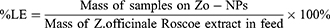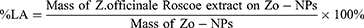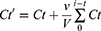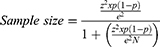Back to Journals » International Journal of Nanomedicine » Volume 19
Breast Cancer Chemoprevention from Nano Zingiber officinale Roscoe
Authors Wardana AP , Kristanti AN, Aminah NS , Fahmi MZ, Raoov M, Indriani
Received 15 July 2024
Accepted for publication 8 October 2024
Published 1 November 2024 Volume 2024:19 Pages 11039—11053
DOI https://doi.org/10.2147/IJN.S474611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sachin Mali
Andika Pramudya Wardana,1 Alfinda Novi Kristanti,2,3 Nanik Siti Aminah,2,3 Mochamad Zakki Fahmi,2 Muggundha Raoov,4 Indriani5
1Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Negeri Surabaya, Surabaya, East Java, Indonesia; 2Department of Chemistry, Faculty of Science and Technology, Universitas Airlangga, Surabaya, East Java, Indonesia; 3Biotechnology of Tropical Medicinal Plants Research Group, Universitas Airlangga, Surabaya, Surabaya, East Java, Indonesia; 4Department of Chemistry, Faculty of Science, University of Malaya, Kuala Lumpur, Malaysia; 5Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Tadulako, Palu, Central Sulawesi, Indonesia
Correspondence: Alfinda Novi Kristanti, Department of Chemistry, Faculty of Science and Technology, Universitas Airlangga, Surabaya, East Java, Indonesia, Tel +62-31-5936501, Fax +62-31-5936502, Email [email protected]
Background: After cardiovascular disease, cancer is one of the leading causes of death due to uncontrolled cell growth. Breast cancer is among the most prevalent types of cancer. Zingiber officinale Roscoe. rich in phenolic compounds, which can stimulate and function as endogenous antioxidants.
Purpose: Investigation of the in vivo chemopreventive has the potential of nano Z. officinale Roscoe (Zo-NPs) in breast cancer.
Study Design: Using female Mus musculus Balb/c induced with benzo[α]pyrene, the chemopreventive action of Z. officinale Roscoe. nanoencapsulated using κ-carrageenan was assessed.
Results: Z. officinale Roscoe Extract. contains 58 compounds, with the main component being [6]-gingerol with [6]-gingerol content being 697.65 ± 8.52 mg/g extract. Nanoencapsulation of Z. officinale Roscoe. has been successfully prepared with a particle size of 483.30 ± 11.23 nm. Zo-NPs are generally resistant to pH, temperature, and salt content variations. Compared to group C1, which underwent ductular dilatation, the administration of Zo-NPs (group T2) to female Mus musculus Balb/c, induced by benzo[α]pyrene, revealed no histological alterations in breast tissue. Moreover, administering Zo-NPs can raise blood serum levels of CAT, GSH, and SOD. In addition, it showed a greater ability to lower TNF-α levels than the T1 group, which received Z. officinale Roscoe extract. (Zo).
Keywords: breast cancer, chemopreventive, nanoencapsulation, red ginger, Zingiber officinale Roscoe
Introduction
One of the deadliest diseases in the world, cancer harms public health everywhere it occurs.1 In 2022, there were 19,976,499 cancer cases worldwide, with 9,743,832 deaths. In Indonesia, the same year saw 408,661 new cancer cases and 242,988 deaths.2 Breast cancer is among the most prevalent types of cancer. Oxidative stress is one cause of cancer.3 The condition known as oxidative stress is brought on by an imbalance between the generation and build-up of reactive oxygen species (ROS) in tissues and cells and a diminished capacity of biological systems to eliminate these reactive byproducts.4
The body has an inbuilt antioxidant system that includes superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT), which is always helpful in preventing or lowering ROS.5 Elevated intracellular ROS levels in cancer cells may be caused by damage to or a decrease in endogenous antioxidants. Cancer cells may proliferate more quickly if they have higher levels of oxidative stress.6 In addition to their direct antioxidant properties, exogenous antioxidants (such as flavonoids, phenolics, etc). can also indirectly raise the expression of endogenous antioxidant genes, hence raising endogenous antioxidant levels.7
Natural dietary ingredients like ginger have anti-inflammatory and anti-carcinogenic qualities.8 Bioactive substances such as phenolic compounds and terpenes are abundant in ginger.9 Zingiber officinale Roscoe, or red ginger, is a plant frequently utilized as a component in conventional medicine. Ginger’s anticancer properties have been extensively studied and published. Dried ginger powder can inhibit the growth of gastric adenocarcinoma and colorectal cancer cells.10 Ginger extract significantly reduced the increased expression of NF-κB and TNF-α in mice with liver cancer. Ginger may have anti-inflammatory and anticancer effects by inhibiting pro-inflammatory TNF-α and deactivating NF-ĸB.11 The expression of genes involved in the Ras/extracellular signal-regulated kinase (ERK) and PI3K/Akt pathways, such as v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), ERK, Akt, and B-cell lymphoma-extralarge (Bcl-xL), which increases the expression of caspase-9 and causes HT-29 colorectal cancer cells to undergo apoptosis, is also increased by ginger extract.12
Herbal medications have promising results in in vitro experiments, but their limited solubility and non-specific effects make for less-than-ideal in vivo test outcomes.13 For this reason, using herbs for treatment usually takes a while. There is currently much interest in creating novel methods to lessen the scarcity of herbal substances for the pharmaceutical sector.14,15 Creating a drug delivery system (DDS) based on nanotechnology can lessen the drawbacks of herbal products. The benefit of DDS based on nanotechnology is that it can reduce the dosage required while improving herbal medicines’ solubility, selectivity, efficacy, and safety and delivering them to the intended organs. Furthermore, by using this procedure, the therapeutic impact of herbal medications can be increased and their toxicity reduced.16 Some research in drug delivery for therapeutic applications includes the development of branched glycopolymer prodrug-derived nanoparticles for anti-PD-L1 antibody therapy,17 as well as branched polymeric drug delivery systems aimed at reducing metabolic competition in tumor cells.18 The production of nano Z. officinale Roscoe and its assessment as a chemopreventive agent for breast cancer are the main focus of this research project.
Materials and Methods
Material
The rhizome of Zingiber officinale Roscoe was collected from Mount Lawu, East Java, Indonesia. The collection site is located at coordinates 7°30’59”S 111°15’15”E and has an altitude of 322 masl. This plant was determined by The Indonesian Biology Generation Foundation (“Yayasan Generasi Biologi Indonesia [YGBI]”) with No. 002/03.235.JI.Genbinesia and the voucher specimen (UA-ZZo02032022) was deposited at Herbarium Center, Institute of Life Science, Technology and Engineering - Universitas Airlangga. There are no additional approvals required to conduct research with this species.
The substances used in the experiment include κ-carrageenan, distilled water, methanol, benzo[α]pyrene (Merck), ethanol, Neutral Buffered Formalin (NBF) 10%, buffer solutions with pH values of pH 2 (HCl (Merck) – KCl (Merck)), 7 (Phosphate buffer (Merck)), and 8.5 (H3BO3 (Merck) – NaOH (Merck)), phosphate buffer saline (PBS) (Merck), CAT kit (BT-Lab E0075Mo), GSH kit (BT-Lab EA0104Mo), SOD kit (BT-Lab E0290Mo), TNF-α kit (BT-Lab E0117Mo), and Na-CMC (Sigma).
Extraction of Z. officinale Roscoe
The rhizomes of Z. officinale Roscoe were extracted by maceration using methanol in a ratio of 1:2 for 24 hours, utilizing approximately 1 kilogram of dry powder. The extract of Z. officinale Roscoe was concentrated using a rotary vacuum evaporator and analyzed using LC-MS (Shimadzu LCMS-8040 LC/MS) for characterization).
Nanoencapsulation of Z. officinale Roscoe
Zo-NPs refer to nanoparticles derived from Z. officinale Roscoe, obtained by nanoencapsulation. This involves coating the extract of Z. officinale Roscoe with κ-carrageenan.19,20 The ratio of Z. officinale Roscoe. extract and carrageenan was 1:4 w/w. The mixture was then ultrasonicated for 5 min, to obtain Zo-NPs. The characterization of Zo-NPs involves the analysis of their physicochemical properties using techniques such as Zetasizer Nano ZS (Malvern), TGA (Perkin Elmer TGA 4000), AFM (Bruker Nanoscan), and FTIR (Shimadzu IRTracer-100).
Stability Analysis of Zo-NPs
The stability of Zo-NPs is seen based on the parameters of heating temperature, salt concentration and changes in pH. The test results measured the turbidity values and UV-Vis spectra of Zo-NPs.20
Loading and Release of Zo-NPs
The loading value of Zo-NPs was determined to find the amount of Z. officinale Roscoe bioactive components trapped in the nanocapsules. Loading consists of loading efficiency (LE) and loading amount (LA) according to equations (1) and (2). The percentage release of bioactive components in Zo-NPs was measured at pH 2, 7, and 8.5 (equation (3)).19,20
Where Ct’: concentration correction at t time
Ct: measured concentration at t time
V: total volume of buffer used
v: volume of aliquots
Chemopreventive Potency
Chemopreventive evaluation of Zo-NPs breast cancer was carried out in vivo, using a female Mus musculus Balb/c, 8 weeks old with a body weight of ± 25 g (Ethical Clearance Certificate, No. 844/HRECC.FODM/VII/2023). Mus musculus Balb/c mice were obtained from an experimental animal farm. The animals were placed in cages with the room temperature maintained at 20–25°C and humidity at 45–55%. The carcinogenic agent used was benzo[α]pyrene, which was induced in the abdominal mammary of female Mus musculus Balb/c. Experimental animals are grouped into 4 groups consisting of:
C0: Control, mice were treated with water only.
C1: Cancer control
T1: Treatment 1, mencit diberi 100 mg/kg BB ekstrak Z. officinale Roscoe secara peroral
T2: Treatment 2, mencit diberi 100 mg/kg BB Zo-NPs secara peroral
Calculation of experimental animal samples using the formula:
Where N: size population
e: margin of error
z: z-score
At the end of treatment, female Mus musculus Balb/c will be analyzed for the histology of breast tissue, kidney, liver, pancreas, spleen, and blood serum (SOD, GSH, CAT, and TNF-α).20 Measurement of blood serum SOD, GSH, CAT, and TNF levels using the calorimetry method with an ELISA kit.
Statistical Analysis
Statistical analysis in this study used one-way ANOVA. Tukey’s test was also used to conduct post-hoc analysis to identify group differences. In this instance, P < 0.05 was deemed significant.
Results
Phytochemical Profiling of Z. officinale Roscoe
LC-MS analysis of the methanol extract of Z. officinale Roscoe identified 58 compounds consisting of benZoic acid derivatives, diaryl heptanoids, flavonoids, methoxyphenols, terpenoids and phenylpropanoids (Figures 1–6) (Table S1). The content of the main component in Z. officinale Roscoe, [6]-gingerol was 697.65 ± 8.52 mg / g extract.
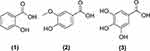 |
Figure 1 Structure of the compound group benZoic acid derivative from Z. officinale Roscoe. |
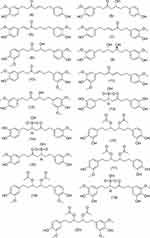 |
Figure 2 Structure of diarylheptanoid group compounds from Z. officinale Roscoe. |
 |
Figure 3 Structure of flavonoid compounds from Z. officinale Roscoe. |
 |
Figure 4 Structure of methoxy phenol group compounds from Z. officinale Roscoe. |
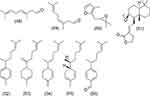 |
Figure 5 Structure of terpene group compounds from Z. officinale Roscoe. |
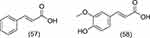 |
Figure 6 Structure of phenylpropanoid group compounds from Z. officinale Roscoe. |
Nano Z. officinale Roscoe Characterization
Zo-NPs are nano Z. officinale Roscoe results from nanoencapsulation using κ-carrageenan. The choice of κ-carrageenan as a coating matrix is widely used in the food and pharmaceutical industries.21 Besides that, carrageenan can also act as a drug delivery system22,23 that is biocompatible and biodegradable.24,25
The particle size of Zo-NPs is 483.30 ± 11.23 d.nm, which is a measure of hydrodynamic diameter. This value refers to how a particle diffuses in a fluid.26 In general, the size of nanoparticles ranges from 1–100 nm,27 but particles with a size of 50–500 nm can still be used in drug delivery systems.28 The size of nanoparticles for pharmaceuticals (drugs) in a matrix tends to be larger because it requires a sufficient number of drug components.27 Based on the AFM topography results, the Zo-NPs are ovoid in shape (Figure 7).
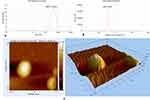 |
Figure 7 (A) Size Distribution, (B) Zeta Potential Distribution, and (C) AFM topography of Zo-NPs. |
The polydispersity index (PDI) value is a physicochemical property that describes the non-uniformity of particle size distribution.29 This property influences the mechanism of endocytosis of particles into cells.30 According to the standards ISO 22412:2017 and ISO 22412:2017, a PDI value < 0.05 is a monodisper sample, while a PDI value > 0.7 is for a polydisperse sample or has a wide particle size distribution.31 Zo-NPs’ PDI value was 0.43 ± 0.02; this falls within the range of the particle size distribution algorithm (0.05–0.7).26
One of the nanomaterials is stabilized by electrostatic repulsion on the particle surface, which can be expressed by the Zeta potential. The stability of nanomaterials in solution tends to be stable at a Zeta potential value of ± 25 mV.32,33 Zo-NPs have a Zeta potential value of −44.47 ± 11.91 mV, so they are considered a stable system that prevents aggregation of nanomaterials. The balance between van der Waals forces and electrostatic repulsion at the interface charges keeps the system stable.34,35
The FTIR absorption bands of Zo-NPs show the presence of wave numbers of Z. officinale Roscoe extract at 2923–2857, 1513, 1432–1371, and 1161–1128 cm−1 which are the C-H sp2 stretching, C=C in the ring, C-H bending, and C-O stretching. The characteristics of κ-carrageenan in Zo-NPs can be observed at wave numbers 921 and 843 cm−1, which are the functional groups of 3.6-anhydrogalactose and galactose-4-sulfate that make up κ-carrageenan (Figure 8). 36,37
 |
Figure 8 (A) FTIR and (B) TGA spectra of Zo-NPs. Abbreviation: a) Z. officinale Roscoe (Zo), b) Zo-NPs, and c) κ-Carrageenan |
Based on the TGA thermogram, Zo-NPs experienced a weight loss at temperatures of 63 °C, 259 °C and 783 °C by 18%, 65%, and 20%. The weight loss at temperatures of 63 °C and 259 °C indicates degradation of the κ-carrageenan component. Degradation at 783 °C is thought to be the degradation of Z. officinale Roscoe extract components. This shows that the nanoencapsulation process of Z. officinale Roscoe extract with κ-carrageenan was successful (Figure 8).
Stability of Nano Z. officinale Roscoe
[6]-gingerol is one of the main components of Z. officinale Roscoe. The stability of Zo-NPs was tested based on changes in pH, heating temperature, and salt (NaCl) to see if the nanoencapsulation process prevented damage to Z. officinale Roscoe components (Figure 9). Based on observations of the UV-Vis spectra of Zo and Zo-NPs, the spectra of both samples have a λmax around 279 nm. Changes in pH do not result in shifts in the absorption bands, either bathochromic or hypsochromic shifts. In alkaline conditions above basic pH (pH 6), Zo experiences a slight hypochromic shift, and at pH 2–4, there is a relatively high hyperchromic shift, namely around 0.1–0.2. This is also supported by a drastic decrease in turbidity values of around 10 NTU. The decrease in the turbidity value of Zo at pH 2–4 is due to the Zo component experiencing coagulation, which is characterized by the formation of a crust at the bottom of the container. This shows that Zo is unstable in acidic conditions. This is related to the stability of the phenolic compounds in Zo. At alkaline pH, phenolic compounds will form stable phenoxide ions. The formation of a resonance-stabilized phenoxide ion is followed by the release of a proton from the -OH group. The more added, the more phenolic compounds are converted into phenoxide ions and dissolve in water. Phenoxide salts are more polar compared to phenolic compounds because phenolic compounds can form hydrogen bonds with water molecules. In addition, most of the phenolic compound molecules consist of non-polar phenyl groups, so their solubility in water is limited. Meanwhile, the addition of acid shifts the pH of the system to an acidic level, causing the equilibrium to favor the formation of keto-tautomeric cyclohexadiene compounds, which are unstable and poorly soluble in water. In Zo-NPs, the UV-Vis absorption band tends to be more stable in all pH ranges. Changing the basic pH of Zo-NPs to become more acidic increased the turbidity value. The more acidic the pH of Zo-NPs, the higher the turbidity value. The turbidity value of Zo-NPs in alkaline conditions decreased. This is because, at acidic pH, κ-carrageenan undergoes hydrolysis, while at alkaline pH, it is not hydrolyzed.38 The glycosidic bonds of κ-carrageenan under acidic conditions will be hydrolyzed, and depolymerization (aggregation) may occur to a larger size, thereby increasing the turbidity value.39
In general, relatively changing the heating temperature of Zo and Zo-NPs does not result in a shift in the UV-Vis absorption band. Based on the results of turbidity measurements from Zo, it shows that at a temperature of 30 °C, it is 354 NTU and increases to 430 NTU at a temperature of 40 °C. The Zo turbidity value at a heating temperature of 40–100 °C tends to be stable in the 420–430 NTU range. This is thought to be because the bioactive components of Zo tend to aggregate so that they have relatively high turbidity values. The turbidity value of Zo-NPs, influenced by changes in heating temperature of 30–100 °C, tends to be stable in the range of 25–30 NTU.
In Zo, adding 0.1 M NaCl resulted in a decrease in turbidity caused by coagulation, characterized by the formation of sediment (crust) at the bottom of the container and the clear solution. The greater concentration of NaCl added also results in the formation of coagulation at the bottom of the container. This is due to the salting-out effect, which causes the solubility of the bioactive components to decrease due to increasing NaCl concentrations.40 In Zo-NPs, increasing the concentration of NaCl added results in an increase in the turbidity value. The higher the NaCl concentration, the higher the turbidity value. This is due to the stability of the Zo-NPs coating, namely κ-carrageenan, which undergoes gelation with Na+ cations from NaCl, which results in molecular aggregation so that the molecular size becomes more significant and the turbidity value increases. In general, the spectra of Zo and Zo-NPs do not experience a shift in the absorption bands, either bathochromic or hypsochromic shifts, with λmax around 279 nm. Both samples experienced a hypochromic shift with increasing NaCl concentration. However, Zo-NPs retain bioactive components better than Zo due to the influence of NaCl concentration.
Loading and Release of Zo-NPs
Nanoencapsulation of Z. officinale Roscoe with κ-carrageenan showed Loading Efficiency (LE) and Loading Amount (LA) values of 80.58 ± 1.82% and 23.02 ± 0.52%. Release of bioactive components in nanoherbal Zo-NPs at pH 2, 7, and 8.5. The choice of pH release is based on the oral administration of nano herbal preparations from the Zingiberaceae family so that the pH of the release is adjusted to the pH of the digestive system. Release of [6]-gingerol in Zo-NPs was most significant at pH 8.5 and most minor at pH 2 after 7 hours (Figure 10). This is thought to be influenced by the stability of κ-carrageenan used as a coating on Zo-NPs. At acidic pH, Zo-NPs aggregate to form a gel caused by the electrostatic decrease between the particles. The gel formation causes the bioactive components to be trapped in the micelles and cannot be released properly.19,20
 |
Figure 10 Release [6]-gingerol in Zo-NPs. Abbreviation: a; pH 2, b; pH 7, and c; pH 8.5. |
Chemopreventive Potency of Zo-NPs
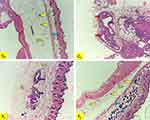
Histological observations of female Mus musculus breast tissue that had been given herbal and nano preparations from the Zingiberaceae family and induced with benzo[α]pyrene can be seen in Figure 11. The parameters observed were the histology changes in the ductuli and inflammatory cells in the breast tissue. Group C0 is a control group of normal female Mus musculus. Breast histology in the group C0 showed no histological changes in the ductuli or inflammatory cells. This is different from group C1, which is a cancer control group. Group C1 experienced benign ductal ectasia or an inflammatory disorder that caused the milk ducts to widen.41 Ductular dilatation is characterized by a build-up of ductular epithelial layers that are flat to cuboidal in shape, which is thought to be due to the benzo[α]pyrene fluid that is induced to fill the ductular cavity, which irritates and compresses the walls of the ductules and causes inflammation. Apart from that, the group T1 also showed ductular hyperplasia. Hyperplasia is an overgrowth of cells lining the breast’s lobules (milk-producing glands) or ductuli. This is thought to be due to injury from treatment with benzo[α]pyrene.
The treatment group was divided into 2 groups, namely T1 (red ginger extract, Zo) and P2 (Zo-NPs). In the group T2, the histology of the ductuli did not show the same histological changes as the control group K0. This shows that the administration of Zo-NPs has succeeded in inhibiting the formation of breast cancer in female Mus musculus, which was induced by benzo[α]pyrene. Observation of the ductuli in groups C0 and T2 showed that the ductuli were small or not yet fully developed. Mice puberty occurs at 3–6 weeks of age. Maximum ductal growth occurs until the age of 3 months or 24 weeks.42 In this study, the age of Mus musculus at the end of treatment was around 14–16 weeks. This is thought to be the reason the observed ductules are still small.
Histology of the group T1 given red ginger extract showed mild ductular dilatation and mild inflammation around the ductuli. This is thought to be due to the administration of the Zo formula at T1 not being strong enough to inhibit benzo[α]pyrene carcinogenesis. The degree of histological damage in group C1 was very severe. This can be seen from the inflammatory process that has penetrated the layers of the skin, and a mass of cancer cells was found in the breast tissue of group C1.
Analysis of SOD, GSH and CAT levels in the blood serum of Mus musculus Balb/c in group αgroups T1 and T2 showed an increase in the three endogenous antioxidant enzymes. The highest levels were obtained in the group T2, which was given Zo-NPs. TNF-α levels in the group C0 showed the highest levels. Giving Zo and Zo-NPs to groups T1 and T2 was able to reduce TNF-α levels (Table 1). The results of the statistical analysis showed a p-value of <0.05, indicating that the treatment with Zo and Zo-NPs significantly increased SOD, GSH, and CAT levels, while decreasing TNF- levels (Table S2–S5).
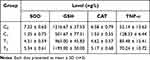 |
Table 1 SOD, GSH, CAT, and TNF-α Levels in Blood Serum of Female Mus Musculus Balb/c |
Discussion
Z. officinale Roscoe is one of the Zingiberaceae family, which is widely known throughout the world with the main bioactive components including [6]-gingerol, [8]-gingerol, [10]-gingerol, etc. Providing Zo and Zo-NPs treatment was proven to increase endogenous antioxidant levels (SOD, GSH, CAT) and reduce TNF-α levels. Increasing endogenous antioxidant levels can inhibit ROS levels produced by benzo[α]pyrene metabolism. Superoxide dismutase (SOD) is an endogenous enzyme that catalyzes the dismutation of O2•¯ to H2O2. This reaction is generally considered the body’s primary antioxidant defence because it prevents further generation of free radicals. In humans, the highest levels of SOD are found in the liver, adrenal glands, kidneys and spleen.43
Catalase (CAT) is an enzyme in peroxisomes containing iron ions. This enzyme is responsible for converting H2O2 into H2O and O2. Another mechanism is that CAT can also carry out catalytic peroxidation by interacting H2O2 with a hydrogen donor compound of one H2O molecule.44
Glutathione is composed of the enzymes glutathione reductase (GR), glutathione peroxidase (GSx), and the cofactor reduced glutathione (GSH). The glutathione enzyme removes the H2O2 compound by forming oxidized glutathione (GSSG) and will be converted back into glutathione by GR with the NADPH cofactor from glucose 6-phosphate dehydrogenase.45
Reduced glutathione (GSH) is a component of the glutathione peroxidase or glutathione reductase system, which functions as a cofactor for the glutathione transferase enzyme, which functions in the detoxification process of drugs and particular chemicals as well as other reactive molecules from cells. In addition, GSH can interact directly with specific ROS (such as, •OH) to detoxify and carry out other essential processes in cells.44
Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine whose expression is increased in various types of cancer. In breast cancer, TNF-α correlates with increased tumour cell proliferation, malignancy, metastatic events, and prognosis. Blocking TNF-α has the potential as a therapy for breast cancer.46
Persistent exposure to benzo[α]pyrene causes excess ROS production. ROS overproduction is associated with the large amount of pro-inflammatory cytokines produced.47 The carcinogenicity of benzo[α]pyrene can be reduced by increasing endogenous enzyme production. SOD and CAT will act to neutralize ROS produced during the benzo[α]pyrene metabolism process. On the other hand, GSH can form a conjugate with the compound benzo[α]pyrene-7,8-oxide, which will later be released through detoxification. Meanwhile, reducing ROS levels can suppress the production of TNF-α so that cell injury can be minimized. When cell injury can be minimized, the formation of cancer cells can indirectly be suppressed. It can be seen that the treatment given to groups P1 and P2 did not reveal histologically severe damage to breast tissue.
Conclusion
Z. officinale Roscoe. is a plant rich in benefits, with one of its main components being [6]-gingerol. Nanoencapsulation process of Z. officinale Roscoe. with κ-carrageenan (Zo-NPs) can increase the chemopreventive activity against breast cancer in female Mus musculus Balb/c induced by benzo[α]pyrene. Apart from preventing breast tissue damage, Zo and Zo-NPs can increase SOD, GSH and CAT levels in blood serum. A pretty drastic reduction in TNF-α levels was shown by the group T2 given Zo-NPs. This has good potential in developing breast cancer chemopreventive supplements from Z. officinale Roscoe.
Acknowledgments
This research was supported by the Universitas Airlangga “Penelitian Unggulan Airlangga 2023 (PUA)” with contract number 293/UNS.15/FT/2023.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gulfishan M, Afzal M, Kazmi I, Quazi AM, Bhat TA, Jahan A. Mechanism of action of anticancer herbal medicines. In: Anticancer Plants: Mechanisms and Molecular Interactions. Springer; 2018:337–360.
2. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today (version 1.1). Lyon, France: International Agency for Research on Cancer; 2024. Available from: https://gco/iarc/who/int/today.
3. Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, LLeonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376–390. doi:10.1016/j.arr.2012.10.004
4. Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017. doi:10.1155/2017/8416763
5. Gupta V, Sharma M. Phytochemical analysis and evaluation of antioxidant activities of methanolic extracts of Maytenus emarginata. Omics. 2012;16(5):257–262. doi:10.1089/omi.2011.0051
6. Shin J, Song M-H, Oh J-W, Keum Y-S, Saini RK. Pro-oxidant actions of carotenoids in triggering apoptosis of cancer cells: a review of emerging evidence. Antioxidants. 2020;9(6):532. doi:10.3390/antiox9060532
7. Sumardika IW, Jawi IM. Ekstrak air daun ubi jalar ungu memperbaiki profil lipid dan meningkatkan kadar SOD darah tikus yang diberi makanan tinggi kolesterol [Purple sweet potato leaf water extract improved lipid profiles and increased blood SOD levels in rats fed a high cholesterol diet]. Medicina. 2012;43(2):67–70.
8. Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1, 2 dimethylhydrazine-induced colon cancer. Clin Chim Acta. 2005;358(1–2):60–67. doi:10.1016/j.cccn.2005.02.018
9. Prasad S, Tyagi AK. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract. 2015;2015:1–11. doi:10.1155/2015/142979
10. Sakulnarmrat K, Srzednicki G, Konczak I. Antioxidant, enzyme inhibitory and antiproliferative activity of polyphenolic‐rich fraction of commercial dry ginger powder. Int J Food Sci Tech. 2015;50(10):2229–2235. doi:10.1111/ijfs.12889
11. Habib SHM, Makpol S, Hamid NAA, Das S, Ngah WZW, Yusof YAM. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics. 2008;63(6):807–813. doi:10.1590/S1807-59322008000600017
12. Tahir AA, Sani NFA, Murad NA, Makpol S, Ngah WZW, Yusof YAM. Combined ginger extract & gelam honey modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29 cells. Nutr J. 2015;14(1):31. doi:10.1186/s12937-015-0015-2
13. Yao H, Liu J, Xu S, Zhu Z, Xu J. The structural modification of natural products for novel drug discovery. Expert Opin Drug Discov. 2017;12(2):121–140. doi:10.1080/17460441.2016.1272757
14. Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today. 2008;13(3–4):161–171. doi:10.1016/j.drudis.2007.10.010
15. Wang C-Z, Calway T, Yuan C-S. Herbal medicines as adjuvants for cancer therapeutics. Am J Chin Med. 2012;40(04):657–669. doi:10.1142/S0192415X12500498
16. Chakraborty K, Shivakumar A, Ramachandran S. Nano-technology in herbal medicines: a review. Int J Herbal Med. 2016;4(3):21–27. doi:10.22271/flora.2016.v4.i3.05
17. Li Z, Zhang Q, Li Z, et al. Branched glycopolymer prodrug-derived nanoassembly combined with a STING agonist activates an immuno-supportive status to boost anti-PD-L1 antibody therapy. Acta Pharmaceutica Sinica B. 2024;14(5):2194–2209. doi:10.1016/j.apsb.2024.02.006
18. Li Y, Duan Z, Pan D, et al. Attenuating metabolic competition of tumor cells for favoring the nutritional demand of immune cells by a branched polymeric drug delivery system. Adv Mater. 2023;35(11):2210161. doi:10.1002/adma.202210161
19. Wardana AP, Aminah NS, Fahmi MZ, et al. Nanoencapsulation of Syzygium polycephalum extract using folate modified κ-carrageenan as vehicles for pronounced anticancer activity. Trop J Nat Product Res. 2020;4(11):945–952. doi:10.26538/tjnpr/v4i11.17
20. Wardana AP, Aminah NS, Kristanti AN, et al. Nano Uncaria gambir as chemopreventive agent against breast cancer. Int J Nanomed. 2023;18:4471–4484. doi:10.2147/IJN.S403385
21. Kirsch P. Carrageenan: a safe additive. Environ Health Perspect. 2002;110(6):A288–A288. doi:10.1289/ehp.110-a288a
22. Kalsoom Khan A, Saba AU, Nawazish S, et al. Carrageenan based bionanocomposites as drug delivery tool with special emphasis on the influence of ferromagnetic nanoparticles. Oxid Med Cell Longev. 2017;2017. doi:10.1155/2017/8158315
23. Sathuvan M, Thangam R, Gajendiran M, et al. κ-carrageenan: an effective drug carrier to deliver curcumin in cancer cells and to induce apoptosis. Carbohydr Polym. 2017;160:184–193. doi:10.1016/j.carbpol.2016.12.049
24. Van de Velde F, Lourenço ND, Pinheiro HM, Bakker M. Carrageenan: a food‐grade and biocompatible support for immobilisation techniques. Adv Synth Catal. 2002;344(8):815–835. doi:10.1002/1615-4169(200209)344:8<815::AID-ADSC815>3.0.CO;2-H
25. Pacheco-Quito E-M, Ruiz-Caro R, Veiga M-D. Carrageenan: drug delivery systems and other biomedical applications. Mar Drugs. 2020;18(11):583. doi:10.3390/md18110583
26. Worldwide MI. Dynamic light scattering, common terms defined. Inform White Paper Malwern Instruments Limited. 2011;2011:1–6.
27. Martien R, Adhyatmika A, Irianto ID, Farida V, Sari DP. Perkembangan teknologi nanopartikel sebagai sistem penghantaran obat [Development of nanoparticle technology as a drug delivery system]. Majalah Farmaseutik. 2012;8(1):133–144.
28. Perera G, Zipser M, Bonengel S, Salvenmoser W, Bernkop-Schnürch A. Development of phosphorylated nanoparticles as zeta potential inverting systems. Eur J Pharm Biopharm. 2015;97:250–256. doi:10.1016/j.ejpb.2015.01.017
29. Bera B. Nanoporous silicon prepared by vapour phase strain etch and sacrificial technique. Int J Comput Appl. 2015;975:42–45.
30. Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):1–17. doi:10.3390/pharmaceutics10020057
31. Mudalige T, Qu H, Van Haute D, Ansar SM, Paredes A, Ingle T. Characterization of nanomaterials: tools and challenges. Nanomater Food Appl. 2019;313–353.
32. Horie M, Fujita K. Toxicity of metal oxides nanoparticles. Adv Mol Toxicol. 2011;5:145–178.
33. Shnoudeh AJ, Hamad I, Abdo RW, et al. Synthesis, characterization, and applications of metal nanoparticles. Biomat and Bionanotech. 2019;527–612.
34. Beck-Broichsitter M, Ruppert C, Schmehl T, et al. Biophysical investigation of pulmonary surfactant surface properties upon contact with polymeric nanoparticles in vitro. Nanomed Nanotechnol Biol Med. 2011;7(3):341–350. doi:10.1016/j.nano.2010.10.007
35. Harush-Frenkel O, Bivas-Benita M, Nassar T, et al. A safety and tolerability study of differently-charged nanoparticles for local pulmonary drug delivery. Toxicol Appl Pharmacol. 2010;246(1–2):83–90. doi:10.1016/j.taap.2010.04.011
36. Pereira L, Amado AM, Critchley AT, Van de Velde F, Ribeiro-Claro PJ. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009;23(7):1903–1909. doi:10.1016/j.foodhyd.2008.11.014
37. Webber V, Carvalho SMD, Ogliari PJ, Hayashi L, Barreto PLM. Optimization of the extraction of carrageenan from Kappaphycus alvarezii using response surface methodology. Food Sci Technol. 2012;32:812–818. doi:10.1590/S0101-20612012005000111
38. Capron I, Yvon M, Muller G. In-vitro gastric stability of carrageenan. Food Hydrocoll. 1996;10(2):239–244. doi:10.1016/S0268-005X(96)80040-3
39. Stanley N. Production, properties and uses of carrageenan. Prod Util Prod Comm Seaweeds FAO Fish Tech Paper. 1987;288:116–146.
40. Noubigh A, Mgaidi A, Abderrabba M, Provost E, Fürst W. Effect of salts on the solubility of phenolic compounds: experimental measurements and modelling. J Sci Food Agric. 2007;87(5):783–788. doi:10.1002/jsfa.2762
41. Kim KW, Cho KR, Seo BK, et al. Sonographic findings of mammary duct ectasia: can malignancy be differentiated from benign disease? J Breast Cancer. 2010;13(1):19–26. doi:10.4048/jbc.2010.13.1.19
42. Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–241. doi:10.1023/A:1026499523505
43. Halliwell B, Zentella A, Gomez EO, Kershenobich D. Antioxidants and human disease: a general introduction. Nutr Rev. 1997;55(1):S44. doi:10.1111/j.1753-4887.1997.tb06100.x
44. Victor VM, McCreath KJ, Rocha M. Recent progress in pharmacological research of antioxidants in pathological conditions: cardiovascular health. Recent Patents Anti-Infective Drug Discov. 2006;1(1):17–31. doi:10.2174/157489106775244136
45. Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–1312. doi:10.1016/s0891-5849(01)00724-9
46. Mercogliano MF, Bruni S, Elizalde PV, Schillaci R. Tumor necrosis factor α blockade: an opportunity to tackle breast cancer. Front Oncol. 2020;10:584. doi:10.3389/fonc.2020.00584
47. Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22(6):1115–1126. doi:10.1016/S0891-5849(96)00501-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.