Back to Journals » International Journal of Nanomedicine » Volume 20
Brain Delivery Strategies for Biomacromolecular Drugs: Intranasal Administration
Authors Wu H, Li C, Yuan H, Zhao J, Li S
Received 5 February 2025
Accepted for publication 3 May 2025
Published 22 May 2025 Volume 2025:20 Pages 6463—6487
DOI https://doi.org/10.2147/IJN.S520768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lijie Grace Zhang
Huanhuan Wu,1,2,* Chenyu Li,1,2,* Hong Yuan,3 Jingyuan Zhao,3,* Shuai Li1
1The First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China; 2Dalian Medical University, Dalian, People’s Republic of China; 3Central Hospital of Dalian University of Technology, Dalian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Yuan, Central Hospital of Dalian University of Technology, Clinical Laboratory Center, Dalian, People’s Republic of China, Email [email protected] Shuai Li, The First Affiliated Hospital of Dalian Medical University, Department of Pharmacy, Dalian, People’s Republic of China, Email [email protected]
Abstract: Macromolecular Drugs (including monoclonal antibodies, recombinant proteins, and nucleic acid therapies) have become a cornerstone strategy for intervening in complex pathological mechanisms such as cancer, autoimmune diseases, and genetic disorders due to their high specificity for disease targets and low off-target toxicity. However, compared to traditional small-molecule drugs, the high molecular weight (> 10 kDa) and structural complexity of macromolecular drugs result in extremely low transmembrane permeability. This is particularly challenging in the treatment of central nervous system (CNS) diseases, where the blood-brain barrier (BBB) imposes stringent selectivity, further limiting drug delivery efficiency. This review focuses on the breakthrough strategy of nose-to-brain (NtB) drug delivery. On one hand, the NtB pathway bypasses the BBB, enabling direct CNS drug delivery. On the other hand, nanocarrier technology can synergistically achieve systemic delivery and brain-targeted transport. Based on the latest research advances, this article systematically examines the feasibility of delivering macromolecular drugs via NtB administration. We comprehensively summarize relevant delivery carriers and discuss the potential advantages of intranasal-brain delivery for CNS disease treatment. Notably, while significant progress has been made in this field, further exploration is still needed regarding the mechanisms of NtB delivery and challenges in clinical translation.
Keywords: macromolecular drugs, BBB, central nervous system disorders, nanocarriers, intranasal administration
Graphical Abstract:

Introduction
At this stage, the hot topic in the field of pharmaceutical technology is how to improve the bioavailability of drugs and achieve a more convenient and faster treatment method based on a balance between toxicity and therapeutic effect of drugs.1 As a result, a number of biomolecular drugs prepared from biomaterials, including proteins, monoclonal antibodies, peptides, nucleic acids, vaccines, etc., have gained widespread interest because of their highly effective specificity.2 Macromolecular drugs are commonly employed in the diagnosis and treatment of tumors, immune disorders, cardiovascular diseases, and particularly central nervous system (CNS) diseases. Biomolecular drugs are often used in the diagnosis and treatment of tumors, immune diseases and cardiovascular diseases. Today, biomolecular drugs have become one of the most promising directions in drug development.3 To date, a variety of delivery methods for macromolecular drugs exist, such as oral, injection, intranasal, etc., and a variety of drug dosage forms are available. The compilation of macromolecular drugs that have entered clinical trials is summarized in Table 1.
 |
Table 1 Biomolecule Drugs Already on the Market, Includes Three Modes of Administration and Different Pharmacological Effects |
Although macromolecular drugs have made great strides and are playing an increasingly important role in the treatment of disease, there are still many difficulties and obstacles to their in vivo delivery.41 For example, large molecule drugs are very unstable, easily degraded by proteases in vivo, difficult to isolate and purify, complex in form, immunogenic and difficult to cross the barrier in vivo.42 The blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) play crucial roles in segregating the CNS from the peripheral system by preventing the entry of foreign substances, including toxins and bioactive drugs.43 Generally, small molecules with molecular weights below 500 Da can traverse the BBB via paracellular or transcellular pathways.44 However, approximately 98% of macromolecules fail to cross this barrier, creating significant challenges for targeted CNS delivery of biologics such as monoclonal antibodies and nucleic acid-based therapeutics. Invasive techniques such as intracerebral injection may be unsuitable for chronic patients requiring long-term treatment, particularly elderly populations.45 Consequently, strategies bypassing the BBB for targeted drug delivery have become critically important. Notably, intranasal administration has emerged as a highly promising alternative approach, offering a non-invasive NtB delivery route.46 Some studies have suggested that nasal administration may be an effective way to address the low bioavailability of large molecule biopharmaceuticals.47–49 In addition, the use of nanomaterials to load macromolecular drugs inside the nasal cavity would further improve drug absorption.50–52 In this review we will discuss the advantages of nasal delivery of macromolecules and the use of nanocarriers in the nasal delivery of macromolecules for the treatment of CNS diseases.
Barriers to the Delivery of Biomacromolecular Drugs in vivo
The Gastrointestinal Barrier
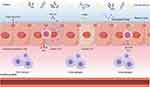
Oral administration is the most common and convenient route for drug delivery in daily medical practice. However, the gastrointestinal tract (GIT) presents a significant barrier to the effective delivery of certain biomacromolecular drugs.53 Since the first attempts to administer insulin orally in humans in the last century, the oral administration of large molecules has not been promising.54 Although a number of biomolecule drugs have made it to market, their bioavailability remains low.55 The gastrointestinal system, comprising the mouth, esophagus, stomach, small intestine, and colon, harbors a complex milieu of tens of thousands of microorganisms and various enzymes. This environment poses significant hurdles to the successful delivery of macromolecules orally. The presence of digestive enzymes and low pH in the stomach can degrade biomolecules, while the mucosal epithelium of the intestine presents a formidable barrier to their absorption56 (Figure 1). Additionally, the GI tract’s immune system and the presence of efflux transporters further limit the bioavailability of orally administered biomolecules.
The most significant difficulty encountered in the oral administration of macromolecules is the problem of stability; drugs are easily degraded by acidic and alkaline environments and by metabolic enzymes abundant in the GIT. To ensure the stability of the drug, it is essential to know the pH of the GIT, which varies throughout the digestive tract during the digestion of the drug57 (Figure 2). The range spans roughly 1 to 8, and changing pH values can cause structural changes in biomolecule drugs (α-helix, β-folding, etc)., as well as ionisation of some amino acids, resulting in a substantial decrease in the stability of the drug.58 In particular, high acidity in the stomach leads to protein defolding, exposing more motifs that can be recognised by degradative enzymes.55 A variety of digestive enzymes in the GIT, such as pepsin, chymotrypsin, trypsin and bile salts, cleave proteins and nucleic acids into fragments causing degradation of macromolecular drugs. Even if these impediments are safely circumvented, the mucosal layer covering the entire GIT prevents the entry of large particulate matters.59,60
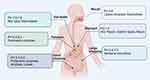 |
Figure 2 Schematic representation of the different pH values of the digestive tract. Created in BioRender. Wu, H. (2025) https://BioRender.com/wb4kzlh. |
The Blood-Brain Barrier
Injectable administration of biomolecules, while effectively bypassing the challenges posed by the GIT, faces a significant barrier in the form of the BBB. This barrier is highly selective, restricting the passage of most biomolecules into the brain. Therefore, despite their therapeutic potential, many biomolecules administered via injection are unable to reach the brain in sufficient quantities to exert their desired effects. The BBB was first discovered back in the 20th century, Paul Ehrlich and his students, while staining animal organs by injecting water-soluble dyes into the peripheral circulation, found that staining failed in the brain and cerebrospinal fluid, and then injected dyes directly into the cerebrospinal fluid only to find that only the brain and spinal cord were stained.61 It was not until the invention of the scanning electron microscope (SEM) that the membrane barrier was truly observed.62 The BBB is situated at the microvascular level within the brain and comprises five essential components that together form a sophisticated barrier63 (Figure 3). These components include pericytes, astrocytes, neurons, basement membranes, and connecting complexes. Functioning as a dynamic gateway, this neurovascular unit exerts dual regulatory effects: establishing physicochemical exclusion of neurotoxic compounds through transendothelial resistance modulation, while orchestrating carrier-mediated translocation of neurotrophic factors. Of particular significance is the astroglial compartment, whose perivascular endfeet not only provide structural reinforcement but also dynamically regulate barrier permeability via calcium-dependent signaling cascades. One of their key functions is to provide nutrients and metabolic support to neurons, ensuring their proper functioning and health. These star-shaped glial cells provide essential support to neurons and help regulate the microenvironment of the brain.61 Additionally, astrocytes are involved in the regulation of the extracellular environment, helping to maintain the balance of ions and neurotransmitters. By actively participating in the BBB, astrocytes contribute to protecting the brain from potentially harmful substances circulating in the bloodstream, thus preserving the delicate neural environment and safeguarding against damage. More importantly, the efflux transport system of the BBB represents another critical mechanism limiting drug penetration into the CNS, primarily mediated by the ATP-binding cassette (ABC) transporter superfamily. These transporters utilize energy derived from ATP hydrolysis to actively pump xenobiotics (eg, therapeutic drugs and toxins) from brain parenchyma back into the bloodstream, thereby maintaining homeostasis of the cerebral microenvironment.64–66
 |
Figure 3 Components of the blood-brain barrier and its internal structure. Created by Figdraw. |
The operational principle of this neuroprotective system inherently restricts macromolecular translocation across the blood-CNS interface. Functioning as a dynamic filtration barrier, the BBB establishes stringent molecular sieving through polarized efflux transporters and tight junction complexes. Structural analyses reveal that transcellular permeation is thermodynamically favored only for low-mass lipophilic compounds,67 creating substantial pharmacokinetic challenges for most neurotherapeutics requiring cerebral bioavailability. The selective permeability of the BBB poses a major pharmacological challenge for CNS-targeted drug development, as the majority of therapeutic compounds fail to achieve sufficient penetration. Consequently, overcoming the BBB remains one of the most critical hurdles in the development of efficacious neurological therapies. Direct CNS delivery methods, including intracerebral, intracerebroventricular, and intrathecal injections or infusions, can circumvent the BBB and facilitate drug distribution within the brain parenchyma. However, these invasive techniques necessitate administration by specialized medical personnel and are associated with elevated risks of infection. Moreover, they are clinically impractical for chronic treatments requiring sustained drug delivery.
The Potential Solution
Drug delivery systems (DDS) enhance drug efficacy by incorporating therapeutic agents with optimized carriers (eg, nanoparticles, NPs).68 The fundamental principle of DDS involves the strategic combination of drugs with tailored carrier materials. Critical parameters for effective delivery systems comprise:1 attaining therapeutic concentrations at target sites, and2 maintaining controlled, sustained release profiles within designated timeframes.69 Recent advancements in nanotechnology have established NPs as promising drug carriers, demonstrating unique advantages derived from their nanoscale dimensions:1 Enhanced surface properties enabling superior drug loading capacity,2 Improved barrier penetration facilitating efficient transport across biological membranes. These distinctive characteristics underscore the significant potential of NPs in biomedical applications.70 In recent decades, researchers have developed various innovative approaches to enhance drug delivery to the brain. Compared to conventional methods that directly compromise BBB integrity, biologically-inspired engineering of drug carriers enables precise targeting of specific BBB receptors for non-invasive brain delivery.71 Growing evidence supports the feasibility of NtB delivery, with numerous macromolecular peptides and proteins demonstrating superior efficacy via intranasal administration compared to intravenous injection.72 This creates a pivotal opportunity for macromolecular NtB delivery. Research demonstrates that peptide or protein drugs administered intranasally can elicit distinct therapeutic effects. Notably, multiple clinical studies have confirmed that intranasal insulin improves cognitive function in patients with Alzheimer’s (AD) and Parkinson’s diseases (PD).73,74 The NtB route offers unique advantages:1 ease of administration;2 pharmacokinetic superiority;3 central targeting specificity;41 formulation versatility. These characteristics make it particularly suitable for delivering macromolecular therapeutics like proteins and peptides, as will be elaborated in subsequent sections.
Physiology of the Nasal Cavity and Nasal Drug Delivery
Human Nasal Anatomy
The nasal structures of humans and rats are different, with the rat’s nasal cavity being more complex, so only the human nasal structure will be briefly described here. Specific differences are presented in Table 2. The nose is one of the smallest organs in the human body, with a length of about 12–14 cm, a volume of 13 mL and a total surface area of up to 160 cm2.75 The nasal cavity spans from the external nares to the nasopharynx, featuring three bony projections (superior, middle, and inferior conchae) that define its lateral architecture. A midline septal partition creates bilateral chambers, comprising three functional zones: vestibular, respiratory, and olfactory regions.76 The nasal vestibule occupies the anterior aspect of the nasal cavity, characterized by limited surface area and low permeability. Its dense mucus secretion and vibrissae filtration system constitute the primary immunological barrier against airborne pathogens.77
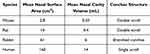 |
Table 2 Differences in the Structure of the Nasal Cavity Between Man and Other Experimental Animals |
Olfactory Region
The olfactory region occupies the superior nasal cavity, positioned dorsal to the superior concha, and comprises approximately 10% of the total human nasal epithelial surface area.75 This region consists of pseudostratified columnar epithelium lining the superior nasal cavity, primarily mediating olfactory signal transduction.78 The olfactory region contains olfactory sensory neurons (OSNs), the sole primary neurons in this area. These neurons feature ciliated dendritic terminals that project through the mucus layer, establishing direct contact with the external environment.79 The olfactory cilia of OSNs contain specialized olfactory receptors. Odorant molecules readily interact with these receptors within the olfactory epithelium’s mucus layer, initiating olfactory transduction. The olfactory epithelium additionally comprises multiple cell types, including sustentacular cells, basal cells, and microvillar cells.80 They have irregular microvilli and the secreted serous can be used as solvents for odor molecules.
Respiratory Region
In contrast, the respiratory region constitutes the predominant surface area within the nasal cavity, encompassing approximately 80–90% of the total nasal mucosa. This region exhibits a tripartite anatomical organization:81 the superior turbinate, the middle turbinate and the inferior turbinate, which cause turbulence through the nasal passage, increasing contact between the mucosa and the inhaled air. The nasal mucosa can remove particles, microbes and allergens, warm and humidify the air inhaled.82 This region comprises a stratified organization of epithelial cells, basement membrane, and lamina propria. The respiratory epithelium features approximately 300 microvilli per cell, with extensive vascularization that enhances surface area and facilitates systemic drug absorption.83 The respiratory epithelium (ciliated pseudostratified columnar epithelium) comprises four principal cellular components: goblet cells, ciliated and non-ciliated columnar cells, and basal cells. Notably, basal cells are exclusively localized to the basement membrane, functioning as defensive elements that mediate epithelial repair.84 Goblet cells are responsible for secreting mucin and closely connect with the other two kinds of cells between epithelial cells to prevent hydrophilic particles from spreading freely through the paracellular transport pathway.85 The lamina propria, situated superior to the respiratory epithelium, facilitates drug absorption through its rich vascular and neural networks. Notably, trigeminal nerve innervation extends throughout the nasal epithelial lining.
The Nasal-CNS Neural Pathways
Advantages of Nasal Delivery
Nasal drug delivery provides numerous advantages compared to other administration routes. The nasal mucosa boasts a large surface area, which, coupled with its rich vascularization, enables efficient drug absorption. This route also offers rapid systemic delivery due to the abundant blood vessels in the submucosal layer, leading to faster drug onset compared to oral administration.86,87 Topical administration of drugs through the nasal route is highly effective. Nasal sprays and drops are well-established for treating inflammation or congestion. Specifically, hydrophobic or low molecular weight drugs are particularly suitable for topical nasal applications due to their ability to easily penetrate the nasal mucosa and achieve therapeutic concentrations locally.
Additionally, direct site administration of drugs can necessitate lower doses than systemic administration, which not only reduces the risk of side effects but also provides faster relief of disease symptoms. Topical nasal delivery significantly reduces sedative effects commonly observed with oral antihistamines, thereby improving therapeutic adherence. This route offers dual pharmacokinetic advantages: circumvention of hepatic first-pass metabolism prevents gastrointestinal and hepatic complications, while minimizing systemic drug exposure. Furthermore, low molecular weight lipophilic agents readily undergo transcellular absorption, achieving systemic distribution via vascular or lymphatic pathways.88
BBB presents a formidable obstacle for most neurotherapeutics, severely limiting drug penetration into the CNS. Intranasal delivery circumvents this barrier through two principal pathways: the olfactory and trigeminal routes. Notably, following respiratory deposition, select compounds undergo axonal transport via trigeminal nerve branches, facilitating direct delivery to brainstem and other CNS regions through receptor-mediated endocytotic mechanisms.89 A greater proportion of drugs are delivered to the brain via the olfactory region, and the olfactory pathway has three different modes of transport: Drugs can be internalised in or transported within neurons; can be transported out of cells through gaps between cells, (especially along pathways near the olfactory nerve); can be transported across cells through the basal epithelium.90 For CNS administration, from the nasal cavity to the brain is the simplest and easiest way to achieve91 (Figure 4).
 |
Figure 4 Diagram of the uptake mechanism of the nasal epithelium for NtB delivery and schematic diagram of the NtB delivery pathway. (A) Schematic diagram of the anatomy of the nasal epithelium and the main pathways across this barrier. (B) Anatomical diagram of the NtB delivery pathway, mainly including the trigeminal nerve pathway and the olfactory nerve pathway. Reprinted from Adv Drug Deliv Rev, 207, Chen Y, Zhang C, Huang Y, et al. Intranasal drug delivery: the interaction between nanoparticles and the nose-to-brain pathway, 115196, Copyright 2024, with permission from Elsevier.92 |
The Olfactory Pathway
The olfactory pathway comprises specialized cellular components, notably OSNs accompanied by sustentacular and progenitor basal cells.77 Olfactory receptor neurons are unique in that they originate from the nasal olfactory epithelium and extend specialized hair-like structures called cilia into the mucus lining the nasal cavity. OSNs transduce airborne odorant molecules into neural impulses, which propagate axonally to the olfactory bulb (OB) for central odor processing. Sustentacular cells maintain neuronal structural integrity and metabolic homeostasis, while resident basal stem cells ensure lifelong neurogenesis. Pharmacologic agents undergo transneuronal transport via this pathway, migrating from nasal epithelium to OB. From there, it progresses to the olfactory cortex, which is responsible for processing olfactory information. Finally, the drug enters the CNS, specifically reaching the cerebrum and cerebellum. This unique pathway offers a direct route for drugs to access the brain, bypassing the BBB and potentially enhancing therapeutic effects for CNS disorders.93 However, this method has the disadvantage of a relatively slow transduction time due to the complex process of neuronal signaling and transmission. Additionally, drugs may also be transported outside the nerve pathway. For example, drugs can traverse the olfactory mucosa through various mechanisms, including diffusion through the intercellular spaces of the epithelial cells or by being carried along with the supporting cells as they migrate toward the surface of the epithelium.94
The Trigeminal Pathway
The trigeminal nerve, representing the most extensive cranial neural pathway, comprises three principal divisions: ophthalmic, maxillary, and mandibular. These axonal projections synapse at the trigeminal ganglion—a pivotal relay nucleus mediating connectivity between peripheral sensory inputs and central brainstem nuclei.95 The maxillary and ophthalmic divisions of the trigeminal nerve form direct neuroanatomical conduits between nasal mucosa and the CNS. This pathway mediates both somatosensory transduction and potential neurotherapeutic delivery, bypassing conventional BBB restrictions.96 The drug can travel along the respiratory epithelium, gaining access to the trigeminal nerve and ultimately reaching the brainstem. This route presents a unique opportunity for drugs to bypass the BBB and directly target the CNS.97 An animal study investigated the potential of intranasally administered insulin-like growth factor-I (IGF-I) to enter the CNS and its related transport mechanisms.98 The results showed that after intranasal and intravenous administration in different groups of rats, the concentration of [125I]-IGF-I in central nervous tissues was significantly higher with intranasal delivery compared to intravenous injection. Specifically, in the OB, the intranasal concentration (3.43±0.53 nM) was approximately 880 times higher than the intravenous concentration (0.0039±0.0002 nM), demonstrating that intranasal administration can effectively bypass the BBB to deliver IGF-I to the CNS.98 Furthermore, the investigation demonstrated that intranasal IGF-I predominantly accesses the CNS via dual neural pathways: the olfactory and trigeminal systems.98 These results empirically validate this pathway’s utility for CNS-targeted therapeutic delivery.
The Systemic Pathway
After intranasal administration, drugs are absorbed by the nasal mucosal capillaries and enter systemic circulation, distributing throughout the body.99 However, to reach the CNS, drugs must cross the highly selective BBB,99 which presents a significant challenge as it restricts the passage of many neurotherapeutic molecules.100 Consequently, this reduces treatment efficacy and limits the quantity of drugs that can effectively reach the brainstem.99
Drug Absorption Barrier
Nevertheless, multiple physiological variables within the nasal cavity modulate drug delivery efficacy. Nasal hemodynamics, enzymatic degradation, mucociliary clearance efficiency, and mucosal integrity collectively determine the pharmacokinetic profile of intranasally administered therapeutics.101 The extensive vascularization of the nasal cavity serves dual physiological functions: thermohumidification of inspired air and mucosal perfusion. This robust circulatory network simultaneously enables efficient systemic drug absorption through enhanced vascular permeability and first-pass metabolism avoidance.102 Enhanced vascular perfusion facilitates systemic drug absorption and distribution, while nasal mucosal enzymes may compromise pharmaceutical stability.
For instance, aminopeptidases and proteases can degrade peptides and proteins, reducing the effectiveness of these drugs when administered nasally.103 Furthermore, the nasal mucosa contains endopeptidases like serine and cysteine proteases, which cleave internal peptide bonds, as well as exopeptidases such as aminopeptidases and carboxypeptidases, which respectively cleave N-terminal and C-terminal peptides.104 These enzymatic activities can degrade peptides and proteins, leading to decreased bioavailability of drugs administered nasally.
The nasal mucus undergoes complete renewal every 10–15 minutes, propelled by ciliary action at 5–6 mm/min to facilitate the clearance of airborne particulates and pathogens.105,106 Consequently, intranasal drugs exhibit brief residence times, necessitating rapid absorption. Hydrophilic compounds demonstrate particularly slow transmucosal penetration due to mucosal solubility, resulting in accelerated ciliary clearance. Notably, mucociliary clearance efficiency displays regional heterogeneity across nasal subsites.107 There are fewer cilia in the first half of the nasal cavity, so active drugs can stay here longer. Continuous nasal administration is also a challenge in the cold season. Due to the change of climate, the physiological environment in the nose will also change, so the conditions of drug absorption will change, and it will also affect the effect of drug absorption.108 In addition, this problem can also be exacerbated by irritation of the nasal mucosa by some other diseases.
Delivery of Macromolecular Drugs by Intranasal Administration
Systemic Drug Delivery
Protein and polypeptide drugs encounter challenges with oral administration due to degradation by gastric acid and barriers in the intestines. Although these therapeutics can be delivered parenterally (intravenously or subcutaneously), hepatic first-pass metabolism persists, significantly reducing systemic bioavailability prior to circulation.109 Nasal administration offers a more direct route, bypassing the first-pass effect and allowing for rapid drug onset, making it a preferable choice for these types of drugs.109 The cell-penetrating peptides (CPPs) have shown promise in delivering a variety of bio-pharmaceuticals,110 which presents a novel approach for intranasal administration of peptides and proteins, offering potential advantages in drug delivery and therapy.110,111 Research findings indicate that insulin is delivered intranasally using CPPs, it can be absorbed by the nasal mucosa and enter the systemic circulation. This administration method leads to a significant decrease in blood glucose levels in normal mice, suggesting its potential effectiveness in managing glucose levels through non-invasive means.112 In addition, Nastech Pharmaceutical has developed a rapidly acting intranasal insulin formulation. This new type of intranasal insulin not only has a bioavailability between 17% and 28%, but also has a comparable effect to subcutaneous insulin aspart in controlling postprandial blood glucose levels and is better than conventional treatments. Moreover, after this intranasal insulin enters the human body, it only takes 30 minutes to reach its peak concentration, which is much faster than insulin aspart (90 minutes), indicating that it can take effect more quickly.113 Various strategies can enhance drug delivery through the nasal route. These include the use of absorption enhancers to improve drug uptake, employing nano-transport systems such as NPs and microspheres for targeted delivery, and incorporating compounds like cyclodextrins and chitosan (CS) to enhance drug stability and bioavailability.78
Nasal Brain-Targeted Delivery for the Treatment of Central Nervous System Diseases
An important feature of intranasal administration is the ability to enable drugs to reach the brain directly through the nasal cavity, a phenomenon known as NtB drug delivery. This pathway takes advantage of the anatomical proximity between the nasal mucosa and the brain.114 By utilizing this pathway, drugs can bypass the blood-brain barrier and enter the brain directly, potentially enhancing the therapeutic effects for nervous system diseases100 (Figure 5).
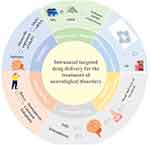 |
Figure 5 Schematic diagram of intranasal targeted drug delivery for the treatment of various neurological disorders. |
Healthcare statistics reveal a rapid increase in the prevalence of CNS disorders worldwide.115 Conditions like PD, AD, and other neurodegenerative disorders are becoming more prevalent.116 While current therapeutic strategies have improved patient survival rates, they still face significant challenges in effectively treating and managing these complex disorder.116 The primary obstacle in treating most CNS disorders is the necessity for drugs to effectively penetrate the BBB to achieve therapeutic levels.117 Research suggests that approximately 98% of low molecular weight active substances and nearly 100% of large molecules are unable to cross the BBB, resulting in notably low bioavailability at the target site. This significant challenge underscores the urgent need for innovative drug delivery approaches to surmount the BBB and improve treatment outcomes for CNS disorders.118 Nasal brain administration is a painless, non-invasive method of drug delivery compared to other delivery methods.119 This route bypasses the BBB, enabling direct cerebral drug delivery. Furthermore, nasal administration avoids first-pass metabolism, typically requiring doses 2–10 times lower than oral regimens.
AD
AD, the predominant form of global dementia,120 is pathologically characterized by β-amyloid (Aβ) plaque accumulation and neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau proteins.121 Currently, the treatment of AD primarily relies on pharmacological interventions.122 Nevertheless, AD therapeutic development encounters substantial obstacles, including dose-limiting toxicity, inadequate BBB penetration to achieve therapeutic drug levels, and prohibitive developmental costs and resource requirements.122 Small-molecule drugs, such as acetylcholinesterase (AChE) inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists, have been commercialized and widely used for treatment. However, they often induce gastrointestinal side effects that compromise therapeutic efficacy.123 Recently developed monoclonal antibodies, such as lecanemab, donanemab, and aducanumab, have shown encouraging clinical trial evidence—achieving >60% clearance of Aβ deposits after 18 months of treatment.124 Immunotherapy may represent the most advanced therapeutic strategy for AD.125 However, biologics such as vaccines and antibodies, which are currently administered peripherally, have limited brain penetration, resulting in inefficient drug delivery.125 Nanocarriers administered intranasally play a crucial role in enhancing drug concentration and bioavailability,122 offering a highly promising alternative therapeutic approach.123 Gaikwad S et al developed a monoclonal antibody, TTCM2, loaded into micelles (TTCM2-ms). Intranasal delivery of TTCM2-ms effectively targeted pathological tau in mouse brains, clearing pathological tau in aged mice after a single dose, increasing synaptic protein levels, and improving cognition.126 Additionally, Yang et al developed a multifunctional nanocarrier (Rapa@DAK/siRNA) for AD treatment. This system was constructed by modifying dendrigraft poly-L-lysines (DGLs) with Aleuria aurantia lectin (AAL) and Aβ-binding peptides (KLVFF). Following intranasal administration, it successfully co-delivered small interfering RNA targeting β-site amyloid precursor protein cleaving enzyme-1 (BACE1 siRNA) and rapamycin to the brain. The intranasal route enabled drug delivery via the NtB pathway, with AAL enhancing brain uptake efficiency and KLVFF facilitating Aβ binding and inhibition. The dual release of these agents reduced BACE1 expression, enhanced autophagy, and decreased Aβ deposition, ultimately improving cognition in transgenic AD mice127 (Figure 6). This combined therapy, integrating gene therapy (siRNA) and pharmacological treatment (rapamycin), may exert synergistic effects through distinct mechanisms of action. It not only provides an effective intranasal delivery strategy for AD treatment but also offers a novel approach for comprehensive disease management.
 |
Figure 6 Schematic diagram of Rapa@DAK/siRNA for intranasal administration in the treatment of AD and the possible mechanism of neuroprotection. Reprinted with permission from Yang X, Yang W, Xia X, et al. Intranasal delivery of BACE1 siRNA and rapamycin by dual targets modified nanoparticles for Alzheimer’s disease therapy. Small. 2022;18(30):e2203182. © 2022 Wiley-VCH GmbH.127 |
PD
PD represents the second most prevalent neurodegenerative disorder worldwide and a predominant etiology of neurological impairment.128 Its core pathological features include the misfolding and aggregation of α-synuclein, forming Lewy bodies and Lewy neurites—neuronal inclusions that lead to cell loss in brain regions such as the substantia nigra and trigger prion-like cell-to-cell propagation.128 The primary pharmacological treatments for PD rely on small-molecule drugs, including levodopa, anticholinergics, and antiglutamatergic agents, typically administered orally or via injection.129 However, these approaches increase systemic exposure risks, often causing diverse complications, and fail to halt disease progression, significantly limiting their therapeutic efficacy.129 Thus, there is an urgent need to explore novel therapeutics that enhance targeting precision, minimize adverse effects, and delay disease progression. Glial cell-derived neurotrophic factor (GDNF) exhibits potent neurotrophic effects on various neurons, particularly dopaminergic neurons (DANs),130 making it a promising candidate for alleviating PD symptoms. However, BBB remains a major obstacle hindering the therapeutic efficacy of such macromolecular drugs.131 An animal study investigated the therapeutic effects of intranasally administered GDNF-loaded extracellular vesicles (EVs) derived from genetically engineered macrophages in PD.131 In wire-hang and rotarod tests, EV-GDNF treated PD mice showed approximately 4 times longer endurance than saline-treated controls after 12 months of treatment, reaching levels comparable to wild-type (WT) mice, demonstrating effective preservation of motor function. EV-GDNF also significantly improved hyperactive behaviors and anxiety-like symptoms in PD mice (p<0.05).131 This study suggests a promising new intranasal delivery strategy for PD treatment.
Epilepsy
Epilepsy is a common neurological disorder affecting approximately 65 million people worldwide. Its primary clinical manifestation is recurrent and persistent epileptic seizures.132 Since the 19th century, over 30 antiseizure medications (ASMs) have been developed for epilepsy treatment.133 However, these drugs are ineffective against drug-resistant epilepsy and fail to modify disease progression.133 There is an urgent need to develop novel therapeutics with improved tolerability, safety, and disease-modifying potential.134 RNA-based drugs represent a promising approach, as they can be designed to target non-coding RNAs and modulate entire disease pathways. Additionally, they can be customized to correct genetic mutations. While these drugs are typically delivered via intrathecal injection for targeted CNS action,133 intranasal administration offers a less invasive alternative with potentially superior therapeutic outcomes.135 A study evaluated the silencing efficacy of intranasally administered siRNA nanoparticle systems targeting the GluN1 subunit in a rat epilepsy model.136 Results showed that GluN1 subunit expression in the hippocampus of the siRNA-treated group significantly decreased (0.45-fold change). The latency of the first tonic-clonic seizure in the siRNA-treated group was notably prolonged at 14 days compared to the control group (p = 0.0001), with no statistical difference between the two groups at 21 days. The experiment indicated that the intranasal administration route, featuring easy operation, good patient tolerance, relative safety and continuous usability, offers a new perspective for treating temporal lobe epilepsy (TLE).136
Other Neurological Disorders
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder with a variety of symptoms and potential causes. 5-hydroxytryptamine and risperidone are medications that can help control certain symptoms of ASD, but they do not address all aspects of the condition. Currently, there is no medication that can completely treat autism.137 Recent studies have highlighted oxytocin as a neurotransmitter of significant interest for potential ASD treatment.138 Intranasal oxytocin delivery has emerged as a prominent research focus, with multiple clinical trials demonstrating its therapeutic potential for ASD treatment in recent years.139 Oxytocin receptors regulating emotional and social cognition are predominantly expressed in the frontal cortex, which exhibits anatomical proximity to the OB. Consequently, increased oxytocin concentrations in the OB enhance therapeutic efficacy for ASD.138 The administration time is shorter and the concentration of the drug in the brain is higher compared to intravenous administration. At the same time, intranasal administration of oxytocin can also induce changes in brain function in response to social stimuli.140,141 Patients with schizophrenia have low oxytocin levels, and oxytocin treatment can reduce psychotic symptoms and improve emotional recognition in schizophrenic patients. In schizophrenic patients, oxytocin decreased amygdala activity in response to emotional faces, whereas in healthy controls, oxytocin increased amygdala activity. Oxytocin nasal spray may be effective in altering neural activity in schizophrenia patients with dysfunction related to emotion processing.142 Transnasal oxytocin can also relieve chronic pain, as Tzabazis A et al have demonstrated by showing that intranasal oxytocin can significantly inhibit the response of specific brainstem regions to head and facial pain stimuli.143 In addition to oxytocin, peptides such as desmopressin, calcitonin and enkephalin can also be administered in a transnasal manner to relieve chronic pain.119,144,145
The nasal-brain targeted drug delivery route provides an effective and feasible solution for treating CNS diseases, offering hope for the cure of some diseases that are otherwise difficult to treat.
Important Nanocarriers for Nasal Drug Delivery
Building upon the effectiveness of the nasal-brain pathway, the choice of drug carrier is crucial. When traditional formulations are used for nasal-brain drug delivery, issues often arise due to their physicochemical properties, such as low bioavailability, hydrophobicity or lipophilicity, ionization, and extensive metabolism.146 Intranasal nano-carrier-based delivery represents one of the most promising approaches for transporting macromolecular drugs to the CNS. This system offers multiple advantages including high drug-loading capacity, enhanced delivery efficiency, low toxicity, minimal immunogenicity, improved drug absorption, increased bioavailability, and superior brain targeting specificity. Furthermore, the versatile nature of these carriers allows for structural modifications to optimize performance.92 These advantages allow macromolecular drugs to overcome physiological barriers and achieve targeted delivery. However, nano-carriers present limitations, including the constrained drug-loading capacity and poor storage/delivery stability of lipid-based nano-carriers (LBNs) due to potential intraliposomal coagulation and crystallization, which may cause premature drug release.147 To address these limitations, researchers have developed effective solutions. CS, a naturally occurring polysaccharide polymer, offers multiple advantages including hydrophilicity, non-toxicity, biocompatibility, and biodegradability.148 Various CS-based nano-carriers have been engineered for intranasal macromolecular drug delivery to the brain.149–152 The benefits of CS nano-carriers have been extensively reviewed.68 Polyethylene glycol (PEG)-modified nano-carriers have been extensively developed. PEGylation alters the physicochemical properties of nano-carriers by reducing surface charge density and enhancing stability.101 More importantly, PEG modification enables NPs to evade mononuclear phagocyte system uptake, thereby improving targeted delivery efficiency in vivo.127 To further enhance nano-carrier delivery and targeting efficacy, researchers have employed CPPs and CPP-modified nano-carriers for NtB delivery.153–155 CPPs enable the transformation from passive delivery systems to active penetrating systems. This review summarizes key nano-carriers for intranasal macromolecular drug delivery (Table 3), including representative studies demonstrating their potential to overcome physiological barriers and achieve effective brain targeting (Figure 7).
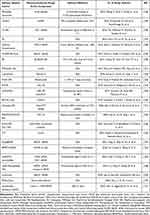 |
Table 3 Summary of Nanocarriers for Delivery of Macromolecular Drugs in the Past Decade |
 |
Figure 7 Schematic diagrams of various nanocarriers for nose-to-brain delivery. (A) Liposomes; (B) Nanoemulsions; (C) Lipid-based nanoparticles; (D) SLN and NLC; (E) Polymeric nanoparticles; (F) Metal-based nanoparticles. Reprinted with permission from Zha S, Wong K-L, All AH. Intranasal delivery of functionalized polymeric nanomaterials to the brain. Adv Healthc Mater. 2022;11(11):e2102610. © 2022 Wiley-VCH GmbH.172 |
Lipid-Based Nanocarriers (LBNs)
In 1965, Bangham AD et al first reported the spontaneous formation of phospholipid bilayers in aqueous solution, forming lamellar liquid crystals with water layers separating each bilayer.173 This discovery established the theoretical foundation for liposomes as drug carriers. Currently, LBNs represent one of the most widely used DDS. For intranasal macromolecular delivery, major LBNs types include: liposomes,154,156,157 solid lipid nanoparticles (SLNs),159 nanostructured lipid carriers (NLCs),149,166 nanoemulsions,158 key advantages of LBNs include: (1) high encapsulation efficiency; (2) excellent biocompatibility; (3) strong biomembrane permeability; (4) targeted delivery capability; (5) low toxicity and biodegradability.172,174
Liposomes
Liposomes are vesicular structures composed of phospholipids and cholesterol, featuring hydrophilic heads and hydrophobic tails. In aqueous environments, phospholipid molecules spontaneously self-assemble into closed bilayers through hydrophobic interactions, subsequently forming NPs.172 These characteristics confer excellent biocompatibility while protecting drug activity and enhancing solubility. Zheng X et al prepared PEGylated liposomes encapsulated with β-sheet breaking peptide (H102 peptide), and then evaluated the improvement of AD symptoms in a rat model after intranasal administration. The encapsulation efficiency of the liposomes was 71.35±0.87%, which could significantly protect the H102 peptide from degradation by plasma enzymes (63% remained after 2 hours). After intranasal administration, the area under the plasma concentration-time curve (AUC) of the liposome group in the hippocampus (HI) of rats was 2.92 times that of the H102 solution group. Meanwhile, it could improve the spatial memory impairment of AD model rats and had low toxicity to the nasal mucosa.156 Another study constructed an 89WP-CLS/R8/siRNA core-shell liposomal complex, featuring an octaarginine (R8) core enveloped by cationic liposomes (CLSs) and surface-modified with Q8W/N9W-penetratin (89WP) for glioblastoma (GBM) targeting (Figure 8A). This lipoplex significantly enhanced siRNA permeability across the nasal mucosa (Figure 8B) and demonstrated a 3-fold increase in tumor siRNA accumulation in glioma-bearing mice (Figure 8C). The system potently suppressed c-Myc expression, achieving 57.7% protein-level and 32.3% mRNA-level reductions. These findings establish a novel therapeutic strategy for GBM treatment.154
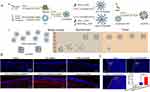 |
Figure 8 Construction of lipoplexes for targeted treatment of glioblastoma. (A) Schematic diagram of the preparation and transportation of lipoplexes. (B) Paraffin sections of nasal mucosa treated with siRNA formulations. Cell nuclei are stained blue with 4’,6-diamidino-2-phenylindole (DAPI), and siRNA is labeled with Cy5 and appears red. (C) Confocal images of the distribution of DiD-labeled lipoplexes in the brains of glioma-bearing mice and the normalized integral optical density (IOD). Cell nuclei are stained blue with DAPI, and the DiD-labeled lipoplexes are red. Reprinted from Acta Biomater, 138, Hu Y, Jiang K, Wang D, et al. Core-shell lipoplexes inducing active macropinocytosis promote intranasal delivery of c-Myc siRNA for treatment of glioblastoma, 478–490, Copyright 2022, with permission from Elsevier.154 |
SLN and NLC
SLNs feature a solid lipid core coated with surfactants. Their physiologically compatible solid matrix remains stable at body temperature, enabling controlled drug release.172 Developed from SLNs, NLCs incorporate both liquid and solid lipids in their core, surrounded by surfactants. This hybrid structure combines the advantages of both lipid phases, demonstrating enhanced drug-loading capacity, improved stability, and tunable release kinetics.175 Rassu et al developed CS-coated, CPP (RVG-9R)-modified SLNs for BACE-1 siRNA delivery in AD treatment. In Caco-2 cell models, the CS-coated group demonstrated optimal permeability at 60 minutes, significantly enhancing siRNA penetration. The BACE1 siRNA effectively reduced Aβ plaque formation, suggesting potential for AD progression delay.159 A recent study explored NtB delivery for AD using NLCs loaded with TRAIL-neutralizing monoclonal antibodies (anti-TRAIL mAb), achieving 99% encapsulation efficiency. Following intranasal administration in mice, immunofluorescence analysis revealed significantly elevated NLC levels in the hippocampus. This system offers a promising non-invasive strategy for CNS delivery of macromolecules, particularly antibodies.166 Further research is needed to evaluate the immunogenicity and toxicity of these nanocarriers.
Polymeric Nanocarriers
Biodegradable polymeric nanocarriers have emerged as one of the most promising strategies for NtB drug delivery. These polymers can spontaneously form nano-sized aggregates (1–1000 nm) under specific environmental or experimental conditions, exhibiting unique physicochemical properties that qualify them as nanobiopolymers.176 Extensive research has utilized both natural polymers (particularly CS) and synthetic polymers (including PEG, PLGA, and polyethyleneimine) as nanocarriers for macromolecular drug delivery.127,151,160,162 Notably, these synthetic polymers undergo biodegradation into oligomers and monomers that can be eliminated through normal metabolic pathways.177 The polymeric nanocarriers demonstrate excellent drug encapsulation and protection capabilities, particularly for stabilizing vulnerable therapeutic agents like proteins and peptides by preventing their premature degradation in vivo.69,178 Sousa F et al pioneered bevacizumab-loaded PLGA NPs that significantly enhanced cerebral bioavailability via intranasal administration, achieving approximately 3-fold higher brain drug concentrations compared to free bevacizumab. This innovative strategy presents substantial clinical potential for glioblastoma treatment.162
Additional Nanocarriers
Other nanocarriers have been developed for macromolecular NtB delivery. Nanoemulsions are composed of two phases, namely water and oil, among which oil-in-water (O/W) nanoemulsions are the predominant type. Lipophilic drugs dissolved in the oil phase can form nanoprecipitates during phase transition to the aqueous phase. These nanoprecipitates exhibit high surface area and rapid dissolution kinetics, thereby enhancing delivery efficiency.123 Yadav S et al developed cationic nanoemulsions (SNEs) encapsulating anti-TNF-α siRNA, achieving 5-fold higher cerebral siRNA concentrations than free siRNA after intranasal administration. The SNEs significantly reduced systemic siRNA exposure and decreased brain TNF-α expression (523% to 351%, p<0.05), demonstrating superior brain targeting. This study provides an innovative therapeutic strategy for neuroinflammatory disorders.158
Recent studies have explored nanomicelles for drug delivery. A multifunctional core-shell nanomicelle system (HA/DP7-C) was developed using CCP DP7-C and hyaluronic acid (HA) for NtB siRNA delivery. Following intranasal administration, HA/DP7-C/siRNA complexes rapidly reached the CNS via trigeminal pathways within hours and accumulated at tumor sites. Both in vitro and in vivo studies demonstrated significant inhibition of glioma growth when targeting VEGF or PLK1 siRNA was delivered through this system167 (Figure 9). This strategy constitutes a robust and biocompatible siRNA delivery system for glioma treatment, especially targeting chemoresistant brain malignancies.
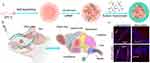 |
Figure 9 Preparation of nanomicelles for the treatment of glioma. (A) Schematic diagram of the preparation process of nanomicelles. (B) Schematic diagram of the nose-to-brain delivery pathway of nanomicelles. (C) Tissue sections of the trigeminal nerve and olfactory bulb 30 minutes after the intranasal administration of the siRNA formulation. Reprinted from J Control Release, 342, ang Y, Zhang X, Wu S, et al. Enhanced nose-to-brain delivery of siRNA using hyaluronan-enveloped nanomicelles for glioma therapy,66-80, Copyright 2022, with permission from Elsevier.167 |
Recent advances have established bioinspired nanovehicles, including exosomal and intranasal microbial carriers, for facilitating neurotherapeutic macromolecule delivery.170,171 Engineered Lactobacillus plantarum WCFS1 (Lp), exhibiting natural olfactory epithelium tropism, efficiently mediated brain-targeted payload delivery. In obesity models, intranasal Lp administration induced prolonged anorexigenic effects and enhanced metabolic parameters compared to recombinant leptin, indicating greater therapeutic durability171 (Figure 10). This innovative approach utilizes endogenous olfactory epithelium-targeting microbiota to circumvent intranasal delivery barriers. Concurrently, inorganic NPs have been engineered for enhanced neurotherapeutic transport.164
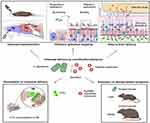 |
Figure 10 Lactobacillus plantarum WCFS1 (Lp) has a natural affinity for the OE, and it is engineered to serve as a brain-targeted drug delivery carrier. After intranasal administration, Lp can release the payload in the OE and be transported to the brain. Reprinted with permission from.171 |
Summary and Outlook
Intranasal delivery offers a non-invasive, low-risk alternative with adjustable dosing and easy discontinuation, making it preferable for patients unsuitable for or declining surgery. Crucially, it bypasses the BBB, enabling direct CNS delivery of macromolecular biologics (eg, proteins, peptides). This approach provides novel therapeutic strategies for CNS disorders including PD, AD, autism, and schizophrenia.
However, the natural clearance mechanism in the nasal cavity poses challenges to drug delivery. To overcome these obstacles, nanocarriers loaded with therapeutic drugs have emerged as a promising solution. Therefore, continuous research efforts are crucial for developing nanocarriers specifically designed for intranasal administration.1 Enhance delivery efficiency and precise targeting: Optimize the structure, size, surface properties, and biocompatibility of nanocarriers to maximize drug loading efficiency, stability, and brain targeting ability. By developing co-delivery systems (such as siRNA + chemotherapeutic drugs/immunomodulators), multi-target delivery can be achieved, and tumor drug resistance can be overcome through synergistic treatment.2 Deepen the delivery mechanism of nasal-to-brain administration: The specific transport mechanisms of NPs in the trigeminal and olfactory nerve pathways can be clarified through in vivo imaging and neural tracing techniques.3 Conduct preclinical and clinical translational research: Evaluate the long-term nasal mucosal toxicity, immunogenicity, and the risk of neuroinflammation in animal models, as well as the effects of repeated administration on olfactory and trigeminal nerve functions. Meanwhile, optimize the preparation process of NPs to achieve large-scale production and stability, making them convenient for clinical use.41 Combine multiple treatment methods, such as gene therapy, immunotherapy, and chemotherapeutic drugs.42 Interdisciplinary integration: Incorporate exosomes or biomimetic NPs to improve biocompatibility and targeting ability. In addition, it can be combined with intranasal drug delivery devices to maximize the deposition of drugs on the olfactory epithelium, thereby achieving better therapeutic effects. The combination of intranasal administration and optimized nanocarriers provides an important opportunity for advancing treatment strategies for a series of CNS diseases. Continuous research and development in this field have the potential to transform the treatment prospects for patients with these challenging diseases.
Acknowledgment
Thanks to Figdraw and BioRender for providing some elements for schematic diagram. Created in BioRender. Wu, H. (2025) https://BioRender.com/5s93vvk.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zelikin AN, Ehrhardt C, Healy AM. Materials and methods for delivery of biological drugs. Nat Chem. 2016;8(11):997–1007. doi:10.1038/nchem.2629
2. Guo Q, Jiang C. Delivery strategies for macromolecular drugs in cancer therapy. Acta Pharm Sin B. 2020;10(6):979–986. doi:10.1016/j.apsb.2020.01.009
3. Tyagi P, Santos JL. Macromolecule nanotherapeutics: approaches and challenges. Drug Discov Today. 2018;23(5):1053–1061. doi:10.1016/j.drudis.2018.01.017
4. Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–117. doi:10.1016/S0140-6736(22)02420-5
5. Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386(3):241–251. doi:10.1056/NEJMoa2112431
6. André F, Hee Park Y, Kim SB, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;401(10390):1773–1785. doi:10.1016/S0140-6736(23)00725-0
7. Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-pantumor02 phase II trial. J Clin Oncol. 2024;42(1):47–58. doi:10.1200/JCO.23.02005
8. Peyrin-Biroulet L, Loftus EV Jr, Colombel J-F, et al. Histologic outcomes with vedolizumab versus adalimumab in ulcerative colitis: results from an efficacy and safety study of vedolizumab intravenous compared to adalimumab subcutaneous in participants with ulcerative colitis (VARSITY). Gastroenterology. 2021;161(4):1156–67.e3. doi:10.1053/j.gastro.2021.06.015
9. Fleischmann R, Swierkot J, Penn SK, et al. Long-term safety and efficacy of upadacitinib versus Adalimumab in patients with rheumatoid arthritis: 5-year data from the phase 3, randomised SELECT-COMPARE study. RMD Open. 2024;10(2):e004007.
10. McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus Adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. 2020;395(10235):1496–1505. doi:10.1016/S0140-6736(20)30564-X
11. Powles T, Valderrama BP, Gupta S, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875–888. doi:10.1056/NEJMoa2312117
12. Diaz LA Jr, Shiu KK, Kim TW, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23(5):659–670. doi:10.1016/S1470-2045(22)00197-8
13. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, Phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi:10.1016/S1470-2045(20)30445-9
14. Geetha D, Dua A, Yue H, et al. Efficacy and safety of avacopan in patients with ANCA-associated vasculitis receiving rituximab in a randomised trial. Ann Rheum Dis. 2024;83(2):223–232. doi:10.1136/ard-2023-224816
15. Atisha-Fregoso Y, Malkiel S, Harris KM, et al. Phase II randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol. 2021;73(1):121–131. doi:10.1002/art.41466
16. Pfisterer J, Shannon CM, Baumann K, et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(5):699–709. doi:10.1016/S1470-2045(20)30142-X
17. Watanabe J, Muro K, Shitara K, et al. Panitumumab vs bevacizumab added to standard first-line chemotherapy and Overall survival among patients with RAS wild-type, left-sided metastatic colorectal cancer: a randomized clinical trial. JAMA. 2023;329(15):1271–1282. doi:10.1001/jama.2023.4428
18. Park S, Kim TM, Han JY, et al. Phase III, randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR- or ALK-mutated non-small-cell lung cancer (ATTLAS, KCSG-LU19-04). J Clin Oncol. 2024;42(11):1241–1251. doi:10.1200/JCO.23.01891
19. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
20. Tykodi SS, Gordan LN, Alter RS, et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase 3b/4 CheckMate 920 trial. J Immunother Cancer. 2022;10(2):e003844. doi:10.1136/jitc-2021-003844
21. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi:10.1001/jamaoncol.2020.1024
22. Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. doi:10.1001/jamaoncol.2020.4564
23. van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med. 2023;389(19):1778–1789. doi:10.1056/NEJMoa2309863
24. Hanauer SB, Sands BE, Schreiber S, et al. Subcutaneous infliximab (CT-P13 SC) as maintenance therapy for inflammatory bowel disease: two randomized phase 3 trials (LIBERTY). Gastroenterology. 2024;167(5):919–933. doi:10.1053/j.gastro.2024.05.006
25. Schreiber S, Ben-Horin S, Leszczyszyn J, et al. Randomized controlled trial: subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology. 2021;160(7):2340–2353. doi:10.1053/j.gastro.2021.02.068
26. Sahni J, Dugel PU, Patel SS, et al. Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration: the AVENUE phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138(9):955–963. doi:10.1001/jamaophthalmol.2020.2685
27. Brown DM, Boyer DS, Do DV, et al. Intravitreal aflibercept 8 mg in diabetic macular oedema (PHOTON): 48-week results from a randomised, double-masked, non-inferiority, phase 2/3 trial. Lancet. 2024;403(10432):1153–1163. doi:10.1016/S0140-6736(23)02577-1
28. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. doi:10.1056/NEJMoa2212948
29. Goswamy VP, Lee KE, McKernan EM, Fichtinger PS, Mathur SK, Viswanathan RK. Omalizumab for treatment of idiopathic angioedema. Ann Allergy Asthma Immunol. 2022;129(5):605–11.e1. doi:10.1016/j.anai.2022.07.017
30. Wood RA, Togias A, Sicherer SH, et al. Omalizumab for the treatment of multiple food allergies. N Engl J Med. 2024;390(10):889–899. doi:10.1056/NEJMoa2312382
31. Jeka S, Dokoupilová E, Kivitz A, et al. Equivalence trial of proposed denosumab biosimilar GP2411 and reference denosumab in postmenopausal osteoporosis: the ROSALIA study. J Bone Miner Res. 2024;39(3):202–210. doi:10.1093/jbmr/zjae016
32. Li H, Huang Y, Chen Z, et al. Efficacy and safety of denosumab biosimilar QL1206 versus denosumab in patients with bone metastases from solid tumors: a randomized phase III trial. BioDrugs. 2023;37(2):259–269. doi:10.1007/s40259-023-00579-5
33. Yuen MF, Lim YS, Yoon KT, et al. VIR-2218 (elebsiran) plus pegylated interferon-alfa-2a in participants with chronic hepatitis B virus infection: a phase 2 study. Lancet Gastroenterol Hepatol. 2024;9(12):1121–1132. doi:10.1016/S2468-1253(24)00237-1
34. Sims EK, Carr ALJ, Oram RA, DiMeglio LA, Evans-Molina C. 100 years of insulin: celebrating the past, present and future of diabetes therapy. Nat Med. 2021;27(7):1154–1164. doi:10.1038/s41591-021-01418-2
35. Imiglucerase. Drugs and Lactation Database (Lactmed®). Bethesda (MD): National Institute of Child Health and Human Development; 2006.
36. Wallace EL, Goker-Alpan O, Wilcox WR, et al. Head-to-head trial of pegunigalsidase alfa versus agalsidase beta in patients with Fabry disease and deteriorating renal function: results from the 2-year randomised phase III BALANCE study. J Med Genet. 2024;61(6):520–530. doi:10.1136/jmg-2023-109445
37. Knop FK, Aroda VR, Do Vale RD, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;402(10403):705–719. doi:10.1016/S0140-6736(23)01185-6
38. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851. doi:10.1056/NEJMoa1901118
39. Galderisi A, Cohen N, Calhoun P, et al. Effect of Afrezza on glucose dynamics during HCL treatment. Diabetes Care. 2020;43(9):2146–2152. doi:10.2337/dc20-0091
40. Amelina EL, Krasovsky SA, Akhtyamova-Givirovskaya NE, et al. Comparison of biosimilar tigerase and pulmozyme in long-term symptomatic therapy of patients with cystic fibrosis and severe pulmonary impairment (subgroup analysis of a phase III randomized open-label clinical trial (NCT04468100)). PLoS One. 2021;16(12):e0261410. doi:10.1371/journal.pone.0261410
41. Moura RP, Pacheco C, Pego AP, Des Rieux A, Sarmento B. Lipid nanocapsules to enhance drug bioavailability to the central nervous system. J Control Release. 2020;322:390–400. doi:10.1016/j.jconrel.2020.03.042
42. Ding D, Zhu Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater Sci Eng C Mater Biol Appl. 2018;92:1041–1060. doi:10.1016/j.msec.2017.12.036
43. Sonvico F, Clementino A, Buttini F, et al. Surface-modified nanocarriers for nose-to-brain delivery: from bioadhesion to targeting. Pharmaceutics. 2018;10(1):34. doi:10.3390/pharmaceutics10010034
44. Pardridge WM. Why is the global CNS pharmaceutical market so under-penetrated? Drug Discov Today. 2002;7(1):5–7. doi:10.1016/S1359-6446(01)02082-7
45. Haque S, Md S, Fazil M, et al. Venlafaxine loaded chitosan NPs for brain targeting: pharmacokinetic and pharmacodynamic evaluation. Carbohydr Polym. 2012;89(1):72–79. doi:10.1016/j.carbpol.2012.02.051
46. Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337(1–2):1–24. doi:10.1016/j.ijpharm.2007.03.025
47. Al Asmari AK, Ullah Z, Tariq M, Fatani A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des Devel Ther. 2016;10:205–215. doi:10.2147/DDDT.S93937
48. Patel S, Chavhan S, Soni H, et al. Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J Drug Target. 2011;19(6):468–474. doi:10.3109/1061186X.2010.523787
49. Madane RG, Mahajan HS. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: design, characterization, and in vivo study. Drug Deliv. 2016;23(4):1326–1334. doi:10.3109/10717544.2014.975382
50. Mittal D, Md S, Hasan Q, et al. Brain targeted nanoparticulate drug delivery system of rasagiline via intranasal route. Drug Deliv. 2016;23(1):130–139. doi:10.3109/10717544.2014.907372
51. Md S, Khan RA, Mustafa G, et al. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur J Pharm Sci. 2013;48(3):393–405. doi:10.1016/j.ejps.2012.12.007
52. Xiao XY, Zhu YX, Bu JY, Li GW, Zhou JH, Zhou SP. Evaluation of neuroprotective effect of thymoquinone nanoformulation in the rodent cerebral ischemia-reperfusion model. Biomed Res Int. 2016;2016:2571060. doi:10.1155/2016/2571060
53. Moroz E, Matoori S, Leroux JC. Oral delivery of macromolecular drugs: where we are after almost 100 years of attempts. Adv Drug Deliv Rev. 2016;101:108–121. doi:10.1016/j.addr.2016.01.010
54. Wang M, Wang C, Ren S, et al. Versatile oral insulin delivery nanosystems: from materials to nanostructures. Int J Mol Sci. 2022;23(6):3362.
55. Duran-Lobato M, Niu Z, Alonso MJ. Oral delivery of biologics for precision medicine. Adv Mater. 2020;32(13):e1901935. doi:10.1002/adma.201901935
56. Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243. doi:10.1136/gutjnl-2020-322260
57. Han Y, Gao Z, Chen L, et al. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm Sin B. 2019;9(5):902–922. doi:10.1016/j.apsb.2019.01.004
58. Vllasaliu D, Thanou M, Stolnik S, Fowler R. Recent advances in oral delivery of biologics: nanomedicine and physical modes of delivery. Expert Opin Drug Deliv. 2018;15(8):759–770. doi:10.1080/17425247.2018.1504017
59. Xia D, Wood-Yang AJ, Prausnitz MR. Clearing away barriers to oral drug delivery. Sci Robot. 2022;7(70):eade3311. doi:10.1126/scirobotics.ade3311
60. Wright L, Barnes TJ, Prestidge CA. Oral delivery of protein-based therapeutics: gastroprotective strategies, physiological barriers and in vitro permeability prediction. Int J Pharm. 2020;585:119488. doi:10.1016/j.ijpharm.2020.119488
61. Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. doi:10.1016/j.jconrel.2017.12.015
62. Ribatti D, Nico B, Crivellato E, Artico M. Development of the blood-brain barrier: a historical point of view. Anat Rec B New Anat. 2006;289(1):3–8. doi:10.1002/ar.b.20087
63. Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17(1):69. doi:10.1186/s12987-020-00230-3
64. Li W, Sharma M, Kaur P. The DrrAB efflux system of Streptomyces peucetius is a multidrug transporter of broad substrate specificity. J Biol Chem. 2014;289(18):12633–12646. doi:10.1074/jbc.M113.536136
65. On NH, Miller DW. Transporter-based delivery of anticancer drugs to the brain: improving brain penetration by minimizing drug efflux at the blood-brain barrier. Curr Pharm Des. 2014;20(10):1499–1509. doi:10.2174/13816128113199990458
66. Li W, Rao DK, Kaur P. Dual role of the metalloprotease FtsH in biogenesis of the DrrAB drug transporter. J Biol Chem. 2013;288(17):11854–11864. doi:10.1074/jbc.M112.441915
67. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:10.1016/j.nbd.2009.07.030
68. Khodaverdi K, Bakhshi A, Mozafari MR, Naghib SM. A review of chitosan-based nanocarriers as drug delivery systems for brain diseases: critical challenges, outlooks and promises. Int J Biol Macromol. 2024;278(Pt 3):134962. doi:10.1016/j.ijbiomac.2024.134962
69. Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65(3):259–269. doi:10.1016/j.ejpb.2006.11.009
70. Prakash S. Nano-based drug delivery system for therapeutics: a comprehensive review. Biomed Phys Eng Express. 2023;9(5):052002. doi:10.1088/2057-1976/acedb2
71. Yu S, Xu X, Feng J, Liu M, Hu K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm. 2019;560:282–293. doi:10.1016/j.ijpharm.2019.02.012
72. Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–516. doi:10.1038/nn0602-849
73. Novak P, Pimentel Maldonado DA, Novak V. Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: a double-blinded placebo-controlled pilot study. PLoS One. 2019;14(4):e0214364. doi:10.1371/journal.pone.0214364
74. Craft S, Raman R, Chow TW, et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 2020;77(9):1099–1109. doi:10.1001/jamaneurol.2020.1840
75. Costa CP, Moreira JN, Sousa Lobo JM, Silva AC. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies. Acta Pharm Sin B. 2021;11(4):925–940. doi:10.1016/j.apsb.2021.02.012
76. Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi:10.1016/j.lfs.2017.12.025
77. Laffleur F, Bauer B. Progress in nasal drug delivery systems. Int J Pharm. 2021;607:120994. doi:10.1016/j.ijpharm.2021.120994
78. Lobaina Mato Y. Nasal route for vaccine and drug delivery: features and current opportunities. Int J Pharm. 2019;572:118813. doi:10.1016/j.ijpharm.2019.118813
79. Singh RM, Kumar A, Pathak K. Mucoadhesive in situ nasal gelling drug delivery systems for modulated drug delivery. Expert Opin Drug Deliv. 2013;10(1):115–130. doi:10.1517/17425247.2013.746659
80. Giunchedi P, Gavini E, Bonferoni MC. Nose-to-brain delivery. Pharmaceutics. 2020;12(2). doi:10.3390/pharmaceutics12020138
81. Dorrego-Rivas A, Grubb MS. Developing and maintaining a nose-to-brain map of odorant identity. Open Biol. 2022;12(6):220053. doi:10.1098/rsob.220053
82. Martins PP, Smyth HDC, Cui Z. Strategies to facilitate or block nose-to-brain drug delivery. Int J Pharm. 2019;570:118635. doi:10.1016/j.ijpharm.2019.118635
83. Agrawal M, Saraf S, Saraf S, et al. Stimuli-responsive in situ gelling system for nose-to-brain drug delivery. J Control Release. 2020;327:235–265. doi:10.1016/j.jconrel.2020.07.044
84. Brann DH, Datta SR. Finding the brain in the nose. Annu Rev Neurosci. 2020;43:277–295. doi:10.1146/annurev-neuro-102119-103452
85. Kanazawa T, Taki H, Okada H. Nose-to-brain drug delivery system with ligand/cell-penetrating peptide-modified polymeric nano-micelles for intracerebral gliomas. Eur J Pharm Biopharm. 2020;152:85–94. doi:10.1016/j.ejpb.2020.05.001
86. Babazadeh A, Mohammadi Vahed F, Jafari SM. Nanocarrier-mediated brain delivery of bioactives for treatment/prevention of neurodegenerative diseases. J Control Release. 2020;321:211–221. doi:10.1016/j.jconrel.2020.02.015
87. Zhang YT, He KJ, Zhang JB, Ma QH, Wang F, Liu CF. Advances in intranasal application of stem cells in the treatment of central nervous system diseases. Stem Cell Res Ther. 2021;12(1):210. doi:10.1186/s13287-021-02274-0
88. Yokel RA. Direct nose to the brain nanomedicine delivery presents a formidable challenge. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(2):e1767. doi:10.1002/wnan.1767
89. Upadhaya PG, Pulakkat S, Patravale VB. Nose-to-brain delivery: exploring newer domains for glioblastoma multiforme management. Drug Deliv Transl Res. 2020;10(4):1044–1056. doi:10.1007/s13346-020-00747-y
90. Borrajo ML, Alonso MJ. Using nanotechnology to deliver biomolecules from nose to brain - peptides, proteins, monoclonal antibodies and RNA. Drug Deliv Transl Res. 2022;12(4):862–880. doi:10.1007/s13346-021-01086-2
91. Khatri DK, Preeti K, Tonape S, et al. Nanotechnological advances for nose to brain delivery of therapeutics to improve the Parkinson therapy. Curr Neuropharmacol. 2023;21(3):493–516. doi:10.2174/1570159X20666220507022701
92. Chen Y, Zhang C, Huang Y, et al. Intranasal drug delivery: the interaction between nanoparticles and the nose-to-brain pathway. Adv Drug Deliv Rev. 2024;207:115196. doi:10.1016/j.addr.2024.115196
93. Selvaraj K, Gowthamarajan K, Karri V. Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. Artif Cells Nanomed Biotechnol. 2018;46(8):2088–2095. doi:10.1080/21691401.2017.1420073
94. Erdo F, Bors LA, Farkas D, Bajza A, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–170. doi:10.1016/j.brainresbull.2018.10.009
95. Pandit R, Chen L, Gotz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2020;165-166:1–14. doi:10.1016/j.addr.2019.11.009
96. Dhuria SV, Hanson LR, Frey WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–1673. doi:10.1002/jps.21924
97. Correia AC, Monteiro AR, Silva R, Moreira JN, Sousa Lobo JM, Silva AC. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: crossing or circumventing the blood–brain barrier (BBB) to manage neurological disorders. Adv Drug Deliv Rev. 2022;189:114485. doi:10.1016/j.addr.2022.114485
98. Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi:10.1016/j.neuroscience.2004.05.029
99. Bourganis V, Kammona O, Alexopoulos A, Kiparissides C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur J Pharm Biopharm. 2018;128:337–362. doi:10.1016/j.ejpb.2018.05.009
100. Akpinar Adscheid S, Türeli AE, Günday-Türeli N, Schneider M. Nanotechnological approaches for efficient N2B delivery: from small-molecule drugs to biopharmaceuticals. Beilstein J Nanotechnol. 2024;15:1400–1414. doi:10.3762/bjnano.15.113
101. de Oliveira Junior ER, Santos LCR, Salomão MA, et al. Nose-to-brain drug delivery mediated by polymeric nanoparticles: influence of PEG surface coating. Drug Deliv Transl Res. 2020;10(6):1688–1699. doi:10.1007/s13346-020-00816-2
102. Goncalves J, Bicker J, Gouveia F, et al. Nose-to-brain delivery of levetiracetam after intranasal administration to mice. Int J Pharm. 2019;564:329–339. doi:10.1016/j.ijpharm.2019.04.047
103. Ullah I, Chung K, Bae S, et al. Nose-to-brain delivery of cancer-targeting paclitaxel-loaded nanoparticles potentiates antitumor effects in malignant glioblastoma. Mol Pharm. 2020;17(4):1193–1204. doi:10.1021/acs.molpharmaceut.9b01215
104. Jiang Y, Jiang Y, Ding Z, Yu Q. Investigation of the “nose-to-brain” pathways in intranasal HupA nanoemulsions and evaluation of their in vivo pharmacokinetics and brain-targeting ability. Int J Nanomedicine. 2022;17:3443–3456. doi:10.2147/IJN.S369978
105. Tiozzo Fasiolo L, Manniello MD, Banella S, et al. Flurbiprofen sodium microparticles and soft pellets for nose-to-brain delivery: serum and brain levels in rats after nasal insufflation. Int J Pharm. 2021;605:120827.
106. Kim YS, Sung DK, Kim H, Kong WH, Kim YE, Hahn SK. Nose-to-brain delivery of hyaluronate – FG loop peptide conjugate for non-invasive hypoxic-ischemic encephalopathy therapy. J Control Release. 2019;307:76–89. doi:10.1016/j.jconrel.2019.06.021
107. Akita T, Kimura R, Akaguma S, et al. Usefulness of cell-penetrating peptides and penetration accelerating sequence for nose-to-brain delivery of glucagon-like peptide-2. J Control Release. 2021;335:575–583. doi:10.1016/j.jconrel.2021.06.007
108. Du W, Li H, Tian B, et al. Development of nose-to-brain delivery of ketoconazole by nanostructured lipid carriers against cryptococcal meningoencephalitis in mice. Colloids Surf B Biointerfaces. 2019;183:110446. doi:10.1016/j.colsurfb.2019.110446
109. Maeng J, Lee K. Systemic and brain delivery of antidiabetic peptides through nasal administration using cell-penetrating peptides. Front Pharmacol. 2022;13:1068495. doi:10.3389/fphar.2022.1068495
110. Radis-Baptista G. Cell-penetrating peptides derived from animal venoms and toxins. Toxins. 2021;13(2):147. doi:10.3390/toxins13020147
111. Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci. 2017;38(4):406–424. doi:10.1016/j.tips.2017.01.003
112. Kim NA, Thapa R, Jeong SH, et al. Enhanced intranasal insulin delivery by formulations and tumor protein-derived protein transduction domain as an absorption enhancer. J Control Release. 2019;294:226–236. doi:10.1016/j.jconrel.2018.12.023
113. Soares S, Costa A, Sarmento B. Novel non-invasive methods of insulin delivery. Expert Opin Drug Deliv. 2012;9(12):1539–1558. doi:10.1517/17425247.2012.737779
114. Ulusoy S, Bayar Muluk N, Karpischenko S, et al. Mechanisms and solutions for nasal drug delivery - a narrative review. Eur Rev Med Pharmacol Sci. 2022;26(2 Suppl):72–81. doi:10.26355/eurrev_202212_30487
115. Kaushik A, Jayant RD, Bhardwaj V, Nair M. Personalized nanomedicine for CNS diseases. Drug Discov Today. 2018;23(5):1007–1015. doi:10.1016/j.drudis.2017.11.010
116. Singh S, Shukla R. Nanovesicular-mediated intranasal drug therapy for neurodegenerative disease. AAPS PharmSciTech. 2023;24(7):179.
117. Maher R, Moreno-Borrallo A, Jindal D, Mai BT, Ruiz-Hernandez E, Harkin A. Intranasal polymeric and lipid-based nanocarriers for CNS drug delivery. Pharmaceutics. 2023;15(3):746. doi:10.3390/pharmaceutics15030746
118. Bonferoni MC, Rossi S, Sandri G, et al. Nanoemulsions for “”“Nose-to-Brain” drug delivery. Pharmaceutics. 2019;11(2):84. doi:10.3390/pharmaceutics11020084
119. Borchers A, Pieler T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes. 2010;1(3):413–426. doi:10.3390/genes1030413
120. Wang Y, Hu H, Liu X, Guo X. Hypoglycemic medicines in the treatment of Alzheimer’s disease: pathophysiological links between AD and glucose metabolism. Front Pharmacol. 2023;14:1138499. doi:10.3389/fphar.2023.1138499
121. Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43(1):545–584. doi:10.1146/annurev.pharmtox.43.100901.140248
122. Zhang J, Zhang Y, Wang J, Xia Y, Zhang J, Chen L. Recent advances in Alzheimer’Alzheimer’s disease: mechanisms, clinical trials and new drug development strategies. Signal Transduct Target Ther. 2024;9(1):211. doi:10.1038/s41392-024-01911-3
123. Cunha S, Forbes B, Sousa Lobo JM, Silva AC. Improving drug delivery for Alzheimer’s disease through nose-to-brain delivery using nanoemulsions, nanostructured lipid carriers (NLC) and in situ hydrogels. Int J Nanomedicine. 2021;16:4373–4390. doi:10.2147/IJN.S305851
124. Jucker M, Walker LC. Alzheimer’Alzheimer’s disease: from immunotherapy to immunoprevention. Cell. 2023;186(20):4260–4270. doi:10.1016/j.cell.2023.08.021
125. Song C, Shi J, Zhang P, et al. Immunotherapy for Alzheimer’s disease: targeting β-amyloid and beyond. Transl Neurodegener. 2022;11(1):18. doi:10.1186/s40035-022-00292-3
126. Gaikwad S, Puangmalai N, Sonawane M, et al. Nasal tau immunotherapy clears intracellular tau pathology and improves cognitive functions in aged tauopathy mice. Sci Transl Med. 2024;16(754):eadj5958. doi:10.1126/scitranslmed.adj5958
127. Yang X, Yang W, Xia X, et al. Intranasal delivery of BACE1 siRNA and rapamycin by dual targets modified nanoparticles for Alzheimer’s disease therapy. Small. 2022;18(30):e2203182. doi:10.1002/smll.202203182
128. Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson’Parkinson’s disease. Lancet Neurol. 2021;20(5):385–397. doi:10.1016/S1474-4422(21)00030-2
129. Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):795–808. doi:10.1136/jnnp-2019-322338
130. Tong S-Y, Wang R-W, Li Q, et al. Serum glial cell line-derived neurotrophic factor (GDNF) a potential biomarker of executive function in Parkinson’s disease. Front Neurosci. 2023;17:1136499. doi:10.3389/fnins.2023.1136499
131. Zhao Y, Haney MJ, Fallon JK, et al. Using extracellular vesicles released by GDNF-transfected macrophages for therapy of Parkinson disease. Cells. 2022;11(12):1933. doi:10.3390/cells11121933
132. Billakota S, Devinsky O, Kim K-W. Why we urgently need improved epilepsy therapies for adult patients. Neuropharmacology. 2020;170:107855. doi:10.1016/j.neuropharm.2019.107855
133. Hansen SN, Holm A, Kauppinen S, Klitgaard H. RNA therapeutics for epilepsy: an emerging modality for drug discovery. Epilepsia. 2023;64(12):3113–3129. doi:10.1111/epi.17772
134. Perucca E, White HS, Bialer M. New GABA-targeting therapies for the treatment of seizures and epilepsy: II Treatments in Clinical Development. CNS Drugs. 2023;37(9):781–795.
135. Aigner A. Applications of RNA interference: current state and prospects for siRNA-based strategies in vivo. Appl Microbiol Biotechnol. 2007;76(1):9–21. doi:10.1007/s00253-007-0984-y
136. Perri RGB, Mantello AG, Rosa DS, Beleboni RO. Silencing of the GluN1-NMDA glutamate receptor subunit by intranasal siRNA increases the latency time for seizures in the pilocarpine rodent model of epilepsy. Pharmaceuticals. 2022;15(12):1470. doi:10.3390/ph15121470
137. Liu B, Liu J, Wang M, Zhang Y, Li L. From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci. 2017;11:305. doi:10.3389/fncel.2017.00305
138. Tanaka A, Furubayashi T, Arai M, et al. Delivery of oxytocin to the brain for the treatment of autism spectrum disorder by nasal application. Mol Pharm. 2018;15(3):1105–1111. doi:10.1021/acs.molpharmaceut.7b00991
139. Guastella AJ, Gray KM, Rinehart NJ, et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol Psychiatry. 2015;56(4):444–452. doi:10.1111/jcpp.12305
140. Quintana DS, Lischke A, Grace S, Scheele D, Ma Y, Becker B. Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Mol Psychiatry. 2021;26(1):80–91. doi:10.1038/s41380-020-00864-7
141. Cacciotti-Saija C, Langdon R, Ward PB, et al. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophr Bull. 2015;41(2):483–493. doi:10.1093/schbul/sbu094
142. Shin NY, Park HY, Jung WH, et al. Effects of oxytocin on neural response to facial expressions in patients with schizophrenia. Neuropsychopharmacology. 2015;40(8):1919–1927. doi:10.1038/npp.2015.41
143. Tzabazis A, Kori S, Mechanic J, et al. Oxytocin and migraine headache. Headache. 2017;57(Suppl 2):64–75. doi:10.1111/head.13082
144. Fighera TM, Spritzer PM. Effect of intranasal calcitonin in a patient with McCune-Albright syndrome, fibrous dysplasia, and refractory bone pain. Case Rep Endocrinol. 2017;2017:7898713. doi:10.1155/2017/7898713
145. Kropotova ES, Ivleva IS, Karpenko MN, Mosevitsky MI. Design of enkephalin modifications protected from brain extracellular peptidases providing long-term analgesia. Bioorg Med Chem. 2020;28(1):115184. doi:10.1016/j.bmc.2019.115184
146. Rajput A, Pingale P, Dhapte-Pawar V. Nasal delivery of neurotherapeutics via nanocarriers: facets, aspects, and prospects. Front Pharmacol. 2022;13:979682. doi:10.3389/fphar.2022.979682
147. Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288–303. doi:10.4103/1735-5362.235156
148. Román-Doval R, Torres-Arellanes SP, Tenorio-Barajas AY, Gómez-Sánchez A, Valencia-Lazcano AA. Chitosan: properties and its application in agriculture in context of molecular weight. Polymers. 2023;15(13):2867. doi:10.3390/polym15132867
149. Gartziandia O, Herrán E, Ruiz-Ortega JA, et al. Intranasal administration of chitosan-coated nanostructured lipid carriers loaded with GDNF improves behavioral and histological recovery in a partial lesion model of parkinson’s disease. J Biomed Nanotechnol. 2016;12(12):2220–2230. doi:10.1166/jbn.2016.2313
150. Van Woensel M, Wauthoz N, Rosière R, et al. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J Control Release. 2016;227:71–81. doi:10.1016/j.jconrel.2016.02.032
151. Sava V, Fihurka O, Khvorova A, Sanchez-Ramos J. Enriched chitosan nanoparticles loaded with siRNA are effective in lowering Huntington’s disease gene expression following intranasal administration. Nanomedicine. 2020;24:102119. doi:10.1016/j.nano.2019.102119
152. Nojoki F, Ebrahimi-Hosseinzadeh B, Hatamian-Zarmi A, Khodagholi F, Khezri K. Design and development of chitosan-insulin-transfersomes (Transfersulin) as effective intranasal nanovesicles for the treatment of Alzheimer’s disease: in vitro, in vivo, and ex vivo evaluations. Biomed Pharmacother. 2022;153:113450. doi:10.1016/j.biopha.2022.113450
153. Kamei N, Okada N, Ikeda T, et al. Effective nose-to-brain delivery of exendin-4 via coadministration with cell-penetrating peptides for improving progressive cognitive dysfunction. Sci Rep. 2018;8(1):17641. doi:10.1038/s41598-018-36210-9
154. Hu Y, Jiang K, Wang D, et al. Core-shell lipoplexes inducing active macropinocytosis promote intranasal delivery of c-Myc siRNA for treatment of glioblastoma. Acta Biomater. 2022;138:478–490. doi:10.1016/j.actbio.2021.10.042
155. Kanazawa T, Kurano T, Ibaraki H, Takashima Y, Suzuki T, Seta Y. Therapeutic effects in a transient middle cerebral artery occlusion rat model by nose-to-brain delivery of anti-TNF-alpha siRNA with cell-penetrating peptide-modified polymer micelles. Pharmaceutics. 2019;11(9):478.
156. Zheng X, Shao X, Zhang C, et al. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm Res. 2015;32(12):3837–3849. doi:10.1007/s11095-015-1744-9
157. Zhao YZ, Lin M, Lin Q, et al. Intranasal delivery of bFGF with nanoliposomes enhances in vivo neuroprotection and neural injury recovery in a rodent stroke model. J Control Release. 2016;224:165–175. doi:10.1016/j.jconrel.2016.01.017
158. Yadav S, Gandham SK, Panicucci R, Amiji MM. Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammation. Nanomedicine. 2016;12(4):987–1002. doi:10.1016/j.nano.2015.12.374
159. Rassu G, Soddu E, Posadino AM, et al. Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surf B Biointerfaces. 2017;152:296–301. doi:10.1016/j.colsurfb.2017.01.031
160. Cheng YS, Chen ZT, Liao TY, et al. An intranasally delivered peptide drug ameliorates cognitive decline in Alzheimer transgenic mice. EMBO Mol Med. 2017;9(5):703–715. doi:10.15252/emmm.201606666
161. Picone P, Sabatino MA, Ditta LA, et al. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J Control Release. 2018;270:23–36. doi:10.1016/j.jconrel.2017.11.040
162. Sousa F, Dhaliwal HK, Gattacceca F, Sarmento B, Amiji MM. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J Control Release. 2019;309:37–47. doi:10.1016/j.jconrel.2019.07.033
163. Samaridou E, Walgrave H, Salta E, et al. Nose-to-brain delivery of enveloped RNA - cell permeating peptide nanocomplexes for the treatment of neurodegenerative diseases. Biomaterials. 2020;230:119657. doi:10.1016/j.biomaterials.2019.119657
164. Sukumar UK, Bose RJC, Malhotra M, et al. Intranasal delivery of targeted polyfunctional gold-iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials. 2019;218:119342. doi:10.1016/j.biomaterials.2019.119342
165. Hao R, Sun B, Yang L, Ma C, Li S. RVG29-modified microRNA-loaded nanoparticles improve ischemic brain injury by nasal delivery. Drug Deliv. 2020;27(1):772–781. doi:10.1080/10717544.2020.1760960
166. Musumeci T, Di Benedetto G, Carbone C, et al. Intranasal administration of a TRAIL neutralizing monoclonal antibody adsorbed in PLGA nanoparticles and NLC nanosystems: an in vivo study on a mouse model of Alzheimer’s disease. Biomedicines. 2022;10(5):985. doi:10.3390/biomedicines10050985
167. Yang Y, Zhang X, Wu S, et al. Enhanced nose-to-brain delivery of siRNA using hyaluronan-enveloped nanomicelles for glioma therapy. J Control Release. 2022;342:66–80. doi:10.1016/j.jconrel.2021.12.034
168. Li J, Peng H, Zhang W, et al. Enhanced nose-to-brain delivery of combined small interfering RNAs using lesion-recognizing nanoparticles for the synergistic therapy of Alzheimer’s disease. ACS Appl Mater Interfaces. 2023;15(46):53177–53188. doi:10.1021/acsami.3c08756
169. Lee D, Shen AM, Garbuzenko OB, Minko T. Liposomal formulations of anti-Alzheimer drugs and siRNA for nose-to-brain delivery: design, safety and efficacy in vitro. Aaps J. 2024;26(5):99. doi:10.1208/s12248-024-00967-x
170. Wu J, Li A, Shi Y, et al. Intranasal delivery of mesenchymal stem cell-derived exosomes ameliorates experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2025;146:113853. doi:10.1016/j.intimp.2024.113853
171. Shen H, Aggarwal N, Cui B, et al. Engineered commensals for targeted nose-to-brain drug delivery. Cell. 2025;188(6):1545–62.e16. doi:10.1016/j.cell.2025.01.017
172. Zha S, Wong K-L, All AH. Intranasal delivery of functionalized polymeric nanomaterials to the brain. Adv Healthc Mater. 2022;11(11):e2102610. doi:10.1002/adhm.202102610
173. Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–252. doi:10.1016/S0022-2836(65)80093-6
174. Zheng Y, Cui L, Lu H, et al. Nose to brain: exploring the progress of intranasal delivery of solid lipid nanoparticles and nanostructured lipid carriers. Int J Nanomedicine. 2024;19:12343–12368. doi:10.2147/IJN.S497480
175. Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613. doi:10.1016/j.biopha.2018.04.055
176. Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8(24):1112–1120. doi:10.1016/S1359-6446(03)02903-9
177. Calzoni E, Cesaretti A, Polchi A, Di Michele A, Tancini B, Emiliani C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J Funct Biomater. 2019;10(1):4. doi:10.3390/jfb10010004
178. Vhora I, Patil S, Bhatt P, Misra A. Protein- and peptide-drug conjugates: an emerging drug delivery technology. Adv Protein Chem Struct Biol. 2015;98:1–55.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.