Back to Journals » International Journal of Nanomedicine » Volume 20
Cancer-Derived Exosomal LINC01615 Induces M2 Polarization of Tumor-Associated Macrophages via RBMX-EZH2 Axis to Promote Colorectal Cancer Progression
Authors Li A, Hong J, Ma X, Huang Y, Jiang Q, Zhang C, Wang Y, Huang Y
Received 5 October 2024
Accepted for publication 5 June 2025
Published 11 June 2025 Volume 2025:20 Pages 7343—7358
DOI https://doi.org/10.2147/IJN.S499381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Eng San Thian
Anshu Li,1,* Jiaxin Hong,2,* Xianxiong Ma,1,* Yongzhou Huang,1 Qi Jiang,1 Chenggang Zhang,3 Yu Wang,4 Yongming Huang1
1Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 3Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China; 4Department of Gastrointestinal Surgery, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongming Huang, Email [email protected]
Background: LncRNAs have been proved to play an important role in human cancers. The M2 polarization of tumor associated macrophages (TAMs) is also reported to promote cancer progression. However, the specific role of cancer derived exosomal lncRNA in the M2 polarization of macrophages remains largely unknown.
Methods: Bioinformatic analysis was used to screen out the differentially expressed lncRNAs in colorectal cancer (CRC). Single-cell RNA sequencing was conducted to investigate the different distribution of cell type in tumor and para-tumor tissues. Function gain and loss assays were performed both in vitro and in vivo to verify the specific role of target genes. The involvement of exosomes was verified by transmission electron microscopy, nano-sight particle tracking analysis and Cre-LoxP system. RNA immunoprecipitation, RNA pull-down, truncation experiment, dual-luciferase reporter assay, chromatin immunoprecipitation, qRT-PCR and Western blot were used to explore the interactions between LINC01615, RBMX and EZH2.
Results: LINC01615 was highly expressed in CRC and contributed to the M2 polarization of TAMs and progression of CRC. Mechanistically, LINC01615 could be transported from CRC cells to TAMs via exosomes. The exosomal LINC01615 acted as a scaffold to mediate the combination between RBMX and EZH2 mRNA and EZH2 promoter, which promoted EZH2 expression and M2 polarization of TAMs, thus promoting CRC progression.
Conclusion: Cancer-derived exosomal LINC01615 induces M2 polarization of TAMs via RBMX-EZH2 axis to promote CRC progression, which may be a reliable diagnostic marker and potential therapeutic target for CRC.
Keywords: colorectal cancer, LINC01615, EZH2, RBMX, exosome, macrophage
Introduction
Colorectal cancer (CRC) poses a great threat to human health and life. According to statistics, it ranks third in men and second in women in terms of incidence of human cancers.1 Furthermore, CRC tends to occur at a younger age.2,3 With the application of immune checkpoint inhibitors in recent years, the prognosis of CRC patients has been improved. However, due to the situation that immune checkpoint inhibitors are only appropriate for CRC patients that are mismatch-repair-deficient (dMMR) or have high levels of microsatellite instability (MSI-H),4 there is still an urgent need to find new effective treatment targets for CRC.
CRC mostly originates from chronic inflammation in the intestines. The occurrence and development are closely related to the immune cell infiltration in the microenvironment. As important immune cells in the tumor microenvironment (TME), tumor-associated macrophages (TAMs) play a vital regulatory role in CRC initiation and progression.5,6 Macrophages represent an exceptionally adaptable and heterogeneous group of immune cells, pivotal to nearly every facet of tumor development. Initially recognized for their role in immune surveillance, research has since revealed that macrophages within the majority of solid tumors often act as facilitators rather than barriers. The simplistic characterization of M2 macrophages has evolved to acknowledge the existence of various subsets of tumor-infiltrating macrophages, each distinguished by their unique distribution, phenotypic traits, and functional capacities. Furthermore, while the prevailing narrative has often emphasized their pro-tumorigenic influences, emerging evidence indicates that certain macrophages with anti-tumor properties persist within the tumor microenvironment. Our understanding of tumor-associated macrophages (TAMs) has significantly advanced over time, paralleling the persistent endeavors aimed at targeting these crucial cellular players.7–9
LncRNA is the abbreviation of long noncoding RNA, which is a kind of functional RNAs that are longer than 200 nucleotides and do not encode proteins.10 In recent years, an increasing number of lncRNAs have been discovered and reported to play an important role in tumor origination and development.11,12 Abnormal expression of lncRNAs is closely associated with tumor occurrence and progression, making them promising diagnostic markers and therapeutic targets for cancers.13 With the advancement of omics technology, more and more RNA-binding proteins (RBPs) have been identified. The interaction between lncRNAs and RBPs has also been reported to play a significant role in cancers.14 However, the role of lncRNA-RBP interaction in the M2 polarization of TAMs remains unclear.
Extracellular vesicles, which are secreted vesicles that mediate intercellular communication, have attracted extensive attention in recent years. With a diameter ranging from 30 to 200 nm, these vesicles encapsulate substances such as proteins, nucleic acids, and lipids, playing important roles in various biological processes.15 There have been reports suggesting the significant role of exosome-mediated communication between tumor cells and TAMs in promoting cancer progression. For example, tumor-derived exosomes promoted bladder cancer progression by facilitating M2 polarization of TAMs.16 M2 polarized TAM-derived exosomes promoted CRC migration and invasion.17 However, whether CRC-derived exosomes play a role in promoting TAM M2 polarization and their specific mechanisms are still to be explored.
In our study, we found that LINC01615 was highly expressed in CRC and there was a significant infiltration of M2 polarized macrophages in the TME by single-cell RNA sequencing. Through a series of in vitro and in vivo experiments, we have demonstrated that tumor-derived exosomes promoted M2 polarization of TAMs through LINC01615, thereby promoting the progression of CRC. These results may provide new insights for the development of targeted therapies for CRC.
Materials and Methods
Bioinformatic Analysis
Gene Expression Omnibus (GEO) database was adopted to search for the differentially expressed lncRNAs (DELs) of CRC (accession numbers: GSE115856 and GSE70880). The data were analyzed with R software (version 4.2.0) with the threshold set as log2 |fold change| ≥ 2. The single-cell RNA sequencing was performed to explore the cell type distribution of the tumor microenvironment. Principal Component Analysis (PCA) was used to evaluate the cell composition with unbiased clustering across all cells and T-distributed Stochastic Neighbor Embedding (TSNE) was adopted to visualize the results. The survival data of CRC patients from The Cancer Genome Atlas (TCGA) database was analyzed to investigate the correlation between M2 polarization score and CRC prognosis. The CatRAPID and RBPdb databases were employed to predict the potential interacted protein of target lncRNA. RNA sequencing was conducted after overexpression of LINC01615 or RBMX and the differentially expressed genes (DEGs) were screened with R software with the threshold set as log2 |fold change| ≥ 2. The analysis results were overlapped to investigate the downstream genes.
Cell Culture and Tissue Samples
Human normal colon epithelial cell line NCM460, CRC cell lines LoVo, HCT116, SW480 and human monocytic leukemia cell line THP-1 were purchased from Sunncell (Wuhan, China). Mouse bone marrow-derived macrophages (BMDMs) were extracted from C57BL/6 mice as previously described.18 NCM460 and THP-1 cells were cultured in 1640 medium. LoVo cells were cultured in F12K medium and HCT116 cells were cultured in McCoy’s 5A medium. All the mediums were supplemented with 10% fetal bovine serum (FBS). The cell incubator was adopted to culture these cells under 37 °C, 5% CO2. All tissue samples were collected from CRC patients who underwent surgical resection in Union Hospital, Huazhong University of Science and Technology. The procedures were approved by the Ethics Committee of Union Hospital, Huazhong University of Science and Technology and were performed according to the principles of the Declaration of Helsinki. All tissue donors provided written informed consent.
Quantitative Real‑time Polymerase Chain Reaction (qRT‑PCR) and Transfection
Total RNA was extracted using an RNA extraction kit (Vazyme, Nanjing) according to the manufacturer’s instructions. qRT-PCR was performed using a SYBR Green PCR kit (TaKaRa, Kyoto, Japan) as previously described.19 Bio-rad quantitative PCR system (California, USA) was used for content determination, with the relative expression calculated with 2−ΔΔCT method. Details of primer sequences are given in Supplementary Table S1. Plasmids of LINC01615-OE, sh-LINC01615, RBMX-OE and RBMX-KD were purchased from GeneChem (Shanghai, China). Lipofectamine 3000 (Invitrogen, MA, USA) was adopted for the transfection of plasmids into cells. Sequence details for gene knockdown are provided in Supplementary Table S2.
Western Blot (WB)
Previous protocol was adopted to perform WB analysis.20 RIPA lysate kit (Beyotime, Shanghai, China) was used for protein extraction. Cellular total proteins were isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred into PVDF membranes, which were incubated with primary antibodies at 4 °C overnight. The next day, the membranes were rinsed with PBS and incubated with second antibodies. After rinsed with PBS for three times, they were finally visualized with gel imaging system (Bio-rad, USA). Antibody details are presented in Supplementary Table S3.
Cell Counting Kit-8 (CCK8) and Colony Formation Assay
CCK8 assay was performed to evaluate cell viability. 2×103 cells were planted in 96-well plates, which were incubated with CCK8 reagent (Dojondo Laboratories, Japan) for 2h after cultured for 1d, 2d, 3d, 4d and 5d. The optical density (OD) value was detected at 450nm. As for colony formation assay, six-well plates were planted with 800 cells per well, which were added with adequate complete mediums and cultured for 2 weeks. The mediums were changed for fresh mediums at 5d and 10d. 0.1% crystal violet was used for the staining of cell colony.
Transwell Assay
Transwell chamber (Corning, Shanghai, China) was employed for transwell assay. 4×104 CRC cells in 200μL serum-free medium were added to the upper chamber. 600μL 25%-FBS medium was added to the lower chamber. After cultured for 24h, the cells remaining on the upper side of the membrane were removed gently by cotton swab. The cells migrating to the lower side of the membrane were stained with 0.1% crystal violet. The images were collected with optical microscope at 200x.
Flow Cytometry Analysis
CD11b-FITC and CD206-PE antibodies were used for the mark of CD11b+CD206+ macrophages. The cells were digested, washed, and incubated with above antibodies away from light at room temperature for 15 mins, which were finally subjected to BD flow cytometer (NJ, USA) to evaluate the cell percentage of the samples. The detailed antibody information is provided in Supplementary Table S3.
Exosomes Isolation and Analysis
The exosomes were isolated as previous study reported.19 The cells were cultured in mediums supplemented with 10% exosome-free FBS (Saios, Wuhan, China) for 48h. Then the cell mediums were collected and differentially centrifuged at 500 × g and 3000 × g for 10 min. The supernatants were collected and filtrated with 0.2μm filters, which were subsequently subjected to centrifugation at 1.2×105 × g at 4 °C for 2 h. The sediments were resuspended with PBS to collect the exosomes. Transmission electron microscope (Thermo, MA, USA) was adopted to take pictures of the exosomes, with the number and size recorded by nanoparticle tracking analysis (NTA) (NanoSight NS300 system, NanoSight Technology, Malvern, UK).
RNA-Fluorescence in Situ Hybridization (FISH)
FISH probe kit (RiboBio, China) was utilized to perform RNA-FISH according to the manufacturer’s instructions. Briefly, the cells were fixed and permeated before they were incubated with the probes away from light at 37 °C overnight. The second day, they were washed and dyed with DAPI and finally subjected to Carl Zeiss confocal laser scanning microscope (LSM 780 with Airyscan, Germany) for collection of the fluorescent pictures. LINC01615 probe labelled with biotin was synthesized by RiboBio (Guangzhou, China).
RNA-Immunoprecipitation (RIP)
RIP assay was performed with RIP kit (Millipore, MA, USA) following the manufacturer’s specification. Briefly, the protein A/G magnetic beads were incubated with specific antibodies for 1h. Then the cell lysates were incubated with the antibody-protein A/G magnetic beads complex in shaky circumstance at 4 °C overnight. The next day, the immunoprecipitation was washed and immunoprecipitated RNA was extracted, which was detected by qRT-PCR. Detailed antibody information can be seen in Supplementary Table S3.
RNA Pull-Down
RNA pull-down assay was conducted with RNA-protein pull-down kits (Thermo Scientific, USA) in accordance with the manufacturer’s protocol. Briefly, the target RNA was labelled with biotin and incubated with streptavidin magnetic beads at 4 °C overnight. The second day, the cell lysates were incubated with RNA-magnetic beads complex. The proteins pulled down by target RNA were washed and detected by WB analysis.
Dual-Luciferase Reporter Assay
Wildtype EZH2, mutant EZH2, LINC01615 overexpression plasmid or sh-LINC01615 plasmid were co-transfected into CRC cells. The dual-luciferase reporter kit (MCE, China) was adopted to perform dual-luciferase reporter assay in accordance with the manufacturer’s instructions after 48h of transfection. The ratio of firefly luciferase activity to renilla luciferase activity was considered as the relative luciferase activity.
Chromatin Immunoprecipitation (ChIP)
ChIP assay was performed as our previous study reported.21 Magna ChIP™ G Assay Kit was used for the experiment according to the manufacturer’s instructions. First, 37% formaldehyde was used for the crosslinking of protein and DNA at room temperature for 10mins. Then the chromatin was smashed by sonication. Finally, the DNA-protein complexes were immunoprecipitated by corresponding antibodies. The detail of used antibodies is showed in Supplementary Table S3. The immunoprecipitated DNA was detected by DNA electrophoresis or PCR.
Enzyme Linked Immunosorbent Assay (ELISA)
Sandwich ELISA was performed to determine the cytokines secreted by macrophages. IL 1β (MLbio, China), TNFα (MLbio, China), Arginase-1 (MultiSciences, China) and IL 10 (MLbio, China) ELISA kits were utilized to conduct the detection following the manufacturer’s instructions. Briefly, the solid phase carrier of the reaction hole was coated with specific antibody. After incubated with supernatant samples, the reaction hole was added with enzyme labelled specific antibody and substrate. The OD value was detected at 450nm after the color reaction was terminated.
M2 Score
Single-sample Gene Set Enrichment Analysis (ssGSEA) using GSVA R packages was applied to calculate the M2 score of each TCGA samples,22 The TCGA expression matrix and M2 macrophage molecular markers from cellmarker2.023 were used as input files.
Animal Experiments
The Ethics Committee of Union Hospital, Huazhong University of Science and Technology approved the animal experiments. The care and use of mice were performed according to the guidelines of the Institutional Animal Care and Use Committee of the Huazhong University of Science and Technology. BALB/c nude mice (male, 4 weeks old) were purchased from Huafukang (Beijing, China) and managed by professional animal keepers in sterile animal rooms. All mice were separated into four groups randomly. To develop subcutaneous xenograft models, 5×106 cells suspended in 100μL PBS were injected into the groin of mice. The tumor length and width were recorded every 5 days after implantation and the tumor volumes were calculated. For peritoneal metastasis models, 5×106 cells suspended in 100μL PBS were injected into the peritoneum of mice. After 25 days, the mice were sacrificed. The subcutaneous xenografts and peritoneal metastatic tumors were separated and the tumor weights were recorded.
Statistical Analysis
All in vitro experiments were repeated at least three times. Data are presented as mean ± standard deviation (SD). The differences were compared by Student’s t-test. Statistical analysis was conducted with GraphPad Prism (version 8.0). P < 0.05 was considered significant.
Results
LINC01615 Is Highly Expressed in CRC and Promotes CRC Proliferation, Invasion and Migration
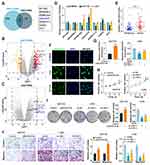
The DELs between CRC and normal tissues were searched from the microarray datasets of GEO database (Accession number: GSE115856 and GSE70880) and seven lncRNAs were identified (Figure 1A–C). Their expression was detected in NCM460 and CRC cell lines of LoVo and HCT116, and only LINC01615 was found to be consistently highly expressed in CRC cells (Figure 1D). The genetic locus of LINC01615 is located on chromosome 6q27 (Figure S1A). The total length of LINC01615 amplified by the RACE technique was 647 bp and Sanger sequencing verified the sequence of LINC01615 (Figure S1B). Further analysis with CRC tumor and peritumor tissues showed that LINC01615 had higher expression in CRC tumor tissues (Figure 1E). RNA-FISH results indicated that LINC01615 was mainly located in cytoplasm (Figure 1F). To further explore the role of LINC01615 in CRC, phenotypic experiments were conducted after LINC01615 overexpression or knockdown (Figure 1G). It was found that overexpression of LINC01615 promoted CRC proliferation, invasion and migration, and inverse effects were observed after LINC01615 knockdown (Figure 1H–J).
LINC01615 Promotes CRC Proliferation, Invasion and Migration via Facilitating M2 Polarization of TAMs
To investigate the potential role of LINC01615 on the tumor environment of CRC, Single-cell RNA sequencing was conducted to investigate the distribution of cell type in tumor and para-tumor tissues (Figure 2A). Macrophages were found to be largely abundant in tumor tissues, especially for M2 polarized macrophages (Figure 2B and C), which were reported to promote cancer progression.24,25 To explore the correlation between LINC01615 and M2 polarization of TAMs, we used IL-4 to induce M2 polarization of TAMs or cocultured them with LoVo-sh-LINC01615 cells. The expression of M2 polarization markers (Ariginase-1, IL-1ra, IL-4 and TGFβ) in macrophages were detected and were found to be increased when induced by IL-4. Meanwhile, compared with CRC cells treated by negative control shRNA (sh-NC), the expression of M2 markers were significantly decreased in macrophages co-cultured with CRC cells treated with sh-LINC01615 (Figure 2D and E). Besides, the proliferation, invasion and migration of CRC cells was significantly elevated with IL4-activated M2 macrophages in comparison to that of PBS-treated macrophages, knockdown of LINC01615 in co-cultured macrophages obviously suppressed the proliferation, invasion and migration of CRC cells (Figure 2F and G). Analysis between M2 score and the survival of CRC patients from TCGA database showed that higher M2 score was related to worse overall survival of CRC patients (Figure 2H). All above results indicate that LINC01615 promotes CRC progression by facilitating M2 polarization of TAMs.
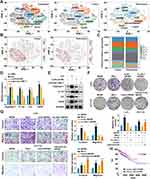
LINC01615 Promotes M2 Polarization of TAMs by Exosomes
Previous studies reported that exosome-packed lncRNAs could promote M2 polarization of macrophages.26,27 To explore whether exosomes participated in this process, we isolated the extracellular vesicles from LoVo-sh-NC and LoVo-sh-LINC01615 cells. Electron microscopy proved the typical size of exosomes and NTA revealed that the distribution of diameters mainly concentrated on 50–150 nm (Figure 3A). The results of WB with markers of exosomes (CD63, CD81 and TSG101) showed that knockdown of LINC01615 in LoVo cells did not affect the number of excretive exosomes (Figure 3B), but reduced the expression of LINC01615 in exosomes (Figure 3C). We then used the Cre-LoxP system28 to investigate the transportation of exosomal LINC01615 into macrophages (Figure S1C). The macrophages were transfected with pLV-CMV-Loxp-DsRed-stop-LoxP-eGFP virus, and were added with the exosomes of LoVo cells transfected with CD63-Cre. The results showed that the fluorescence of macrophages transformed from red to green, suggesting that CD63-Cre packed in exosomes was taken up by macrophages and successfully expressed (Figure 3D). qRT-PCR results indicated that the expression of LINC01615 in macrophages was significantly reduced by the exosomes of LoVo-sh-LINC01615 cells (Figure 3E). FISH results showed that macrophages co-cultured with LoVo exosomes had higher fluorescence of LINC01615 and Arginase-1 (a marker of M2 polarized macrophages) (Figure 3F). WB analysis with markers of M2 polarized macrophages (Arginase-1 and IL-4) revealed that co-culture with LoVo exosomes promoted M2 polarization of macrophages, while knockdown of LINC01615 in LoVo cells weakened the promotion of M2 phenotype (Figure 3G). Flow cytometry analysis results showed that co-culture with LoVo-sh-LINC01615 exosomes decreased the percentage of CD206+CD11b+ macrophages (M2 polarized macrophages) while co-culture with HCT116-LINC01615 exosomes had the inverse effects (Figure 3H). In addition, colony formation and transwell assays showed that co-culture with macrophages promoted the proliferative, invasive and migratory capacities of LoVo cells. However, these effects were weakened attenuated when the macrophages were added with LoVo-sh-LINC01615 exosomes (Figure 3I–J), and were further enhanced when the macrophages were added with HCT116-LINC01615 exosomes (Figure S2A and B). Taken together, these results proved that LINC01615 could be wrapped in exosomes and transported from CRC cells into macrophages, which promoted M2 polarization of TAMs.
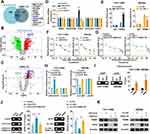
LINC01615 Directly Combines with RBMX
To investigate the cancer-promoting effect of LINC01615, we predicted the RBPs of LINC01615 from CatRAPID and RBPdb databases. The results were overlapped and three RBPs were screened out (Figure 4A). RIP was conducted with antibodies of these three proteins and RBMX was found to directly bind with LINC01615. Moreover, overexpression of LINC01615 promoted the combination between RBMX and LINC01615 (Figure 4B and C). RNA pull-down assay showed that LINC01615 could pull down RBMX (Figure 4D). To explore the specific domain of RBMX that combines with LINC01615, truncation experiment was performed and three truncated proteins were generalized (GST-RRM, GST-RRM1CTR and GST-C-RBD) (Figure 4E). RIP was conducted with these three truncated proteins and DNA electrophoresis was carried out with the reverse transcription products of immunoprecipitated RNA. The results indicated that RBMX could bind with LINC01615 in domain RRM1CTR (Figure 4F). Furthermore, we found that overexpression or knockdown of LINC01615 did not affect the expression of RBMX both in RNA and protein levels (Figure 4G).
RBMX Promotes EZH2 Expression by Combining with EZH2 mRNA and EZH2 Promoter
To explore the downstream target of LINC01615 and RBMX, we conducted RNA sequencing after overexpressing LINC01615 and RBMX, and eleven DEGs were screened out (Figure 5A). As shown in Figure 5B and C, five genes were found to be consistently expressed after overexpressing LINC01615 and RBMX. qRT-PCR was performed to verify the expression of these five genes after overexpression or knockdown of LINC01615, and only EZH2 was found to be consistent with the expression of LINC01615 (Figure 5D). RIP results confirmed the combination between RBMX and EZH2 mRNA in macrophages. Overexpression of LINC01615 promoted their combination while knockdown of LINC01615 inhibited it (Figure 5E). Furthermore, overexpression of LINC01615 promoted the stability of EZH2 mRNA, and knockdown of RBMX could weaken the promotion. Similarly, overexpression of RBMX could rescue the reduced stability of EZH2 mRNA (Figure 5F and G). Dual-luciferase reporter assay showed that overexpression of LINC01615 promoted the combination between RBMX and EZH2 mRNA while knockdown of LINC01615 inhibited it (Figure 5H). Meanwhile, ChIP was conducted with RBMX antibody and DNA electrophoresis was adopted to detect the enrichment of EZH2 promoter. The results showed that RBMX combined with EZH2 promoter (Figure 5I). In addition, we found that overexpression of LINC01615 promoted the combination between RBMX and EZH2 promoter and knockdown of RBMX could weaken the promotion. Similarly, overexpression of RBMX could rescue the reduced combination by knockdown of LINC01615 (Figure 5J). WB results showed that overexpression of LINC01615 promoted the protein expression of EZH2, which could be rescued by the knockdown of RBMX. And overexpression of RBMX could rescue the reduced protein expression of EZH2 by LINC01615 knockdown (Figure 5K). Above all, our results indicated that RBMX combined with EZH2 mRNA and promoted its stability. On the other hand, RBMX could bind with EZH2 promoter and promoted the transcription of EZH2.
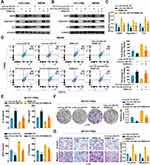
LINC01615 Induces M2 Polarization of TAMs by RBMX-EZH2 Axis to Promote CRC Progression
Rescue experiments were conducted to investigate whether LINC01615 promoted M2 polarization of TAMs by RBMX-EZH2 axis. The results showed that co-culture with LoVo-sh-LINC01615 EV decreased the protein and RNA expression of EZH2 and M2 polarization markers (Ariginase-1 and IL-4) in macrophages, while RBMX overexpression could rescue these changes (Figure 6A and C). And knockdown of RBMX could also rescue the increased expression of EZH2 and M2 polarization markers in macrophages induced by the co-culture with HCT116-LINC01615 EV (Figure 6B). Similarly, rescue experiments were performed with flow cytometry to detect the percentages of M2 polarized macrophages and showed the same results (Figure 6D). Cytokines of M1 and M2 polarized macrophages were detected to measure their functions. M1 polarized macrophages associated cytokines (IL-1β and TNFα) were found to be upregulated after co-culture with LoVo-sh-LINC01615 EV but reduced following the overexpression of RBMX. The inverse effects were observed with M2 polarized macrophages associated cytokines (Ariginase-1 and IL-10) (Figure 6E). The LoVo cells were cocultured with macrophages which were treated with LoVo-sh-LINC01615 EV and overexpression of RBMX to explore the specific role of LINC01615-RBMX-EZH2 axis in CRC progression. The proliferation, invasion and migration of LoVo cells were inhibited after co-culture with macrophages treated with LoVo-sh-LINC01615 EV, but were rescued after overexpression of RBMX in macrophages (Figure 6F and G), the opposite effects were observed in HCT116 cells after co-culture with macrophages treated with HCT116-LINC01615 EV and knockdown of RBMX (Figure S2C and D). To augment clinical relevance, a microsatellite stable (MSS) colorectal cancer (CRC) cell line, SW480, was employed to corroborate our research findings. The outcomes indicated that following co-culture with SW480 cell extracellular vesicles (SW480-sh-LINC01615 EV), characterized by low expression of LINC01615, there was a notable decrease in the protein and RNA expression levels of EZH2 and M2 polarization markers (arginase-1 and IL-4) in macrophages, changes that could be reversed through RBMX overexpression (Figure S3A and B). Flow cytometry rescue experiments conducted to ascertain the proportion of M2-polarized macrophages yielded consistent findings (Figure S3C). Furthermore, the analysis of cytokines in M1- and M2-polarized macrophages revealed that co-culture with SW480 cell EVs exhibiting low LINC01615 expression resulted in an upregulation of M1-polarized macrophage-associated cytokines (IL-1β and TNFα), which were subsequently downregulated following RBMX overexpression. Conversely, M2-polarized macrophage-associated cytokines (arginase-1 and IL-10) displayed an opposing pattern of alterations (Figure S3D). To investigate the specific role of the LINC01615-RBMX-EZH2 axis in the progression of SW480, SW480 cells were co-cultured with macrophages treated with SW480-sh-LINC01615 EV and overexpressing RBMX. The results demonstrated that co-culture with macrophages treated with SW480-sh-LINC01615 EV inhibited the proliferation, invasion, and migration capabilities of SW480 cells, although these abilities were reinstated following RBMX overexpression in macrophages (Figure S3E and F). In vivo experiments confirmed that co-culture with macrophages which were treated with LoVo-sh-LINC01615 EV inhibited the proliferation and peritoneal metastasis of HCT-116 cells, while overexpression of RBMX in macrophages could rescue the inhibition (Figure 7A and B). In conclusion, our results indicate that cancer-derived exosomal LINC01615 induces M2 polarization of TAMs by RBMX-EZH2 axis to promote CRC progression.
Discussion
LncRNAs are one of the key regulatory factors in biological processes. Their abnormal expression is related to the onset and development of many diseases including cancer.29,30 LINC01615 has been reported to be highly expressed in breast cancer and CRC, promoting their occurrence and progression.31,32 In Head and neck squamous cell carcinoma (HNSCRC), LINC01615 was reported to be related to its immune suppression state, and could serve as a prognostic indicator for the metastasis of HNSCRC and hepatocellular carcinoma.33,34 These previous studies suggest that LINC01615 may play an important role in cancer. Through bioinformatics analysis and single-cell RNA sequencing, we also found high expression of LINC01615 and abundant infiltration of M2 polarized macrophages in CRC. Further exploration revealed that CRC cells interacted with TAMs through exosomal LINC01615, which promoted M2 polarization of TAMs and thus facilitating CRC development. Notably, the pro-metastatic effects of LINC01615 were consistently observed in both LoVo and HCT116 cell lines. The latter, known for its aggressive phenotype, exhibited heightened metastatic potential in in vivo models when exposed to LINC01615-enriched exosomes. This aligns with our in vitro findings where HCT116 cells displayed increased invasion and migration upon co-culture with reprogrammed TAMs. Orthotopic data will be reported separately due to ongoing technical optimization. Our study first discovered that LINC01615 could be transmitted to the TME by exosomes and reshaped it towards a more beneficial direction for tumor progression, thus promoting CRC development, which provides new research directions for treating CRC.
Tumors are the result of interactions between tumor cells and the surrounding TME. TAMs are an important type of immune cells infiltrated in the TME and play a crucial role in tumor occurrence and metastasis, extracellular matrix remodeling, tumor immune escape, and drug resistance.35 Therefore, TAMs are a promising target for cancer therapy. Our study has demonstrated the key role of M2 polarization of TAMs in CRC progression and proved that inhibiting M2 polarization of TAMs could effectively suppress CRC progression, which provides a theoretical basis for targeting TAMs in CRC treatment.
As an important mediator of communication between cells in the TME, exosomes also play a crucial role in tumor progression.36 Our research showed that CRC delivered LINC01615 to TAMs through exosomes, thereby promoting M2 polarization of TAMs and CRC progression. As biological carriers, exosomes were reported to be applied for anti-tumor treatment by delivering therapeutic drugs.37 Our study demonstrated that knockdown of LINC01615 in CRC cells could reduce its content in exosomes, thus inhibiting M2 polarization of TAMs and CRC progression, which also proves the therapeutic potential of exosomes. In addition, as a subset of EVs, exosomes have an average diameter of approximately 100 nm and contain nucleic acids, proteins, amino acids, metabolites and other substances. Depending on the type and amount of contents in exosomes, it is possible to determine the type and status of source cell; therefore they may become one of the indicators for tumor diagnosis.38
Previous studies reported that lncRNA regulated gene expression mainly by competing with mRNAs for miRNA binding sites, combining with the enhancers and 3′-UTRs of genes, or recruiting the transcription factors to gene promoters.39,40 With the discovery of more RBPs, the role of lncRNA-RBP interaction network in cancer has also attracted widespread attention.41 After binding with RBPs, lncRNA regulates its stability, localization, and the expression of downstream genes, which plays an important role in various biological processes like tumor proliferation, metastasis, drug resistance and tumor metabolism.14 Our study found that LINC01615 acted as a scaffold after being transported to TAMs through exosomes. It mediated the combination between RBMX and EZH2 mRNA as well as EZH2 promoter to promote EZH2 expression, which facilitated M2 polarization of TAMs. This also suggests the important role of lncRNA-RBP interaction network in reshaping TME.
In conclusion, our study found that LINC01615 was highly expressed in CRC and promoted its progression. Mechanistically, LINC01615 could be transported from CRC cells to TAMs via exosomes and promoted M2 polarization of TAMs through the RBMX-EZH2 axis, thereby promoting CRC progression. These results suggest that LINC01615 may be a reliable diagnostic marker and potential therapeutic target for CRC.
Data Sharing Statement
All datasets and raw data generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the ethics committee of the Huazhong University of Science and Technology, and all included patients provided written informed consent. All methods were carried out in accordance with relevant guidelines and regulations. All animal experiments were carried out according to the guidelines and experiments were approved by the Institutional Animal Care Use Committee of Huazhong University of Science and Technology.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was supported by National Natural Science Foundation of China (82000512, 82403220).
Disclosure
The authors declare no competing interests in this work.
References
1. Taieb J, André T, Auclin E. Refining adjuvant therapy for non-metastatic colon cancer, new standards and perspectives. Cancer Treat Rev. 2019;75:1–11. doi:10.1016/j.ctrv.2019.02.002
2. Lancet Oncology T. The Lancet O. Colorectal cancer: a disease of the young? Lancet Oncol. 2017;18(4):413. doi:10.1016/S1470-2045(17)30202-4
3. Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–2185. doi:10.1136/gutjnl-2019-319511
4. Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375.
5. Cheng Y, Zhu Y, Xu J, et al. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumor-associated macrophages via regulating DUSP6-Erk1/2 pathway. Mol Cancer. 2018;17(1):13. doi:10.1186/s12943-017-0747-z
6. Liu M, Fu X, Jiang L, et al. Colon cancer cells secreted CXCL11 via RBP-Jκ to facilitated tumour-associated macrophage-induced cancer metastasis. J Cell & Mol Med. 2021;25(22):10575–10590. doi:10.1111/jcmm.16989
7. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi:10.1038/nrclinonc.2016.217
8. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382.
9. Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904.
10. Li J, Meng H, Bai Y, Wang K. Regulation of lncRNA and its role in cancer metastasis. Oncol Res. 2016;23(5):205–217. doi:10.3727/096504016X14549667334007
11. Smolarz B, Zadrożna-Nowak A, Romanowicz H. The role of lncRNA in the development of tumors, including breast cancer. Int J Mol Sci. 2021;22(16):8427. doi:10.3390/ijms22168427
12. Chen Y, Li Z, Chen X, Zhang S. Long non-coding RNAs: from disease code to drug role. Acta pharmaceutica Sinica B. 2021;11(2):340–354.
13. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–3981.
14. Yao ZT, Yang YM, Sun MM, et al. New insights into the interplay between long non-coding RNAs and RNA-binding proteins in cancer. Cancer Commun. 2022;42(2):117–140.
15. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
16. Jiang Z, Zhang Y, Zhang Y, Jia Z, Zhang Z, Yang J. Cancer derived exosomes induce macrophages immunosuppressive polarization to promote bladder cancer progression. Cell Commun Signal. 2021;19(1):93.
17. Lan J, Sun L, Xu F, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79(1):146–158.
18. Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci U S A. 2013;110(35):14408–14413. doi:10.1073/pnas.1306376110
19. Zhang C, Wei G, Zhu X, et al. Exosome-delivered circSTAU2 inhibits the progression of gastric cancer by targeting the miR-589/CAPZA1 axis. Int J Nanomed. 2023;18:127–142.
20. Zhang C, Ma X, Wei G, et al. Centrosomal protein 120 promotes centrosome amplification and gastric cancer progression via USP54-mediated deubiquitination of PLK4. iScience. 2023;26(1):105745.
21. Ma X, Chen H, Li L, Yang F, Wu C, Tao K. CircGSK3B promotes RORA expression and suppresses gastric cancer progression through the prevention of EZH2 trans-inhibition. J Exp Clin Cancer Res. 2021;40(1):330.
22. Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 2013;14:7. doi:10.1186/1471-2105-14-7
23. Hu C, Li T, Xu Y, et al. CellMarker 2.0: an updated database of manually curated cell markers in human/mouse and web tools based on scRNA-seq data. Nucleic Acids Res. 2023;51(D1):D870–D6. doi:10.1093/nar/gkac947
24. Xiao M, Bian Q, Lao Y, et al. SENP3 loss promotes M2 macrophage polarization and breast cancer progression. Mol Oncol. 2022;16(4):1026–1044. doi:10.1002/1878-0261.12967
25. Chen S, Morine Y, Tokuda K, et al. Cancer‑associated fibroblast‑induced M2‑polarized macrophages promote hepatocellular carcinoma progression via the plasminogen activator inhibitor‑1 pathway. Int J Oncol. 2021;59(2). doi:10.3892/ijo.2021.5239
26. Liang ZX, Liu HS, Wang FW, et al. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019;10(11):829. doi:10.1038/s41419-019-2077-0
27. Xin L, Wu Y, Liu C, et al. Exosome-mediated transfer of lncRNA HCG18 promotes M2 macrophage polarization in gastric cancer. Mol Immunol. 2021;140:196–205.
28. Zomer A, Steenbeek SC, Maynard C, van Rheenen J. Studying extracellular vesicle transfer by a Cre-loxP method. Nat Protocols. 2016;11(1):87–101. doi:10.1038/nprot.2015.138
29. Hao L, Wu W, Xu Y, et al. LncRNA-MALAT1: a key participant in the occurrence and development of cancer. Molecules. 2023;28(5):2126. doi:10.3390/molecules28052126
30. Guo K, Qian K, Shi Y, Sun T, Wang Z. LncRNA-MIAT promotes thyroid cancer progression and function as ceRNA to target EZH2 by sponging miR-150-5p. Cell Death Dis. 2021;12(12):1097. doi:10.1038/s41419-021-04386-0
31. Hu Z, Yang C, Guo S, Li Y, Li Y. LINC01615 activates ZEB2 through competitively binding with miR-3653-3p to promote the carcinogenesis of colon cancer cells. Cell Cycle. 2022;21(3):228–246.
32. Xiang Y, Feng L, Liu H, et al. SIPA1 regulates LINC01615 to promote metastasis in triple-negative breast cancer. Cancers. 2022;14(19):4815.
33. Yin X, Wang J, Bian Y, Jia Q, Shen Z, Zhang H. Comprehensive analysis of LINC01615 in head and neck squamous cell carcinoma: a hub biomarker identified by machine learning and experimental validation. J Oncol. 2022;2022:5039962. doi:10.1155/2022/5039962
34. Ji D, Chen GF, Liu X, et al. Identification of LINC01615 as potential metastasis-related long noncoding RNA in hepatocellular carcinoma. J Cell Physiol. 2019;234(8):12964–12970. doi:10.1002/jcp.27963
35. Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21(11):799–820.
36. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871(2):455–468.
37. Zhao X, Wu D, Ma X, Wang J, Hou W, Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother. 2020;128:110237.
38. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
39. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407.
40. Yang M, Lu H, Liu J, Wu S, Kim P, Zhou X. lncRNAfunc: a knowledgebase of lncRNA function in human cancer. Nucleic Acids Res. 2022;50(D1):D1295–d306.
41. Shaath H, Vishnubalaji R, Elango R, et al. Long non-coding RNA and RNA-binding protein interactions in cancer: experimental and machine learning approaches. Semin Cancer Biol. 2022;86(Pt 3):325–345.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.