Back to Journals » International Journal of Nanomedicine » Volume 19
Bioactive Materials Facilitate the Restoration of Neurological Function Post Cerebral Ischemic Stroke
Authors Wang C, Sun C, Ding Z, Wu X, Liu K, Cao J
Received 31 August 2024
Accepted for publication 10 December 2024
Published 31 December 2024 Volume 2024:19 Pages 14171—14191
DOI https://doi.org/10.2147/IJN.S493987
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Chunyan Wang,1,* Chao Sun,2,* Ziyan Ding,1 Xiujuan Wu,1 Kangding Liu,1 Jie Cao1
1Department of Neurology, Neurology Specialist Hospital, The First Hospital of Jilin University, Jilin University, Changchun, People’s Republic of China; 2Department of Orthopedic Surgery, Orthopedic Center, The First Hospital of Jilin University, Jilin University, Changchun, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kangding Liu; Jie Cao, Department of Neurology, Neurology specialist hospital, The First Hospital of Jilin University, Jilin University, Changchun, People’s Republic of China, Email [email protected]; [email protected]
Abstract: The recovery process following ischemic stroke is a complex undertaking involving intricate cellular and molecular interactions. Cellular dysfunction or aberrant pathways can lead to complications such as brain edema, hemorrhagic transformation, and glial scar hyperplasia, hindering angiogenesis and nerve regeneration. These abnormalities may contribute to long-term disability post-stroke, imposing significant burdens on both families and society. Current clinical interventions primarily focus on endovascular therapy, overlooking the protection of brain cells themselves. However, the use of bioactive materials in stroke management has shown notable safety and efficacy. By precisely targeting the ischemic site at a cellular and molecular level, this therapeutic approach mitigates ischemia-induced brain tissue damage and promotes site repair. This review examines the protective benefits of bioactive materials in reducing cell damage and facilitating nerve restoration in accordance with the pathophysiological basis of ischemic stroke. Enhanced understanding of ischemic stroke mechanisms has the potential to advance the targeted and efficient clinical use of bioactive materials.
Keywords: angiogenesis, bioactive materials, inflammation, ischemic stroke, nerve regeneration, oxidative stress
Introduction
Ischemic stroke denotes a clinical syndrome resulting from the interruption of cerebral blood flow, leading to ischemic and hypoxic necrosis of localized brain tissue, thereby causing corresponding neurological deficits.1,2 The increasing prevalence and incidence of stroke are closely associated with the rise in common risk factors, for instance, diabetes, hypertension, obesity, hyperlipidemia, substance abuse, and smoking.3,4 Globally, the number of ischemic stroke cases rose from 4.07 million in 1990 to 7.86 million in 2020, projected to reach 9.62 million by 2030.5 The subsequent cognitive and motor impairments impose a significant burden on families and society, with the estimated global cost of stroke exceeding $891 billion, constituting 1.12% of global Gross Domestic Product.6 Therefore, an urgent need exists to innovate viable therapeutic approaches for ischemic stroke.
Conventional therapeutic modalities primarily include intravascular intervention (intravenous thrombolysis and thrombectomy) and pharmacotherapy. Recombinant tissue plasminogen activator (rt-PA), an endogenous or exogenous plasminogen activator, catalyzes the conversion of plasminogen to plasmin, leading to the degradation of fibrin in thrombi and their subsequent dissolution. Since the Food and Drug Administration approved rt-PA in 1996, intravenous thrombolysis has become the standard intervention for acute ischemic stroke.7 However, stringent temporal constraints and exclusion criteria limit its efficacy for most patients. A national stroke registry analysis showed that only 12.6% of 11,675 documented stroke patients qualified for thrombolytic therapy, with just 1.6% receiving intravenous rt-PA.8 In addition, among those who underwent intravenous thrombolysis, 34% experienced early reocclusion after initial recanalization, leading to neurological deterioration and increased in-hospital mortality.9 Moreover, bleeding complications constitute a substantial limitation of intravenous thrombolysis using rt-PA in ischemic stroke patients.10–12 The underlying mechanisms may be linked to immune response,10 inflammation11 and disruption of the blood-brain barrier (BBB).12,13
In 2015, several seminal trials demonstrated the superiority of endovascular thrombectomy over conservative medical management for anterior circulation macrovascular occlusive stroke.14 Nevertheless, endovascular thrombectomy requires rigorous prerequisites concerning patients’ physiological status, institutional capabilities, and healthcare professionals’ proficiency.15 Another limitation of thrombectomy arises in patients with severe vascular occlusion.10 Both intravenous thrombolysis and endovascular thrombectomy face challenges in achieving effective recanalization. Over 50% of ischemic stroke patients treated with rt-PA thrombolysis do not show clinical improvement, a condition known as ‘ineffective recanalization.16,17 Notably, despite successful reperfusion, the infarct often expands, exacerbating neurological deficits due to ischemia-reperfusion injury, excessive brain edema, and hemorrhagic transformation.17,18
Initially, pharmacotherapy for ischemic stroke concentrated on neuronal protection. Following the introduction of the neurovascular unit paradigm by the National Institute of Neurological Disorders and Stroke in 2001, the scope of pharmacotherapy was broadened to encompass the protection of various neuronal subtypes, including astrocytes, pericytes, and endothelial cells.19,20 Macromolecules endowed with anti-inflammatory and antioxidant attributes encounter challenges in traversing the BBB, demonstrating insufficient dissemination within the infarct core. Additionally, numerous small molecule drugs encounter diverse challenges in efficient absorption, including the BBB obstruction and abbreviated half-life. Furthermore, constrained antioxidant and anti-inflammatory efficacy, in conjunction with a lack of precise targeting capabilities, have also contributed to the inefficacy of certain clinical trials involving neuroprotective agents.18
A fundamental tenet in the clinical management of ischemic stroke is the timely restoration of blood perfusion to the ischemic penumbra to salvage compromised neurons. Nevertheless, survivors remain vulnerable to significant risks of disability, neurological deficits, and other complications post ischemia-reperfusion.21 Regrettably, conventional treatment methods have been inadequate in mitigating the risk of these complications and in the long-term management of patients. Considering the significant constraints of conventional therapies, bioactive substances such as hydrogels,22–24 polymer nanoparticles,25,26 liposomes,27 and micelles28,29 offer innovative and promising solutions due to their inherent biochemical and biophysical attributes, encompassing biocompatibility, biodegradability and targeting capabilities.30,31 Additionally, bioactive materials address the challenges of short plasma half-life and insufficient BBB permeability of drugs through various technologies, such as polyethylene glycol functionalization and targeted ligand binding on nanomedicine surfaces. Concurrently, these materials induce specific responses in microenvironment to accelerate ischemic tissue repair, facilitating effective drug distribution to ischemic brain tissue and improving stroke prognosis.32
Hence, this review elucidates the pivotal role of bioactive materials in mitigating cellular damage and promoting blood vessel and nerve regeneration by engaging with the cellular and molecular mechanisms of cerebral ischemia and hypoxia.33–36 Scheme 1 provides a graphical representation to enhance understanding of these interrelated processes. Notably, it is crucial to recognize and address existing constraints and challenges to fully exploit the potential of bioactive materials in the treatment of ischemic stroke. By thoroughly examining these aspects, our objective is to enrich the current discourse and facilitate future progress in this field.
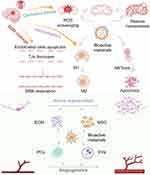 |
Scheme 1 Treatment of ischemic stroke mediated by bioactive materials. The Scheme was created in BioRender. Wang, C. (2025) https://BioRender.com/t09f458. |
Bioactive Materials Inhibit Neuronal Necrosis by Regulating Mitochondrial Homeostasis and Cellular Behavior
Following cerebral ischemia, a cascade of events ensues, encompassing mitochondrial impairment, rapid elevation of reactive oxygen species (ROS), inflammatory cascade activation, augmented BBB permeability, culminating in neuronal demise.2 ROS instigate cellular damage, disrupting cytoplasmic membrane integrity, inducing ion dysregulation and precipitating mitochondrial dysfunction. Furthermore, Cellular debris and necrotic remnants can initiate the inflammatory cascade, stimulating microglia to secrete a plethora of pro-inflammatory cytokines, chemokines and upregulate the expression of cellular adhesion molecules. Subsequently, neutrophils adhere to endothelial cells in response to inflammatory cytokines, migratory cues from chemokines prompt their excessive infiltration into the ischemic locus, exacerbating cerebral injury. Following this, endothelial cell apoptosis ensues and the expression of tight junction proteins (TJPs) between endothelial cells decreases. This compromises the integrity of the BBB, contributing to local brain edema and hemorrhage, exacerbating functional decline. Hence, imperative strategies in ischemic stroke management entail mitigating the aforementioned pathological cascade to enhance neurological recovery.
Regulating Oxidative Stress and Homeostasis in Mitochondria
Mitochondrial dysfunction stands prominently as a pivotal mechanism in ischemic stroke, marked by mitochondrial oxidative stress and disruption of mitochondrial quality control.37 Upon mitochondrial damage, they undergo membrane encapsulation, forming autophagosomes to eliminate dysfunctional mitochondria.38,39 Oxygen depletion constrains mitochondrial oxidative phosphorylation, diminishing adenosine 5’-triphosphate (ATP) synthesis and yielding a large amount of ROS such as superoxide anion, hydroxyl radical, and hydrogen peroxide. The equilibrium between ROS generation and scavenging upholds redox homeostasis. Post-cerebral ischemia, this equilibrium falters, precipitating oxidative stress. The repercussions of oxidative stress hinge upon the magnitude of fluctuations in ROS and their derivatives. Physiological levels of mitochondrial ROS serve as redox mediators in intracellular signaling, while excessive ROS disrupt cellular equilibrium, culminating in mitochondrial dysfunction.40,41 This steady-state dynamic fluctuation may be countered by the endogenous antioxidant system, yet profound oxidative stress can induce irreversible harm to the organism.42 Hence, the elimination of surplus ROS to reinstate redox homeostasis and the facilitation of mitochondrial function recovery are imperative for enhancing the neurological function of ischemic brain tissue.
Concerning the regulation of ROS levels, Dl-3-n-butylphthalide (NBP), a compound derived from celery seeds, has been authorized by the National Medical Products Administration for the clinical management of ischemic stroke since 2002.43 NBP directly stimulates cytochrome C oxidase 7c (Cox7c) within endothelial cell mitochondria, augmenting ATP synthesis, diminishing ROS emission, and aiding in the stabilization of mitochondrial membrane potential. Furthermore, NBP potentially enhances the expression of Zonula occludens and occludin in endothelial cells through Cox7c upregulation, thereby preserving the integrity of the BBB.44 NBP may additionally foster mitochondrial fusion and ultimately ameliorate cerebral ischemia/reperfusion symptoms by modulating the Adenosine 5‘-monophosphate-activated protein kinase -mediated mitofusin 1 pathway.43 A study assessing the efficacy and safety of NBP in patients experiencing acute cerebral infarction undergoing revascularization demonstrated that NBP substantially enhanced short-term outcomes at 90 days.45 Moreover, the precise administration of NBP via Ceria nanoparticles as conveyors eradicates ROS in brain endothelium and hippocampal neurons (Figure 1), thereby restoring mitochondrial membrane potential, morphology, and functionality. Consequently, in vitro BBB impairment and neuronal apoptosis were mitigated.46 To enhance blood concentration at the ischemic site, biomaterials have undeniably assumed an indispensable role. Yang et al devised a ROS-responsive, transformable, and triple-targeted NBP nanotherapy, which notably augmented cellular NBP uptake and elevated plasma NBP concentration at ischemic sites.47
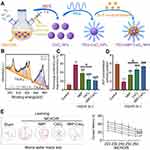 |
Figure 1 Cerium oxide nanoparticles are utilized for targeted drug delivery to ischemic regions in stroke therapy. (A) The synthesis protocol for NBP-CeO2 NPs. (B) XPS analysis of Ce 3 d showed the binding energy level of Ce (III) in NBP-CeO2 NPs. (C) Quantitative representation of the proportion of BMVECs containing mitochondrial fragments (n = 3; ***P < 0.001, &P < 0.05, ##P < 0.01, ###P < 0.001). (D) Assess the ATP content in BMVECs to elucidate mitochondrial function (n = 3; ***P < 0.001, &P < 0.05, ##P < 0.01, ###P < 0.001). (E) Representative swim trajectories in the spatial learning evaluation segment of Morris water maze test. Mice locate the underwater platform as the escape latency period 22–26 days after MCAO/R (n = 12; ***P < 0.001, ###P < 0.001, &&&P < 0.001). Reprinted from Biomaterials, Li X, Han Z, Wang T, et al. Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials. 2022;291:121904. doi:10.1016/j.biomaterials.2022.121904. Copyright 2022, with permission from Elsevier.46 Abbreviations: ATP, Adenosine 5’-triphosphate; BMVECs, brain microvascular endothelial cells; MCAO/R, middle cerebral artery occlusion/reperfusion; NBP-CeO2 NPs, Dl-3-n-butylphthalide-cerium oxide nanoparticles; XPS, X-ray photoelectron spectroscopy. |
Following cerebral ischemia, alterations occur in mitochondrial membrane permeability. To adapt to ischemia-induced changes, mitochondria selectively eliminate dysfunctional counterparts via autophagy, a pivotal process for sustaining mitochondrial homeostasis.48 Wang et al engineered an inhalable nanotherapeutic agent, denoted as P/D @ Mn/Co3O4, synthesized from an artificial platelet membrane and Mn/Co3O4 encapsulated in 2.3-(dioxy propyl)-trimethylammonium chloride. This bioactive material depolarizes mitochondrial membrane potential by depleting the H+ surrounding the mitochondria, prompts mitochondrial autophagy, removes aberrant mitochondria.49
As previously documented, specific bioactive substances can mitigate ROS levels and facilitate mitochondrial function restoration. These bioactive materials comprise polyphenol nanoparticles,50 recombinant human heavy chain ferritin nanoparticles51 and Cyclosporine A nanoparticles.52 Additionally, a cutting-edge two-dimensional (2D) nanomaterial, namely layered transition metal carbon/nitrogen compound (MXene), has garnered significant attention owing to its exceptional antioxidant attributes. Fan et al devised a nanotherapeutic agent, 2D MXene-loaded isoquercetin, demonstrating its capability to further ameliorate ROS-induced cellular activity decline by scavenging surplus ROS.53
These findings reaffirm the viability of mitigating surplus ROS and fostering mitochondrial homeostasis through the utilization of bioactive substances. Hence, this strategy holds significant promise for the advancement and clinical translation of ischemic stroke therapy.
Regulating the Activation and Polarization of Microglia
Microglia, as resident macrophages within central nervous system (CNS), are pivotal in neuroinflammation and the pathological progression of ischemic tissue.54,55 Following cerebral ischemia, cellular demise releases damage-associated molecular patterns, activating inflammatory pathways and triggering an inflammatory storm. Upon ischemic insult, microglia undergo activation, exerting a dual effect of neurotoxicity and neuroprotection, with the balance of these two effects dictating neuronal fate.56 Post-cerebral ischemia, microglia swiftly transition from a surveilling state to an “activated” state within minutes, manifesting phenotypic changes encompassing morphological transformation, proliferation, and polarization.57 These activated microglia migrate to injury sites, engaging in phagocytosis.58 Remarkably, microglia and macrophages exhibit dynamic responses to ischemic injury. Local microglia and newly recruited macrophages display an M2 phenotype during the early phases of ischemic stroke, transitioning gradually to an M1 phenotype within the peri-infarct region. 59 M1 microglia secrete proinflammatory cytokines and chemokines, including tumor necrosis factor, Interleukin 6 (IL-6), IL-1β, IL-12, and C-C motif chemokine ligand 2, exacerbating ischemia-induced nerve inflammation. Conversely, M2 microglia activation fosters the release of anti-inflammatory cytokines like IL-10 and transforming growth factor β, aiding neural function recovery.60 Therefore, manipulating microglia activation and polarization emerges as a promising strategy against cerebral ischemia.
Inhibiting microglial activation is a crucial neuroprotective strategy that can salvage neurons in the ischemic penumbra.26,61 Rapamycin (RAPA), an established inhibitor of mechanistic target of rapamycin complex 1 (mTORC1), attenuates microglial activation by suppressing the phosphatidylinositol-3-kinase (PI3K)/protein kinase (AKT)/mTORC1 pathway, thereby ameliorating the neuroinflammatory response following ischemia.62,63 Due to its hydrophobic characteristics, the amalgamation of bioactive substances with RAPA emerges as a favorable option.64,65 Gao et al formulated a nanocarrier for the specific transport of RAPA (Figure 2). The nanocarrier comprised a sulfated chitosan (SCS) polymer core, functionalized with a ROS-responsive boronic ester, encased in a red blood cell membrane shell incorporating a stroke-targeting peptide. Upon exposure to elevated intracellular ROS levels in ischemic brain tissue, the nanocarrier promotes the release of SCS and RAPA, thereby aiding in the restoration of cerebral function.65
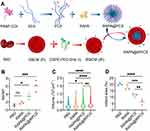 |
Figure 2 Polysaccharide sulfate-based nanocarriers deliver targeted neuroprotective agent rapamycin in the management of cerebral infarction. (A) The schematic design of RAPA @ tRPCS. (B) Phenotypic changes in microglia upon exposure to various nanoparticles (n = 3; *P < 0.05, ***P < 0.005). (C) The effect of different nanoparticles on microglia size (n = 3; *P < 0.05, **P < 0.01, ****P < 0.001). (D) The infarct volumes at 7 days after tMCAO were measured with ImageJ in different groups (n = 3; **P < 0.01, ***P < 0.005, ****P < 0.001). Reprinted with permission from Cao Y, Yu Y, Pan L, et al. Sulfated polysaccharide-based nanocarrier drives microenvironment-mediated cerebral neurovascular remodeling for ischemic stroke treatment. Nano Lett. 2024;24(17):5214–5223. Copyright 2024, American Chemical Society.65 Abbreviations: ns, not significant; tMCAO, transient middle cerebral artery occlusion. |
Microglia exert a pivotal role in the evolution of injury and tissue reorganization post-ischemic stroke.66 Given the deleterious impact of M1 microglia on ischemic brain tissue, modulating microglial polarization holds considerable significance.67–69 Edaravone dexborneol (Eda-Dex) is a neuroprotective agent sanctioned for ischemic stroke therapy in China as of 2020. It mitigates the synthesis of pro-inflammatory cytokines and chemokines by impeding the polarization of microglia/macrophages and astrocytes towards the M1 phenotype.55,70 Additionally, it prompts the transformation of lipopolysaccharide-stimulated microglia from the M1 to M2 phenotype by negatively regulating the Toll-like receptor 4/myeloid differentiation marker 88 /nuclear factor kappa B (NF-κB) signaling cascade.71 The TASTE-SL randomized clinical trial demonstrated that among patients experiencing acute ischemic stroke within 48 hours, sublingual administration of Eda-Dex yields superior short-term prognosis compared to placebo.72 The clinical application of this substance is constrained due to its abbreviated biological half-life and inadequate water solubility.73 As previously stated, Yin et al reported an engineered nanoerythrocytes modified with MG1 peptide and RVG29 peptide, which has the ability to effectively penetrate the BBB and accurately recognize M1 microglia. The platform reprograms microglia from classical M1 to alternative M2 by activating heme oxygenase-1 within microglia, stimulating the signaling pathway of Notch1/Hes1/ transcription 3, and further inhibiting NF- κB p65 translocation.74
Furthermore, High mobility group box 1 (HMGB1) serves as a potent pro-inflammatory mediator that promotes M1 polarization in microglia. Eighteen β-Glycyrrhetinic acid acts as a potent intracellular inhibitor of HMGB1. Jin et al developed a ROS-responsive 18 β-Glycyrrhetinic acid-conjugated polymer nanoparticle system to modulate microglial polarization by inhibiting the translocation of nuclear HMGB1.75
Regulating the Activation and Infiltration of Neutrophils
Neutrophils, integral constituents of the innate immune system and regulators of the adaptive immune response, wield significant influence within ischemic brain tissue.76 The neutrophil extracellular trap (NET) is a reticular DNA structure comprising double-stranded DNA, histones, and granular proteins released by activated neutrophils, tightly regulated for production and clearance. NETosis represents a distinctive form of neutrophil demise, concomitant with the liberation of NETs. NETs compromise the integrity of the BBB, instigate thrombosis, and subsequently exacerbate neuronal injury and neurological impairment following ischemia.21,77 Increased plasma NET biomarkers are linked to poorer stroke outcomes.78 Following cerebral ischemia, neutrophils undergo rapid activation and transmigrate across the endothelium towards the ischemic region. Upon reaching the site, they adhere to endothelial cells via integrin β2 on neutrophils and intercellular adhesion molecule-1 expressed on endothelial cells. Subsequently, the count of rolling or adherent white blood cells escalates, with excessive neutrophil infiltration leading to cerebral tissue damage.79,80 In summary, neutrophils exacerbate ischemic cerebral injury through diverse mechanisms, such as inducing capillary congestion, secreting inflammatory mediators, and augmenting thrombosis via the formation of neutrophil-platelet aggregates and NETs.81,82 Hence, regulating the activation and infiltration of neutrophils represents a promising therapeutic avenue for ischemic stroke management.
Neutrophil activation generates a substantial quantity of NETs. Hence, the primary approach to controlling neutrophil activation involves averting the release of NETs. Yin et al studied and designed a neutrophil hijacking/reprogramming nanoplatform, termed APTS (Figure 3). This platform was synthesized by modifying polydopamine-coated A151/PEI nanoparticles with a targeted peptide (TP peptide) and sialic acid. TP peptide facilitates the recognition of neutrophils adhering to inflammatory endothelial cells, thereby inducing the uptake of APTS by neutrophils and reprogramming them from NETosis to apoptosis via ROS-mediated citrulline histone inhibition pathway, significantly reducing the formation of NETs.83 Notably, Cl-amidine, an inhibitor of peptidylarginine deiminase 4, is encapsulated within self-assembled liposomal nanocarriers, which are modified with ROS-responsive polymers and fibrin-binding peptide. This formulation aims to suppress the NETs release process to enhance the reduction in mortality associated with cerebral infarction.21
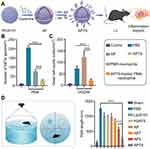 |
Figure 3 A APTS significantly reduces the formation of NETs by reprogramming neutrophil NETosis to cell apoptosis. (A) Preparation process of APTS. (B) The number of NETs after different treatments (n = 6; ***P < 0.001). (C) Quantitative analysis of dead cell counts in different treatments (n = 6; ***P < 0.001). (D) Schematic diagram of Morris water maze test. Path length in different processing (n = 8; ***P < 0.001). Reproduced from Yin N, Wang W, Pei F, et al. A neutrophil hijacking nanoplatform reprograming NETosis for targeted microglia polarizing mediated ischemic stroke treatment. Adv Sci. 2024;5:e2305877. http://creativecommons.org/licenses/by/4.0/.83 Copyright 2024, The Authors. Advanced Science published by Wiley‐VCH GmbH. Abbreviations: APTS, neutrophil hijacking nanoplatform; NETs, neutrophil extracellular traps. |
The aggregation and clearance of neutrophils are pivotal determinants influencing neuroinflammation in acute ischemic stroke. Resolvin D2 (RvD2), a lipid-lowering hormone, has the ability to reprogram energy metabolism from glycolysis to oxidative phosphorylation. This, in turn, facilitates the phagocytic clearance of neutrophils by microglia, diminishing local neutrophil accumulation and mitigating neuroinflammation in the ischemic brain.84,85 Given that RvD2 easily binds to proteins, Dong et al elucidated a drug delivery framework comprising neutrophil membrane-derived RvD2-loaded nanovesicles.86 Mechanistically, RvD2 instigates nitric oxide production in the endothelium, consequently attenuating neutrophil-endothelium interactions.87 RvD2 additionally interacts with the G protein-coupled receptor on neutrophils to impede neutrophil infiltration and provoke neutrophil apoptosis, thus expediting inflammation resolution.88 Furthermore, specific biomaterials, such as platelet-mimicking nanoparticles, possess the capacity to diminish neutrophil infiltration.89
Furthermore, the swift and efficient migration of neutrophils into the brain following cerebral ischemia has garnered significant interest. Their inflammatory homing characteristics render them an appealing conduit for targeted drug administration.90,91 Additionally, neutrophils play an indispensable role in penetrating the BBB to facilitate drug delivery to the brain.92
Regulating Apoptosis and Connectivity of Endothelial Cells
BBB represents a distinctive and dynamic regulatory interface comprising capillaries, pericytes, basement membranes and astrocytes.93 Endothelial cells, along with their tight junctions (TJs), constitute the primary barrier to permeability, while pericytes and astrocytes assume significant regulatory functions.94 In physiological homeostasis, BBB serves as a pivotal “Gatekeeper”, orchestrating essential roles in the regulation of paracellular permeability, ion balance, nutrient trafficking, and cerebral blood flow.95
Endothelial cells, pivotal constituents of BBB, envelop the entire microvasculature. Following a stroke, these cells experience oxygen deprivation, leading to BBB dysfunction and increased permeability. EPALRESTAT, an Aldose reductase (AR) inhibitor, mitigates endothelial cell apoptosis by modulating mTOR phosphorylation via the AR/AKT/mTOR signaling pathway. It also sustains the expression levels of TJPs in endothelial cells, thus safeguarding BBB.96
TJs govern solute flux between contiguous endothelial cells, establishing a seamless and impermeable barrier termed paracellular diffusion, quantifiable by high transendothelial resistance.97 The modulation of TJPs expression is governed by various intrinsic signaling cascades, encompassing phosphorylation, matrix metalloproteinases, and microRNAs.98 Following cerebral ischemia, the TJs among endothelial cells deteriorate, culminating in heightened paracellular permeability. This phenomenon contributes to angiogenic edema, hemorrhagic transformation, and elevated mortality rates.99 Consequently, preventing TJPs degradation and enhancing its expression emerge as promising strategies to mitigate BBB permeability. These bioactive materials include c(RGDyK) peptide modified, caffeic acid phenethyl ester (a NF-κB inhibitor)-loaded and reactive nitrogen species stimuli-responsive liposomal nanocarrier,100 neutrophil membrane-fused nanoliposomal leonurine92 and Dietary Fe3O4 nanozymes.101
It is noteworthy that Liu et al devised a cerium-doped myricetin oligomer nanostructure, which modulates TJPs expression through the activation of protective autophagy, thus showcasing its capability in BBB restoration.102 Moreover, Gao et al devised an M2 microglia-targeting lipid nanoparticle (Figure 4). This nanoparticle was observed to stimulate IL-10 production and amplify the anti-inflammatory response through the Janus kinase-STAT pathway, thus augmenting the integrity of the BBB.103
 |
Figure 4 Targeting mRNA nanoparticles to improve BBB damage after ischemic stroke. (A) Schematic diagram of targeting mIL-10@MLNPs. (B) The average particle size of mIL-10@LNPs and mIL-10@MLNPs (left). Size of mIL-10@LNPs in 10% serum condition at 37 °C for up to 24 h (right). (C) Representative histograms of CD206+microglia isolated from the ischemic hemisphere of the designated treatment group. (D) Quantitative analysis of IgG leakage MFI in each group (n = 4; ****P < 0.0001). (E) Quantitative assessment of SMI32/MBP MFI ratio in the outer capsule of the indicator group (n = 4; ****P < 0.0001). Reprinted with permission Gao M, Li Y, Ho W, et al. Targeted mRNA nanoparticles ameliorate blood-brain barrier disruption postischemic stroke by modulating microglia polarization. ACS Nano. 2024;18(4):3260–3275. Copyright 2024, American Chemical Society.103 Abbreviations: BBB, blood−brain barrier; MFI, mean fluorescence intensity; mIL-10@MLNPs, mIL-10-loaded M2 microglia-targeting lipid nanoparticle; n.s., not significant; SMI32/MBP, non-phosphorylated neurofilament H/myelin basic protein. |
Bioactive Materials Promote Nerve Tissue Repair by Regulating Angiogenesis and Nerve Regeneration
The cerebrovascular system not only delivers glucose and oxygen to brain tissue but also shields the brain from neurotoxin harm.104 Following cerebral ischemia, the brain initiates a cascade of endogenous reparative mechanisms to accommodate to this perturbation, primarily involving angiogenesis and neurogenesis. Following cerebral ischemia, nerve cells migrate to regions where early vascular remodeling and increased vascular density persist.105 Nonetheless, this innate self-repair mechanism is notably limited.106 Hence, breaching limitations and advocating for restoration are imperative in enhancing the prognosis of ischemic stroke patients. Stem cell therapy demonstrates distinctive therapeutic potential for ischemic stroke, with the cellular homing, differentiation, and paracrine capabilities of stem cells instilling optimism for neuroprotection.15,107 Recently, the utilization of bioactive materials has augmented the capacity of stem cells to stimulate vascular and nerve regeneration in ischemic locales. Hence, a thorough comprehension of bioactive material-mediated mechanisms underlying vascular and nerve regeneration is imperative for ischemic stroke therapy.
Regulating the Recruitment and Paracrine Secretion of Progenitor Cells
Angiogenesis represents a pivotal stage in brain tissue regeneration post-stroke. Endothelial progenitor cells (EPCs) serve as precursors to vascular endothelial cells, possessing the ability to differentiate into endothelial cells and adhere to active angiogenic loci, thus fostering the genesis of new blood vessels subsequent to tissue ischemia.108 Concurrently, through the secretion of extracellular vesicles (EVs) and diverse pro-angiogenic factors in a paracrine manner, EPCs expedites the maturation of endothelial cells and the proliferation, differentiation, and migration of peripheral EPCs, thus facilitating the restoration of impaired vascular endothelium.109 Elevated levels of circulating EPCs post-acute ischemic stroke correlate with enhanced functional outcomes and diminished infarct volume.110 The diminished quantity of EPCs constitutes an independent risk determinant for unfavorable prognosis among individuals afflicted by acute ischemic stroke.111,112 Increasing the abundance and viability of progenitor cells (PCs) and EVs at the site of localized infarction emerges as a novel objective for fostering angiogenesis.
Intrinsic EPCs engage in neovascularization through the C-X-C chemokine receptor 4 / stromal cell-derived factor (SDF-1) axis subsequent to cerebral ischemia.113 SDF-1, recognized as Chemokine C-X-C motif chemokine ligand 12, represents a chemotactic cytokine delineated by two variants, α and β, with SDF-1α being the predominant variant. Given its capacity to attract EPCs, neural PCs, and mesenchymal stem cells to the infarcted region, SDF-1α has garnered significant interest in ischemic stroke therapy in recent years (Figure 5). 114 In vitro studies have demonstrated that SDF-1α mitigates EPC apoptosis under ischemic conditions through the PI3K/AKT/ endothelial nitric oxide synthase (eNOS) pathway.115 The localized administration of SDF-1 augments angiogenesis by bolstering EPC recruitment in ischemic tissues.116 Nevertheless, the therapeutic efficacy of chemokines is significantly compromised by adverse BBB permeability and systemic side effects.117 Thus, targeted administration of SDF-1α employing bioactive materials presents a promising approach. Kim et al devised and synthesized a dual ionic pH-responsive copolymer poly (urethane amino sulfamethazine). Their findings demonstrate that targeted delivery of SDF-1α can efficiently modulate the microenvironment and augment angiogenesis.117 Furthermore, Wilson et al formulated SDF-1 bound Heparin Nanoparticles. The findings indicated a significant increase in perfusion vessels within 10 days post-stroke.114
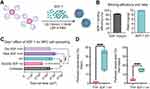 |
Figure 5 SDF-1 bound nH promote angiogenesis after stroke by recruiting progenitor cells for differentiation. (A) Covalent binding of SDF-1 soluble growth factor with nH. (B) ELISA data combining SDF-1 and nH measurements and the ratio of SDF-1 to nH. (C) Quantification of NPC dissemination following 24 hours of exposure to nH, soluble SDF-1, bound and unbound SDF-1 nH at 200 ng SDF-1 (n = 3; *P < 0.05, ** P < 0.01, ***P < 0.005). (D) The perfusion vascular area in the infarcted area (left). The perfusion vascular area around the peri-infarct area (right) (n = 5; ****P < 0.001). Reproduced with permission from Wilson KL, Joseph NI, Onweller LA, et al. SDF-1 bound heparin nanoparticles recruit progenitor cells for their differentiation and promotion of angiogenesis after stroke. Adv Healthc Mater. 2023;27:e2302081. © 2023 Wiley-VCH GmbH.114 Abbreviations: nH, heparin nanoparticles; NPC, neural progenitor cell; SDF-1, Stromal cell-derived factor 1. |
EVs are diminutive phospholipid bilayer structures secreted via paracrine action by cells, encapsulating essential proteins, lipids, and genetic material for intercellular communication. EPC-derived EVs (EPC-EVs) express α4 integrins and CD29 (β1 integrin), facilitating their internalization into human microvascular endothelial cells and human umbilical vein endothelial cells (HUVECs). EPC-EVs transport mRNA to endothelial cells, activate the PI3K/Akt/eNOS signaling pathway, and induce angiogenesis. Simultaneously, EVs upregulate the expression of the anti-apoptotic protein Bcl-xL in endothelial cells, exerting an anti-apoptotic effect. Additionally, EVs harbor polymerase genes responsible for mRNA synthesis in eukaryotes.118 Hu et al discovered that miR-21-5p is abundantly enriched in EPC- EVs and selectively suppresses the expression of the angiogenesis inhibitor thrombospondin-1 in recipient endothelial cells, thereby promoting endothelial repair.
Furthermore, exogenous EPC-EV uptake enhances the proliferation and migration capacities of HUVECs.119 A study elucidating the regenerative efficacy of cord blood (CB) EPC-EVs on CB-EPCs in vitro demonstrated that EPC-EVs augmented the regenerative capacity of EPCs without altering their endothelial characteristics even at elevated concentrations.120 And a comparative investigation contrasting stem cell therapy with stem cell-derived EV therapy revealed both modalities enhancing angiogenesis in ischemic stroke, with no discernible disparity between the two.121,122 These findings suggest that EVs possess therapeutic potential as a substitute for stem cells. Thus, EV-based treatment is poised to emerge as an ideal approach for promoting post-stroke brain repair.
Nevertheless, EVs-based therapy encounters challenges including low production, variable biological efficacy, and reduced tissue retention.123 Hence, it is crucial to engineer novel EVs using bioactive materials to generate high-yield EVs with enhanced biological activity for clinical implementation.124,125 Jiang et al developed a dual-responsive hydrogel sensitive to glucose and ROS. These hydrogels exhibit responsiveness to the cerebral microenvironment post-stroke in type 2 diabetes mice, enabling controlled release. Intracerebral injection of these hydrogels in ischemic mice enhances EVs retention and facilitates sustained release, thereby promoting angiogenesis and enhancing neural recovery. These findings underscore the safety and efficacy of this microenvironment-responsive, sustained-release EVs hydrogel system as a therapeutic approach for diabetic stroke.126 In the future, biomaterials enhanced EVs-based therapy is poised to make a substantial impact in ischemic stroke.
Regulating the Local Ecological Microenvironment of Stem Cells
The microenvironment refers to the physical and chemical milieu that surrounds cells, comprising the extracellular matrix (ECM), oxygen concentration, growth factors, and other components. It exerts influence on the proliferation, differentiation, migration, and homing behavior of cells. ECM is a sophisticated three-dimensional network produced by cells, akin to soil, that enmeshes cells within tissues and organs throughout the body. It is pivotal in neural development and regeneration, including the maintenance of the stem cell niche, neural cell migration, and axonal growth.127 During ischemic stroke, the autophagy of damaged tissues and the degradation of the ECM by metalloproteinases lead to the gradual formation of a stroke cavity in locally affected brain tissue. This cavity lacks physical support structures necessary for cell infiltration and tissue regeneration, consequently leading to impaired angiogenesis and neurogenesis.128 The grafting of neural stem cells (NSCs) enhances functional recovery following an ischemic stroke. Nevertheless, following cerebral ischemia, the local microenvironment is disrupted, characterized by ECM degradation, elevated levels of inflammatory factors, and excessive ROS, all of which diminish NSC survival and differentiation rates, thus limiting the therapeutic efficacy of NSC transplantation.129 Hence, orchestrating the microenvironment encompassing the niche of stem cells assumes a pivotal role in fostering neural regeneration.
The natural ECM furnishes essential biomechanical and biochemical cues within the extracellular milieu, rendering it a promising candidate for bioactive material development.130–132 Bioactive materials mimicking the ECM can be categorized as follows:130 1) decellularized matrix sourced from tissues/organs, 2) matrix derived from cells, and 3) materials comprising purified ECM constituents. ECM mimics biomaterials, particularly decellularized matrix, which can mend tissue defects and stimulate in situ tissue regeneration. It has been effectively employed to facilitate constructive remodeling of neural tissue.133–135 An essential strategy for CNS regeneration involves fabricating an artificial scaffold that mimics the physiological ECM, guiding nerve regeneration spatially.136
Notably, Álvarez et al devised an ECM emulation platform utilizing supramolecular nanoscale fibril scaffolds derived from peptide amphiphile (PA) molecules.137 They observe that nanofibers displaying enhanced intensity of internal supramolecular motion demonstrate heightened bioactivity toward motor and cortical neurons derived from human induced pluripotent stem cells. Highly mobile PA scaffolds were shown to elicit augmented activation of the β1-integrin pathway and enhance the matured electrophysiological activity of neurons. Furthermore, Moshayedi et al formulated a self-polymerizing hydrogel utilizing hyaluronic acid as its base.138 This hydrogel serves as a platform for structural motif adhesion and for the storage and controlled release of growth factors, thereby fostering the survival and differentiation of transplanted stem cells.
Following cerebral infarction, the local microenvironment harbors elevated levels deleterious substances, exacerbating the viability of transplanted stem cells within the stroke-inflicted region. In light of this, Xu et al developed a dual-buffered arm mesenchymal stem cell bioengineering system to manipulate the local microenvironment (Figure 6), enhance the biological activity of mesenchymal stromal cells, and facilitate neuronal regeneration.139 Similar bioactive materials also encompass surface-bound ROS-responsive micellar carriers140 and Polylactic-co-glycolic acid-polyethylene glycol micelle biomaterials enriched with Reelin and embryonic NSC.28
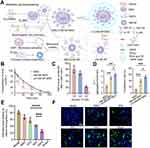 |
Figure 6 The bioorthogonal MSC bioengineering system promotes neuronal regeneration after ischemic stroke through hormone effects. (A) Schematic diagram for preparing LA-HM-NP-MSC. (B) The ability of LA-HM-NP-MSC to remove ROS. (C) The PC absorption capacity of LA-HM-NP-MSC relative to natural MSC. (D) Quantitative evaluation of mitochondrial membrane potential (left) (n = 3; *P < 0.05, ***P < 0.001). Quantitative analysis of cell proliferation (right) (n = 3; *P < 0.05, ***P < 0.001). (E) Infarct size in stroke rats 28 days after MSC treatment (n = 5; ***P < 0.001, ****P < 0.0001). (F) Representative NeuN immunofluorescence images of the infarcted region following 2 months of MSC therapy (scale bar = 100 µm). Reproduced from Xu J, Sun Y, You Y, et al. Bioorthogonal microglia-inspired mesenchymal stem cell bioengineering system creates livable niches for enhancing ischemic stroke recovery via the hormesis. Acta Pharm Sin B. 2024;14(3):1412–1427. (http://creativecommons.org/licenses/by-nc-nd/4.0/).139 Copyright 2024, The Authors. Published by Elsevier B.V. on behalf of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Abbreviations: LA-HM-NP-MSC, lipoic acid- activated HAPI microglia derived membrane-nanoparticle-mesenchymal stem cell; MSC, mesenchymal stem cell; PC, proinflammatory cytokines; ROS, reactive oxygen species. |
Conclusion and Perspectives
At present, the clinical management of ischemic stroke mainly depends on endovascular treatment and the use of neuroprotective drugs. Nevertheless, the increasing incidence and prevalence of stroke pose significant challenges to its treatment. As medical technology advances, novel neuroprotective medications continue to emerge. A thorough assessment of the merits and drawbacks of these medications is imperative to foster the development of more efficacious bioactive compounds. Hence, we undertook a comprehensive review of diverse drugs employed in ischemic stroke treatment and delineated their individual mechanisms of action, detailed in Table 1.
 |
Table 1 Conspectus of Anti-Ischemia Tactics and Repair Mechanisms in Management of Ischemic Stroke |
Reestablishing mitochondrial homeostasis, exerting anti-inflammatory effects, reducing BBB permeability, and fostering brain tissue regeneration represent prevalent pharmacological approaches in the treatment of ischemic stroke. These methodologies seek to impede ischemic cell apoptosis, regulate the ischemic microenvironment, mobilize stem cells, and enhance their viability and differentiation, thereby ameliorating neurological function post-cerebral ischemia.
Maintaining moderate ROS levels and mitochondrial equilibrium are pivotal for cell viability. Following cerebral ischemia, this equilibrium is disrupted. Bioactive materials can alleviate brain tissue damage by scavenging excessive ROS and reinstating mitochondrial dynamics equilibrium. Moderate inflammation and repair are crucial for maintaining homeostasis. The inflammatory response in the local microenvironment following cerebral ischemia is heterogeneous. Early microglia exacerbate inflammation by releasing pro-inflammatory cytokines and chemokines, whereas late microglia facilitate tissue repair by altering their phenotype and secreting anti-inflammatory cytokines. Concurrently, neutrophils exacerbate the inflammatory cascade through the release of NETs and local infiltration. Excessive inflammation and inadequate repair during ischemia can disrupt homeostasis. Bioactive materials can mitigate brain tissue damage by suppressing microglia and neutrophil activation, promoting microglial polarization towards the M2 phenotype and inhibiting the infiltration of neutrophil. The integrity of the BBB is compromised by Ischemic microenvironment, while the bioactive material enhances neural function by mitigating endothelial cell apoptosis and reinstating TJPs expression between endothelial cells.
Hence, modulation of the aforementioned cellular behaviors represents a pivotal therapeutic strategy. Lastly, bioactive materials are instrumental in advancing neural function repair by modulating progenitor cells and their corresponding EVs and enhancing the local microenvironment for stem cells. These modalities constitute a crucial facet of contemporary pharmacotherapy for ischemic stroke.
Despite the plethora of available medications, effective clinical management of ischemic stroke remains elusive. Following cerebral ischemia, the pathophysiological cascade is intricate, defying remediation through mono-modal pharmacotherapy. Moreover, neuronal regeneration post-cerebral ischemia is severely constrained. Thus, mere antioxidative or anti-inflammatory interventions are unlikely to yield favorable outcomes in ischemic stroke management. Employing multi-modal bioactive materials with targeted delivery mechanisms, holds promise in elevating local drug concentrations, mitigating systemic adverse effects and enhancing prognostic outcomes. Concurrently, such interventions may complement endovascular therapies, fostering enhanced cerebral tissue recovery. Furthermore, harnessing bioactive materials to augment stem cell recruitment, viability, and differentiation represents a focal point and a formidable challenge in advancing stroke therapeutics.
Abbreviations
AKT, protein kinase; ATP, adenosine 5’-triphosphate; BBB, blood-brain barrier; CNS, central nervous system; Cox7c, cytochrome C oxidase 7c; 2D, two-dimensional; Eda-Dex, edaravone dexborneol; IL-6, Interleukin 6; mTORC1, mechanistic target of rapamycin complex 1; MXene, layered transition metal carbon/nitrogen compound; NBP, dl-3-n-butylphthalide; PI3K, phosphatidylinositol-3-kinase; RAPA, Rapamycin; ROS, reactive oxygen species; rt-PA, recombinant tissue plasminogen activator; SCS, Sulfated chitosan; TJs, tight junctions; TJPs, tight junction proteins.
Data Sharing Statement
Data available on request from the corresponding author.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Walter K. What is acute ischemic stroke? JAMA. 2022;327(9):885. doi:10.1001/jama.2022.1420
2. Qin C, Yang S, Chu YH, et al. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7(1):215. doi:10.1038/s41392-022-01064-1
3. Singhal AB, Biller J, Elkind MS, et al. Recognition and management of stroke in young adults and adolescents. Neurology. 2013;81(12):1089–1097. doi:10.1212/WNL.0b013e3182a4a451
4. Zhao Y, Hua X, Ren X, et al. Increasing burden of stroke in China: A systematic review and meta-analysis of prevalence, incidence, mortality, and case fatality. Int J Stroke. 2023;18(3):259–267. doi:10.1177/17474930221135983
5. Pu L, Wang L, Zhang R, Zhao T, Jiang Y, Han L. Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke. 2023;54(5):1330–1339. doi:10.1161/strokeaha.122.040073
6. Feigin VL, Brainin M, Norrving B, et al. World stroke organization (WSO): Global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi:10.1177/17474930211065917
7. Fugate JE, Rabinstein AA. Update on intravenous recombinant tissue plasminogen activator for acute ischemic stroke. Mayo Clin Proc. 2014;89(7):960–972. doi:10.1016/j.mayocp.2014.03.001
8. Wang Y, Liao X, Zhao X, et al. Using recombinant tissue plasminogen activator to treat acute ischemic stroke in China: Analysis of the results from the Chinese National Stroke Registry (CNSR). Stroke. 2011;42(6):1658–1664. doi:10.1161/strokeaha.110.604249
9. Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59(6):862–867. doi:10.1212/wnl.59.6.862
10. Shi K, Zou M, Jia DM, et al. tPA mobilizes immune cells that exacerbate hemorrhagic transformation in stroke. Circ Res. 2021;128(1):62–75. doi:10.1161/circresaha.120.317596
11. Jiang W, Zhao Y, Liu R, et al. Histidine-rich glycoprotein modulates neutrophils and thrombolysis-associated hemorrhagic transformation. EMBO Mol Med. 2024;16(9):2146–2169. doi:10.1038/s44321-024-00117-y
12. Liu J, Pang SY, Zhou SY, et al. Lipocalin-2 aggravates blood-brain barrier dysfunction after intravenous thrombolysis by promoting endothelial cell ferroptosis via regulating the HMGB1/Nrf2/HO-1 pathway. Redox Biol. 2024;76:103342. doi:10.1016/j.redox.2024.103342
13. Geng YQ, Qiu LN, Cheng YQ, et al. Alleviating recombinant tissue plasminogen activator-induced hemorrhagic transformation in ischemic stroke via targeted delivery of a ferroptosis inhibitor. Adv Sci. 2024;11(24):e2309517. doi:10.1002/advs.202309517
14. Jadhav AP, Desai SM, Jovin TG. Indications for mechanical thrombectomy for acute ischemic stroke: Current guidelines and beyond. Neurology. 2021;97(20 Suppl 2):S126–s136. doi:10.1212/wnl.0000000000012801
15. Sun Y, Jiang X, Gao J. Stem cell-based ischemic stroke therapy: Novel modifications and clinical challenges. Asian J Pharm Sci. 2024;19(1):100867. doi:10.1016/j.ajps.2023.100867
16. Deng G, Chu YH, Xiao J, et al. Risk factors, pathophysiologic mechanisms, and potential treatment strategies of futile recanalization after endovascular therapy in acute ischemic stroke. Aging and Disease. 2023;14(6):2096–2112. doi:10.14336/ad.2023.0321-1
17. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke. 2007;38(3):967–973. doi:10.1161/01.Str.0000258112.14918.24
18. Yuan J, Li L, Yang Q, et al. Targeted treatment of ischemic stroke by bioactive nanoparticle-derived reactive oxygen species responsive and inflammation-resolving nanotherapies. ACS Nano. 2021;15(10):16076–16094. doi:10.1021/acsnano.1c04753
19. Chamorro Á, Lo EH, Renú A, van Leyen K, Lyden PD. The future of neuroprotection in stroke. J Neurol Neurosurg Psych. 2021;92(2):129–135. doi:10.1136/jnnp-2020-324283
20. Iadecola C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi:10.1016/j.neuron.2017.07.030
21. Sun S, Lv W, Li S, et al. Smart liposomal nanocarrier enhanced the treatment of ischemic stroke through neutrophil extracellular traps and cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING) pathway inhibition of ischemic penumbra. ACS Nano. 2023;17(18):17845–17857. doi:10.1021/acsnano.3c03390
22. Zenych A, Jacqmarcq C, Aid R, et al. Fucoidan-functionalized polysaccharide submicroparticles loaded with alteplase for efficient targeted thrombolytic therapy. Biomaterials. 2021;277:121102. doi:10.1016/j.biomaterials.2021.121102
23. Jin Y, Kim IY, Kim ID, et al. Biodegradable gelatin microspheres enhance the neuroprotective potency of osteopontin via quick and sustained release in the post-ischemic brain. Acta Biomater. 2014;10(7):3126–3135. doi:10.1016/j.actbio.2014.02.045
24. Jin YC, Kim SW, Cheng F, et al. The effect of biodegradable gelatin microspheres on the neuroprotective effects of high mobility group box 1 A box in the postischemic brain. Biomaterials. 2011;32(3):899–908. doi:10.1016/j.biomaterials.2010.09.054
25. Mei T, Kim A, Vong LB, et al. Encapsulation of tissue plasminogen activator in pH-sensitive self-assembled antioxidant nanoparticles for ischemic stroke treatment - synergistic effect of thrombolysis and antioxidant. Biomaterials. 2019;215:119209. doi:10.1016/j.biomaterials.2019.05.020
26. Choi SG, Shin J, Lee KY, et al. PINK1 siRNA-loaded poly(lactic-co-glycolic acid) nanoparticles provide neuroprotection in a mouse model of photothrombosis-induced ischemic stroke. Glia. 2023;71(5):1294–1310. doi:10.1002/glia.24339
27. Wang H, Xu X, Guan X, et al. Liposomal 9-aminoacridine for treatment of ischemic stroke:From drug discovery to drug delivery. Nano Lett. 2020;20(3):1542–1551. doi:10.1021/acs.nanolett.9b04018
28. Shabani Z, Rahbarghazi R, Karimipour M, et al. Transplantation of bioengineered Reelin-loaded PLGA/PEG micelles can accelerate neural tissue regeneration in photothrombotic stroke model of mouse. Bioeng Transl Med. 2022;7(1):e10264. doi:10.1002/btm2.10264
29. Lee J, Hyun H, Kim J, et al. Dexamethasone-loaded peptide micelles for delivery of the heme oxygenase-1 gene to ischemic brain. J Control Release. 2012;158(1):131–138. doi:10.1016/j.jconrel.2011.11.001
30. Nitta SK, Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 2013;14(1):1629–1654. doi:10.3390/ijms14011629
31. Lin X, Li N, Tang H. Recent advances in nanomaterials for diagnosis, treatments, and neurorestoration in ischemic stroke. Front Cell Neurosci. 2022;16:885190. doi:10.3389/fncel.2022.885190
32. Chen W, Jiang L, Hu Y, et al. Nanomedicines, an emerging therapeutic regimen for treatment of ischemic cerebral stroke: A review. J Control Release. 2021;340:342–360. doi:10.1016/j.jconrel.2021.10.020
33. Petro M, Jaffer H, Yang J, Kabu S, Morris VB, Labhasetwar V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials. 2016;81:169–180. doi:10.1016/j.biomaterials.2015.12.009
34. Hosoo H, Marushima A, Nagasaki Y, et al. Neurovascular unit protection from cerebral ischemia-reperfusion injury by radical-containing nanoparticles in mice. Stroke. 2017;48(8):2238–2247. doi:10.1161/strokeaha.116.016356
35. Zheng S, Bai YY, Liu Y, et al. Salvaging brain ischemia by increasing neuroprotectant uptake via nanoagonist mediated blood brain barrier permeability enhancement. Biomaterials. 2015;66:9–20. doi:10.1016/j.biomaterials.2015.07.006
36. Liu X, An C, Jin P, Liu X, Wang L. Protective effects of cationic bovine serum albumin-conjugated PEGylated tanshinone IIA nanoparticles on cerebral ischemia. Biomaterials. 2013;34(3):817–830. doi:10.1016/j.biomaterials.2012.10.017
37. She R, Liu D, Liao J, Wang G, Ge J, Mei Z. Mitochondrial dysfunctions induce PANoptosis and ferroptosis in cerebral ischemia/reperfusion injury: From pathology to therapeutic potential. Front Cell Neurosci. 2023;17:1191629. doi:10.3389/fncel.2023.1191629
38. Tian H, Chen X, Liao J, et al. Mitochondrial quality control in stroke: From the mechanisms to therapeutic potentials. J Cell Mol Med. 2022;26(4):1000–1012. doi:10.1111/jcmm.17189
39. Charmpilas N, Fang EF, Palikaras K. Mitophagy and neuroinflammation: A compelling interplay. Curr Neuropharmacol. 2023;21(7):1477–1481. doi:10.2174/1570159x20666220628153632
40. Yun HR, Jo YH, Kim J, Shin Y, Kim SS, Choi TG. Roles of autophagy in oxidative stress. Int J Mol Sci. 2020;21(9). doi:10.3390/ijms21093289
41. Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and autophagy: Interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 2015;35(5):615–621. doi:10.1007/s10571-015-0166-x
42. Huang J, Chen L, Yao ZM, Sun XR, Tong XH, Dong SY. The role of mitochondrial dynamics in cerebral ischemia-reperfusion injury. Biomed Pharmacother. 2023;162:114671. doi:10.1016/j.biopha.2023.114671
43. Zhu T, Dong S, Qin N, Liu R, Shi L, Wan Q. Dl-3-n-butylphthalide attenuates cerebral ischemia/reperfusion injury in mice through AMPK-mediated mitochondrial fusion. Front Pharmacol. 2024;15:1357953. doi:10.3389/fphar.2024.1357953
44. Jia J, Deng J, Jin H, et al. Effect of Dl-3-n-butylphthalide on mitochondrial Cox7c in models of cerebral ischemia/reperfusion injury. Front Pharmacol. 2023;14:1084564. doi:10.3389/fphar.2023.1084564
45. Wang A, Jia B, Zhang X, et al. Efficacy and safety of butylphthalide in patients with acute ischemic stroke: A randomized clinical trial. JAMA Neurol. 2023;80(8):851–859. doi:10.1001/jamaneurol.2023.1871
46. Li X, Han Z, Wang T, et al. Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials. 2022;291:121904. doi:10.1016/j.biomaterials.2022.121904
47. Yang Q, Pu W, Hu K, et al. Reactive oxygen species-responsive transformable and triple-targeting butylphthalide nanotherapy for precision treatment of ischemic stroke by normalizing the pathological microenvironment. ACS Nano. 2023;17(5):4813–4833. doi:10.1021/acsnano.2c11363
48. Yamashita SI, Sugiura Y, Matsuoka Y, et al. Mitophagy mediated by BNIP3 and NIX protects against ferroptosis by downregulating mitochondrial reactive oxygen species. Cell Death Differ. 2024;22. doi:10.1038/s41418-024-01280-y
49. Wang D, Li B, Wang S, et al. Engineered inhaled nanocatalytic therapy for ischemic cerebrovascular disease by inducing autophagy of abnormal mitochondria. NPJ Regen Med. 2023;8(1):44. doi:10.1038/s41536-023-00315-1
50. Meng W, Ma Z, Ye H, Liu L, Han Q, Shi Q. Polyphenolic oligomer-derived multienzyme activity for the treatment of ischemic stroke through ROS scavenging and blood-brain barrier restoration. J Mater Chem B. 2024;12(8):2123–2138. doi:10.1039/d3tb02676k
51. Qi M, Cheng Y, Liu K, et al. Recombinant human heavy chain ferritin nanoparticles serve as ROS scavengers for the treatment of ischemic stroke. Int J Nanomed. 2024;19:2285–2299. doi:10.2147/ijn.S449606
52. Liu D, Ji Q, Cheng Y, et al. Cyclosporine A loaded brain targeting nanoparticle to treat cerebral ischemia/reperfusion injury in mice. J Nanobiotechnology. 2022;20(1):256. doi:10.1186/s12951-022-01474-x
53. Fan L, Lin X, Hong L, et al. Simultaneous antioxidant and neuroprotective effects of two-dimensional (2D) MXene-loaded isoquercetin for ischemic stroke treatment. J Mater Chem B. 2024;12(11):2795–2806. doi:10.1039/d3tb01973j
54. Zhang S. Microglial activation after ischaemic stroke. Stroke Vasc Neurol. 2019;4(2):71–74. doi:10.1136/svn-2018-000196
55. Hu R, Liang J, Ding L, et al. Edaravone dexborneol provides neuroprotective benefits by suppressing NLRP3 inflammasome-induced microglial pyroptosis in experimental ischemic stroke. Int Immunopharmacol. 2022;113(Pt A):109315. doi:10.1016/j.intimp.2022.109315
56. Zeng J, Bao T, Yang K, et al. The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: A review. Front Immunol. 2022;13:1047550. doi:10.3389/fimmu.2022.1047550
57. Zhang Y, Li J, Zhao Y, et al. Arresting the bad seed: HDAC3 regulates proliferation of different microglia after ischemic stroke. Sci Adv. 2024;10(10):eade6900. doi:10.1126/sciadv.ade6900
58. Zhou S, Zhu W, Zhang Y, Pan S, Bao J. S100B promotes microglia M1 polarization and migration to aggravate cerebral ischemia. Inflamm Res. 2018;67(11–12):937–949. doi:10.1007/s00011-018-1187-y
59. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. doi:10.1161/strokeaha.112.659656
60. Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi:10.1146/annurev-immunol-051116-052358
61. Zhu Z, Lu H, Jin L, et al. C-176 loaded Ce DNase nanoparticles synergistically inhibit the cGAS-STING pathway for ischemic stroke treatment. Bioact Mater. 2023;29:230–240. doi:10.1016/j.bioactmat.2023.07.002
62. Villa-González M, Rubio M, Martín-López G, et al. Pharmacological inhibition of mTORC1 reduces neural death and damage volume after MCAO by modulating microglial reactivity. Biol Direct. 2024;19(1):26. doi:10.1186/s13062-024-00470-5
63. Zhang Y, Li D, Gao H, Zhao H, Zhang S, Li T. Rapamycin alleviates neuronal injury and modulates microglial activation after cerebral ischemia. Mol Neurobiol. 2024. doi:10.1007/s12035-023-03904-9
64. Cheng Y, Cheng A, Jia Y, et al. pH-responsive multifunctional theranostic rapamycin-loaded nanoparticles for imaging and treatment of acute ischemic stroke. ACS Appl Mater Interfaces. 2021;13(48):56909–56922. doi:10.1021/acsami.1c16530
65. Cao Y, Yu Y, Pan L, et al. Sulfated polysaccharide-based nanocarrier drives microenvironment-mediated cerebral neurovascular remodeling for ischemic stroke treatment. Nano Lett. 2024;24(17):5214–5223. doi:10.1021/acs.nanolett.4c00650
66. Raffaele S, Thougaard E, Laursen CCH, et al. Microglial TNFR2 signaling regulates the inflammatory response after CNS injury in a sex-specific fashion. Brain Behav Immun. 2024;116:269–285. doi:10.1016/j.bbi.2023.12.025
67. Liu Z, Zhang S, Ran Y, et al. Nanoarchitectonics of tannic acid based injectable hydrogel regulate the microglial phenotype to enhance neuroplasticity for poststroke rehabilitation. Biomater Res. 2023;27(1):108. doi:10.1186/s40824-023-00444-0
68. Wang Z, Pan J, Yuan R, Chen M, Guo X, Zhou S. Shell-sheddable polymeric micelles alleviate oxidative stress and inflammation for enhanced ischemic stroke therapy. Nano Lett. 2023;23(14):6544–6552. doi:10.1021/acs.nanolett.3c01567
69. Zhao Y, Li Q, Niu J, et al. Neutrophil membrane-camouflaged polyprodrug nanomedicine for inflammation suppression in ischemic stroke therapy. Adv Mater. 2024;22:e2311803. doi:10.1002/adma.202311803
70. Wang D, Wang Y, Shi J, et al. Edaravone dexborneol alleviates ischemic injury and neuroinflammation by modulating microglial and astrocyte polarization while inhibiting leukocyte infiltration. Int Immunopharmacol. 2024;130:111700. doi:10.1016/j.intimp.2024.111700
71. Huang J, Hu X, Li J, Gong D. Edaravone dexborneol promotes M2 microglia polarization against lipopolysaccharide-induced inflammation via suppressing TLR4/MyD88/NF-κB pathway. Naunyn Schmiedebergs Arch Pharmacol. 2024;15. doi:10.1007/s00210-024-03045-3
72. Fu Y, Wang A, Tang R, et al. Sublingual edaravone dexborneol for the treatment of acute ischemic stroke: The TASTE-SL randomized clinical trial. JAMA Neurol. 2024. doi:10.1001/jamaneurol.2023.5716
73. Lu Y, Wang JT, Li N, et al. Intranasal administration of edaravone nanoparticles improves its stability and brain bioavailability. J Control Release. 2023;359:257–267. doi:10.1016/j.jconrel.2023.06.001
74. Yin N, Zhao Y, Liu C, et al. Engineered nanoerythrocytes alleviate central nervous system inflammation by regulating the polarization of inflammatory microglia. Adv Mater. 2022;34(27):e2201322. doi:10.1002/adma.202201322
75. Jin L, Zhu Z, Hong L, Qian Z, Wang F, Mao Z. ROS-responsive 18β-glycyrrhetic acid-conjugated polymeric nanoparticles mediate neuroprotection in ischemic stroke through HMGB1 inhibition and microglia polarization regulation. Bioact Mater. 2023;19:38–49. doi:10.1016/j.bioactmat.2022.03.040
76. Gao X, Zhao X, Li J, et al. Neutrophil extracellular traps mediated by platelet microvesicles promote thrombosis and brain injury in acute ischemic stroke. Cell Commun Signal. 2024;22(1):50. doi:10.1186/s12964-023-01379-8
77. Li C, Xing Y, Zhang Y, Hua Y, Hu J, Bai Y. Neutrophil extracellular traps exacerbate ischemic brain damage. Mol Neurobiol. 2022;59(1):643–656. doi:10.1007/s12035-021-02635-z
78. Denorme F, Portier I, Rustad JL, et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. 2022;132(10). doi:10.1172/jci154225
79. Price CJ, Menon DK, Peters AM, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: An imaging-based study. Stroke. 2004;35(7):1659–1664. doi:10.1161/01.Str.0000130592.71028.92
80. Kataoka H, Kim SW, Plesnila N. Leukocyte-endothelium interactions during permanent focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2004;24(6):668–676. doi:10.1097/01.Wcb.0000117812.35136.5b
81. Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: Friend and foe for ischemic stroke. J Neuroinflam. 2019;16(1):142. doi:10.1186/s12974-019-1516-2
82. Dhanesha N, Patel RB, Doddapattar P, et al. PKM2 promotes neutrophil activation and cerebral thromboinflammation: Therapeutic implications for ischemic stroke. Blood. 2022;139(8):1234–1245. doi:10.1182/blood.2021012322
83. Yin N, Wang W, Pei F, et al. A neutrophil hijacking nanoplatform reprograming NETosis for targeted microglia polarizing mediated ischemic stroke treatment. Adv Sci. 2024;5:e2305877. doi:10.1002/advs.202305877
84. Li L, Cheng SQ, Sun YQ, et al. Resolvin D1 reprograms energy metabolism to promote microglia to phagocytize neutrophils after ischemic stroke. Cell Rep. 2023;42(6):112617. doi:10.1016/j.celrep.2023.112617
85. Otxoa-de-Amezaga A, Miró-Mur F, Pedragosa J, et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 2019;137(2):321–341. doi:10.1007/s00401-018-1954-4
86. Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13(2):1272–1283. doi:10.1021/acsnano.8b06572
87. Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi:10.1038/nature08541
88. Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212(8):1203–1217. doi:10.1084/jem.20150225
89. Tang C, Wang C, Zhang Y, et al. Recognition, intervention, and monitoring of neutrophils in acute ischemic stroke. Nano Lett. 2019;19(7):4470–4477. doi:10.1021/acs.nanolett.9b01282
90. Mu Q, Yao K, Syeda MZ, et al. Ligustrazine nanoparticle hitchhiking on neutrophils for enhanced therapy of cerebral ischemia-reperfusion injury. Adv Sci. 2023;10(19):e2301348. doi:10.1002/advs.202301348
91. Zhang C, Ling CL, Pang L, et al. Direct macromolecular drug delivery to cerebral ischemia area using neutrophil-mediated nanoparticles. Theranostics. 2017;7(13):3260–3275. doi:10.7150/thno.19979
92. Tang Z, Meng S, Song Z, et al. Neutrophil membrane fusogenic nanoliposomal leonurine for targeted ischemic stroke therapy via remodeling cerebral niche and restoring blood-brain barrier integrity. Mater Today Bio. 2023;20:100674. doi:10.1016/j.mtbio.2023.100674
93. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi:10.1016/j.nbd.2003.12.016
94. Jiang X, Andjelkovic AV, Zhu L, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163-164:144–171. doi:10.1016/j.pneurobio.2017.10.001
95. Díaz-Castro B, Robel S, Mishra A. Astrocyte endfeet in brain function and pathology: Open questions. Annu Rev Neurosci. 2023;46:101–121. doi:10.1146/annurev-neuro-091922-031205
96. Zhang T, Wu J, Yao X, et al. The aldose reductase inhibitor epalrestat maintains blood-brain barrier integrity by enhancing endothelial cell function during cerebral ischemia. Mol Neurobiol. 2023;60(7):3741–3757. doi:10.1007/s12035-023-03304-z
97. Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315(3):C343–c356. doi:10.1152/ajpcell.00095.2018
98. Zheng X, Ren B, Gao Y. Tight junction proteins related to blood-brain barrier and their regulatory signaling pathways in ischemic stroke. Biomed Pharmacother. 2023;165:115272. doi:10.1016/j.biopha.2023.115272
99. Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13 Suppl 1):S52–7. doi:10.1212/WNL.0b013e3182697e70
100. Lu H, Li S, Dai D, et al. Enhanced treatment of cerebral ischemia-reperfusion injury by intelligent nanocarriers through the regulation of neurovascular units. Acta Biomater. 2022;147:314–326. doi:10.1016/j.actbio.2022.05.021
101. Yan BC, Cao J, Liu J, et al. Dietary Fe3O4 nanozymes prevent the injury of neurons and blood-brain barrier integrity from cerebral ischemic stroke. ACS Biomater Sci Eng. 2021;7(1):299–310. doi:10.1021/acsbiomaterials.0c01312
102. Liu L, Ma Z, Han Q, et al. Myricetin oligomer triggers multi-receptor mediated penetration and autophagic restoration of blood-brain barrier for ischemic stroke treatment. ACS Nano. 2024;18(14):9895–9916. doi:10.1021/acsnano.3c09532
103. Gao M, Li Y, Ho W, et al. Targeted mRNA nanoparticles ameliorate blood-brain barrier disruption postischemic stroke by modulating microglia polarization. ACS Nano. 2024;18(4):3260–3275. doi:10.1021/acsnano.3c09817
104. He W, Zhang Z, Sha X. Nanoparticles-mediated emerging approaches for effective treatment of ischemic stroke. Biomaterials. 2021;277:121111. doi:10.1016/j.biomaterials.2021.121111
105. Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–3039. doi:10.1161/strokeaha.107.488445
106. Shafiee A, Kehtari M, Zarei Z, et al. An in situ hydrogel-forming scaffold loaded by PLGA microspheres containing carbon nanotube as a suitable niche for neural differentiation. Mater Sci Eng C Mater Biol Appl. 2021;120:111739. doi:10.1016/j.msec.2020.111739
107. Nistor-Cseppentö DC, Jurcău MC, Jurcău A, Andronie-Cioară FL, Marcu F. Stem cell- and cell-based therapies for ischemic stroke. Bioengineering. 2022;9(11). doi:10.3390/bioengineering9110717
108. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. doi:10.1126/science.275.5302.964
109. Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–742. doi:10.1016/j.yjmcc.2005.07.003
110. Sobrino T, Hurtado O, Moro MA, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38(10):2759–2764. doi:10.1161/strokeaha.107.484386
111. Yip HK, Chang LT, Chang WN, et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39(1):69–74. doi:10.1161/strokeaha.107.489401
112. Tsai NW, Hung SH, Huang CR, et al. The association between circulating endothelial progenitor cells and outcome in different subtypes of acute ischemic stroke. Clin Chim Acta. 2014;427:6–10. doi:10.1016/j.cca.2013.09.029
113. Mao L, Huang M, Chen SC, et al. Endogenous endothelial progenitor cells participate in neovascularization via CXCR4/SDF-1 axis and improve outcome after stroke. CNS Neurosci Ther. 2014;20(5):460–468. doi:10.1111/cns.12238
114. Wilson KL, Joseph NI, Onweller LA, et al. SDF-1 bound heparin nanoparticles recruit progenitor cells for their differentiation and promotion of angiogenesis after stroke. Adv Healthc Mater. 2023;27:e2302081. doi:10.1002/adhm.202302081
115. Zheng H, Dai T, Zhou B, et al. SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis. 2008;201(1):36–42. doi:10.1016/j.atherosclerosis.2008.02.011
116. Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107(9):1322–1328. doi:10.1161/01.cir.0000055313.77510.22
117. Kim DH, Seo YK, Thambi T, et al. Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1α using a dual ionic pH-sensitive copolymer. Biomaterials. 2015;61:115–125. doi:10.1016/j.biomaterials.2015.05.025
118. Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. doi:10.1182/blood-2007-03-078709
119. Hu H, Wang B, Jiang C, Li R, Zhao J. Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21-5p to modulate thrombospondin-1 expression. Clin Sci. 2019;133(14):1629–1644. doi:10.1042/cs20190188
120. Ben Fraj S, Naserian S, Lorenzini B, et al. Human umbilical cord blood endothelial progenitor cell-derived extracellular vesicles control important endothelial cell functions. Int J Mol Sci. 2023;24(12). doi:10.3390/ijms24129866
121. Moon GJ, Sung JH, Kim DH, et al. Application of mesenchymal stem cell-derived extracellular vesicles for stroke: Biodistribution and microRNA study. Transl Stroke Res. 2019;10(5):509–521. doi:10.1007/s12975-018-0668-1
122. Doeppner TR, Herz J, Görgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–1143. doi:10.5966/sctm.2015-0078
123. Liu B, Lee BW, Nakanishi K, et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018;2(5):293–303. doi:10.1038/s41551-018-0229-7
124. Yu B, Li H, Zhang Z, et al. Extracellular vesicles engineering by silicates-activated endothelial progenitor cells for myocardial infarction treatment in male mice. Nat Commun. 2023;14(1):2094. doi:10.1038/s41467-023-37832-y
125. Chen CW, Wang LL, Zaman S, et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc Res. 2018;114(7):1029–1040. doi:10.1093/cvr/cvy067
126. Jiang Y, Wang R, Wang C, et al. Brain microenvironment responsive and pro-angiogenic extracellular vesicle-hydrogel for promoting neurobehavioral recovery in type 2 diabetic mice after stroke. Adv Healthc Mater. 2022;11(22):e2201150. doi:10.1002/adhm.202201150
127. Jain D, Mattiassi S, Goh EL, Yim EKF. Extracellular matrix and biomimetic engineering microenvironment for neuronal differentiation. Neural Regen Res. 2020;15(4):573–585. doi:10.4103/1673-5374.266907
128. Liu X, Yang M, Lei F, Wang Y, Yang M, Mao C. Highly effective stroke therapy enabled by genetically engineered viral nanofibers. Adv Mater. 2022;34(20):e2201210. doi:10.1002/adma.202201210
129. Zhang R, Mao W, Niu L, et al. NSC-derived exosomes enhance therapeutic effects of NSC transplantation on cerebral ischemia in mice. Elife. 2023;12. doi:10.7554/eLife.84493
130. Xing H, Lee H, Luo L, Kyriakides TR. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv. 2020;42:107421. doi:10.1016/j.biotechadv.2019.107421
131. Zhang X, Chen X, Hong H, Hu R, Liu J, Liu C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater Apr. 2022;10:15–31. doi:10.1016/j.bioactmat.2021.09.014
132. Badylak SF, Freytes DO, Gilbert TW. Reprint of: Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2015;23:S17–26. doi:10.1016/j.actbio.2015.07.016
133. Zhang Y, Luo H, Zhang Z, et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials. 2010;31(20):5312–5324. doi:10.1016/j.biomaterials.2010.03.029
134. Agrawal V, Brown BN, Beattie AJ, Gilbert TW, Badylak SF. Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J Tissue Eng Regen Med. 2009;3(8):590–600. doi:10.1002/term.200
135. Crapo PM, Medberry CJ, Reing JE, et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33(13):3539–3547. doi:10.1016/j.biomaterials.2012.01.044
136. Raspa A, Gelain F. Mimicking extracellular matrix via engineered nanostructured biomaterials for neural repair. Curr Neuropharmacol. 2021;19(12):2110–2124. doi:10.2174/1570159x18666201111111102
137. Álvarez Z, Ortega JA, Sato K, et al. Artificial extracellular matrix scaffolds of mobile molecules enhance maturation of human stem cell-derived neurons. Cell Stem Cell. 2023;30(2):219–238.e14. doi:10.1016/j.stem.2022.12.010
138. Moshayedi P, Nih LR, Llorente IL, et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016;105:145–155. doi:10.1016/j.biomaterials.2016.07.028
139. Xu J, Sun Y, You Y, et al. Bioorthogonal microglia-inspired mesenchymal stem cell bioengineering system creates livable niches for enhancing ischemic stroke recovery via the hormesis. Acta Pharm Sin B. 2024;14(3):1412–1427. doi:10.1016/j.apsb.2023.11.009
140. You Y, Liu Y, Ma C, et al. Surface-tethered ROS-responsive micelle backpacks for boosting mesenchymal stem cell vitality and modulating inflammation in ischemic stroke treatment. J Control Release. 2023;362:210–224. doi:10.1016/j.jconrel.2023.08.039
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

