Back to Journals » International Journal of Nanomedicine » Volume 20
Biological and Bioinspired Vesicles for Wound Healing: Insights, Advances and Challenges
Authors Zhao Y, Zhang T, Zhao G, Lu J, Zhang X, Lai Y, Chen Z , Ding X, Tai Z
Received 26 February 2025
Accepted for publication 16 May 2025
Published 30 June 2025 Volume 2025:20 Pages 8497—8528
DOI https://doi.org/10.2147/IJN.S522067
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Yingchao Zhao,1,* Tingrui Zhang,1,* Guanlin Zhao,2,* Jiaye Lu,1 Xinyue Zhang,1 Yongxian Lai,1 Zhongjian Chen,1 Xiaofeng Ding,1 Zongguang Tai1
1Shanghai Skin Disease Hospital, School of Medicine, Tongji University, Shanghai, 200443, People’s Republic of China; 2Yangpu Hospital, School of Medicine, Tongji University, Shanghai, 200070, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaofeng Ding, Shanghai Skin Disease Hospital, School of Medicine, Tongji University, 1278 Baode Road, Shanghai, 200443, People’s Republic of China, Email [email protected] Zongguang Tai, Shanghai Skin Disease Hospital, School of Medicine, Tongji University, 1278 Baode Road, Shanghai, 200443, People’s Republic of China, Email [email protected]
Abstract: Millions of people suffer traumatic injuries and surgical procedures each year, making effective wound management very important. Benign wound repair is essential for structural and functional recovery of damaged tissues. Natural biological vesicles have emerged as attractive candidates for accelerating wound healing due to their endogenous nature and distinctive biological properties. However, obstacles such as low separation yields, potential safety concerns, and unsatisfactory production restrict their continuous application. In recent years, bioinspired materials and technologies have played an important role in the field of remodeling wound healing. Bioinspired vesicles function by effectively transporting therapeutic substances through mimicking the chemical and biological properties in nature, and can be modified and prepared through a variety of advanced techniques such as genetic engineering, chemical modification, and physical techniques, and are good substitutes for natural vesicles. Therefore, integrating bioinspired design into vesicles to promote wound healing can improve the curative effect while maintaining good safety, which helps to accelerate the clinical transformation of vesicles. This review discusses the latest progress, barriers to clinical transformation, and future development directions of biological and bioinspired vesicles for wound healing.
Keywords: wound healing, biological vesicles, bioinspired vesicles, extracellular vesicles, preparation
Introduction
Skin, serving as the body’s largest multifunctional barrier organ, provides essential protection for internal organs against environmental threats. Numerous endogenous pathological conditions and exogenous mechanical insults that compromise cutaneous tissue integrity or architecture can result in skin wounds, posing significant life-threatening risks to patients.1 Current epidemiological estimates indicate that dermatological pathologies affect approximately one-third of the global population, with wound management imposing substantial healthcare and socioeconomic burdens on both individuals and societies.2 Beyond localized wound characteristics (including depth, dimensions, and infection status), the healing trajectory may be further complicated by systemic factors such as immunological/nutritional deficiencies, advanced age, and chronic comorbidities.3 Despite extensive research efforts to enhance cutaneous wound healing, current clinical therapeutic strategies frequently demonstrate suboptimal efficacy across diverse clinical scenarios.
Biological ingredients possess the remarkable capacity to alter the cellular milieu and impart a diverse array of stimuli to host cells. Such active ingredients can exist as biological vesicles, synthetic bioactive vesicles, or live host cells. Cell therapy entails the utilization of transplanted cells to participate in various stages of wound healing, suppress excessive local immune responses, secrete diverse growth factors, facilitate vascular regeneration and wound re-epithelialization, and ultimately expedite wound closure and enhance the quality of wound healing.4 Nevertheless, their sustained clinical application has been hindered by immune rejection, non-biocompatible scaffold materials, cell sources and processing techniques, maintaining cell viability throughout therapy, individual patient variations, and exorbitant costs.5 Biological vesicles are secreted by cells, such as extracellular vesicles, and their importance in intercellular signaling communication has been firmly established. The events mediated by biological vesicles are associated with cell proliferation, differentiation, migration, apoptosis, immunomodulation, and the ability to cross barriers, such as plasma membrane, blood-brain barrier, and gastrointestinal tract.6–8 Therefore, biovesicles are considered a highly promising alternative strategy for cell therapy.9,10 These biological vesicles have been shown to retain the efficacy of the maternal cells while avoiding problems of immune rejection, tumorigenicity, teratogenicity, and ethical perspectives. Biological vesicles are ideally suited for long-term storage due to their high stability, controllability for monitoring and assessing the healing process, precision for quantitative application, and high efficiency for enriching the wounded area.11,12 Furthermore, biological vesicles contain a wide range of proteins and RNAs that can stimulate cell migration, proliferation, and differentiation; they can also speed up the formation of blood vessels and extracellular matrix reconstruction by inducing inflammation in the wound; and they can function as a dual agent, promoting wound healing while preventing the formation of epileptic scars, which has good potential for regeneration and repair.13
Bioinspired vesicles represent a class of artificial or semi-artificial nanoscale carrier systems engineered through biomimetic principles, deriving structural and functional characteristics from natural biological vesicles (eg, extracellular vesicles, liposomes).14 Their core design paradigm involves constructing functionalized vesicles with tailored biocompatibility, targeting specificity, or environmental responsiveness by mimicking critical biological membrane features - including compositional architecture, dynamic behaviors, and signaling mechanisms of cellular/organelle membranes.15 Compared to natural counterparts, these engineered systems exhibit superior performance in structural stability, drug-loading efficiency, and functional programmability, while effectively circumventing inherent challenges of natural vesicles such as source-dependent heterogeneity and scalability limitations in manufacturing.16 Bioinspired vesicles show significant market potential for application in the field of wound healing. In terms of production cost economics, the donor is widely available and inexpensive. With the continuous optimization of the preparation process, the application of genetic engineering and chemical modification technology has increased the vesicle yield and reduced the production cost. From the point of view of market application value, bio-inspired vesicles have more significant therapeutic effects and a wider application scope. With the improvement of the quality of wound healing and the increasing demand for new therapeutic technologies, bio-inspired vesicles have a huge market potential, and their wide application will promote the development of related medical devices and pharmaceuticals and expand the market space.
The challenges such as large-scale manufacturing, purification, modification, drug loading, storage, and heterogeneity persistently impede the further transformation of biological vesicles. To circumvent the limitations of biological vesicles, synthetic nanoparticles that mimic the therapeutic properties and drug delivery capabilities of biological vesicles—such as their nanoscale size, targeting ability, cellular internalization efficiency, and stability—have been made possible by biomimetic nanotechnology in recent years. These nanoparticles also make up for the drawbacks of biological vesicles, which include low yields, complicated isolation and purification procedures, and high costs.17 The advancement of embedded sensors and intelligent materials has driven the evolution of smart dressings for wound management, with these integrated sensors and responsive materials demonstrating the capability to detect specific biological triggers (eg, pH variations, temperature fluctuations, enzymatic activities) through precise physicochemical responses, thereby offering innovative platforms for efficient delivery of bioactive vesicles.18 On the other hand, there are still many challenges that need to be addressed before active vesicles can be used in clinical applications.19 The production process of active vesicles needs to be further optimized to increase yield and reduce cost. Also, their stability and biodistribution in vivo need to be studied in greater depth to ensure their safety and efficacy during treatment. In addition, the long-term storage conditions and transport requirements of active vesicles need to be clarified to ensure their feasibility and convenience in practical applications. In view of the significant advantages of bioinspired vesicles in terms of biocompatibility, therapeutic efficacy, drug delivery efficiency, production cost control, and market potential, bioinspired vesicles are becoming increasingly important in wound healing and other medical applications, and have become a preferred option that has attracted a great deal of attention.
We first systematically reviewed cutting-edge research on diverse sources and isolation techniques of biological vesicles, while highlighting the distinct characteristics of the associated strategies. Furthermore, this comprehensive analysis examines recent advancements and current limitations of bioinspired vesicle systems engineered through multiple technologies for wound healing enhancement. Finally, we synthesized data from ongoing and completed vesicles-based clinical trials, identifying multifaceted challenges in clinical translation. Our analysis suggests that enhanced mechanistic understanding of both biological and bioinspired vesicles could yield novel therapeutic candidates and precision strategies for advanced wound management.
The Origin of Biological Vesicles
Biological vesicles exhibit diverse biological origins encompassing mammalian organisms, botanical species, and specialized biological groups, each demonstrating unique therapeutic advantages in wound management due to their distinct compositional profiles and functional characteristics.20,21 Mammalian-derived vesicles have emerged as pivotal therapeutic agents for complex wound pathologies (eg, diabetic ulcers, burn injuries) through their superior bioactivity and precise regulatory capabilities, while plant- and insect-derived counterparts address critical gaps in conventional therapies via their distinctive components (antioxidant molecules, antimicrobial peptides) and enhanced delivery efficiency.22 This section systematically catalogs currently available vesicle sources, investigates their mechanisms of action, and elucidates their distinct therapeutic benefits in wound healing processes (Figure 1).
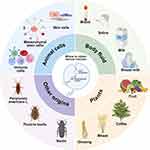 |
Figure 1 Natural sources of biological vesicles. The figure was created with https://app.biorender.com/. |
Animal Cells
Animal-derived biological vesicles originate from diverse cellular sources, with mesenchymal stem cells (MSCs) representing the most extensively characterized source to date.23 MSC-derived EVs (MSC-EVs) have garnered significant scientific attention due to their demonstrated therapeutic potential in tissue regeneration processes.24 Notably, cutaneous cellular components (keratinocytes and fibroblasts) and immune effector cells (macrophages) constitute additional biologically significant EV sources.25 This section systematically examines the differential functional profiles and therapeutic characteristics exhibited by these cellularly derived EVs during wound healing progression.26
Resident Skin Cells
Skin cell-derived biological vesicles have emerged as native therapeutic agents in wound repair, distinguished by their direct cellular origin, multifunctional efficacy, and inherent compatibility with the wound microenvironment. Unlike vesicles from non-cutaneous sources, these biological vesicles originate from peri-wound cells, demonstrating superior microenvironmental adaptation and efficient cellular uptake. Scientific consensus confirms their wound-healing enhancement through four coordinated mechanisms: promoting cellular proliferation/migration, accelerating angiogenesis/re-epithelialization, modulating immune responses, and reducing scar formation. Specifically, keratinocyte-derived exosomes mitigate pro-inflammatory cytokine release (eg, TNF-α, CD74, and inducible nitric oxide synthase [iNOS]) while enhancing anti-inflammatory mediator synthesis, thereby controlling macrophage migration/activation.27 Fibroblast-derived exosomes promote aged skin wound healing through miR-125b release and fibroblast-to-myofibroblast transition induction.28 During tissue remodeling, exosomes from fibroblasts and endothelial cells regulate extracellular matrix (ECM) synthesis, maturation, and reconstruction.29,30 Additionally, exosomes from keratinocytes and fibroblasts prevent scar formation by transferring fibroblast-developmental miRNAs to myofibroblasts and modulating ECM deposition.9,31 As a result, skin resident cells are considered to be one of the reliable sources of biological vesicles.
The synergistic integration of biomaterials with skin-derived biological vesicles presents a transformative strategy for wound repair, leveraging material engineering to optimize vesicle stability, targeted delivery efficiency, and functional durability, thereby maximizing therapeutic potential.32 To address the challenge of biomolecular leakage—a critical limitation in conventional delivery systems—researchers developed microneedles (MNs) encapsulating tazarotene and exosomes for localized wound application, demonstrating their dual functionality in spatiotemporally controlled release.33 Experimental validation revealed that this MN-based platform significantly enhances cellular proliferation, migration, and angiogenesis across both in vitro and in vivo models, with murine wound studies confirming synchronized delivery of therapeutic agents and exosomes.34 The elucidation of molecular mechanisms underlying skin-derived biological vesicles remains a pivotal research focus,35 with metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) identified as a critical regulatory factor functioning through binding interactions with miR-124 or miR-378a-8 to exert therapeutic effects and promote tissue regeneration.36 Building on this discovery, researchers engineered MALAT1-overexpressing exosomes from human keratinocytes, demonstrating that this combinatorial strategy not only activates MFGE8 via suppression of miR-1914-3p but also enhances macrophage phagocytic capacity (Figure 2), thereby establishing a dual-axis repair mechanism integrating molecular silencing with immunomodulatory functions.37 However, skin-derived biological vesicles face unique challenges in clinical translation for wound therapy. Primary skin cells demonstrate limited in vitro expansion efficiency and accelerated senescence under conventional two-dimensional (2D) culture conditions, resulting in vesicle yields significantly lower than those from mesenchymal stem cells.38 Psoriasis patient-derived vesicles may exacerbate inflammatory cascades, necessitating stringent donor screening or compositional modification.39 Furthermore, the absence of FDA-established quality control metrics specific to skin-derived vesicles underscores the urgent need to develop quantitative standards based on skin-specific biomarkers such as keratin 14 (KRT14) and loricrin (LOR).40
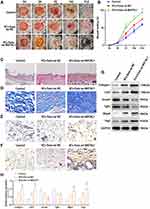 |
Figure 2 KCs -Exo carrying MALAT1 promoted wound healing in mice with diabetes mellitus. (A) Representative images of full thickness defects in mice at days 0, 3, 7, 14, and 21 days postoperatively. (B) Wound healing closure rates were calculated among the different groups using the ImageJ software. (C) H&E staining among the different groups at day 14, Scale bar: 200μm. (D) Masson’s trichrome staining among the different groups at day 14, Scale bar: 20μm. (E) Representative immunohistochemical images of CD31, Scale bar: 20μm. (F) Representative immunohistochemical images of vascular endothelial growth factor (VEGF), Scale bar: 20μm. (G and H) Relative protein expression of Collagen I, Cd31, Smad3, Tgfb1, Mfge8, Vegf determined by Western blot analysis. *p < 0.05, **p < 0.01 versus the control group; #p < 0.05 versus the KCs-Exo oe-NC group. Measurement data were expressed as mean ± SD. Comparison among multiple groups was conducted using one-way ANOVA, followed by Tukey’s post hoc test. (A–H) adapted from Kuang L, Zhang C, Li B, Deng H, Chen R, Li G. Human keratinocyte-derived exosomal MALAT1 promotes diabetic wound healing by upregulating MFGE8 via microRNA-1914-3p. Int J Nanomed. 2023;18:949. © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, 3.0) License.37 |
Mesenchymal Stem Cell
Mesenchymal stem cells (MSCs) are multifunctional, self-renewing cells that can secrete growth factors or differentiate into skin cells to enhance proliferation and facilitate tissue healing.41,42 Macrophage polarization, myofibroblast formation, and extracellular matrix reconstitution are all influenced by a variety of cytokines secreted by MSCs, including insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), MMP-1, keratinocyte growth factor, and type I collagen.43,44 Because of their low immunogenicity, ease of storage, and highly efficient biological activity, MSC-derived exosomes, or MSC-exos, have drawn the attention of more researchers in light of the limited number of endogenous MSCs present during wound self-repair and the numerous adverse events that occur during MSC transplantation.45 Adipose-derived stem cells (ADSCs), bone marrow-derived mesenchymal stem cells (BMSCs), human umbilical cord mesenchymal stem cells (hUC-MSCs), and other stem cell types serve as viable mesenchymal stem cell (MSC) sources, all demonstrating therapeutic potential in diabetic wound healing, inflammatory wound repair, and keloid formation.46,47
Comparative analyses of MSC sources have revealed that human ADSCs (hADSCs) exhibit superior longevity, proliferative capacity, and resistance to senescence compared to BMSCs, therefore constituting the preferred biological vesicle source.48 In foundational studies, the application of hADSC-derived exosomes (hADSC-Exos) encapsulated in thermosensitive PF-127 hydrogel demonstrated enhanced wound closure rates in murine models.49 Furthermore, extensive evidence indicates that preconditioning strategies can amplify the paracrine activity of MSCs, exemplified by melatonin-pretreated MSC-derived exosomes (MT-Exo), which suppress excessive inflammation, stimulate angiogenesis and collagen deposition, and ultimately accelerate diabetic wound healing.50 The combinatorial use of MSC-derived vesicles from multiple sources presents synergistic advantages, capitalizing on their distinct molecular profiles. Researchers developed an innovative dual-crosslinked hydrogel composed of sericin and fibroin, co-loaded with platelet-rich plasma exosomes (PRP-Exo) and MSC-derived exosomes (MSC-Exos), which accelerated diabetic wound repair through coordinated upregulation of growth factor (GF) expression while downregulating matrix metalloproteinase-9 (MMP-9), thereby promoting angiogenesis, re-epithelialization, and anti-necrotic effects (Figure 3).51
 |
Figure 3 Schematic illustration of (A) the synthetic process for dual-crosslinked hydrogels (SP@PRP, SP@PRP, and SP@PRP) and their use as diabetic wound dressings; (B) Factor XIIIa catalyzes a chemical reaction resulting in insoluble fibrin highly covalently crosslinked through glutamine and lysine residues; (C) Ca+2-silk protein interactions in SP@PRP activated with calcium gluconate, and (D) formation of exosome-loaded genipin-crosslinked silk protein hydrogels. (A–D) adapted from Bakadia BM, Ahmed AAQ, Lamboni L, et al. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact Mater. 2023;28:74. © 2023 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd. CC BY-NC-ND 4.0.51 |
In vivo, double-cross-linked hydrogels accelerate wound healing by upregulating the expression of growth factors (GFs), downregulating the expression of matrix metalloproteinase-9, and promoting anti-NETotic actions, angiogenesis, and re-epithelialization. Recent research attention has extended to specialized bio-derived vesicles generated under specific physiological conditions. Notably, unlike exosomes, apoptotic bodies inherently possess dual signaling capabilities - employing “find-me” signals (eg, chemokine CX3CL1) and “eat-me” signals (eg, phosphatidylserine exposure) to recruit macrophages for targeted phagocytosis.52 Building upon these mechanisms, researchers have successfully developed ABs@GMSs through integrated liquid-phase microfluidic technology and biosynthetic engineering, creating an injectable localized biomaterial comprising biodegradable microspheres and releasable apoptotic vesicles.53 In rat models of chronic diabetic wounds, ABs@GMSs demonstrated superior efficacy in macrophage polarization and wound closure compared to conventional treatments (Figure 4).
 |
Figure 4 AB@GMSs induce the polarization of M2 macrophages and promote angiogenesis during wound healing. *p < 0.05. Data are expressed as mean ±SEM. (A–K) adapted from Mao J, Qian S, Zhao Q, et al. Balancing macrophage polarization via stem cell-derived apoptotic bodies for diabetic wound healing. Med. 2024;5(2):148. © 2024 The Author(s). Published by Elsevier Inc. Creative Commons CC-BY-NC-ND.53 |
With the advancement of research and clinical experience, clinical trials investigating MSC-Exos have demonstrated a progressive annual increase. In a recent clinical trial (NCT05475418), hADSC-Exos were isolated and applied in wound therapy research, though experimental outcomes remain pending publication. Furthermore, Exogenus Therapeutics (Portugal) is developing ExoWound, a therapeutic product combining extracellular vesicles (EVs) from human umbilical cord blood mononuclear cells (Exo-101) with sustained-release hydrogel to modulate release kinetics, achieving threefold EV yield enhancement for wound healing applications. Despite these advancements, MSC-derived biological vesicles still encounter unique clinical translation barriers in wound management, particularly the marked functional heterogeneity observed among vesicles originating from different tissue sources (bone marrow, umbilical cord, adipose). Nevertheless, MSC-derived vesicles undeniably constitute a pivotal component in future clinical strategies for cutaneous injury repair.
Immune Cells
The wound healing process typically involves infection and tissue damage triggering immune responses that recruit neutrophils, macrophages, and lymphocytes to the injury site, facilitating wound bed clearance and tissue repair.4,54 Immune cell-derived biological vesicles demonstrate unique advantages in wound regeneration through their inherent immunomodulatory capacities. Specifically, neutrophil-derived EVs (N-EVs) directly eradicate pathogens via myeloperoxidase while mitigating inflammatory storms through let-7 miRNA-mediated TLR4 pathway inhibition, with their spatiotemporal recruitment dynamics proving particularly advantageous for acute wound intervention.55 Specifically, macrophage-derived EVs (M-EVs) exhibit dual functionality based on M1/M2 phenotypic polarization.56 M1-EVs eliminate pathogens via pro-inflammatory mediators like TNF-α, while M2-EVs promote collagen deposition through TGF-β, with engineered miR-21 loading further enhancing their anti-inflammatory and pro-angiogenic effects.57
Recent breakthroughs address diabetic wound complexities through hydrogel-based portable bioactive ink technology (PAINT), which significantly enhances macrophage M2 polarization and endothelial angiogenesis via M2-exosome (M2-Exos) delivery, achieving over 50% improvement in diabetic wound closure rates.58 Mechanistic studies reveal monocytes’ critical regulatory role:59 monocyte deficiency induces pathological leptin overproduction causing vascular hyperdilation at infection sites, thereby delaying healing and exacerbating scarring, whereas polynuclear cells counterbalance this through ghrelin-mediated suppression of leptin-induced vascular hyperplasia while promoting collagen alignment and epithelial regeneration.60 Immune cell precision regulation further inhibits scarring, exemplified by M2-EVs upregulating TGF-β3 and miR-29b to reduce type I collagen deposition while maintaining skin elasticity. Innovative research on re-epithelialization demonstrates dendritic epidermal T cells (DETCs) regulate epidermal stem cell (EpSC) proliferation through exosomal communication:61 Tcrδ-knockout models show 40% EpSC population reduction and re-epithelialization delay upon DETC exosome depletion, while exogenous DETC-EV supplementation restores EpSC function via Wnt/β-catenin pathway activation. Moreover, the integration of photothermal therapy with MN carriers expands therapeutic dimensions through localized thermal effects that suppress inflammatory cytokine storms, while enabling deep transdermal delivery of EVs via microneedle arrays, demonstrating multimodal regenerative potential in infected wounds62 (Figure 5).
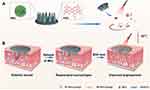 |
Figure 5 (A) Schematic illustration of MEs@PMN for diabetic wound healing. (B) MEs@PMN for treating diabetic wound based on anti-inflammation and angiogenesis promotion. (A and B) adapted from Zeng J, Sun Z, Zeng F, Gu C, Chen X. M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mater Today Bio. 2023;20:100649. © 2023 The Authors. Published by Elsevier Ltd. license.63 |
The clinical translation of immune cell-derived biological vesicles remains in early exploratory stages, with allogeneic compatibility issues posing unique translational challenges distinct from other cell-derived EVs. Immune cells (eg, macrophages, dendritic cells) inherently exhibit heightened immunological activity, with their secreted EVs carrying parent cell-specific antigen-presenting molecules (MHC-I/II) on surface membranes, potentially triggering recipient immune rejection. For instance, unmodified dendritic cell-derived EVs (DC-EVs) have been shown to induce CD8+ T cell activation and IgG antibody production in murine allotransplantation models, resulting in an 80% EV clearance rate within 24 hours. Further complicating this landscape, immunogenicity varies substantially across EV subtypes: activated M1 macrophage-derived EVs demonstrate elevated surface expression of CD40 and CD86 co-stimulatory molecules compared to M2-EVs, exhibiting greater propensity for host T cell activation, while neutrophil-derived EVs may trigger alternative complement pathway activation through myeloperoxidase enrichment.
Other Cells
In addressing the critical challenge of low yield in vesicle production for regenerative applications, researchers have systematically explored alternative biological sources with enhanced productivity and accessibility. Oral mucosa has emerged as an ideal candidate for cell sheet engineering due to its unique regenerative properties, demonstrating scarless healing characteristics analogous to fetal wound repair mechanisms,64–66 with subsequent studies utilizing in vivo wound models to validate the pro-regenerative potential of exosomes derived from oral mucosal epithelial cells (OMECs).67 Concurrently, placental amniotic membrane serves as a prominent source of human amniotic epithelial cells (hAECs), which offer advantages including high yield extraction efficiency, low immunogenicity, and non-tumorigenic properties - researchers have successfully isolated high-purity exosomes (hAECs-Exos) from this source,68 demonstrating their capacity to accelerate wound healing through fibroblast activation and angiogenesis stimulation via PI3K-Akt-mTOR pathway modulation.69 Furthermore, activated platelets have been shown to enhance tissue repair through dual mechanisms of angiogenesis promotion and cellular proliferation stimulation,70 while plant-derived resveratrol, a non-flavonoid polyphenolic compound from Vitis vinifera, exhibits complementary therapeutic effects by reducing inflammatory responses and promoting vascularization during cutaneous wound healing. This pharmacological synergy has been strategically harnessed through the development of a composite hydrogel incorporating both platelet-derived extracellular vesicles (PDEVs) and mesoporous silica nanoparticle-loaded resveratrol (MSN-Res),71 creating a multifunctional therapeutic platform that simultaneously addresses angiogenesis enhancement and inflammation regulation (Figure 6).
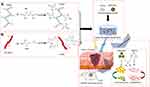 |
Figure 6 Schematic diagram of GelMA/SFMA/MSN-RES/PDEVs hydrogel preparation and application. (A) Schematic of the modification of the gelatin molecule with MA. (B) Schematic modification of a silk fibroin molecule with GMA. (C) Schematic showing the preparation of GelMA/SFMA/MSN-RES/PDEVs hydrogels used as a dressing for wounds in a diabetic mouse model. PDEVs were drawn using Figdraw (www.fgdraw.com). Figure adapted with permission Zhu W, Dong Y, Xu P, et al. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022;154:212. © 2022 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.71 |
Vesicles of Body Fluid Origin
Biological vesicles constitute ubiquitous nanoscale communication systems within biological fluids such as bile, milk, saliva, urine, and blood, with their diagnostic and therapeutic exploitation leveraging the inherent accessibility of these liquid biopsy sources.72 Serum-derived exosomes (serum-Exos), the most prevalent and quantitatively dominant exosomal population in circulation, have demonstrated therapeutic efficacy in enhancing wound healing outcomes in diabetic patients. To elucidate the specific mechanistic contributions of serum-Exos to diabetic wound repair, researchers isolated substantial quantities of these nanovesicles from murine serum and administered them in vivo, revealing accelerated wound closure kinetics in diabetic murine models.73 Post-treatment analyses confirmed that serum-Exos upregulated CD31 expression, augmented fibronectin and collagen-α synthesis, and stimulated granulation tissue formation. Further mechanistic investigations identified enhanced serine-threonine kinase Akt phosphorylation in burn-injured dermal tissues following serum-Exos administration, suggesting activation of pro-regenerative signaling pathways.74 Emerging evidence posits that exercise-induced systemic adaptations involve the generation of “exercise factors” packaged into exosomes and released into circulation,75 with preclinical studies achieving successful therapeutic outcomes in cutaneous wound models through exercise-modulated exosomal delivery.76 Salivary exosomes (saliva-Exos) have garnered attention as optimal candidates for tissue regeneration due to their rich proteomic profile and growth factor content,77 with experimental validation confirming their pro-angiogenic capacity and wound-healing acceleration.78 Mammalian milk, a nutritionally complex biological fluid containing developmentally critical biomolecules,79 has been shown to promote fibroblast proliferation and collagen biosynthesis during dermal repair.80,81 Notably, milk-derived exosomes (mi-Exos) exhibit multifunctional regulatory properties encompassing immunomodulation, metabolic regulation, and neurodevelopmental support,82 establishing their potential as engineered therapeutic carriers for trauma management.83 Preclinical validation studies have consistently documented mi-Exos’ therapeutic efficacy in wound healing applications, further enhanced by their high yield and accessibility.84,85 Mechanistic evaluations revealed mi-Exos’ antioxidant capacity through free radical scavenging assays and modulation of key inflammatory mediators, while their scarless healing potential was attributed to selective upregulation of TGF-β3/TGF-β1 ratio in regenerating tissues.86 Fluid-derived biological vesicles face two predominant clinical translation barriers: heterogeneity control and isolation/purification technical constraints. The substantial heterogeneity of biological vesicles derived from bodily fluids manifests in dimensional variability (30–1000 nm), membrane protein compositional differences (eg, coexistence of exosomes and apoptotic bodies in plasma), and biomolecular cargo diversity, resulting in ambiguous functional definitions and inter-batch quality fluctuations (coefficient of variation >35% in critical biomarkers). Current isolation technologies are challenged by complex biological matrices containing serum protein aggregates and lipoprotein particles, with conventional ultracentrifugation methods causing structural damage to vesicle membranes while achieving suboptimal recovery rates of 5–25% as quantified by nanoparticle tracking analysis.87
Plant-Derived Vesicles
Biomedical research has increasingly focused on plant-derived exosome-like nanovesicles (PENs) as natural intercellular communicators enriched with bioactive compounds, with systematic investigations now identifying key botanical sources including Triticum aestivum (wheat), Panax ginseng, Aloe barbadensis, Citrus paradisi (grapefruit), Vitis vinifera (grape), Citrus limon (lemon), Brassica oleracea (broccoli), Daucus carota (carrot), Malus domestica (apple), Zingiber officinale (ginger), Coffea arabica, and Citrus species through standardized multi-omics characterization.88–93 Functional validation studies demonstrate species-specific therapeutic advantages: Grapefruit-derived vesicles (GEVs) enhance HaCaT keratinocyte migration rates by 2.3-fold through coordinated upregulation of wound-associated genes (COL1A1, MMP9), proteins (fibronectin, laminin-5), and cytokines (IL-6, TGF-β1), as quantified via transcriptomic and proteomic profiling.94 Comparative analyses reveal PENs’ superior anti-inflammatory capacity and scalable production yields, positioning them as cost-effective alternatives for translational applications.95 Current research prioritizes systematic screening of plant species using high-content wound healing assays, with Triticum aestivum emerging as a lead candidate through multi-parametric evaluation—wheat PENs induce 2.1-fold fibroblast proliferation, accelerate angiogenesis (1.8-fold CD31+ vessel density increase), and upregulate 17 core healing-related genes (FGF2, VEGF-A, TIMP1) in full-thickness wound models.96 Parallel investigations of Panax ginseng demonstrate pleiotropic benefits beyond wound repair, including metabolic regulation and neurological protection,97 while ginseng-derived nanoparticles (GDNPs) exhibit dual therapeutic action—2.4-fold collagen deposition enhancement coupled with 57% IL-1β/IL-6 suppression in senescent skin models.98 These findings collectively establish PENs as a pharmacologically versatile platform offering enhanced biosafety, cost efficiency, and therapeutic multifunctionality for next-generation wound management strategies.
Other Sources of Vesicles
The natural world harbors diverse biological resources with vesicle-producing potential, among which Periplaneta americana L. (PA)—an environmentally resilient insect with historical medicinal applications—has demonstrated therapeutic relevance.99 PA-derived glycoproteins accelerate diabetic wound healing by modulating macrophage polarization dynamics, while exosome-like nanoparticles isolated from PA (PA-ELNs) exhibit tripartite therapeutic efficacy: enhanced re-epithelialization rates, autophagy pathway regulation, and anti-inflammatory activity, collectively contributing to improved diabetic wound closure.100 These findings underscore the broad phylogenetic distribution of vesicle sources and suggest that species-specific exosomal components may harbor unique therapeutic properties warranting systematic investigation.
Isolation of Vesicles
Vesicles are nowadays heavily used for direct treatment of diseases or delivery of multiple drugs.101 It is crucial to understand how to produce vesicles with high purity and throughput for later clinical applications.102 Numerous methods, including size exclusion chromatography, differential ultracentrifugation, ultrafiltration, immunoaffinity capture, polymer precipitation, and microfluidics, have been used to isolate vesicles—particularly exosomes—to date (Table 1).72,103 To choose the best separation techniques for future uses, a thorough analysis of factors including speed, efficiency, purity, dependability, automation level, and flexibility must be made. The benefits and drawbacks of widely used separation techniques are outlined in this section (Figure 7).
 |
Figure 7 Methods for isolating biological vesicles (exosomes as an example). Numbers 1–6 in sequence are ultracentrifugation, ultrafiltration, immunoaffinity separation, volume exclusion chromatography, polymer precipitation, and microfluidics. The figure was created with https://app.biorender.com/. |
 |
Table 1 Overview of Isolation of Vesicles |
Ultracentrifugation
Ultracentrifugation (including differential centrifugation and density gradient ultracentrifugation) is one of the most widely used exosome separation methods, which is called the “gold standard” for vesicles separation.101 Because ultracentrifugation does not need expensive or complicated sample preparation, it is commonly utilized in exocrine processing.104 Regretfully, the ultracentrifugation technique yields only moderately pure samples and is time-consuming.105 Furthermore, exosomes may sustain mechanical damage as a result of repeated ultracentrifugation procedures. Apoptotic fragments, protein aggregates, and other non-exosomal contaminants are often eliminated using a number of purification processes used in differential ultracentrifugation.105,106 The purification process includes a low-speed centrifugation phase (300 to 400×g) to remove cell and apoptotic debris, a high-speed centrifugation phase (2000×g) to eliminate larger vesicles, and an ultra-fast centrifugation phase to harvest exosomes. Exosomes with a modest yield and purity have been produced via differential ultracentrifugation, as demonstrated by numerous tests. Although the above complex centrifugation process can ensure higher purity exosomes, it is difficult to avoid the loss of exosomes during this period.
A gradient medium, such as sucrose, is needed for density gradient ultracentrifugation in order to enrich vesicles in density-specific layers. Density gradient technique can extract exosomes in a given size range with higher purity but lesser yield when compared to differential ultrafast centrifugation. Nevertheless, density gradient ultracentrifugation is costly and unsuitable for the large-scale separation of exosomes because of the intricate structure of gradient media.
Ultrafiltration
Using commercial membrane filters with predetermined pore sizes, the ultrafiltration technique isolates biological fluids from vesicles.107 As a result, the separation is accomplished using the molecular weight and size. When compared to other methods, ultrafiltration is more straightforward and has a higher output.108 However, poor vesicle production will arise from vesicle buildup and pore blockage caused by vesicle retention in the pore canal.109 Damage may result from shear tension between the filter membrane and the vesicles brought on by the pressure of separation. To address these issues, techniques like membrane cleaning have been developed.
Direct current filtration (DFF) and tangential flow filtration (TFF) are the two categories into which the current vesicles ultrafiltration techniques can be separated. DFF has several drawbacks, including contaminated membranes and poor particle separation. DFF is also limited to the separation of small volume samples. The technique of tangentially moving sample fluid to filter the membrane and prevent cake formation or clogging is called TFF. TFF transfers the sample tangentially to the membrane as opposed to applying orthogonal pressure, as the aforementioned methods do. This technique prevents the buildup of particles on the membrane that causes blockage. TFF is gentler on the sample and can manage higher sample volumes than DFF. TFF will take longer to process, though, in order to obtain more pure exosomes.
Polymer Precipitation Method
Vesicles and other tiny particles are precipitated from biological fluids through polymer precipitation, which modifies the solubility or dispersity of hydrophilic polymers.110 One of the most often used polymers for polymer precipitation is polyethylene glycol (PEG). After centrifugation, vesicles made of hydrophilic PEG with a certain molecular weight were separated from the incubated cell culture medium or bodily fluid.111 The researchers came up with an ideal plan by modifying the precipitation, salt, and polymer levels. Certain exosome extraction kits sold commercially have also used this technique.112 However, exosomes produced with this technique contain some protein impurities.113
Immunoaffinity Separation
Through the interaction of vesicles’ surface proteins—such as CD9, CD63, and CD81—with particular antibodies, immunoaffinity isolation is able to collect vesicles.114 To facilitate separation, antibodies can be attached to a variety of support surfaces, such as magnetic beads, chromatographic substrates, plates, and microfluidic devices.115 To eliminate protein aggregates and other big particles, immunoaffinity capture is therefore frequently employed in conjunction with pretreatment techniques like volumetric exclusion chromatography or centrifugation. It goes without saying that immunoaffinity separation is a gentle and highly specialized technique that can keep vesicles’ biological activity mostly intact after separation. However, this technique is limited by the antibodies that can be supported, the application scope is limited, and the processing volume is small.116 Additionally, a longer incubation period is needed for immunoaffinity isolation. For instance, when used for separation, each magnetic Dynabead from Thermo Fisher needs to be incubated for 12 hours. Longer incubation times are caused by the magnetic beads’ large size (1.0–4.5 µm), limited mobility in solution, and low surface area to volume ratio. Using pH-or temperature-responsive magnetic nanoparticles can speed up this process. Because the nanoparticles have a larger surface-area-to-volume ratio and higher magneto electrophoretic mobility, after aggregation caused by changes in temperature or pH to allow for rapid magnetic separation. It is possible to shorten the periods of incubation and separation to a few minutes. Magnetism is also used in immunoaffinity techniques based on Raman scattering to separate and analyze vesicles. Molecules can be recognized by Raman scattering due to their distinctive chemical fingerprints. High sensitivity and specificity have been achieved in the detection of breast cancer in patient samples using magnetic surface-enhanced Raman scattering. The integration of characterisation and sample processing is essential in streamlining the therapeutic and diagnostic applications of exosomes and sets them apart from alternative immunoaffinity methodologies.
Volume Exclusion Chromatography
Vesicles can be separated using a size-based technique called volumetric exclusion chromatography.117 This easy-to-use, low-cost vesicle separation technique prevents shear stress damage and does not require any specialized equipment. In general, this technique does a good job of preserving the integrity and structure of vesicles. It is frequently employed as a crucial stage in the purification of small EVs so that plasma impurities may be removed from them.118 To achieve high resolution particle size, processing parameters such as resin type, flow rate, column size, microbead loading, and system volume must be taken into account. To get a sample free of lipoprotein and protein contaminants, the material must first undergo ultracentrifugation or ultrafiltration. Size-exclusion chromatography, like other size-based separation methods, is not a practical way to separate exosomes from other particles of the same size.119 This issue might be resolved by combining immunoaffinity capture and separation techniques.120
Microfluidic Technology
Microfluidic technology is a high-throughput method, which can be compatible with a variety of vesicles separation methods.121 A high-throughput technique that can work with a range of vesicles separation techniques is microfluidic technology.122 Microfluidic devices offer a number of benefits.123 First, quicker separation times and reduced sample losses are made possible by microfluidic technology. Second, it can boost productivity and purity in a synergistic way when used in conjunction with other vesicle separation techniques. Furthermore, microfluidic devices can be expanded in accordance with actual needs and have a more compact structure than conventional separation devices. Microfluidic instruments are ideal for vesicle identification due to their small sample size and portability.
Nevertheless, there are certain issues with using microfluidic devices.124 First, samples may obstruct the path during examination, lengthening the time needed for identification and separation. Second, the method’s large-scale application is limited by the need for sophisticated equipment. In order to produce quick, effective, and high-purity vesicles separation procedures, researchers are attempting to integrate microfluidic devices with a variety of external pressures. These forces can be broadly classified into two categories: passive isolation techniques (membrane-based, column-based, and fluid dynamics) and active isolation techniques (electrical, centrifugal, and dynamical). Using acoustic dynamics as an example, it is widely accepted that larger particles in the sound field would experience stronger acoustic forces, causing them to separate from one another.125 Researchers combined acoustic flow and droplet rotation techniques to separate and identify exosomes.126 Experiments conducted later indicate that the gadget can handle data in less than a minute and achieve a high separation efficiency of 80–86%.
Vesicles have significantly advanced the fields of medication administration and non-invasive illness diagnostics and therapy because of their therapeutic potential and distinct biological activities. One of the main obstacles to vesicles’ therapeutic evolution in the last few decades has been their isolation.127 Currently, there are many established methods for separating vesicles that make use of cutting-edge technology like electricity, centrifugal force, microfluidics, and acoustics.128 Ultracentrifugation (UC), the most widely utilized separation technique, exhibits recovery rates significantly influenced by sample types and operational parameters. A systematic comparative study demonstrates that UC achieves exosome recovery rates below 30% from plasma, with high centrifugal forces potentially causing partial degradation of vesicular membrane proteins such as CD63.118 Immunoaffinity methods (eg, antibody-conjugated magnetic bead capture) achieve high-purity isolation (>90%) but incur substantial reagent costs (antibody consumption reaching hundreds of dollars per experiment) and risk compromising vesicle integrity during elution.129 Microfluidic platforms, despite their high-throughput processing capabilities and low shear stress characteristics, face clinical-scale implementation challenges: studies on inertial focusing chips reveal mandatory pre-filtration requirements for complex biological samples like serum to remove large particulate contaminants, accompanied by progressively increasing channel clogging rates during prolonged operation.130 Regarding scalability, UC maintains dominance due to equipment accessibility but struggles to meet therapeutic-grade dosage demands, while parallelized microfluidic designs (eg, multi-channel chips) theoretically enhance processing capacity yet require optimization of standardization protocols and cost-effectiveness in manufacturing.131 Hybrid strategies combining UC pre-concentration with microfluidic sorting have demonstrated improved recovery rates and purity profiles, though clinical translation necessitates rigorous GMP certification and large-scale validation.132
Design and Development of Biological Vesicles
Bioinspired vesicles demonstrate optimized biomedical application potential through the integration of biomimetic principles and engineering technologies,133 while preserving core functionalities of natural biological vesicles (eg, extracellular vesicles, exosomes).134 First, enhanced stability and controllability: Whereas natural vesicles exhibit fragile membrane structures susceptible to enzymatic degradation or oxidative damage with short in vivo circulation half-lives, bioinspired counterparts achieve extended circulation durations through synthetic lipid incorporation (eg, DSPC), polymer modification (eg, polyethylene glycol), or crosslinking strategies, coupled with environment-responsive materials (eg, pH-sensitive lipids, thermosensitive polymers) for targeted drug release at lesion sites.135 Second, breakthroughs in drug-loading efficiency and functional programmability: Natural vesicles typically exhibit drug-loading capacities below 10% due to endogenous content limitations and membrane permeability constraints, whereas bioinspired systems achieve 30%-50% loading efficiencies for small-molecule drugs, nucleic acids (eg, siRNA, mRNA), and proteins through electroporation, microfluidic manipulation, or membrane reconstitution techniques, enabling multi-drug co-loading and sequential release.136 Third, reduced immunogenicity and allogeneic risks: While natural vesicles (particularly xenogeneic sources) may carry donor-specific antigens or pro-inflammatory factors triggering immune clearance or toxicity, bioinspired variants minimize immunogenicity through purified membrane components (eg, non-target protein removal), surface ligand engineering (eg, CD47 overexpression for immune evasion), or fully synthetic material construction, thereby improving biocompatibility.137 Fourth, scalable manufacturing and standardization feasibility: Contrasting with natural vesicle production limited by cell culture conditions and batch-to-batch variability, bioinspired systems enable standardized large-scale manufacturing via microfluidic assembly, liposome extrusion, or membrane protein reconstitution technologies, meeting clinical-grade quality control requirements (eg, size uniformity, drug-loading consistency).15 Overall, it is anticipated that synthetic vesicles mimics would increase the effectiveness of bioactive compounds’ distribution to therapeutic targets while producing scalable preparation methods and desired results.138 The field of oncology medicines has extensively investigated this synthetic technique.139 The blood-brain barrier’s (BBB) poor drug penetration significantly reduces the effectiveness of treatment for glioblastoma multiforme (GBM). Bionic nanoparticles (NPs) embedded in GBM cell membranes have been used by researchers to successfully transport medications to GBM cells across the blood-brain barrier. In the meanwhile, a number of investigations have demonstrated that, in contrast to biological vesicles, synthetic vesicles offer larger yields, consistent efficacy, and possible therapeutic uses.140 Currently, there are three types of synthetic strategies: top-down, bottom-up, and biohybrid (Figure 8). We also provided a summary of the artificial vesicles here (Table 2).
 |
Figure 8 Strategies for artificial synthesis of bionic vesicles. Number 1–3 in sequence are bottom-up, top-down, and biohybridisation strategies. A bottom-up approach would include developing larger, more complicated structures gradually by beginning with the assembly of smaller, simpler elements. Top-down procedures begin with larger biological structures, which are subsequently broken down into smaller pieces using different techniques to create smaller vesicles. Through the process of biohybridization, natural and synthetic vesicles can be combined to create vesicles. The figure was created with https://app.biorender.com/. |
 |
Table 2 Summarization of the Synthetic Vesicles |
Bottom-Up
A bottom-up approach would include developing larger, more complicated structures gradually by beginning with the assembly of smaller, simpler elements.148 This is comparable to the architectural construction process.149 Therefore, it is evident that bottom-up approaches necessitate a thorough comprehension of the essential elements of natural exosomes. Liposomes are believed to have exceptional biocompatibility because of their ability to replicate the membrane shape of exosomes or natural cells.150 These days, the focus of research is on adding lipids with certain compositions to exosome-like nanoparticles. Then, the necessary surface proteins are added to the manufactured lipid bilayers utilizing methods such as chemical biocoupling, simple incubation, and cell-free protein synthesis.151,152 The resultant particles, which resemble exosomes, have the benefits of controllability and clean components.153 Bottom-up synthetic exosomes are more compliant with market rules and production standards, and they have a better acceptability rate for drugs from an industrial production perspective.154 Nevertheless, there are a lot of drawbacks to using this method to make synthetic exosomes. For the present being, the loading structure and constituents of natural exosomes cannot be completely replicated using bottom-up techniques.155 The active components necessary for natural exosomes to operate properly in the treatment of disease and drug delivery have not yet been identified, and a better comprehension of exosome biology is still lacking in the clinical translation process.156 It would also be challenging to adequately characterize the active components and functional mechanisms of exosomes derived from various cellular sources due to these differences.157,158 To guarantee the efficacy of the final manufactured exosomes, functional molecular integration of exosome-like nanoparticles even necessitates exact manufacturing and purification processes. Therefore, for bottom-up tactics to be further translated into clinical practice, several technological advances may be needed.151,159
Top-Down
Typically, top-down procedures begin with larger, more complicated biological structures, which are subsequently broken down into smaller pieces using different techniques to create smaller vesicles. For instance, it is possible to produce tailored vesicles by disassembling cell membranes into tiny membrane fragments and then assembling those fragments into membrane vesicles. Top-down methods, as opposed to bottom-up ones, convert cell membranes into nanoparticles while preserving proteins or other physiologically active molecules in their native state, guaranteeing the maximum amount of biological complexity preservation.160–162 A top-down method of functionalizing the cell membrane guarantees that the essential characteristics of the vesicles are preserved and makes it easier to maximize their therapeutic and targeted potential. Thus, the top-down strategy helps to reduce non-specific uptake, improve delivery efficiency, isotype targeting and circulating half-life, and achieve superior therapeutic efficacy.163 As a result, the top-down approach contributes to better therapeutic efficacy, isotype targeting, improved delivery efficiency, decreased non-specific absorption, and circulation half-life. Top-down strategy-based preparation techniques have been extensively developed, with extrusion and microfluidics technologies in the forefront.
Microfluidics
Microfluidics’ automation, quick analysis, large throughput, and customizable detection make it a popular tool for vesicle isolation, detection, analysis, and modification.164 Engineered vesicles have started to be produced on a massive scale using microfluidics in recent years.165 The vesicles that are produced using microfluidic platforms exhibit great reproducibility, high drug-carrying efficiency, consistent size, shape, rigidity, and surface charge. Numerous biomimetic vesicles generated from cells and cell membranes have shown specific therapeutic efficacy, and their yield (100-fold) is substantially larger than that of natural exosomes.141,166,167 Potential clinical uses of microfluidics include the successful simplification of the bioinspired vesicle preparation and medication loading procedure.168 In order to prepare macrophage-derived nanomedicines, a staggered herringbone micro-mixer with multiple inlets was used to precisely control the vesicle preparation process and drug loading. This allowed for enhanced in situ glioma targeting of drugs and demonstrated the great potential of microfluidics for nanomedicine preparation.141 Regretfully, rather than bioinspired vesicles, microfluidics has been used more in the field of wound healing to synthesize multifunctional wound healing dressings. Diverse possibilities for cell-free wound therapy may be presented by microfluidic systems that may simultaneously synthesize therapeutic dressings and vesicles. Furthermore, even though precise and effective microfluidic chips have many advantages, their application scope and clinical prices are constrained.169 All things considered, microfluidics is a promising preparation technique that may offer an effective manufacturing platform for bioinspired medication delivery and treatment systems.170
Extrusion
To homogenize the size distribution of colloidal formulations (such as liposome suspensions), extrusion techniques are frequently employed. Owing to its benefits, which include excellent control and repeatability, this approach has been employed extensively in recent years to produce bioinspired vesicles. Cultured cells can be crushed and then passed through a series of progressively smaller pore-sized polycarbonate membrane filters to create vesicles that resemble biological vesicles in both their biological and physical characteristics.171,172 Since proteins and membrane fragments have the same physical characteristics as biological vesicles, density gradient ultracentrifugation is typically used to purify vesicles in order to increase size uniformity and sample purity.173,174 The yield of vesicles derived by extrusion technology is much higher than the yield of exosomes purified from the same number of cells (ie >100-fold).171 Furthermore, extrusion technology offers easier procedures and less equipment needed compared to other techniques. Extrusion technology is currently frequently employed to produce huge quantities of bioinspired vesicles.175,176 Researchers have prepared functionalized artificial vesicles by using a liposome extruder and demonstrated their high yield and efficiency properties for effective targeted drug delivery.142 To achieve evenly sized vesicles, extrusion-produced vesicles still need to go through an extra purification phase, which could add complexity and time to the entire production process.
Biohybridisation
Through the process of biohybridization, natural and synthetic vesicles can be combined to create vesicles. Biohybridisation strategies offer batch production, process control, engineered preparation, ideal stability and suitable drug loading, in addition to targeting, biological barrier penetration, recyclability, low immunogenicity and high biocompatibility.177 Many investigations focused on the combination of liposomes and exosomes, with an emphasis on the biohybridization of nanomaterials and biological vesicles.147,178,179 Numerous techniques for biohybridization have been developed.
Freeze-Thawing Strategy
With a wealth of success, freeze-thawing-based biohybridization techniques have been used with liposome production. As a result, scientists were motivated to use it to prepare exosome-liposome hybrids and load different medications. The researchers demonstrated that the synthesized hybrids not only have similar morphology and biological properties as exosomes, but also have desirable circulating properties and targeting properties.180 Moreover, by adding liposomes with various characteristics (pH-sensitive, photosensitive, and thermosensitive), hybrids can be created multipurpose. Researchers have combined heat-sensitive liposomes and gene-engineered exosomes from CT26 cells overexpressing CD47 to create hybrid nanoparticles (NPs).143 In order to achieve targeted delivery, the hybrid NPs can evade in vivo clearance and release medications in response to temperature.
Incubation
Several biological reactions and processes can be gently and successfully induced during incubation. Researchers loaded dopamine into blood exosomes by incubation and validated its potential for brain-targeted delivery for the treatment of central nervous disorders.144 In addition, the researchers constructed biologically responsive self-assembling exosomes by incubation and used them for early cancer diagnosis.181 It was also determined that because both liposomes and exosomes had a lipid bilayer structure, the fusion of these two types of membranes might be accomplished through incubation techniques.150 Researchers created high-drug-carrying hybrid exosomes by combining liposomes and stem cell-derived exosomes through incubation. These exosomes were then utilized to distribute analgesics across biological barriers.145 A promising method for targeted medicine delivery and cancer diagnostics is hybridized nanoparticles. However, issues like particle aggregation and excessive size may result from incubation-induced spontaneous fusion. More research is required to determine how the fusion process is impacted by the incubation conditions.
Co-Extrusion
The goal of the co-extrusion technique is to physically push exosomes and liposomes to break apart and reassemble into hybrid vesicles as they go through the membrane pores that decrease gradient. When it comes to controlling yield, the co-extrusion method outperforms both freeze-thaw and incubation. Liposomes are frequently used as drug delivery vehicles because of their exceptional engineering and functional modification flexibility. However, biological vesicles are naturally advantageous for endogenous cellular processes. Exosomes and liposomes together organically have the potential to increase medication delivery yield and capacity. Additionally, it can enhance permeability and general biocompatibility.
Hybrid vesicles were created by co-extruding lung-targeted liposomes in a 1:1 ratio with exosomes from bispecific Chimeric Antigen Receptor T-cell Immunotherapy (CAR-T) cells that target mesothelin and programmed death ligand-1.146 The hybrid vesicles demonstrated superior anti-tumor properties in addition to their good lung accumulation capacity. By co-extrusion of clodronate (CLD) liposomes and fibroblast-derived exosomes in a 5:1 ratio,147 a hybrid drug delivery system (EL-CLD) with non-specific phagocytic suppression and fibroblast homing capabilities was created, and it was effectively used to treat pulmonary fibrosis. Bionic nanocarriers, which are produced through the co-extrusion of photo thermo sensitive liposomes and platelet exosomes, have good anti-tumor properties and enable the simultaneous administration of chemodynamic and photothermal therapy.182 Unfortunately, this strategy has not yet made its mark in the field of wound healing. When taking into account the whole strategy’s economics, liposomes and exosome-like nanovesicles together might make for a more effective therapeutic approach.
 |
Table 3 Ongoing and Completed Clinical Trials of Vesicle-Based Wound Treatment |
Four Hurdles to Overcome: Safety, Biodistribution, Degradability, Clinical Evaluation Criteria
Over the past decade, biological vesicles have gone from being a relatively unknown topic of study to one that is gaining prominence due to the rapid advancements in nanobiotechnology. According to Grand View Research (2024), the global EV-based wound therapy market has reached 320 million (35.4780 million by 2031 at a 13.1% CAGR, primarily driven by clinical demands for refractory wounds including diabetic foot ulcers, burns, and chronic venous ulcers. We have compiled a comprehensive summary of both ongoing and completed clinical trials investigating vesicle-based therapeutic interventions for wound healing (Table 3). In a 2023 clinical investigation by Gibello et al,183 repetitive topical administration of autologous serum-derived EVs significantly enhanced vascular density (+38%) and collagen deposition (+25%) in chronic venous ulcers, with mechanistic analyses linking these effects to the pro-angiogenic activity of platelet- and endothelial-derived vesicle subpopulations; however, the limited cohort size (n=24) and interindividual response variability necessitate expanded validation to confirm therapeutic generalizability. A 2020 double-blind trial by Kwon’s team established that mesenchymal stem cell (MSC)-derived EVs accelerated epidermal regeneration post-laser therapy, reducing erythema resolution time by 4.2 days and improving scar quality (Vancouver Scar Scale score reduction: 1.8 points), though the functional contributions of specific EV subtypes (exosomes vs microvesicles) remain undefined.184 Johnson et al conducted a randomized controlled trial using allogeneic platelet EVs isolated via ligand-exosome affinity purification (LEAP) chromatography,185 which failed to demonstrate statistically significant differences in healing time (p=0.12) compared to controls but validated the GMP compliance of LEAP technology (purity >95%, 22% improvement in CD63 retention) and clinical safety profile (no severe adverse events), thereby providing foundational infrastructure for scalable manufacturing. Patent analyses reveal three innovative directions in wound-specific EV technologies: 1) Targeted delivery systems (integrin α5β1 modification in US11992527B2 enhancing inflammatory site accumulation), 2) Stability enhancement processes (EP2666459A1 extending EV storage stability at 4°C from 7 to 30 days through lipid formulation optimization), and 3) Intelligent release control (CN110563829A thermosensitive hydrogel-EV composite enabling on-demand antimicrobial peptide release during wound infection). However, there are four obstacles in the way of clinical translation that both artificial and biological vesicles must get past: sustainability, biodistribution, safety, and clinical evaluation standards (Figure 9).
 |
Figure 9 Four hurdles in the way of clinical translation of both artificial and biological vesicles. The figure was created with https://app.biorender.com/. |
Safety
This issue can be successfully resolved by natural exosomes due to their biological activity, low immunogenicity, and biodegradability. For instance, compared to artificial nanocarriers like liposomes, polymer nanoparticles, and inorganic nanoparticles, naturally occurring exosomes are more physiologically benign. Similarly, clinical results demonstrate that although natural exosomes lack sufficient efficacy in clinical trials, they have consistently demonstrated a favorable in vivo safety profile. Biological vesicles, on the other hand, include a number of intricate ligands and receptors that control a broad range of physiological functions. Since the underlying mechanisms are still being investigated, there may be some safety concerns. The content and function of biological vesicles may also be impacted by the kind and physiological condition of the generating cells, isolation procedures, and purification techniques, which could lead to changed therapeutic efficacy. Through regulated manufacturing procedures, artificial vesicles can alleviate the issues at hand; nevertheless, this may have unintended consequences as well, such as convoluted preparation and characterization procedures. Bioactive proteins may undergo permanent conformational changes as a result of artificial alteration of biological vesicles. Another matter of safety to take into account is immune tolerance. These problems should be resolved by cutting-edge bioengineering technologies like cell-free protein production systems. This technology can translate, fold, and incorporate a range of membrane proteins with distinct functions into liposomes in a single step, doing away with the requirement for a DNA or RNA transfection procedure. Due to advancements in cell growth and metabolic constraints, the cell-free protein synthesis system may complete the synthesis process in 3–5 hours, whereas standard mammalian cells require 3–7 days to complete the expression process. The advantages of a cell-free protein synthesis system also include flexibility, ease of operation, high throughput, and controllability across time, space, reaction conditions, and scale.
Biodistribution
The biodistribution properties of bioengineered vesicles—such as tissue distribution, blood levels, and urine clearance—determine a number of factors, including the therapeutic efficacy and possible toxicity of these extremely effective therapeutic vehicles. Following intravenous injection, it was discovered that the liver and spleen contained the greatest concentration of stem cell-derived exosomes. The distribution of tissue in vivo will be affected differently by various injection techniques. Researchers looked examined the impact of six different means of administration, such as intravenous, intraperitoneal, and subcutaneous injections, on tissue distribution in vivo to learn more about the biodistribution of exosomes. This was accomplished by combining fluorescence molecular labeling of exosomes produced from HEK293 cells with radioactive zirconium 89, along with immunofluorescence and positron emission tomography imaging.186 Exosomes from HEK293 cells that overexpress PTGFRN were employed as experimental subjects, and the researchers’ primary animal models included mice, rats, and non-human primates. Exosomes were primarily injected subcutaneously into the injection site or lymph nodes, intraperitoneally into the gastrointestinal tract and lymph nodes, and intravenously into the liver and spleen. Furthermore, in the context of administering cerebrospinal fluid, exosomes are dispersed via intrathecal injection along the neuraxis to the base of the skull and via intraoccipital pool and ventricular injection around the skull and cervicothoracic spine. This could raise important concerns for the long-term effectiveness and security of vesicles. Researchers have discovered that exosomes have a dense distribution that is localized to the site while treating it. This may imply that vesicle bioactivity within the wound region needs to be maintained and rapidly absorbed by researchers.
Sustainability
The primary method of biovesicle therapy for wound healing is injectable drug delivery, which is assumed to cause a quick in vivo clearance of vesicles, hence impacting therapeutic efficacy. As a result, more and more scientists are starting to mix bioactive carriers in an effort to improve vesicles’ therapeutic effectiveness. In addition to facilitating vesicle sustained release, hydrogels act as efficient vesicle transporters by offering therapeutic advantages that vesicles cannot. Hydrogels and vesicles together have emerged as a hotspot of therapeutic research in the field of wound healing. Researchers used oxygen nanobubbles and polyvinyl alcohol/gelatin hybrid hydrogels to improve exosome delivery and reduce wound hypoxia because vesicles had a poor delivery effect in hypoxic tissues.187 Furthermore, encasing exosomes in biocompatible bioinks and using 3D bioprinting to create exosome-loaded 3D scaffolds can both efficiently increase the vesicles’ lifespan and enhance their sustained distribution in wound tissues.188 Despite producing physiochemically stable three-dimensional structures, 3D printing technology does not yield bioactive structures. To increase scaffold bioactivity, these structures ought to be enhanced with particular cell types, bioactive compounds, or exosomes.
Clinical Evaluation Criteria
With the increasing recognition of the role biological vesicles play in the development of diseases and in possible therapies, the industry sector is seeing exponential expansion. Global sales of exosome diagnostics are predicted to reach USD 321.9 million by 2026, up from USD 57.1 million in 2021, while sales of exosome therapeutics are anticipated to reach USD 169.2 million by 2026, up from USD 33.1 million in 2021.189 The potential economic utility of these particles has not been completely realized, despite the noteworthy advancements in the field of vesicle biology and its applications. For clinical applications, there needs to be standardization in the production and analysis of biological vesicles. Regarding vesicle-related items intended for clinical use, there is disagreement about criteria for release and isolation techniques. The FDA has only authorized a limited number of EV products for therapeutic use thus far. Good Manufacturing Practices (GMP) are essential to guaranteeing the best possible clinical results. Biological vesicle have emerged as innovative biological agents for wound therapy, with their regulatory requirements demonstrating both differentiation and increasing stringency across major global pharmaceutical regulatory systems.190 The US Food and Drug Administration (FDA) categorizes EV therapies as biological products (21 CFR 600.3), necessitating market approval through the Biologics License Application (BLA) pathway. For combination products (eg, smart dressings integrated with sensors), joint review by the Center for Drug Evaluation and Research (CDER) and Center for Devices and Radiological Health (CDRH) under 21 CFR Part 4 is required. Core regulatory mandates include: 1) Manufacturing processes must comply with Current Good Manufacturing Practice (cGMP) standards, with explicit identification methods for EV source cells (eg, mesenchymal stem cells per ISEV2018 guidelines) and purification protocols (eg, ultracentrifugation coupled with size-exclusion chromatography). Documentation must include data from ≥3 production batches demonstrating particle size distribution (80–200 nm), surface markers (CD9/CD63/TSG101), and exogenous contaminants (host cell protein <50 ng/dose); 2) Preclinical validation requires standardized animal models (eg, db/db diabetic mice) demonstrating ≥30% reduction in epithelialization time, complemented by biocompatibility (ISO 10993–5/10) and immunogenicity testing (complement activation rate <5%); 3) Clinical trials must adopt multicenter randomized controlled trial (RCT) designs with primary endpoints of complete epithelialization time and secondary endpoints encompassing infection rates, scar assessment scores, and patient-reported outcomes (PROs). The FDA mandates ≥1 year follow-up to evaluate long-term safety risks, particularly antibody generation against allogeneic EVs. The European Medicines Agency (EMA) regulates EV therapies under the Advanced Therapy Medicinal Products (ATMP) framework through centralized Marketing Authorization Applications (MAAs). Products targeting refractory wounds may qualify for Priority Medicines (PRIME) designation, requiring early clinical efficacy evidence (eg, Phase II trial hazard ratio HR≥1.5) and compliance with EU GMP Annex 2 specifications (cleanroom grade ≥B with viral inactivation validation). EMA emphasizes orthogonal characterization methods (eg, nanoparticle tracking analysis [NTA] combined with flow cytometry) and establishment of critical quality attribute (CQA) control standards (eg, miRNA-21 content coefficient of variation CV<15%). Internationally, the ISO/TC276 draft guideline for EV characterization (2024) mandates testing of ≥20 parameters including particle size, zeta potential, and proteomic profiles. ICH Q5A/Q12 guidelines specify requirements for viral safety evaluation (retrovirus detection limit <1 IU/dose) and manufacturing process change control (comparability studies for microfluidic parameter adjustments).
However, the establishment of robust GMP standards for bioinspired vesicles remains in its nascent stage, necessitating coordinated multidisciplinary efforts to address three critical challenges: (1) standardization of production processes compromised by technical complexities in synthetic biology modifications (eg, controlled insertion of artificial transmembrane proteins), (2) quality control bottlenecks arising from heterogeneous vesicle populations (typically 50–200 nm size distribution with ±15% batch variability), and (3) regulatory alignment for novel characterization requirements beyond conventional EV paradigms (including engineered surface topology quantification and synthetic cargo verification). Progress demands synergistic collaboration across material scientists (for functional module design), bioengineers (for scalable manufacturing development), and regulatory specialists (for QbD framework implementation), ideally through international consortia modeled after the ISO/TC276 working group on synthetic vesicles. Emerging solutions include AI-driven process optimization platforms (reducing parameter optimization time from months to weeks) and blockchain-enabled data sharing ecosystems for global GMP knowledge integration, as demonstrated by the Horizon Europe Strategic Plan establishing 23 cross-border GMP validation centers.
Conclusion and Future Direction
Chronic wound healing, particularly in refractory cases such as diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs), remains a critical challenge in modern medicine. The global burden of chronic wounds has escalated due to aging populations and the rising prevalence of multidrug-resistant pathogens. These wounds are characterized by a self-perpetuating pathological triad of “sustained inflammation-oxidative stress-angiogenic dysfunction”, posing formidable challenges to conventional therapeutic approaches. A retrospective cohort study involving 889 hospitalized patients with chronic refractory cutaneous wounds revealed an all-cause mortality rate of 0.8%, with all fatalities attributed to cardiopulmonary failure secondary to systemic infections.191 Notably, DFU patients face disproportionately high risks of limb amputation, which independently increases mortality by 2.4-fold. Current therapeutic limitations are multifaceted: (1) Over 75% of chronic wounds develop polymicrobial biofilms, reducing antibiotic penetration by >90% and facilitating immune evasion through quorum sensing mechanisms.192 (2) Recombinant growth factor therapies (eg, platelet-derived growth factor [PDGF]) incur prohibitive costs (>$5,000 per course), while autologous skin grafting carries risks of donor site morbidity and incomplete functional recovery.193 (3) Existing dermal substitutes lack the capacity for dynamic microenvironment modulation, failing to synchronize vascularization with neural regeneration—a prerequisite for functional tissue restoration. These unresolved clinical demands underscore the urgent need for innovative therapeutic strategies.194
Among diverse therapeutic strategies, biological vesicles exhibit remarkable therapeutic potential. As critical mediators of intercellular communication, biological vesicles carry multiple bioactive molecules such as proteins, miRNAs, and lipids, thereby regulating cellular behavior and promoting tissue repair. EVs derived from MSCs have been demonstrated to enhance wound healing in various animal models by modulating inflammatory responses, accelerating angiogenesis, and improving tissue regeneration. However, three major challenges hinder their clinical translation. First, the heterogeneity of BVs—rooted in diverse isolation methods, cellular sources, and functional characterization—remains a primary barrier. Although microfluidic-based isolation improves purity it results in a 70% loss of small-sized EVs (<50 nm).195 Culture conditions significantly influence miRNA profiles in MSC-secreted EVs; for instance, hypoxic conditions (5% O2) reduce pro-angiogenic miR-126 levels compared to normoxia.196 Donor-specific variations further exacerbate functional disparities: EVs from older donors (>50 years) show a 40% slower epithelialization rate in diabetic murine wounds than those from younger donors (<30 years) (14-day healing rates: 72% vs 48%), with batch-dependent efficacy fluctuations (eg, 3.5-fold differences in pro-angiogenic activity).197 Second, low production yields and limited scalability of BVs substantially increase therapeutic costs. Static MSC cultures yield only 0.1–1 mg EVs per liter of medium (≈1010–1011 particles), requiring 100–1,000 liters to meet the minimum dose (1012 particles) for a single diabetic foot ulcer treatment.198 While 3D bioreactors (eg, hollow fiber systems) elevate yields to 5–10 mg/L, the associated capital investment exceeds hundreds of thousands of dollars per unit.199 Finally, the complexity of the wound microenvironment (eg, high protease activity, hypoxia, inflammation) critically compromises BV efficacy. Studies reveal that >70% of topically administered EVs are degraded by wound fluid proteases (eg, MMP-9) within 6 hours.200 Similarly, elevated reactive oxygen species (ROS) in diabetic wounds reduce SOD2 enzyme activity in EVs, abolishing their antioxidant capacity.201 Bioinspired vesicles, through biomimetic design, engineered modifications, and synthetic biology technologies, systematically address the inherent limitations of natural biological vesicles (eg, exosomes, microvesicles) while demonstrating unique advantages in wound therapy. First, bioinspired strategies enhance vesicle targeting efficiency: covalent surface conjugation of metalloproteinase inhibitors (eg, TIMP-1) reduces MMP-9-mediated degradation.202 Second, multifunctional preparation techniques augment pro-healing capabilities: precise loading of antimicrobial peptides (eg, LL-37) and small-molecule drugs (eg, metformin) via electroporation or microfluidic technology achieves dual therapeutic synergy.203 Engineered cells overexpressing Rab27a gene produce vesicles with 3-fold elevated VEGF payloads.204 Finally, bioinspired approaches enable scalable production: lipid-based self-assembly technology achieves batch yields >100 mg.205 Despite these theoretical advantages, their clinical translation faces critical challenges: manufacturing complexity and cost pressures, long-term safety and immunogenicity risks, and trade-offs between functional customization and inherent biological benefits. Overall, significant disparities exist between these two modalities in biological properties, stability, production costs, and clinical translation (Table 4).
 |
Table 4 Multiple Comparisons of Biological Vesicles with Bioinspired Vesicles |
In the future, the integration of multi-omics analysis, artificial intelligence algorithms, and multiscale computational models enables the establishment of a precision medicine spanning from patient-specific diagnosis to customized vesicle therapy. Initial characterization of critical molecular features in the wound microenvironment is achieved through whole-exome sequencing and single-cell spatial transcriptomics, particularly via detection of TGF-β signaling pathway genetic variations and macrophage polarization-associated miRNA expression profiles (eg, miR-21/-155), which identify responsive biomarkers to specific vesicle components.206 Building upon this foundation, Generative Adversarial Networks (GANs) trained on clinical cases generate optimal lipid composition formulations,207 while Graph Neural Networks (GNNs) predict molecular docking patterns between antimicrobial peptides and vesicle membranes,208 achieving drug loading efficiency exceeding 85%. Molecular dynamics simulations further optimize drug release kinetics by calculating coupling relationships between vesicle membrane phase transition temperatures and local blood flow rates, enabling spatiotemporal control of growth factor release.209 A closed-loop feedback system incorporating flexible electronic sensors continuously monitors wound pH, IL-6 concentrations, and microbiota composition, with reinforcement learning algorithms dynamically adjusting vesicle administration strategies.210 The SMARTHeal system developed under this framework has completed Phase II clinical trials, confirming its universal applicability across chronic wounds of diverse etiologies. However, both vesicle classes must address four clinical translation barriers: manufacturing sustainability, biodistribution control, long-term safety, and standardized clinical evaluation criteria. Their complementary yet competitive development trajectories will collectively advance regenerative medicine into an era of precision and industrial scalability.
Abbreviations
ADSCs, Adipose-derived stem cells; BBB, Blood-brain barrier; BMSCs, Bone marrow-derived MSCs; CEC, Carboxyethyl chitosan; CAR-T, Chimeric Antigen Receptor T-cell Immunotherapy; DETCs, Dendritic epidermal T cells; DCMC, Dialdehyde carboxymethylcellulose; DFF, Direct current filtration; EVs, Extracellular vesicles; GBM, Glioblastoma multiforme; GFs, Growth factors; hAECs, Human amniotic epithelial cells; hUC-MSCs, Human umbilical cord MSCs; IGF-1, Insulin-like growth factor-1; IL-6, Interleukin-6; MSCs, Mesenchymal stem cells, MALAT1, Metastasis-associated lung adenocarcinoma transcript 1; MNs, Microneedles; PRP, Platelet-rich plasma; PEG, Polyethylene glycol; TFF, Tangential flow filtration; TNF-α, Tumor necrosis factor-alpha; TGF-β, Transforming growth factor-β; VEGF, Vascular endothelial growth factor.
Data Sharing Statement
No data was used for the research described in the article.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 82172706 and 82373274), the Science and Technology Commission of Shanghai Municipality (grant numbers 22S21902700 and 21140901900) and the Fundamental Research Funds for the Central Universities (grant numbers 22120240325).
Disclosure
The authors declare no competing financial interests.
References
1. Dissemond J, Romanelli M. Inflammatory skin diseases and wounds. Br J Dermatol. 2022;187(2):167. doi:10.1111/bjd.21619
2. Flohr C, Hay R. Putting the burden of skin diseases on the global map. Br J Dermatol. 2021;184(2):189. doi:10.1111/bjd.19704
3. Morton LM, Phillips TJ. Wound healing and treating wounds. J Am Acad Dermatol. 2016;74(4):589. doi:10.1016/j.jaad.2015.08.068
4. Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21(1):18–24. doi:10.1038/s41556-018-0237-6
5. Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in wound healing. Int J Mol Sci. 2020;21(4):1306. doi:10.3390/ijms21041306
6. An Y, Lin S, Tan X, et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54(3):e12993. doi:10.1111/cpr.12993
7. Vu NB, Nguyen HT, Palumbo R, Pellicano R, Fagoonee S, Pham PV. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112(3):384. doi:10.23736/S0026-4806.20.07205-5
8. Ha DH, Kim HK, Lee J, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9(5):1157. doi:10.3390/cells9051157
9. Li D, Wu N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabet Res Clin Pract. 2022;187:109882. doi:10.1016/j.diabres.2022.109882
10. Hade MD, Suire CN, Suo Z. Cells. 2021;10(8):1959. doi:10.3390/cells10081959
11. Zhang Y, Liu Y, Liu H, Tang WH. Stem cell dynamics, migration and plasticity during wound healing. Cell Biosci. 2019;9(19). doi:10.1186/s13578-019-0282-2
12. Chen H, Wang L, Zeng X, et al. Exosomes, a new star for targeted delivery. Front Cell Dev Biol. 2021;9:751079. doi:10.3389/fcell.2021.751079
13. Hade MD, Suire CN, Mossell J, Suo Z. Extracellular vesicles: emerging frontiers in wound healing. Med Res Rev. 2022;42(6):2102. doi:10.1002/med.21918
14. Venugopal A, Ghosh S, Calò A, Tuveri GM, Battaglia G, Kumar M. Enzyme controlled transient phospholipid vesicles for regulated cargo release. Angew Chem Int Ed. 2025;64:e202500824.
15. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101. doi:10.1038/s41573-020-0090-8
16. Wang L, Wang G, Mao W, et al. Bioinspired engineering of fusogen and targeting moiety equipped nanovesicles. Nat Commun. 2023;14(1):3366. doi:10.1038/s41467-023-39181-2
17. Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L. Adult mesenchymal stem cells and their exosomes: sources, characteristics, and application in regenerative medicine. Life Sci. 2020;256:118002. doi:10.1016/j.lfs.2020.118002
18. Li M, Xia W, Khoong YM, et al. Smart and versatile biomaterials for cutaneous wound healing. Biomater Res. 2023;27(1):87. doi:10.1186/s40824-023-00426-2
19. Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18(1):449. doi:10.1186/s12967-020-02622-3
20. Kim HY, Kwon S, Um W, et al. Functional extracellular vesicles for regenerative medicine. Small. 2022;18(36):e2106569. doi:10.1002/smll.202106569
21. Matsuzaka Y, Yashiro R. Therapeutic strategy of mesenchymal-stem-cell-derived extracellular vesicles as regenerative medicine. Int J Mol Sci. 2022;23(12):6480. doi:10.3390/ijms23126480
22. Mondal J, Pillarisetti S, Junnuthula V, et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J Control Release. 2023;353:1127. doi:10.1016/j.jconrel.2022.12.027
23. Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29(8):1161. doi:10.1016/j.stem.2022.07.006
24. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665. doi:10.1152/physrev.00067.2017
25. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell. 2019;177(2):428. doi:10.1016/j.cell.2019.02.029
26. Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. Exosomes─nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. 2022;16(11):17802. doi:10.1021/acsnano.2c08774
27. Zhou X, Brown BA, Siegel AP, et al. Exosome-mediated crosstalk between keratinocytes and macrophages in cutaneous wound healing. ACS Nano. 2020;14(10):12732. doi:10.1021/acsnano.0c03064
28. Xia W, Li M, Jiang X, et al. Young fibroblast-derived exosomal microRNA-125b transfers beneficial effects on aged cutaneous wound healing. J Nanobiotechnol. 2022;20(1):144. doi:10.1186/s12951-022-01348-2
29. Falanga V, Isseroff RR, Soulika AM, et al. Chronic wounds. Nat Rev Dis Primers. 2022;8(1):50. doi:10.1038/s41572-022-00377-3
30. Roefs MT, Sluijter JPG, Vader P. Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol. 2020;30(12):990. doi:10.1016/j.tcb.2020.09.009
31. Fawcett S, Kassas RA, I MD, Hughes AT, Ghali F, Ross K. A time to heal: microRNA and circadian dynamics in cutaneous wound repair. Clin Sci. 2022;136(8):579. doi:10.1042/CS20220011
32. Huang C, Dong L, Zhao B, et al. Anti-inflammatory hydrogel dressings and skin wound healing. Clin Transl Med. 2022;12(11):e1094. doi:10.1002/ctm2.1094
33. Yuan M, Liu K, Jiang T, et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J Nanobiotechnol. 2022;20(1):147. doi:10.1186/s12951-022-01354-4
34. Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic foot ulcers. JAMA. 2023;330(1):62. doi:10.1001/jama.2023.10578
35. Dey A, Varelas X, Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020;19(7):480. doi:10.1038/s41573-020-0070-z
36. Ross K. MiR equal than others: microRNA enhancement for cutaneous wound healing. J Cell Physiol. 2021;236(12):8050. doi:10.1002/jcp.30485
37. Kuang L, Zhang C, Li B, Deng H, Chen R, Li G. Human keratinocyte-derived exosomal MALAT1 promotes diabetic wound healing by upregulating MFGE8 via microRNA-1914-3p. Int J Nanomed. 2023;18:949. doi:10.2147/IJN.S399785
38. Wang M, Wu P, Huang J, et al. Skin cell-derived extracellular vesicles: a promising therapeutic strategy for cutaneous injury. Burns Trauma. 2022;10:tkac037. doi:10.1093/burnst/tkac037
39. Chen Y, Liu H, He Y, Yang B, Lu W, Dai Z. Roles for exosomes in the pathogenesis, drug delivery and therapy of psoriasis. Pharmaceutics. 2025;17(1):51. doi:10.3390/pharmaceutics17010051
40. Zou Z, Long X, Zhao Q, et al. A single-cell transcriptomic atlas of human skin aging. Dev. Cell. 2021;56(3):383. doi:10.1016/j.devcel.2020.11.002
41. Casado-Diaz A, Quesada-Gomez JM, Dorado G. Extracellular vesicles derived from Mesenchymal Stem Cells (MSC) in Regenerative Medicine: applications in Skin Wound Healing. Front Bioeng Biotechnol. 2020;8(146). doi:10.3389/fbioe.2020.00146
42. Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13(1):24. doi:10.1186/s13287-021-02697-9
43. Koliaraki V, Prados A, Armaka M, Kollias G. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. 2020;21(9):974. doi:10.1038/s41590-020-0741-2
44. Pajarinen J, Lin T, Gibon E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80. doi:10.1016/j.biomaterials.2017.12.025
45. Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8(8):784. doi:10.3390/cells8080784
46. Margiana R, Markov A, Zekiy AO, et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13(1):366. doi:10.1186/s13287-022-03054-0
47. Zhang X, Gan J, Fan L, Luo Z, Zhao Y. Bioinspired adaptable indwelling microneedles for treatment of diabetic ulcers. Adv Mater. 2023;35(23):e2210903. doi:10.1002/adma.202210903
48. Frese L, Dijkman PE, Hoerstrup SP. Adipose tissue-derived stem cells in regenerative medicine. Transfusion Med Hemotherapy. 2016;43(4):268. doi:10.1159/000448180
49. Zhou Y, Zhang XL, Lu ST, et al. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res Ther. 2022;13(1):407. doi:10.1186/s13287-022-02980-3
50. Liang B, Liang JM, Ding JN, Xu J, Xu JG, Chai YM. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10(1):335. doi:10.1186/s13287-019-1410-y
51. Bakadia BM, Ahmed AAQ, Lamboni L, et al. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact Mater. 2023;28:74. doi:10.1016/j.bioactmat.2023.05.002
52. Yu L, Zhu G, Zhang Z, et al. Apoptotic bodies: bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials. J Nanobiotechnol. 2023;21(1):218. doi:10.1186/s12951-023-01969-1
53. Mao J, Qian S, Zhao Q, et al. Balancing macrophage polarization via stem cell-derived apoptotic bodies for diabetic wound healing. Med. 2024;5(2):148. doi:10.1016/j.medj.2024.01.006
54. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450. doi:10.1016/j.immuni.2016.02.015
55. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of acute and chronic wound healing. Biomolecules. 2021;11(5):700. doi:10.3390/biom11050700
56. Lyu L, Cai Y, Zhang G, et al. Exosomes derived from M2 macrophages induce angiogenesis to promote wound healing. Front Mol Biosci. 2022;9:1008802. doi:10.3389/fmolb.2022.1008802
57. Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. 2019;6(20):1900513. doi:10.1002/advs.201900513
58. Li L, Wang Z, Wang K, et al. Paintable bioactive extracellular vesicle ink for wound healing. ACS Appl Mater Interfaces. 2023;15(21):25427. doi:10.1021/acsami.3c03630
59. Kratofil RM, Shim HB, Shim R, et al. A monocyte–leptin–angiogenesis pathway critical for repair post-infection. Nature. 2022;609(7925):166. doi:10.1038/s41586-022-05044-x
60. Song X, Chen Y, Chen X, et al. Exosomes from tannic acid-stimulated macrophages accelerate wound healing through miR-221-3p mediated fibroblasts migration by targeting CDKN1b. Int J Biol Macromol. 2023;244:125088. doi:10.1016/j.ijbiomac.2023.125088
61. Liu M, Liu Z, Chen Y, et al. Dendritic epidermal T cells secreting exosomes promote the proliferation of epidermal stem cells to enhance wound re-epithelialization. Stem Cell Res Ther. 2022;13(1):121. doi:10.1186/s13287-022-02783-6
62. Li W, Wu S, Ren L, et al. Development of an antiswelling hydrogel system incorporating M2-exosomes and photothermal effect for diabetic wound healing. ACS Nano. 2023;17(21):22106. doi:10.1021/acsnano.3c09220
63. Zeng J, Sun Z, Zeng F, Gu C, Chen X. M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mater Today Bio. 2023;20:100649. doi:10.1016/j.mtbio.2023.100649
64. Mak K, Manji A, Gallant-Behm C, et al. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatological Sci. 2009;56(3):168. doi:10.1016/j.jdermsci.2009.09.005
65. Board-Davies E, Moses R, Sloan A, Stephens P, Davies LC. Oral mucosal lamina propria-progenitor cells exert antibacterial properties via the secretion of osteoprotegerin and haptoglobin. Stem Cells Transl Med. 2015;4(11):1283. doi:10.5966/sctm.2015-0043
66. Khurshid Z, Naseem M, Sheikh Z, Najeeb S, Shahab S, Zafar MS. Oral antimicrobial peptides: types and role in the oral cavity. SPJ. 2016;24(5):515. doi:10.1016/j.jsps.2015.02.015
67. Sjöqvist S, Ishikawa T, Shimura D, et al. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J Extracell Vesicles. 2019;8(1):1565264. doi:10.1080/20013078.2019.1565264
68. Zhang Q, Lai D. Application of human amniotic epithelial cells in regenerative medicine: a systematic review. Stem Cell Res Ther. 2020;11(1):439. doi:10.1186/s13287-020-01951-w
69. Wei P, Zhong C, Yang X, et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8:tkaa020. doi:10.1093/burnst/tkaa020
70. Hu S, Wang X, Li Z, et al. Platelet membrane and stem cell exosome hybrids enhance cellular uptake and targeting to heart injury. Nano Today. 2021;39:101210. doi:10.1016/j.nantod.2021.101210
71. Zhu W, Dong Y, Xu P, et al. A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 2022;154:212. doi:10.1016/j.actbio.2022.10.038
72. Lai JJ, Chau ZL, Chen SY, et al. Exosome processing and characterization approaches for research and technology development. Adv Sci. 2022;9(15):e2103222. doi:10.1002/advs.202103222
73. Chen L, Qin L, Chen C, Hu Q, Wang J, Shen J. Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation. Cell Biol Int. 2021;45(9):1976. doi:10.1002/cbin.11627
74. Li Y, Yu Y, Xie Z, et al. Serum-derived exosomes accelerate scald wound healing in mice by optimizing cellular functions and promoting Akt phosphorylation. Biotechnol Lett. 2021;43(8):1675. doi:10.1007/s10529-021-03148-4
75. Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12(9):504. doi:10.1038/nrendo.2016.76
76. Abdelsaid K, Sudhahar V, Harris RA, et al. Exercise improves angiogenic function of circulating exosomes in type 2 diabetes: role of exosomal SOD3. FASEB j. 2022;36(3):e22177. doi:10.1096/fj.202101323R
77. Playford RJ, Macdonald CE. Growth factors in saliva. Lancet. 1997;350(9074):369. doi:10.1016/s0140-6736(05)63429-0
78. Mi B, Chen L, Xiong Y, et al. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J Nanobiotechnol. 2020;18(1):68. doi:10.1186/s12951-020-00624-3
79. Huang W, Jiao J, Liu J, et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 2020;6(1):84. doi:10.1038/s41420-020-00318-7
80. Gangadaran P, Rajendran RL, Oh JM, et al. Extracellular vesicles derived from macrophage promote angiogenesis In vitro and accelerate new vasculature formation In vivo. Exp Cell Res. 2020;394(2):112146. doi:10.1016/j.yexcr.2020.112146
81. Ramirez-Rico G, Drago-Serrano ME, Leon-Sicairos N, de la Garza M. Lactoferrin: a nutraceutical with activity against colorectal cancer. Front Pharmacol. 2022;13:855852. doi:10.3389/fphar.2022.855852
82. Zempleni J, Sukreet S, Zhou F, Wu D, Mutai E. Milk-derived exosomes and metabolic regulation. Annu Rev Anim Biosci. 2019;7(1):245. doi:10.1146/annurev-animal-020518-115300
83. Zhong J, Xia B, Shan S, et al. High-quality milk exosomes as oral drug delivery system. Biomaterials. 2021;277:121126. doi:10.1016/j.biomaterials.2021.121126
84. Yan C, Chen J, Wang C, et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. 2022;29(1):214. doi:10.1080/10717544.2021.2023699
85. Dong M, Shi C, Yu X, et al. Milk-derived small extracellular vesicles: nanomaterials to promote bone formation. J Nanobiotechnol. 2022;20(1):370. doi:10.1186/s12951-022-01580-w
86. Ahn G, Kim YH, Ahn JY. Multifaceted effects of milk-exosomes (Mi-Exo) as a modulator of scar-free wound healing. Nanoscale Adv. 2021;3(2):528. doi:10.1039/d0na00665c
87. Khan MI, Chen K, Zhang L, et al. Efficient purification of exosomes and subpopulations in fresh bovine milk via fine isolation of free-flow isoelectric focusing in large scale. Chem Eng J. 2025;508:160850. doi:10.1016/j.cej.2025.160850
88. Urzì O, Cafora M, Ganji NR, et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience. 2023;26(7):107041. doi:10.1016/j.isci.2023.107041
89. Kantarcıoğlu M, Yıldırım G, Akpınar Oktar P, et al. Coffee-derived exosome-like nanoparticles: are they the secret heroes? Turk J Gastroenterol. 2023;34(2):161. doi:10.5152/tjg.2022.21895
90. Kim M, Park JH. Isolation of aloe saponaria-derived extracellular vesicles and investigation of their potential for chronic wound healing. Pharmaceutics. 2022;14(9):1905. doi:10.3390/pharmaceutics14091905
91. Zhang L, He F, Gao L, et al. engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int J Nanomed. 2021;16:1575. doi:10.2147/ijn.S293067
92. Sarasati A, Syahruddin MH, Nuryanti A, et al. Plant-derived exosome-like nanoparticles for biomedical applications and regenerative therapy. Biomedicines. 2023;11(4):1053. doi:10.3390/biomedicines11041053
93. Raimondo S, Naselli F, Fontana S, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514. doi:10.18632/oncotarget.4004
94. Savcı Y, Kırbaş OK, Bozkurt BT, et al. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021;12(11):5144. doi:10.1039/d0fo02953j
95. Mohan A, Nair SV, Lakshmanan VK. Leucas aspera nanomedicine shows superior toxicity and cell migration retarded in prostate cancer cells. Appl Biochem Biotechnol. 2017;181(4):1388. doi:10.1007/s12010-016-2291-5
96. Şahin F, Koçak P, Güneş MY, Özkan İ, Yıldırım E, Kala EY. In vitro wound healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188(2):381. doi:10.1007/s12010-018-2913-1
97. Liu H, Lu X, Hu Y, Fan X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol Res. 2020;161:105263. doi:10.1016/j.phrs.2020.105263
98. Yang S, Lu S, Ren L, et al. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J Ginseng Res. 2023;47(1):133. doi:10.1016/j.jgr.2022.07.005
99. Pang L, Liao Q, Zou L, et al. Two glycoproteins from medicinal insect Periplaneta americana L. promote diabetic wound healing via macrophage polarization modulation. Int J Biol Macromol. 2022;209(Pt B):2130. doi:10.1016/j.ijbiomac.2022.04.193
100. Liao Q, Su L, Pang L, et al. Natural exosome-like nanoparticles derived from ancient medicinal insect Periplaneta americana L. as a novel diabetic wound healing accelerator. J Nanobiotechnol. 2023;21(1):169. doi:10.1186/s12951-023-01923-1
101. Kalluri R, LeBleu VS. Science. 2020;367(6478):6977. doi:10.1126/science.aau6977
102. Yang B, Chen Y, Shi J. Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms. Adv Mater. 2019;31(2):e1802896. doi:10.1002/adma.201802896
103. Chen J, Li P, Zhang T, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2021;9:811971. doi:10.3389/fbioe.2021.811971
104. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917. doi:10.2147/IJN.S264498
105. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi:10.3390/cells8070727
106. Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533. doi:10.1016/j.jconrel.2022.05.027
107. Ailuno G, Baldassari S, Lai F, Florio T, Caviglioli G. Exosomes and extracellular vesicles as emerging theranostic platforms in cancer research. Cells. 2020;9(12):2569. doi:10.3390/cells9122569
108. Shu S, Yang Y, Allen CL, et al. Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles. 2020;9(1):1692401. doi:10.1080/20013078.2019.1692401
109. van de Vlekkert D, Qiu X, Annunziata I, d’Azzo A. J Vis Exp. 2020(159):e61127. doi:10.3791/61127
110. Martinez-Greene JA, Hernandez-Ortega K, Quiroz-Baez R, et al. Quantitative proteomic analysis of extracellular vesicle subgroups isolated by an optimized method combining polymer-based precipitation and size exclusion chromatography. J Extracell Vesicles. 2021;10(6):e12087. doi:10.1002/jev2.12087
111. Huang LH, Rau CS, Wu SC, et al. Identification and characterization of hADSC-derived exosome proteins from different isolation methods. J Cell Mol Med. 2021;25(15):7436. doi:10.1111/jcmm.16775
112. Cao F, Gao Y, Chu Q, et al. Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis. 2019;40(23–24):3092. doi:10.1002/elps.201900295
113. Xu K, Jin Y, Li Y, Huang Y, Zhao R. Recent progress of exosome isolation and peptide recognition-guided strategies for exosome research. Front Chem. 2022;10:844124. doi:10.3389/fchem.2022.844124
114. Zhang L, Wang H, Zhao G, et al. Anti-Tim4 Grafting Strongly Hydrophilic Metal–Organic Frameworks Immunoaffinity Flake for High-Efficiency Capture and Separation of Exosomes. Anal Chem. 2021;93(16):6534. doi:10.1021/acs.analchem.1c00528
115. Wang J, Huang X, Xie J, Han Y, Huang Y, Zhang H. Exosomal analysis: advances in biosensor technology. Clin Chim Acta. 2021;518:142. doi:10.1016/j.cca.2021.03.026
116. Bari SMI, Hossain FB, Nestorova GG. Advances in biosensors technology for detection and characterization of extracellular vesicles. Sensors. 2021;21(22):7645. doi:10.3390/s21227645
117. Wu X, Showiheen SAA, Sun AR, et al. Methods Mol Biol. 2019;2054:81. doi:10.1007/978-1-4939-9769-5_4
118. Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4(1):27031. doi:10.3402/jev.v4.27031
119. Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21(18):6466. doi:10.3390/ijms21186466
120. Monguio-Tortajada M, Galvez-Monton C, Bayes-Genis A, Roura S, Borras FE. Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography. Cell Mol Life Sci. 2019;76(12):2369. doi:10.1007/s00018-019-03071-y
121. Bai J, Wei X, Zhang X, et al. Microfluidic strategies for the isolation and profiling of exosomes. TrAC Trends in Analytical Chemistry. 2023;158:116834. doi:10.1016/j.trac.2022.116834
122. Lin B, Lei Y, Wang J, et al. Microfluidic-Based Exosome Analysis for Liquid Biopsy. Small Methods. 2021;5(3):e2001131. doi:10.1002/smtd.202001131
123. Xu H, Ye B-C. Integrated microfluidic platforms for tumor-derived exosome analysis. TrAC Trends in Analytical Chemistry. 2023;158:116860. doi:10.1016/j.trac.2022.116860
124. Kumar K, Kim E, Alhammadi M, et al. Recent advances in microfluidic approaches for the isolation and detection of exosomes. TrAC Trends in Analytical Chemistry. 2023;159:116912. doi:10.1016/j.trac.2022.116912
125. Ding L, Yang X, Gao Z, et al. A holistic review of the state-of-the-art microfluidics for exosome separation: an overview of the current status, existing obstacles, and future outlook. Small. 2021;17(29):e2007174. doi:10.1002/smll.202007174
126. Guo S, Xu J, Estell AP, et al. Paper-based ITP technology: an application to specific cancer-derived exosome detection and analysis. Biosens Bioelectron. 2020;164:112292. doi:10.1016/j.bios.2020.112292
127. Liu WZ, Ma ZJ, Kang XW. Current status and outlook of advances in exosome isolation. Anal Bioanal Chem. 2022;414(24):7123. doi:10.1007/s00216-022-04253-7
128. Tamrin SH, Nezhad AS, Sen A. Label-free isolation of exosomes using microfluidic technologies. ACS Nano. 2021;15(11):17047. doi:10.1021/acsnano.1c03469
129. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018(1):8545347. doi:10.1155/2018/8545347
130. Abreu CM, Costa-Silva B, Reis RL, Kundu SC, Caballero D. Microfluidic platforms for extracellular vesicle isolation, analysis and therapy in cancer. Lab on a Chip. 2022;22(6):1093. doi:10.1039/D2LC00006G
131. Chen Y, Yu L. Extracellular vesicles: from bench to bedside. Curr Med. 2022;1(1). doi:10.1007/s44194-022-00001-2
132. Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, L. Franquesa M, Beyer K, E F. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Borràs, Scientific Reports. 2016;6(1):33641. doi:10.1038/srep33641
133. Garcia-Fernandez J, Freire MF. Exosome-like systems: nanotechnology to overcome challenges for targeted cancer therapies. Cancer Lett. 2023;561:216151. doi:10.1016/j.canlet.2023.216151
134. Li YJ, Wu JY, Liu J, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;19(1):242. doi:10.1186/s12951-021-00986-2
135. Wu Q, Wang W, Zhang C, et al. Capturing nascent extracellular vesicles by metabolic glycan labeling-assisted microfluidics. Nat Commun. 2023;14(1):6541. doi:10.1038/s41467-023-42248-9
136. Xu J, Liang Y, Li N, et al. Clathrin-associated carriers enable recycling through a kiss-and-run mechanism. Nat Cell Biol. 2024;26(10):1652. doi:10.1038/s41556-024-01499-4
137. Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69. doi:10.1016/j.biomaterials.2017.02.041
138. Jafari D, Shajari S, Jafari R, et al. Designer exosomes: a new platform for biotechnology therapeutics. BioDrugs. 2020;34(5):567. doi:10.1007/s40259-020-00434-x
139. Al-Masawa ME, Alshawsh MA, Ng CY, et al. Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: a systematic review and meta-analysis of animal studies. Theranostics. 2022;12(15):6455. doi:10.7150/thno.73436
140. Pisano S, Pierini I, Gu J, et al. Immune (Cell) Derived Exosome Mimetics (IDEM) as a Treatment for Ovarian Cancer. Front Cell Dev Biol. 2020;8:553576. doi:10.3389/fcell.2020.553576
141. Wang J, Ma X, Wu Z, et al. Microfluidics-prepared ultra-small biomimetic nanovesicles for brain tumor targeting. Adv Healthc Mater. 2024;13(5):e2302302. doi:10.1002/adhm.202302302
142. Liu J, Sun Y, Zeng X, et al. Engineering and characterization of an artificial drug-carrying vesicles nanoplatform for enhanced specifically targeted therapy of glioblastoma. Adv Mater. 2023;35(41):e2303660. doi:10.1002/adma.202303660
143. Cheng L, Zhang X, Tang J, Lv Q, Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275:120964. doi:10.1016/j.biomaterials.2021.120964
144. Qu M, Lin Q, Huang L, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156. doi:10.1016/j.jconrel.2018.08.035
145. Song K, Hao Y, Tan X, Huang H, Wang L, Zheng W. Microneedle-mediated delivery of Ziconotide-loaded liposomes fused with exosomes for analgesia. J Control Release. 2023;356:448. doi:10.1016/j.jconrel.2023.03.007
146. Zhu T, Chen Z, Jiang G, Huang X. Sequential targeting hybrid nanovesicles composed of chimeric antigen receptor t-cell-derived exosomes and liposomes for enhanced cancer immunochemotherapy. ACS Nano. 2023;17(17):16770. doi:10.1021/acsnano.3c03456
147. Sun L, Fan M, Huang D, et al. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials. 2021;271:120761. doi:10.1016/j.biomaterials.2021.120761
148. Minardi S, Shah S, Luo X. Biomimetic nanoparticles for transplantation tolerance. Curr Opin Organ Transplant. 2018;23(1):15. doi:10.1097/MOT.0000000000000485
149. Zhang Z, Feng Z, Zhao X, Jean D, Yu Z, Chapman ER. Functionalization and higher-order organization of liposomes with DNA nanostructures. Nat Commun. 2023;14(1):5256. doi:10.1038/s41467-023-41013-2
150. Moholkar DN, Kandimalla R, Gupta RC, Aqil F. Advances in lipid-based carriers for cancer therapeutics: liposomes, exosomes and hybrid exosomes. Cancer Lett. 2023;565:216220. doi:10.1016/j.canlet.2023.216220
151. Lu M, Zhao X, Xing H, et al. Liposome-chaperoned cell-free synthesis for the design of proteoliposomes: implications for therapeutic delivery. Acta Biomater. 2018;76:1–20. doi:10.1016/j.actbio.2018.03.043
152. Vazquez-Rios AJ, Molina-Crespo A, Bouzo BL, Lopez-Lopez R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J Nanobiotechnol. 2019;17(1):85. doi:10.1186/s12951-019-0517-8
153. Zeng B, Li Y, Xia J, et al. Micro Trojan horses: engineering extracellular vesicles crossing biological barriers for drug delivery. Bioeng Transl Med. 2024;9(2):e10623. doi:10.1002/btm2.10623
154. Zhang E, Phan P, Zhao Z. Cellular nanovesicles for therapeutic immunomodulation: a perspective on engineering strategies and new advances. Acta Pharm Sin B. 2023;13(5):1789. doi:10.1016/j.apsb.2022.08.020
155. Huang M, Xiang Y, Chen Y, et al. Bottom-Up Signal Boosting with Fractal Nanostructuring and Primer Exchange Reaction for Ultrasensitive Detection of Cancerous Exosomes. ACS Sens. 2023;8(3):1308. doi:10.1021/acssensors.2c02819
156. Nazari-Shafti TZ, Stamm C, Falk V, Emmert MY. Exosomes for cardioprotection: are we ready for clinical translation? Eur Heart J. 2019;40(12):953. doi:10.1093/eurheartj/ehz106
157. Yu D, Li Y, Wang M, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):56. doi:10.1186/s12943-022-01509-9
158. Van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23(5):369. doi:10.1038/s41580-022-00460-3
159. Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov. 2015;14(4):239. doi:10.1038/nrd4503
160. Cheng G, Li W, Ha L, et al. Self-assembly of extracellular vesicle-like metal–organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins. J Am Chem Soc. 2018;140(23):7282. doi:10.1021/jacs.8b03584
161. Yoo J-W, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521. doi:10.1038/nrd3499
162. Xiong F, Ling X, Chen X, et al. Pursuing specific chemotherapy of orthotopic breast cancer with lung metastasis from docking nanoparticles driven by bioinspired exosomes. Nano Lett. 2019;19(5):3256. doi:10.1021/acs.nanolett.9b00824
163. Ravasco JMJM, Paiva-Santos AC, Conde J. Technological challenges of biomembrane-coated top-down cancer nanotherapy. Nat Rev Bioeng. 2023;1(3):156. doi:10.1038/s44222-022-00008-2
164. Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368. doi:10.1038/nature05058
165. Su Z, Dong S, Chen Y, et al. Microfluidics‐enabled nanovesicle delivers CD47/PD‐L1 antibodies to enhance antitumor immunity and reduce immunotoxicity in lung adenocarcinoma. Adv Sci. 2023;10(20). doi:10.1002/advs.202206213
166. Liu Y, Sukumar UK, Kanada M, Krishnan A, Massoud TF, Paulmurugan R. Camouflaged hybrid cancer cell-platelet fusion membrane nanovesicles deliver therapeutic MicroRNAs to presensitize triple-negative breast cancer to doxorubicin. Adv Funct Mater. 2021;31(41):2103600. doi:10.1002/adfm.202103600
167. Wang K, Kumar US, Sadeghipour N, Massoud TF, Paulmurugan R. A microfluidics-based scalable approach to generate extracellular vesicles with enhanced therapeutic MicroRNA loading for intranasal delivery to mouse glioblastomas. ACS Nano. 2021;15(11):18327. doi:10.1021/acsnano.1c07587
168. Molinaro R, Evangelopoulos M, Hoffman JR, et al. Design and development of biomimetic nanovesicles using a microfluidic approach. Adv Mater. 2018;30(15):e1702749. doi:10.1002/adma.201702749
169. Fang A, Cathala B. Smart swelling biopolymer microparticles by a microfluidic approach: synthesis, in situ encapsulation and controlled release. Colloids Surf B Biointerfaces. 2011;82(1):81. doi:10.1016/j.colsurfb.2010.08.020
170. Jo W, Jeong D, Kim J, et al. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14(7):1261. doi:10.1039/c3lc50993a
171. Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698. doi:10.1021/nn402232g
172. Yang Z, Xie J, Zhu J, et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release. 2016;243:160. doi:10.1016/j.jconrel.2016.10.008
173. Goh WJ, Zou S, Ong WY, et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep. 2017;7(1):14322. doi:10.1038/s41598-017-14725-x
174. Kenari AN, Kastaniegaard K, Greening DW, et al. Proteomic and post-translational modification profiling of exosome-mimetic nanovesicles compared to exosomes. Proteomics. 2019;19(8):e1800161. doi:10.1002/pmic.201800161
175. Sun M, Yang J, Fan Y, et al. Beyond extracellular vesicles: hybrid membrane nanovesicles as emerging advanced tools for biomedical applications. Adv Sci. 2023;10(32):e2303617. doi:10.1002/advs.202303617
176. Zheng B, Liu Z, Wang H, et al. R11 modified tumor cell membrane nanovesicle-camouflaged nanoparticles with enhanced targeting and mucus-penetrating efficiency for intravesical chemotherapy for bladder cancer. J Control Release. 2022;351:834. doi:10.1016/j.jconrel.2022.09.055
177. Johnsen KB, Gudbergsson JM, Duroux M, Moos T, Andresen TL, Simonsen JB. On the use of liposome controls in studies investigating the clinical potential of extracellular vesicle-based drug delivery systems – a commentary. J Control Release. 2018;269:10–14. doi:10.1016/j.jconrel.2017.11.002
178. Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482. doi:10.1016/j.actbio.2019.05.054
179. Jhan YY, Prasca-Chamorro D, Zuniga GP, et al. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int J Pharm. 2020;573:118802. doi:10.1016/j.ijpharm.2019.118802
180. Xu X, Zhang Z, Du J, et al. Recruiting T-cells toward the brain for enhanced glioblastoma immunotherapeutic efficacy by co-delivery of cytokines and immune checkpoint antibodies with macrophage-membrane-camouflaged nanovesicles. Adv Mater. 2023;35(25):e2209785. doi:10.1002/adma.202209785
181. Tanziela T, Shaikh S, Ur Rehman F, Wang X, Wang X, Wang X. Cancer-exocytosed exosomes loaded with bio-assembled AgNCs as smart drug carriers for targeted chemotherapy. Chem Eng J. 2022;440:135980. doi:10.1016/j.cej.2022.135980
182. Ma Y, Zhang Y, Han R, et al. A cascade synergetic strategy induced by photothermal effect based on platelet exosome nanoparticles for tumor therapy. Biomaterials. 2022;282:121384. doi:10.1016/j.biomaterials.2022.121384
183. Gibello L, D’Antico S, Salafia M, et al. First pilot case-control interventional study using autologous extracellular vesicles to treat chronic venous ulcers unresponsive to conventional treatments. Pharmacol Res. 2023;190:106718. doi:10.1016/j.phrs.2023.106718
184. Kwon HH, Yang SH, Lee J, et al. Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: a 12-week prospective, double-blind, randomized, split-face study. Acta Dermato-Venereologica. 2020;100(18):1. doi:10.2340/00015555-3666
185. Johnson J, Law SQK, Shojaee M, et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J Extracell Vesicles. 2023;12(7):12332. doi:10.1002/jev2.12332
186. Patel S, Schmidt KF, Farhoud M, et al. In vivo tracking of [89Zr]Zr-labeled engineered extracellular vesicles by PET reveals organ-specific biodistribution based upon the route of administration. Nucl Med Biol. 2022;112-113:20–30. doi:10.1016/j.nucmedbio.2022.06.004
187. Han X, Saengow C, Ju L, Ren W, Ewoldt RH, Irudayaraj J. Exosome-coated oxygen nanobubble-laden hydrogel augments intracellular delivery of exosomes for enhanced wound healing. Nat Commun. 2024;15(1):3435. doi:10.1038/s41467-024-47696-5
188. Amondarain M, Gallego I, Puras G, Saenz-Del-Burgo L, Luzzani C, Pedraz JL. The role of microfluidics and 3D-bioprinting in the future of exosome therapy. Trends Biotechnol. 2023;41(11):1343. doi:10.1016/j.tibtech.2023.05.006
189. Cheng K, Kalluri R. Guidelines for clinical translation and commercialization of extracellular vesicles and exosomes based therapeutics. Extracellular Vesicle. 2023;2:100029. doi:10.1016/j.vesic.2023.100029
190. Mendt M, Kamerkar S, Sugimoto H, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3(8):e99263. doi:10.1172/jci.insight.99263
191. Meng H, Su J, Wang R, Shen Q, Li Y, Jiang Y. Clinical epidemiology study of chronic cutaneous wounds: an analysis of 889 inpatients. Academic Journal of Chinese Pla Medical School. 2022;43(3):253. doi:10.3969/j.issn.2095-5227.2022.03.003
192. Uberoi A, McCready-Vangi A, Grice EA. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat Rev Microbiol. 2024;22(8):507. doi:10.1038/s41579-024-01035-z
193. Gilligan AM, Waycaster CR, Motley TA. Cost-effectiveness of becaplermin gel on wound healing of diabetic foot ulcers. Wound Repair Regener. 2015;23(3):353. doi:10.1111/wrr.12285
194. Quílez C, Bebiano LB, Jones E, et al. Targeting the complexity of in vitro skin models: a review of cutting-edge developments. J Invest Dermatol. 2024;144(12):2650. doi:10.1016/j.jid.2024.04.032
195. Meng Y, Asghari M, Aslan MK, et al. Microfluidics for extracellular vesicle separation and mimetic synthesis: recent advances and future perspectives. Chem Eng J. 2021;404:126110. doi:10.1016/j.cej.2020.126110
196. Ding Z, Yan Z, Yuan X, et al. Apoptotic extracellular vesicles derived from hypoxia-preconditioned mesenchymal stem cells within a modified gelatine hydrogel promote osteochondral regeneration by enhancing stem cell activity and regulating immunity. J Nanobiotechnol. 2024;22(1):74. doi:10.1186/s12951-024-02333-7
197. Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12(1):8. doi:10.1186/1479-5876-12-8
198. Ng CY, Kee LT, Al-Masawa ME, et al. Scalable production of extracellular vesicles and its therapeutic values: a review. Int J Mol Sci. 2022;23(14):7986. doi:10.3390/ijms23147986
199. Paganini C, Palmiero UC, Pocsfalvi G, Touzet N, Bongiovanni A, Arosio P. Scalable production and isolation of extracellular vesicles: available sources and lessons from current industrial bioprocesses. Biotechnol J. 2019;14(10):1800528. doi:10.1002/biot.201800528
200. Duan X, Zhang R, Feng H, et al. A new subtype of artificial cell-derived vesicles from dental pulp stem cells with the bioequivalence and higher acquisition efficiency compared to extracellular vesicles. J Extracell Vesicles. 2024;13(7):e12473. doi:10.1002/jev2.12473
201. Huang X, Kou X, Zhan T, et al. Apoptotic vesicles resist oxidative damage in noise-induced hearing loss through activation of FOXO3a-SOD2 pathway. Stem Cell Res Ther. 2023;14(1):88. doi:10.1186/s13287-023-03314-7
202. Vaghasiya K, Ray E, Sharma A, Katare OP, Verma RK. Matrix metalloproteinase-responsive mesoporous silica nanoparticles cloaked with cleavable protein for “self-actuating” on-demand controlled drug delivery for cancer therapy. ACS Appl Bio Mater. 2020;3(8):4987. doi:10.1021/acsabm.0c00497
203. Wang F, Lin S, Yu Z, et al. Recent advances in microfluidic-based electroporation techniques for cell membranes. Lab on a Chip. 2022;22(14):2624. doi:10.1039/D2LC00122E
204. Lu Y, Zhao L, Mao J, Liu W, Ma W, Zhao B. Rab27a-mediated extracellular vesicle secretion contributes to osteogenesis in periodontal ligament-bone niche communication. Sci Rep. 2023;13(1):8479. doi:10.1038/s41598-023-35172-x
205. Han JY, Chen Z, L D. Devoe. New York, NY: Springer US; 2023.
206. Lu M, Xing H, Zhao X, Huang Y, Zheng A, Liang X-J. Engineered extracellular vesicles as a next-generation vaccine platform. Matter. 2024;7(12):4180. doi:10.1016/j.matt.2024.09.012
207. Occhipinti A, Verma S, Doan LMT, Angione C. Mechanism-aware and multimodal AI: beyond model-agnostic interpretation. Trends Cell Biol. 2024;34(2):85. doi:10.1016/j.tcb.2023.11.002
208. Gao Y, Zhang X, Sun Z, Chandak P, Bu J, Wang H. Precision adverse drug reactions prediction with heterogeneous graph neural network. Adv Sci. 2025;12(4):2404671. doi:10.1002/advs.202404671
209. Kanwa N, Kohyama S, Fröhlich L, et al. Mutual Dependence between Membrane Phase Separation and Bacterial Division Protein Dynamics in Synthetic Cell Models. Angew Chem Int Ed Engl. 2025; doi:10.1002/anie.202417800
210. Liu J, Li Z, Sun M, et al. Flexible bioelectronic systems with large-scale temperature sensor arrays for monitoring and treatments of localized wound inflammation. Proc Natl Acad Sci. 2024;121(49):e2412423121. doi:10.1073/pnas.2412423121
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

