Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Blood Uric Acid in Mild Cognitive Impairment: A Cross-Sectional Study of Older Chinese Adults
Authors Xue L, Xiao X, Mao X, Zhang X, Liu Y, Wu B
Received 7 February 2025
Accepted for publication 23 June 2025
Published 28 June 2025 Volume 2025:21 Pages 1307—1314
DOI https://doi.org/10.2147/NDT.S521296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yu-Ping Ning
Lingling Xue,1 Xifeng Xiao,1 Xin’e Mao,2 Xiaoli Zhang,1 Yongbing Liu,3 Beibei Wu1
1Department of Nursing, Yangzhou Maternal and Child Health Care Hospital Affiliated to Yangzhou University, Yangzhou, People’s Republic of China; 2Geriatric Unit, Northern Jiangsu People’s Hospital, Yangzhou, People’s Republic of China; 3Department of Nursing, School of Nursing and public Health, Yangzhou University, Yangzhou, People’s Republic of China
Correspondence: Beibei Wu, Yangzhou Maternal and Child Health Care Hospital Affiliated to Yangzhou University, Yangzhou, People’s Republic of China, Email [email protected]
Introduction: Previous studies focused on the association between uric acid (UA) as an antioxidant and cognitive impairment have been limited in scope and obtained contradictory results. Therefore, we investigated whether low blood UA levels were related to mild cognitive impairment (MCI) in a cross-sectional study.
Methods: This study included 231 elderly Chinese adults 60 years and over. We used the MMSE and MoCA to evaluate cognitive function, and fasting venous blood to measure UA concentration. The relationship between blood UA and cognitive impairment was analyzed with multivariate analysis of variance, controlling for demographic information, physical exercise, lifestyle and laboratory results.
Results: A total of 90 (38.96%) participants were healthy and 141 (61.04%) had MCI. Compared with the healthy group, MCI patients were more likely to have fewer years of education, inactivity and lower UA levels. UA levels were significantly lower in MCI patients than healthy individuals (P < 0.05). After adjusting for these variables, we found that among MCI patients, lower UA levels were associated with worse cognitive function in the MMSE. Multivariate logistic regression models demonstrated that UA was a protective factor for MCI. Multivariate analysis comparing the high and low quartile group, which was the reference group, indicated that differences in cognition among groups were statistically significant.
Conclusion: Lower UA levels were associated with worse cognitive function; therefore, controlling UA levels within a suitable range may slow the progression of cognitive disorders.
Keywords: uric acid, mild cognition impairment, factors, differences between groups
Introduction
Cognitive impairment is a general problem in the elderly population; approximately 15.4% of people over 55 have mild cognitive impairment in China.1 Mild cognitive impairment (MCI) was recognized as a clinical stage between natural aging and dementia.2,3 MCI is a condition defined by an objective and subjective decline in cognition that is faster than expected for the patient’s age and education. However, these patients do not meet the diagnostic criteria for dementia.4,5 Some studies have shown that MCI is more likely to switch to dementia,6–8 causing more researchers to begin to pay attention to the risks of MCI.9–12 Although MCI has a greater risk of turning into dementia, sometimes these patients recover from their cognition dysfunction.13–15 Recently, a recent study found that the heterogeneity of MCI allows risk stratification in clinical trials of the intervention and suggests gender-based early intervention for targeted treatment of patients at risk of developing AD,16 supporting that the notion that MCI at any time is a significant clinical condition. Currently, there are no definitive treatments for MCI. Therefore, it is important to carry out early interventions that are directed against the MCI risk factors.
Uric acid (UA) is an antioxidant that has important protective effects by eliminating free radicals; UA is one of the most significant natural antioxidants in people.17 At the same time, UA has many other effects, such as pro-oxidant, pro-inflammatory, immune system interactions, nitric oxide regulation, and anti-aging properties. UA can mediate the innate immune response, which can release inflammatory mediators and activate the renin-angiotensin system.18 UA is both an inflammatory mediator and an antioxidant, so it has a contradictory role in cognitive function. Thus, recent studies have investigated UA levels and cognitive dysfunction, to determine the relationship between the two. Previous studies have indicated an association, particularly in MCI, between higher UA and slower cognitive decline.19–21 However, other studies have found inconsistent results in different patient populations.22–24 These contradictory results may result from interference factors between when blood UA and cognitive function were evaluated. For example, participants with higher UA levels are more likely to have cardiovascular disease,25 which is another risk factor for MCI;26 other confounding factors can also affect UA levels, such as the use of certain medicines, eating habits and lifestyle. Different groups have different habits that can affect the final findings. Therefore, the relationship between UA and MCI needs to be explored in the different populations more nuancedly.
In this study we examined whether low UA is related to MCI in a cross-sectional study of older Chinese adults.
Methods and Materials
Study Population
This study included 141 MCI patients admitted to Northern Jiangsu People’s Hospital, Yangzhou, Jiangsu Province, China between October 2023 and October 2024. MCI was diagnosed by neurological doctors and nurses according to the diagnostic criteria set out by Peterson et al4 and China’s expert consensus on the prevention and treatment of cognitive impairment.27 Concurrently, 90 healthy controls who had independent function and normal cognition were chosen from other departments of the same hospital. Healthy controls were recruited such that their age, sex and eating habits matched the patients. They undergo routine laboratory screening and medical record review. We excluded patients who had difficulty with verbal expression, serious mental disorders, kidney disease, depression, illiteracy, drug usage that affected UA levels or refusal to sign informed consent. We recorded demographic characteristics including age, sex, education years, smoking status, alcohol intake, body mass index (BMI), hypertension and diabetes. All 231 participants underwent a face-to-face investigation with a questionnaire performed by trained nurses and graduate students.
Assessment of Cognitive Function
The Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were used to assess cognitive function. The MMSE is the most widely used tool to estimate cognitive decline; we use the version written by Folstein et al,28 the total score of which is 30 points and includes five dimensions: orientation, memory, computation, language, and visual space, with a maximum number of 19 questions. Studies have found that this test detects dementia with a sensitivity of 0.8–0.9 and specificity of 0.7–0.8, but its sensitivity is only 0.2–0.6 for detecting MCI.29 Therefore, we used the MoCA as an auxiliary tool to diagnose MCI. The MoCA also includes parts to evaluate cognition function, such as naming, language and attention.30 In addition to these overlapping sections with the MMSE, some more challenging sections are added, such as executive function, complex visuospatial processing and higher-level language.31 A recent meta-analysis demonstrated that the MoCA is better than the MMSE scale for screening MCI patients.32 The final combination of MMSE (≤27) and MOCA (<26) was used as the diagnostic criteria for MCI.4
Assessment of UA and Other Clinical Characteristics
After an 8-h fast, we collected 3-mL peripheral venous blood samples from all participants, and the samples were sent to the hospital laboratory center within 30 min. UA and other clinical characteristics were measured using an enzyme-labelled method on a Hitachi 7170 automatic biochemical analyzer (Hitachi, Tokyo, Japan). The normal range of UA concentration was 148–463 mmol/L.
Statistical Analyses
To distinguish differences between the MCI and control groups, we used t-tests and chi-square tests. We applied means, standard deviations (SDs) and frequencies to describe continuous and categorical variables. Multiple logistic regression models were used to examine all variables, which represented the connection between the variables and MCI. Some potential confounders were considered in each model: step I was controlled with demographic characteristics (age, sex and education level); step II was controlled with behavior and lifestyle (physical activity); and step III was controlled with the study variable UA. Sex was classified as male or female; education levels were categorized as primary, junior, senior, college and above, and we recorded the years of education.
To explore the relationship between UA and cognitive function (here we used the MMSE score), multivariate analyses of variance were performed using UA as the group basis. According to the quartile of UA, we divided the MCI patients into four groups and using the bottom quartile group as a reference group. Similarly, the demographic characteristics (age, sex and education level) were adjusted in model I, adding behavior and lifestyle (smoking, drinking and activity) into model II, and model III was adjusted for all variables (BMI, cognitive function and disease history). Smoking was categorized as smoker or non-smoker, and drinking was categorized as drinker or non-drinker. Physical activity and disease history (diabetes and hypertension) were described as yes or no. The other variables (age, BMI, cognitive function and UA) were continuous variables. All statistical analyses were conducted using SPSS software (v26.0; SPSS Inc., Chicago, IL, USA). P<0.05 was used to determine if differences were statistically significant.
Results
Differences Among Variables between the MCI and Control Groups
Between October 2023 and October 2024, 141 MCI patients [mean age ± SD: 69.11 ± 5.99, 73 males (51.8%)] and 90 controls [mean age ± SD: 68.08±5.78, 48 males (53.3%)] were evaluated. The comparison of baseline demographics, cognitive function and UA is summarized in Table 1. The mean years of education for MCI patients were dramatically lower than controls (P=0.011). The rates of smoking and diabetes in MCI patients were higher than the healthy subjects, but the rate of activity was lower; these differences were statistically significant (P<0.05). Both MMSE and MoCA scores of the MCI group were lower than controls; UA levels were also lower for the MCI group.
 |
Table 1 Comparison of Variables Between the MCI and NC Groups (n=231) |
Factors That Influence Cognitive Function
We predicted cognitive function among MCI patients with a binary logistic regression (Table 2). Demographic information (age, sex, education and smoking) were controlled in step I, where education and smoking was associated with cognitive function. Activity was added in step II, indicating that education, smoking and activity were related to cognitive function. UA was added in step III, which showed that education, smoking, activity and UA are linked to cognitive function.
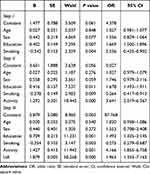 |
Table 2 Logistic Analysis of Factors That Influence MCI |
Correlation Between UA and MMSE Score
There were significant positive correlations between UA and MMSE score in all participants, including orientation, memory, computation, language and visual space five dimensions (P <0.05) (Table 3)
 |
Table 3 Correlation Between MMSE Score and UA |
Comparison of Cognitive Function According to UA Level
Lower UA levels were associated with worse cognitive function in the MMSE score among MCI patients (Table 4). After adjusting for demographic characteristics (age, sex and education level) in model I, comparisons between the high quartile group and the low quartile group, which was the reference group, indicated that the difference in cognition among groups was statistically significant (P=0.001, 0.006 and 0.018, respectively). Similar results were found in model II (adjusting for demographic characteristics, behavior and lifestyle) and model III (adjusting for all variables).
 |
Table 4 Cognitive Differences According to the Quartile of UA Among MCI Patients |
Discussion
In this cross-sectional study of older Chinese adults, we found that lower UA concentrations were associated with worse cognitive function, independent of many common factors, including education, behavior, lifestyle and disease history.
The results of previous studies regarding the relationship between UA and cognitive function have been varied. Studies have demonstrated an inverse relationship between atherosclerotic carotid artery disease and cognitive function in the elderly.33 In this case, and that cardiovascular risk factors may potentially restrain the protective effect of higher UA levels in the elderly. The present study examined the relationship between UA and cognitive function after excluding cardiovascular risk factors, but most studies consider cardiovascular risk factors as covariates. A longitudinal study reported that higher UA levels were linked with a slower cognitive decline, especially in MCI and dementia populations.19 A previous study in Mexico indicated that lower serum UA levels were related to cognitive impairment and higher UA levels were linked with a reduced risk of dementia;34 our study confirmed these results in MCI patients. Additionally, cross-sectional studies of serum UA have reported lower UA concentrations in MCI and Alzheimer's disease(AD) and Parkinson’s disease(PD) subjects compared with healthy controls,35,36 although another study found no significant difference.37 Furthermore, other studies have concluded that higher UA levels could increase the risk of cognitive impairment in the elderly.9,38 These controversial findings are in opposition to our results. Together, these divergent findings have triggered controversy on this issue.
The existing literature demonstrates a relationship between UA and cognitive impairment, although various results have been reported.9,19,33–38 The neuroprotective effects of UA are mainly related to the antioxidant effects of UA, which can alleviate oxidative stress-induced neurodegeneration in patients with cognitive impairment.39 A more recent study examined the UA-cognition relationship across a broad cognitive spectrum,40 the UA-cognition relationship showed a strong association in participators. This relationship is more pronounced in men. It has also been shown that UA has an anti-inflammatory effect at physiological concentrations. Our study explored this relationship only in MCI patients with gender distinction. Our major result was that serum UA levels played an important role in mild cognitive dysfunction, suggesting UA acts as an antioxidant in this process. This result confirms the conclusions of some studies; oxidative stress is an important factor in the progression of neurodegenerative diseases.41 Some studies have shown that high blood UA levels can cause increased white matter lesions, which due to cerebral ischemia, can lead to cognitive impairment.23,24 The reason for the different conclusions in these studies may be that different patient populations were selected. Moreover, differences in Chinese and Western diets and lifestyles may also result in different outcomes.
The prevention and treatment of Alzheimer’s disease has made significant progress in recent years, but the results are not satisfactory. One of the main reasons is that the MCI stage is often considered part of normal aging, and is thus neglected. General treatments for serious cognitive impairment are difficult, so it is necessary to carry out more predictive and early interventions for MCI. We have focused on the role of UA as an antioxidant during cognitive decline, as it is expected that changes in cognitive function can be controlled at the MCI stage by adjusting UA levels to within the appropriate range. However, the pro-oxidation and pro-inflammatory effects of elevated UA also need to be considered,18 UA levels are kept within the optimal range. Currently, we are in the initial stages of this exploration; future studies will investigate this topic at a deeper mechanistic level to clinically define the relationship between UA and MCI.
Our study had some limitations. First, we selected a small sample size with limited coverage, so whether these results can be applied to all groups will require further study. At the same time, dietary structure as an influencing factor was not discussed in this paper due to the small sample size, but in the next study, it is still necessary to increase the sample size of different regions for analysis and explore the influence of dietary structure in more detail. Second, we did not consider the dynamics of UA results. Although we applied strict controls to the effects of other factors before testing, averaging a number of tests will be more credible. Third, we did not record current medications in detail. We excluded only drug users who affected UA, and did not take other medications, such as drugs for diabetes, hypertension, etc. Therefore, we cannot rule out the impact of certain drugs on our results.
Conclusion
Our findings suggest that lower UA levels are associated with worse cognitive function. Further longitudinal studies are needed to determine whether maintaining UA levels within an optimal range could influence cognitive decline.
Abbreviations
MCI, mild cognitive impairment; BMI, body mass index; UA, uric acid; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination.
Ethical Statement and Copyright Permission
This study was performed in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Northern Jiangsu People’s Hospital. The Mini-Mental State Examination (MMSE) is copyrighted by PAR Inc. (www.parinc.com). The MMSE items referenced herein are used strictly for academic analysis under the principle of fair use, without commercial intent. Should any copyright concerns arise, the authors will cooperate fully with PAR Inc. and the publisher to resolve such matters. Any disputes related to the MMSE shall be governed by the laws of the United States, where PAR Inc. is domiciled. The responsibility for any copyright infringement rests solely with the authors, and neither the journal nor the publisher assumes liability related to the use of this instrument.
Disclosure
None of the authors have any conflicts of interest to declare, and all authors are involved in research program design, data collection and thesis writing.
References
1. Deng Y, Zhao S, Cheng G, et al. The prevalence of mild cognitive impairment among Chinese people: a meta-analysis. Neuroepidemiology. 2021;55(2):79–91. PMID: 33756479. doi:10.1159/000512597
2. Yuan L, Ye J, Wang W, et al. Research of arithmetic and drawing writing in improving communication and cognitive function in patients with mild-to-moderate dementia: a cluster randomized controlled trial. Alpha Psychiatry. 2024;25(2):262–268. PMID: 38798802; PMCID: PMC11117424. doi:10.5152/alphapsychiatry.2023.1470
3. Kim HG, Kim Y, Edmonds EC, Bondi MW. Comparison of cognitive impairment diagnosis criteria in clinical settings: conventional vs. Neuropsychological Alpha Psychiatry. 2024;25(2):212–219. PMID: 38798819; PMCID: PMC11117417. doi:10.5152/alphapsychiatry.2024.231448
4. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999. 56(3):303–308. Erratum in: Arch Neurol 1999 Jun;56(6):760. PMID: 10190820. doi:10.1001/archneur.56.3.303
5. Wang KN, Page AT, Etherton-Beer CD. Mild cognitive impairment: to diagnose or not to diagnose. Australas J Ageing. 2021;40(2):111–115. PMID: 33604998. doi:10.1111/ajag.12913
6. Guevara JE, Kurniadi NE, Duff K. Assessing longitudinal cognitive change in mild cognitive impairment using estimated standardized regression-based formulas. J Alzheimers Dis. 2023;95(2):509–521. PMID: 37545235. doi:10.3233/JAD-230160
7. Wang X, Zhou S, Ye N, et al. Predictive models of Alzheimer’s disease dementia risk in older adults with mild cognitive impairment: a systematic review and critical appraisal. BMC Geriatr. 2024;24(1):531. PMID: 38898411; PMCID: PMC11188292. doi:10.1186/s12877-024-05044-8
8. Pourzinal D, Yang JHJ, Byrne GJ, et al. Identifying subtypes of mild cognitive impairment in Parkinson’s disease using cluster analysis. J Neurol. 2020;267(11):3213–3222. PMID: 32535681. doi:10.1007/s00415-020-09977-z
9. Loessner L, Matthes C, Haussmann R, et al. Predictors of subjective cognitive deficits in patients with mild cognitive impairment. Psychogeriatrics. 2022;22(2):210–217. PMID: 34939254. doi:10.1111/psyg.12802
10. Wang F, Li D, Wang L, Zhu J, Zhao M, Lei P. Mild hypertension protects the elderly from cognitive impairment: a 7-year retrospective cohort study. Psychogeriatrics. 2020;20(4):412–418. PMID: 31972899. doi:10.1111/psyg.12519
11. Martin J, Reid N, Ward DD, King S, Hubbard RE, Gordon EH. Investigating sex differences in risk and protective factors in the progression of mild cognitive impairment to dementia: a systematic review. J Alzheimers Dis. 2024;97(1):101–119. PMID: 38143350. doi:10.3233/JAD-230700
12. Kang SH, Chung SJ, Lee J, Koh SB. Independent effect of neurogenic orthostatic hypotension on mild cognitive impairment in Parkinson’s disease. Clin Auton Res. 2022;32(1):43–50. PMID: 34841452. doi:10.1007/s10286-021-00841-2
13. Asaoka D, Xiao J, Takeda T, et al. Effect of probiotic bifidobacterium breve in improving cognitive function and preventing brain atrophy in older patients with suspected mild cognitive impairment: results of a 24-week randomized, double-blind, placebo-controlled trial. J Alzheimers Dis. 2022;88(1):75–95. PMID: 35570493; PMCID: PMC9277669. doi:10.3233/JAD-220148
14. Wang T, Yan S, Lu J. The effects of noninvasive brain stimulation on cognitive function in patients with mild cognitive impairment and Alzheimer’s disease using resting-state functional magnetic resonance imaging: a systematic review and meta-analysis. CNS Neurosci Ther. 2023;29(11):3160–3172. PMID: 37349974; PMCID: PMC10580344. doi:10.1111/cns.14314
15. Romero Reyes YY, Andrade Valbuena LP. Is it possible to prevent the progression of mild cognitive impairment through non-pharmacological treatments? Rev Colomb Psiquiatr. 2023. 52(4):372–379. PMID: 38040542. English, Spanish. doi:10.1016/j.rcpeng.2023.11.001
16. Katabathula S, Davis PB, Xu R. Alzheimer’s disease neuroimaging initiative. sex-specific heterogeneity of mild cognitive impairment identified based on multi-modal data analysis. J Alzheimers Dis. 2023;91(1):233–243. PMID: 36404544; PMCID: PMC11391386. doi:10.3233/JAD-220600
17. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi:10.1073/pnas.78.11.6858
18. Du L, Zong Y, Li H, et al. Hyperuricemia and its related diseases: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9(1):212. PMID: 39191722; PMCID: PMC11350024. doi:10.1038/s41392-024-01916-y
19. Su C, Hu D, Wang X, et al. Association between mild cognitive impairment and serum uric acid levels among people aged 55 and above in 4 provinces of China. Wei Sheng Yan Jiu. 2021. 50(1):8–14. Chinese. PMID: 33517955. doi:10.19813/j.cnki.weishengyanjiu.2021.01.003
20. Ren X, Wang P, Wu H, et al. Relationships between serum lipid, uric acid levels and mild cognitive impairment in Parkinson’s disease and multiple system atrophy. J Integr Neurosci. 2024;23(9):168. PMID: 39344238. doi:10.31083/j.jin2309168
21. Chen C, Li X, Lv Y, et al. High blood uric acid is associated with reduced risks of mild cognitive impairment among older adults in china: a 9-year prospective cohort study. Front Aging Neurosci. 2021;13:747686. PMID: 34720995; PMCID: PMC8552040. doi:10.3389/fnagi.2021.747686
22. Tong XW, Zhang YT, Li X, et al. Uric acid index is a risk for mild cognitive impairment in type 2 diabetes. Hormones. 2023;22(3):425–439. PMID: 37523135. doi:10.1007/s42000-023-00465-3
23. Shi C, Guo H, Liu X. High uric acid induced hippocampal mitochondrial dysfunction and cognitive impairment involving intramitochondrial NF-κB inhibitor α/nuclear factor-κB pathway. Neuroreport. 2022;33(3):109–115. PMID: 35139059. doi:10.1097/WNR.0000000000001762
24. Khaled Y, Abdelhamid AA, Al-Mazroey H, et al. Higher serum uric acid is associated with poorer cognitive performance in healthy middle-aged people: a cross-sectional study. Intern Emerg Med. 2023. 18(6):1701–1709. PMID: 37330420. PMCID: PMC10504193. doi:10.1007/s11739-023-03337-1
25. Baratta F, Moscucci F, Ettorre E, et al. Influence of uric acid on vascular and cognitive functions: evidence for an ambivalent relationship. Metabolites. 2024;14(11):642. PMID: 39590878; PMCID: PMC11596799. doi:10.3390/metabo14110642
26. Vázquez-de Sebastián J, Ortiz-Zuñiga AM, Ciudin A, et al. The dialcat consortium. Cognitive profile and cardiovascular risk factors in older adults with mild cognitive impairment. Int J Environ Res Public Health. 2024;21(4):500. PMID: 38673411; PMCID: PMC11050679. doi:10.3390/ijerph21040500
27. YanSheng L. China’s experts consensus on prevention and treatment of cognitive impairment. Chin J Gerontol. 2006;25(7):485–487.
28. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. PMID: 1202204. doi:10.1016/0022-3956(75)90026-6
29. Piccinin AM, Muniz-Terrera G, Clouston S, et al. Coordinated analysis of age, sex, and education effects on change in MMSE scores. J Gerontol B Psychol Sci Soc Sci. 2013;68(3):374–390. PMID: 23033357; PMCID: PMC3693608. doi:10.1093/geronb/gbs077
30. Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Alzheimer’s disease neuroimaging initiative. relationship between the Montreal cognitive assessment and mini-mental state examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15:107. PMID: 26346644; PMCID: PMC4562190. doi:10.1186/s12877-015-0103-3
31. Tiffin-Richards FE, Costa AS, Holschbach B, et al. The Montreal Cognitive Assessment (MoCA) - a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One. 2014;9(10):e106700. PMID: 25347578; PMCID: PMC4209968. doi:10.1371/journal.pone.0106700
32. Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kędziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016. 50(5):1039–1052. PMID: 27992895. English, Polish. doi:10.12740/PP/45368
33. Mastroiacovo D, Mengozzi A, Dentali F, et al. Enhanced carotid plaque echolucency is associated with reduced cognitive performance in elderly patients with atherosclerotic disease independently on metabolic profile. Metabolites. 2023;13(4):478. PMID: 37110137; PMCID: PMC10144528. doi:10.3390/metabo13040478
34. Méndez-Hernández E, Salas-Pacheco J, Ruano-Calderón L, et al. Lower uric acid linked with cognitive dysfunction in the elderly. CNS Neurol Disord Drug Targets. 2015;14(5):564–566. PMID: 25925000. doi:10.2174/1871527314666150430161659
35. Kunitskaya NA, Ariev AL. The role of hyperuricemia in the development of cognitive changes in the elderly. Adv Gerontol. 2022;35(5):775–782. PMID: 36617333. Russian.
36. Zhai RX, Yu H, Ma H, Liu TT, Zhong P. Progression of cognitive impairment in Parkinson’s disease correlates with uric acid concentration. Front Neurol. 2024;15:1378334. PMID: 38872819; PMCID: PMC11169608. doi:10.3389/fneur.2024.1378334
37. Liu Q, Peng M, Yang T, Si G. Uric acid levels and risk of cognitive impairment: dose-response meta-analysis of prospective cohort studies. PLoS One. 2023;18(11):e0293832. PMID: 37917590; PMCID: PMC10621826. doi:10.1371/journal.pone.0293832
38. Aerqin Q, Jia SS, Shen XN, et al. Serum uric acid levels in neurodegenerative disorders: a cross-sectional study. J Alzheimers Dis. 2022;90(2):761–773. PMID: 36189590. doi:10.3233/JAD-220432
39. Alrouji M, Al-Kuraishy HM, Al-Gareeb AI, et al. Role of uric acid in neurodegenerative diseases, focusing on Alzheimer and Parkinson disease: a new perspective. Neuropsychopharmacol Rep. 2024;44(3):639–649. PMID: 39075837; PMCID: PMC11544450. doi:10.1002/npr2.12445
40. Subasi B, Esnafoglu E. The relationship between uric acid levels, cognition and inflammation in a cohort of elderly subjects. J Neural Transm. 2024;131(9):1059–1065. PMID: 39026034. doi:10.1007/s00702-024-02804-z
41. Iazzolino B, Grassano M, Moglia C, et al. High serum uric acid levels are protective against cognitive impairment in amyotrophic lateral sclerosis. J Neurol. 2024;271(2):955–961. PMID: 37880536; PMCID: PMC10827906. doi:10.1007/s00415-023-12056-8
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

