.critrevonc.2015.07.005
103. Torrealba N, Vera R, Fraile B, Martinez-Onsurbe P, Paniagua R, Royuela M. TGF-beta/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer. Aging Male. 2020;23(5):801–811. doi:10.1080/13685538.2019.1597840
104. Ghoneum A, Gonzalez D, Afify H, et al. Compound C inhibits ovarian cancer progression via PI3K-AKT-mTOR-NFkappaB pathway. Cancers (Basel). 2022;14(20):5099. doi:10.3390/cancers14205099
105. Mazzoletti M, Broggini M. PI3K/AKT/mTOR inhibitors in ovarian cancer. Curr Med Chem. 2010;17(36):4433–4447. doi:10.2174/092986710794182999
106. Mortazavi M, Moosavi F, Martini M, Giovannetti E, Firuzi O. Prospects of targeting PI3K/AKT/mTOR pathway in pancreatic cancer. Crit Rev Oncol Hematol. 2022;176:103749. doi:10.1016/j.critrevonc.2022.103749
107. Stanciu S, Ionita-Radu F, Stefani C, et al. Targeting PI3K/AKT/mTOR signaling pathway in pancreatic cancer: from molecular to clinical aspects. Int J Mol Sci. 2022;23(17):10132. doi:10.3390/ijms231710132
108. Song M, Zhang N, Cao F, Liu J. PKNOX2 suppresses lung cancer cell proliferation by inhibiting the PI3K/AKT/mTOR axis. Exp Ther Med. 2023;25(5):217. doi:10.3892/etm.2023.11917
109. Cheng H, Shcherba M, Pendurti G, Liang Y, Piperdi B, Perez-Soler R. Targeting the PI3K/AKT/mTOR pathway: potential for lung cancer treatment. Lung Cancer Manag. 2014;3(1):67–75. doi:10.2217/lmt.13.72
110. Morgos DT, Stefani C, Miricescu D, et al. Targeting PI3K/AKT/mTOR and MAPK signaling pathways in gastric cancer. Int J Mol Sci. 2024;25(3):1848. doi:10.3390/ijms25031848
111. Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: epigenetics and beyond. Life Sci. 2020;262:118513. doi:10.1016/j.lfs.2020.118513
112. Golob-Schwarzl N, Krassnig S, Toeglhofer AM, et al. New liver cancer biomarkers: PI3K/AKT/mTOR pathway members and eukaryotic translation initiation factors. Eur J Cancer. 2017;83:56–70. doi:10.1016/j.ejca.2017.06.003
113. Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. 2020;19(1):145. doi:10.1186/s12943-020-01258-7
114. Bernitsa S, Dayan R, Stephanou A, Tzvetanova ID, Patrikios IS. Natural biomolecules and derivatives as anticancer immunomodulatory agents. Front Immunol. 2022;13:1070367. doi:10.3389/fimmu.2022.1070367
115. Dong F, Tian H, Yan S, et al. Dihydroartemisinin inhibits endothelial cell proliferation through the suppression of the ERK signaling pathway. Int J Mol Med. 2015;35(5):1381–1387. doi:10.3892/ijmm.2015.2140
116. Shen K, Ji L, Lu B, Wang Z. c-Jun N-terminal kinase mediated VEGFR2 sustained phosphorylation is critical for VEGFA-induced angiogenesis in vitro and in vivo. Cell Biochem Biophys. 2012;64(1):17–27. doi:10.1007/s12013-012-9363-0
117. Miura S, Matsuo Y, Saku K. Jun N-terminal kinase inhibitor blocks angiogenesis by blocking VEGF secretion and an MMP pathway. J Atheroscler Thromb. 2008;15(2):69–74. doi:10.5551/jat.e496
118. Grossi V, Peserico A, Tezil T, Simone C. p38alpha MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol. 2014;20(29):9744–9758. doi:10.3748/wjg.v20.i29.9744
119. Li Z, Meng D, Li G, Xu J, Tian K, Li Y. Celecoxib combined with diacerein effectively alleviates osteoarthritis in rats via regulating JNK and p38MAPK Signaling Pathways. Inflammation. 2015;38(4):1563–1572. doi:10.1007/s10753-015-0131-3
120. Ma X, Liu Y, Wang Q, et al. Tamoxifen induces the development of hernia in mice by activating MMP-2 and MMP-13 expression. Biochim Biophys Acta. 2015;1852(5):1038–1048. doi:10.1016/j.bbadis.2015.02.006
121. Sato Y, Kanno S, Oda N, et al. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann N Y Acad Sci. 2000;902(1):201–205. doi:10.1111/j.1749-6632.2000.tb06314.x
122. Szade A, Grochot-Przeczek A, Florczyk U, Jozkowicz A, Dulak J. Cellular and molecular mechanisms of inflammation-induced angiogenesis. IUBMB Life. 2015;67(3):145–159. doi:10.1002/iub.1358
123. Lu JJ, Meng LH, Shankavaram UT, et al. Dihydroartemisinin accelerates c-MYC oncoprotein degradation and induces apoptosis in c-MYC-overexpressing tumor cells. Biochem Pharmacol. 2010;80(1):22–30. doi:10.1016/j.bcp.2010.02.016
124. Gupta K, Kshirsagar S, Li W, et al. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247(2):495–504. doi:10.1006/excr.1998.4359
125. Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–149. doi:10.1016/j.ceb.2007.02.001
126. Cheng R, Li C, Li C, et al. The artemisinin derivative artesunate inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular endothelial cells. Invest Ophthalmol Vis Sci. 2013;54(5):3400–3409. doi:10.1167/iovs.12-11068
127. Yoon MK, Mitrea DM, Ou L, Kriwacki RW. Cell cycle regulation by the intrinsically disordered proteins p21 and p27. Biochem Soc Trans. 2012;40(5):981–988. doi:10.1042/BST20120092
128. Lu JJ, Meng LH, Cai YJ, et al. Dihydroartemisinin induces apoptosis in HL-60 leukemia cells dependent of iron and p38 mitogen-activated protein kinase activation but independent of reactive oxygen species. Cancer Biol Ther. 2008;7(7):1017–1023. doi:10.4161/cbt.7.7.6035
129. Fu C, Shi H, Chen H, Zhang K, Wang M, Qiu F. Oral bioavailability comparison of artemisinin, deoxyartemisinin, and 10-deoxoartemisinin based on computer simulations and pharmacokinetics in rats. ACS Omega. 2021;6(1):889–899. doi:10.1021/acsomega.0c05465
130. Medhi B, Patyar S, Rao RS, Byrav DSP, Prakash A. Pharmacokinetic and toxicological profile of artemisinin compounds: an update. Pharmacology. 2009;84(6):323–332. doi:10.1159/000252658
131. Liu K, Dai L, Li C, Liu J, Wang L, Lei J. Self-assembled targeted nanoparticles based on transferrin-modified eight-arm-polyethylene glycol-dihydroartemisinin conjugate. Sci Rep. 2016;6(1):29461. doi:10.1038/srep29461
132. Zhang J, Gu L, Jiang Y, et al. Artesunate-nanoliposome-TPP, a novel drug delivery system that targets the mitochondria, attenuates cisplatin-induced acute kidney injury by suppressing oxidative stress and inflammatory effects. Int J Nanomed. 2024;19:1385–1408. doi:10.2147/ijn.S444076
133. Su T, Li F, Guan J, et al. Artemisinin and its derivatives prevent helicobacter pylori-induced gastric carcinogenesis via inhibition of NF-κB signaling. Phytomedicine. 2019;63:152968. doi:10.1016/j.phymed.2019.152968
134. Wang KS, Li J, Wang Z, et al. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol Immunotoxicol. 2017;39(1):28–36. doi:10.1080/08923973.2016.1267744
135. Wang B, Hou D, Liu Q, et al. Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biol Ther. 2015;16(10):1548–1556. doi:10.1080/15384047.2015.1071738
136. Haqq J, Howells LM, Garcea G, Dennison AR. Targeting pancreatic cancer using a combination of gemcitabine with the omega-3 polyunsaturated fatty acid emulsion, Lipidem™. Mol Nutr Food Res. 2016;60(6):1437–1447. doi:10.1002/mnfr.201500755
137. Zhou Z, Lei J, Fang J, et al. Dihydroartemisinin remodels tumor micro-environment and improves cancer immunotherapy through inhibiting cyclin-dependent kinases. Int Immunopharmacol. 2024;139:112637. doi:10.1016/j.intimp.2024.112637
138. Yang Z, Chen J, Xiao Y, et al. Digital barcodes for high-throughput screening. Chem Bio Eng. 2024;1(1):2–12. doi:10.1021/cbe.3c00085
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Bibliometric Analysis of Acupuncture Therapy for Cancer Pain Over the Past 10 Years
Ling F, Qi W, Li X, Zhou J, Xiong J, Zhao Y, Zheng Q, Liang F
Journal of Pain Research 2023, 16:985-1003
Published Date: 20 March 2023
Research Trends of the Research and Development of Acupuncture and Moxibustion Therapy on Lumbar Disc Herniation: A Bibliometric Analysis
Li YX, Xiong J, Zhang Z, Liao K, Zhou XH, Li J, Xiang J, Xu LL
Journal of Pain Research 2023, 16:1835-1853
Published Date: 31 May 2023
Cancer Immunotherapy and Medical Imaging Research Trends from 2003 to 2023: A Bibliometric Analysis

Tang S, Fan T, Wang X, Yu C, Zhang C, Zhou Y
Journal of Multidisciplinary Healthcare 2024, 17:2105-2120
Published Date: 6 May 2024
The Traditional Chinese Medicine in Treating Diabetic Nephropathy: A Bibliometric Analysis
Sun J, Shen J, Liu L, Du J
Journal of Multidisciplinary Healthcare 2024, 17:4627-4636
Published Date: 4 October 2024
Research Hotspots and Trends in Acupuncture for Cancer:A bibliometric analysis from 2004 to 2024
Ji Z, Niu H, He A, Li K, Jia J, Zhang M, Liu G
Journal of Multidisciplinary Healthcare 2025, 18:531-547
Published Date: 31 January 2025



