Back to Journals » Drug Design, Development and Therapy » Volume 18
Bispectral Index-Monitored Anesthesia Induction in Older Adults Undergoing Elective Surgery: Comparing Ciprofol and Propofol in a Prospective, Single-Center, Double-Blind, Randomized Controlled Study
Authors Zou H, Xi F, Fu Y, Xu J, Zhang P, Li D, Luo H
Received 20 August 2024
Accepted for publication 26 October 2024
Published 5 November 2024 Volume 2024:18 Pages 4993—5003
DOI https://doi.org/10.2147/DDDT.S484532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Georgios Panos
Haibin Zou,1 Fangfang Xi,1 Yuanyuan Fu,1 Jinhui Xu,1 Ping Zhang,1 Dongge Li,2 Heguo Luo1
1Department of Anesthesiology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, 330006, People’s Republic of China; 2The Third Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, 330006, People’s Republic of China
Correspondence: Heguo Luo, Department of Anesthesiology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel +86 13807035516, Email [email protected]
Purpose: Ciprofol, a new sedative anesthetic developed in China, offers rapid onset and recovery, reduced injection pain, and stable circulation. However, its effect on blood pressure during anesthesia induction in older adults remains unclear. To compare the effects of propofol and ciprofol on hypotension induced by general anesthesia in older adults.
Patients and Methods: This prospective, single-center, double-blind, randomized, controlled clinical study enrolled 117 older adults undergoing surgery. Patients in the ciprofol group (group C) received an intravenous injection of ciprofol (0.3 mg/kg, n=57), while the propofol group (group P) received an intravenous injection of propofol (1.5 mg/kg, n=58). The primary outcome was the incidence of hypotension (mean arterial pressure (MAP) decreased by > 30% from baseline or MAP< 65 mmHg). Secondary outcomes included induction success rate (bispectral index (BIS) value ≤ 60 and Modified Observer’s Assessment of Alertness/Sedation Scale (MOAA/S) score ≤ 1), injection pain, number of drug additions, time to BIS 60, time to eyelash reflex disappearance, blood pressure changes, incidence of hypertension, tachycardia and BIS values before and after administration.
Results: The incidence of induced hypotension was 26.3% (15/57) in group C and 48.3% (28/58) in group P (OR=0.383, 95% CI:175– 0.837, P =0.015). Group C had significantly lower injection pain incidence (5.3% vs 20.7%, OR=0.213, 95% CI: 0.057– 0.801, p=0.014). Both groups had a 100% induction success rate, with no significant difference in the number of additional doses. Post-intubation hypertension and tachycardia incidence were not significantly different. Group C showed less blood pressure decrease during induction and a deeper anesthesia level.
Conclusion: Compared to propofol, ciprofol reduces the incidence of induced hypotension in older adults and maintains more stable blood pressure during induction. Additionally, ciprofol reduces injection pain and provides a good depth of anesthesia, making it a safe and effective option for anesthesia induction in older adults.
Trial Registration ClinicalTrials.gov Identifier: ChiCTR2200066053.
Keywords: ciprofol, propofol, bispectral index, BIS, hypotension
Introduction
With an aging global population, an increasing number of older adults undergo surgical treatments. These patients often present with preoperative frailty and multiple comorbidities, increasing the risk of postoperative morbidity and mortality.1 Propofol, a widely used general anesthetic, has a major side effect of hypotension. Previous studies have shown that propofol reduces arterial pressure by decreasing systemic vascular resistance (SVR).2 A recent randomized controlled study suggested that propofol lowers patients’ blood pressure by reducing their cardiac index and mean arterial pressure (MAP), thereby causing hypotension, with cardiac index and MAP levels remaining below baseline values even after intubation.3 Severe perioperative hypotension is closely related to postoperative cardiac, brain, and renal dysfunction. Therefore, maintaining intraoperative circulatory stability is crucial for older adults. Individualized systolic blood pressure management has been shown to reduce the risk of postoperative organ dysfunction compared to standard treatment in patients undergoing high-risk abdominal surgery.4 Additionally, greater fluctuations in systolic and pulse pressures may indicate increased vulnerability.5 Thus, perioperative BP stability is particularly beneficial for older adults. Ciprofol, a new sedative anesthetic developed in China, is similar to propofol6 but offers rapid onset and recovery, reduced injection pain, and stable circulation. These characteristics suggest that ciprofol could potentially replace propofol in clinical settings.6,7 A recent randomized controlled double-blind study in gynecological patients showed that 0.4 mg/kg ciprofol induction could reduce the occurrence of hypotension.8 This finding suggests potential benefits for older adults undergoing general anesthesia. Currently, ciprofol is in the early stages of market introduction, and there is limited research on its effects on induced hypotension in older adults. Recent studies on non-cardiac major surgeries in older adults have shown that ciprofol at doses of 0.2,0.3, and 0.4 mg/kg can be safely used for anesthesia induction, with no significant differences in the incidence of induced hypotension,36.7%,26.7%and23.3%, respectively. However, these studies did not compare ciprofol with propofol.9,10
This study aimed to compare the effects of ciprofol and propofol on hypotension during anesthesia induction in older adults. The primary outcome was the incidence of hypotension during general anesthesia induction. Secondary outcomes included the induction success rate, injection pain, the number of drug additions, the time for BIS to reach 60, the time for eyelash reflex disappearance, and blood pressure differences (Δ1, Δ2, Δ3) at specified time points (T0, T1, T2, T3). Additionally, the study examined the incidence of hypertension and tachycardia after intubation. The objective was to determine if ciprofol can reduce the occurrence of anesthesia-induced hypotension in older adults, provide a more stable induction period, and improve patient outcomes.
Material and Methods
Trial Design
This interventional study compared the effects of equivalent doses (ciprofol for 0.3mg/kg and propofol for 1.5mg/kg) on anesthesia-induced hypotension in older adults. We enrolled 120 patients over 60 years of age classified as American Society of Anesthesiologists (ASA) grade I–III, undergoing elective surgery under general anesthesia. The patients were randomly assigned to one of two groups using a computer-generated random number table: the ciprofol group (group C) and the propofol group (group P). This study was approved by the Ethics Committee of Jiangxi Provincial People’s Hospital on November 4, 2022 (Ethics number: Science and Technology Association [2022]11). It was registered with the Chinese Clinical Trials Registry (www.chictr.org.cn) under the registration number ChiCTR2200066053 on November 23, 2022. All participants provided written informed consent.
Participants
Inclusion criteria: Patients over 60 years old; ASA classification I–III; Fasting for at least 8 h; No fluid intake for at least 2 h; Scheduled for elective surgery. Exclusion criteria: allergy to ciprofol or propofol or soybean products; Severe heart failure (NYHA grade III–IV or LVEF<50%); Severe hypertension (grade 3, BP ≥180/110 mmHg); Severe liver dysfunction (Child-Pugh level 3 and above) or kidney dysfunction (based on KIDGO criteria); Mental and nervous system disorders (eg, Alzheimer’s disease, history of cerebral infarction, brain trauma, cerebral hemorrhage, intracranial space-occupying lesions); Long-term use of sedatives or antidepressants; Inability to communicate or cooperate; Participation in other clinical studies.
Interventions
Upon entering the operating room, a peripheral vein was established, and 5–10 mL/kg of Ringer’s solution(Zhejiang Guojing Pharmaceutical Co., Ltd.) was intravenously infused. Relevant vital signs (blood pressure, heart rate, blood oxygen saturation, and BIS) were monitored by an experienced attending anesthesiologist. Local anesthesia was administered for radial artery cannulation to monitor direct arterial pressure. Thirty minutes before anesthesia induction, intravenous midazolam 0.03 mg/kg(Jiangsu Enhua Pharmaceutical Co., Ltd.) was given, followed by intravenous sufentanil 0.3 μg/kg(Yichang Renfu Pharmaceutical Co., Ltd) administered 2 min before induction. Baseline blood pressure and heart rate were recorded 2 min before anesthesia induction. Anesthesia induction commenced with intravenous ciprofol 0.3 mg/kg (Liaoning Hesi Pharmaceutical Co., Ltd.) or propofol 1.5 mg/kg(Sichuan Guorui Pharmaceutical Co., Ltd)., administered over 30s. The time of eyelash reflex disappearance and the attainment of BIS 60 were noted upon initiation of drug titration. Eyelash reflex testing occurred every 5 s from drug initiation until reflex disappearance, recorded as 0 s if reflex vanished before drug titration.
Induction success was defined by a BIS value <60 and MOAA/S sedation score ≤1. If induction failed after 1 min, additional ciprofol 0.15 mg/kg or propofol 0.75 mg/kg was administered once. If still unsuccessful after another minute, midazolam 0.015 mg/kg was added sequentially until successful induction. After successful induction, non-depolarizing neuromuscular blocker rocuronium bromide(Emeishan Tonghui Pharmaceutical Co., Ltd.) was intravenously injected at 0.6 mg/kg. Tracheal intubation, performed by an experienced attending anesthesiologist, occurred 90s after rocuronium bromide injection, with successful intubation within 20s. Post-intubation, ventilation was set at VT 8mL/kg with minimal PEEP, and a respiratory rate of 12–16 times/min. Anesthesia maintenance utilized sevoflurane in 50% oxygen and/or other intravenous anesthetics per surgical requirements or the attending anesthesiologist preference. During anesthesia, dopamine 2 mg or phenylephrine 100 μg was administered if MAP fell below 60 mmHg. Uracil 5 mg or esmolol 10 mg was given if MAP exceeded 100 mmHg or increased >30% from baseline. Atropine 0.5 mg was administered if the heart rate dropped below 50 beats/min, while esmolol 10 mg was administered if heart rate exceeded 100 beats/min, adjusted until satisfactory results were achieved by the attending anesthesiologist.
Outcomes
The primary study outcome was the incidence of hypotension during general anesthesia induction (MAP decreased by > 30% from baseline or < 65 mmHg). Secondary outcomes included the induction success(BIS ≤60 and MOAA/S ≤1) rate, injection pain, the number of additional drug doses required, time to achieve BIS 60, and time for eyelash reflex disappearance. Differences in systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were measured as Δ1 (T0-T1), Δ2 (T2-T1), and Δ3 (T3-T2) at the following time points: T0 (immediately after injection), T1 (immediately before tracheal intubation), T2 (immediately after successful intubation), and T3 (1 min after successful intubation). Additional secondary outcomes included the incidence of hypertension (MAP increase ≥30% from baseline after intubation) and the incidence of tachycardia (heart rate > 100 beats/min) after intubation. Differences in BIS values between the two groups were also assessed before and 10 min after administration.
Sample Size and Power
There is no report on anaesthesia induction with ciprofol and propofol in older adults undergoing surgery. A pilot study was conducted to determine the occurrence of hypotension induced by ciprofol and propofol. Hypotension occurred in 33.3% (3/9) of patients in group C and 60% (6/10) in group P. With a power of 80% and a two-sided significance level of 0.05, and accounting for a 15% dropout rate, a total of 120 patients (60 per group) were required for this study.
Randomization
This study employed a double-blind, randomized controlled design. Patient allocation was achieved using a computer-generated random number table at a 1:1 ratio. The sequence number and corresponding randomization were completed by officials not involved in the study and made into a blind bottom table, and the sequence number was made into an opaque envelope containing the record form and test protocol (drugs to be used by the subjects), and the subjects were numbered in the sequence. The researcher enrolled subjects and provided informed consent on the day before surgery and the officials not involved in the study distributed envelopes to the attending anesthesiologist before anesthesia on the day of surgery. Specifically, the attending anesthesiologist opened the envelope after the patient entered the operating room, retrieved the grouping information, and administered the anesthetic agent accordingly. The attending anesthesiologist was only responsible for intraoperative anesthesia management and did not participate in the study design, data recording, or analysis. All study personnel, including data recorders, follow-up staff, outcome analysts, and patients, remained unaware of the group assignment except for the attending anesthesiologist. The group assignment results were unveiled only after the data analysis was completed.
Statistical Analysis
Categorical data are presented as frequencies (n) and percentages (%). Continuous variables are expressed as mean ± SD or median (interquartile range, IQR). For categorical variables, chi-square tests were used and expressed as percentages, odds ratios (OR), and 95% confidence intervals (CI). The Shapiro–Wilk test confirmed normal distribution for parametric data, analyzed using the two-sample T-test and expressed as mean ± SD. Non-normal distribution for parametric data are presented as median (IQR) and analyzed using the Mann–Whitney U-test. Repeated measures ANOVA with Bonferroni correction was used for multiple comparisons of normally distributed data. BIS values between groups were compared using repeated measures ANOVA with Bonferroni correction statistical tests, with interaction effects analyzed were applicable. Statistical analyses were performed using IBM SPSS Statistics 27 and GraphPad Prism 9.0, with significance set at P < 0.05.
Results
The study enrolled 120 older adults between December 2022 and February 2023, with 115 patients included in the final analysis (57 in group C, 58 in group P), after excluding those with temporary surgery suspensions or patient refusals (Figure 1).
 |
Figure 1 CONSORT flow diagram of the study. |
Demographic and Clinical Characteristics
The baseline characteristics including sex, age, height, weight, ASA classification, comorbidities, and surgical modality were similar between groups C and P (Table 1).
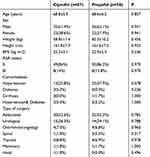 |
Table 1 Patients’ and Surgical Characteristics |
Primary Outcome: Incidence of Hypotension
The total incidence of hypotension in the two groups was 37.4% and the incidence of hypotension was significantly lower in group C (26.3%, 15/57) compared to group P (48.3%, 28/58) (OR=0.383, 95% CI: 0.175–0.837, P =0.015) (Figure 2a and Table 2).
 |
Table 2 Study Outcomes(Binary Outcome Outcomes) |
Secondary Outcomes
Group C exhibited a significantly lower incidence of injection pain during induction compared to group P (5.3% vs 20.7%, OR=0.213, 95% CI: 0.057–0.801, p=0.014) (Figure 2b). Both groups achieved a 100% success rate in anesthesia induction with no significant differences observed. Additionally, there was no significant difference in the frequency of additional drug administrations between the two groups (12.3% vs 6.9%, OR=1.890, 95% CI: 0.522–6.847, p=0.506). The incidence of hypertension (15.8% vs 8.6%) and tachycardia (19.3% vs 8.6%) after intubation was not significantly different between group C (OR=1.988, 95% CI: 0.623–6.346, p=0.240) and group P (OR=2.535, 95% CI: 0.820–7.835, p=0.098) (Figure 2c and Table 2).
Time to BIS 60 and Eyelash Reflex Disappearance
There were no statistically significant differences between groups C and P in the time for BIS to reach 60 (78 [25] s vs 79.5 [33] s, Z = −0.666, P = 0.505) or in the time to eyelash reflex disappearance (46.84 ± 14.311 s vs 45.62 ± 10.063 s, t = 0.530, p=0.597), respectively (Table 3).
 |
Table 3 Study Outcomes(Continuous Variable Outcome Outcomes) |
Hemodynamic Parameters
In the ciprofol group, Δ1SBP, Δ1DBP, Δ1MAP, and Δ3DBP were 23.89 ± 16.295 mmHg, 7.72 ± 7.178 mmHg, 13.77 ± 10.877 mmHg, and 10.21 ± 14.481 mmHg, respectively. In the propofol group, these values were 33.83 ± 19.174 mmHg, 11.66 ± 9.337 mmHg, 20.53 ± 13.219 mmHg, and 5.26 ± 9.918 mmHg, respectively. There were statistically significant differences between the two groups in Δ1SBP, Δ1DBP, Δ1MAP, and Δ3DBP (t=−2.991, p=0.003) (Figure 3a), (t=−2.537, p=0.013) (Figure 3b), (t=−2.992, p=0.003) (Figure 3c), (t=2.143, p=0.034) (Figure 3d). No statistical significance was found in Δ2SBP, Δ2DBP, Δ2MAP, Δ3SBP, and Δ3MAP between the two groups (Table 3).
BIS Values
Using Bonferroni-corrected ANOVA for repeated measures, significant differences in BIS values were observed between groups C and P at 5, 6, 7, 8, 9, and 10 minutes after induction. Simple effect analysis indicated that group C maintained significantly lower BIS values compared to group P at the 10-min mark post-administration (Table 4 and Figure 4).
 |
Table 4 Study Outcomes(Repeated Measurement Data Analysis of Variance for BIS Values) |
Discussion
Propofol is widely recognized globally as a safe and controllable hypnotic. However, its dose-dependent suppression of blood pressure and respiration, especially when combined with other sedatives and analgesics, can lead to severe hypotension. This complication may result in cardiac, cerebral, and renal dysfunction, particularly in older adults, unless treated with a vasoactive and/or an inotropic drug.11,12 Ciprofol, a novel 2, 6-disubstituted phenol derivative and gamma-aminobutyric acid type (GABAa) receptor agonist.13,14 Moreover, ciprofol exerts minimal inhibition on respiration and circulation, potentially reducing lipid exposure and mitigating injection pain.15,16 Theoretically, ciprofol could serve as a superior alternative to propofol for sedation and anesthesia induction; however, robust studies validating its use in older adults are lacking.
Therefore, we conducted a prospective, double-blind, randomized clinical trial to compare ciprofol and propofol for inducing general anesthesia in older adults under BIS monitoring. This study assessed the incidence of hypotension, injection pain, induction success rate, time to disappearance of the lash reflex, and time required for BIS to reach 60 during the induction period. Hemodynamic changes were also evaluated at fixed time points (T0, T1, T2, T3) and within 10 min post-induction to ascertain whether ciprofol offers advantages over propofol in older adults.
The efficacy of ciprofol is reportedly 4–5 times that of propofol, with the recommended adult induction dose at 0.4 mg/kg. For older adults, this dose should be reduced to 75% of the adult induction dose. Studies have demonstrated that a 0.3 mg/kg dose of ciprofol effectively induces general anesthesia in older adults, ensuring safety, efficacy, low incidence of adverse effects, and stable hemodynamics during induction.9,10 In this study, we compared 0.3 mg/kg ciprofol with 1.5 mg/kg propofol and found that ciprofol significantly reduced the incidence of induced hypotension and minimized blood pressure decreases during induction compared to propofol. Consistent with previous findings, ciprofol demonstrated stable hemodynamic profiles, beneficial for older adults. Although no statistically significant difference in post-intubation hypertension incidence was observed between the two groups, hypertension occurred more frequently in the ciprofol group. This may indicate insufficient ciprofol dosage for achieving adequate intubation depth or potential interactions with opioid doses. Future research should consider higher ciprofol doses for comparison. Therefore, we posit that ciprofol provides more stable circulation than propofol.
Both ciprofol and propofol achieved 100% success rates in inducing anesthesia among older adults, with no statistically significant difference observed. This aligns with previous studies indicating their efficacy in procedures such as gynecologic and kidney transplant surgeries.8,17
Propofol alone often induces drug-related injection pain in up to 90% of cases,11,12,18 impacting patient comfort, satisfaction, and hemodynamics. At present, in order to reduce the drug-related injection pain, it is often necessary to inject related adjuvants before injecting propofol. Some studies have shown that lidocaine is commonly used to reduce propofol-related injection pain,18,19 while other drugs include opioids and nonsteroidal anti-inflammatory drugs. Therefore, propofol-related pain is a major clinical concern. Ciprofol can be safely and effectively used for patient induction, and compared to propofol, ciprofol-related injection pain is significantly reduced.8,9,17 In this study, the incidence of ciprofol and propofol drug-related injection pain was relatively low, which may be related to midazolam and sufentanil injections; however, the drug-related injection pain was still statistically significant. Compared to propofol, ciprofold drug-related injection pain decreased significantly. The difference in the incidence of pain at the injection site in this study may be related to differences in the concentrations of ciprofol and propofol in the injection solution. The higher the concentration of propofol, the higher the incidence of injection pain.20 Ciprofol thus holds potential for enhancing comfort in older adults, especially during therapeutic procedures.
The BIS monitor represents a mature technology for assessing anesthesia depth,3,18,21 with a BIS value ≤60 indicating sufficient sedation. The combination of BIS values 40–60 and MOAA/S≤1 improved the accuracy of anesthesia induction in both groups. Notably, ciprofol induced deeper anesthesia depth than propofol, maintaining lower BIS values 10 min post-administration during anesthesia maintenance after intubation.
Limitations
This study is a single-center exploratory study with a small sample size. Future investigations should involve larger, multi-center randomized controlled trials to validate findings. Additionally, comparing different dosages of ciprofol and propofol would enhance understanding of their effects on older adults. This study solely recorded induction effects; future studies should extend to evaluating intraoperative and postoperative recovery under both drugs.
Conclusion
In this study, ciprofol demonstrated reduced induced hypotension and enhanced blood pressure stability compared to propofol during induction in older adults. Moreover, ciprofol effectively minimized injection pain, enhancing patient comfort while providing adequate anesthesia depth. Consequently, ciprofol emerges as a safe choice for anesthesia induction in older adults.
Data Sharing Statement
Data from all subjects were pooled into a single data set, which was maintained by the corresponding author. The datasets generated and/or analyzed during the current study are available from the corresponding author by the Email address [email protected].
Ethics Approval and Consent to Participate
The study was done in accordance with the Helsinki Declaration and followed the CONSORT guidelines. The study was approved by the local institutional Ethics Committee, Jiangxi Provincial People’s Hospital (Ethics number: Science and Technology Association [2022]11) and registered at Chinese Clinical Trial Registry (Registration number: ChiCTR2200066053). All subjects provided written informed consent forms.
Acknowledgments
We sincerely thank all the staff who assisted in this study and all the subjects. We would like to thank Editage (www.editage.com) for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–1782. doi:10.1016/S0140-6736(18)30668-8
2. Claeys MA, Gepts E, Camu F. Haemodynamic changes during anaesthesia induced and maintained with propofol. Br J Anaesth. 1988;60(1):3–9. doi:10.1093/bja/60.1.3
3. Möller Petrun A, Kamenik M. Bispectral index-guided induction of general anaesthesia in patients undergoing major abdominal surgery using propofol or etomidate: a double-blind, randomized, clinical trial. Br J Anaesth. 2013;110(3):388–396. doi:10.1093/bja/aes416
4. Futier E, Lefrant JY, Guinot PG, et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: a Randomized Clinical Trial. JAMA. 2017;318(14):1346–1357. doi:10.1001/jama.2017.14172
5. Rouch L, de Souto Barreto P, Hanon O, et al. Visit-to-Visit Blood Pressure Variability and Incident Frailty in Older Adults. J Gerontol A. 2021;76(8):1369–1375. doi:10.1093/gerona/glab112
6. Bian Y, Zhang H, Ma S, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. 2021;87(1):93–105. doi:10.1111/bcp.14363
7. Teng Y, Ou M, Wang X, et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci. 2021;164:105904. doi:10.1016/j.ejps.2021.105904
8. Chen BZ, Yin XY, Jiang LH, et al. The efficacy and safety of ciprofol use for the induction of general anesthesia in patients undergoing gynecological surgery: a prospective randomized controlled study. BMC Anesthesiol. 2022;22(1):245. doi:10.1186/s12871-022-01782-7
9. Ding YY, Long YQ, Yang HT, et al. Efficacy and safety of ciprofol for general anaesthesia induction in older adults undergoing major noncardiac surgery: a randomised controlled pilot trial. Eur J Anaesthesiol. 2022;39(12):960–963. doi:10.1097/EJA.0000000000001759
10. Duan G, Lan H, Shan W, et al. Clinical effect of different doses of ciprofol for induction of general anesthesia in older adults: a randomized, controlled trial. Pharmacol Res Perspect. 2023;11(2):e01066. doi:10.1002/prp2.1066
11. Wang J, Huang J, Yang S, et al. Pharmacokinetics and Safety of Esketamine in Chinese Patients Undergoing Painless Gastroscopy in Comparison with Ketamine: a Randomized, Open-Label Clinical Study. Drug Des Devel Ther. 2019;13:4135–4144. doi:10.2147/DDDT.S224553
12. ABAD-SANTOS F, GáLVEZ-MúGICA MA, Santos MA, et al. Pharmacokinetics and pharmacodynamics of a single bolus of propofol 2% in healthy volunteers. J Clin Pharmacol. 2003;43(4):397–405. doi:10.1177/0091270003251391
13. Qin L, Ren L, Wan S, et al. Design, Synthesis, and Evaluation of Novel 2,6-Disubstituted Phenol Derivatives as General Anesthetics. J Med Chem. 2017;60(9):3606–3617. doi:10.1021/acs.jmedchem.7b00254
14. Liu Y, Yu X, Zhu D, et al. Safety and efficacy of ciprofol vs. propofol for sedation in intensive care unit patients with mechanical ventilation: a multi-center, open label, randomized, Phase 2 trial. Chinese Med J. 2022;135(9):1043–1051. doi:10.1097/CM9.0000000000001912
15. Li X, Yang D, Li Q, et al. Safety, Pharmacokinetics, and Pharmacodynamics of a Single Bolus of the γ-aminobutyric Acid (GABA) Receptor Potentiator HSK3486 in Healthy Chinese older adults and Non-older adults. Front Pharmacol. 2021;12:735700. doi:10.3389/fphar.2021.735700
16. Hu C, Ou X, Teng Y, et al. Sedation Effects Produced by a Ciprofol Initial Infusion or Bolus Dose Followed by Continuous Maintenance Infusion in Healthy Subjects: a Phase 1 Trial. Adv Ther. 2021;38(11):5484–5500. doi:10.1007/s12325-021-01914-4
17. Qin K, Qin WY, Ming SP, et al. Effect of ciprofol on induction and maintenance of general anesthesia in patients undergoing kidney transplantation. Eur Rev Med Pharmacol Sci. 2022;26(14):5063–5071. doi:10.26355/eurrev_202207_29292
18. Wasinwong W, Termthong S, Plansangkate P, et al. A comparison of ondansetron and lidocaine in reducing injection pain of propofol: a randomized controlled study. BMC Anesthesiol. 2022;22(1):109. doi:10.1186/s12871-022-01650-4
19. Salman AE, Salman MA, Saricaoglu F, et al. Pain on injection of propofol: a comparison of methylene blue and lidocaine. J Clin Anesthesia. 2011;23(4):270–274. doi:10.1016/j.jclinane.2010.09.008
20. Jy S, Lee SH, Park DY, et al. Pain on injection with microemulsion propofol. Br J Clin Pharmacol. 2009;67(3):316–325. doi:10.1111/j.1365-2125.2008.03358.x
21. Chan MT, Cheng BC, Lee TM, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33–42. doi:10.1097/ANA.0b013e3182712fba
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Efficacy and Safety of Ciprofol for Sedation/Anesthesia in Patients Undergoing Hysteroscopy: A Randomized, Parallel-Group, Controlled Trial
Lan H, Shan W, Wu Y, Xu Q, Dong X, Mei P, Duan G, You M, Jin L, Wu J
Drug Design, Development and Therapy 2023, 17:1707-1717
Published Date: 11 June 2023
Ciprofol in Children Undergoing Adenoidectomy and Adenotonsillectomy: A Retrospective Cohort Study
Zeng C, Li L, Wang M, Xiong J, Pang W, Yu H, He J, Wang X, Chen Y, Sun Y
Drug Design, Development and Therapy 2024, 18:4017-4027
Published Date: 6 September 2024
Effects of Ciprofol and Propofol General Anesthesia on Postoperative Recovery Quality in Patients Undergoing Ureteroscopy: A Randomized, Controlled, Double-Blind Clinical Trial
Shi S, Wu J, Wu Y, Han X, Dai H, Chen X, Sun Z, Wang F
Drug Design, Development and Therapy 2025, 19:931-943
Published Date: 11 February 2025
Effect of High Maintenance Dose Versus Low Dose of Remifentanil on Incidence of Postoperative Nausea and Vomiting (PONV) in Patients Under Gynecological Laparoscopic Procedure: A Pilot Study
Liu ZY, Yang XY, Lv C, Ye L, Chen JJ, Chen XY, Wang SP, Wu L, Zhang WQ, Lu M, Guo XW
Drug Design, Development and Therapy 2025, 19:4885-4894
Published Date: 7 June 2025
Efficacy and Safety of Ciprofol Alone versus Ciprofol with Fentanyl for Upper Gastrointestinal Endoscopy: A Randomized, Double-Blind, Controlled Trial
Liu L, Li F, Wei Y, Luo L, Shen L, Li J, Sun N, Qian B, Sun D
Drug Design, Development and Therapy 2025, 19:5231-5241
Published Date: 18 June 2025




