Back to Journals » Drug Design, Development and Therapy » Volume 19
Cannabidiol Ameliorates Doxorubicin-Induced Myocardial Injury via Activating Hippo Pathway
Authors Dong T, Li J, Liang X, Wang W, Chen M, Yang G, Wu D
Received 16 October 2024
Accepted for publication 15 January 2025
Published 24 January 2025 Volume 2025:19 Pages 569—583
DOI https://doi.org/10.2147/DDDT.S497323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Tianwei Dong,1 Jinlian Li,2 Xinfang Liang,3 Wang Wang,3 Meichi Chen,4 Guangyuan Yang,5 Dongmei Wu4
1Key Laboratory of Microecology-Immune Regulatory Network and Related Diseases, School of Basic Medicine, Jiamusi University, Jiamusi, Heilongjiang, 154000, People’s Republic of China; 2College of Pharmacy, Jiamusi University, Jiamusi, Heilongjiang, 154007, People’s Republic of China; 3Department of Cardiology, The First Affiliated Hospital of Jiamusi University, Jiamusi, Heilongjiang, 154002, People’s Republic of China; 4School of Basic Medicine, Jiamusi University, Jiamusi, Heilongjiang, 154000, People’s Republic of China; 5Cardiovascular Medicine, The First Affiliated Hospital of Jiamusi University, Jiamusi, Heilongjiang, 154002, People’s Republic of China
Correspondence: Dongmei Wu; Guangyuan Yang, Email [email protected]; [email protected]
Background: Doxorubicin (DOX) is a chemotherapeutic agent widely used for cancer treatment and has non-negligible cardiotoxicity. Some previous studies have reported that cannabidiol (CBD) has cardioprotective effects. In this study, we evaluated the protective effects of CBD against DOX-induced cardiomyocyte injury, and explored the downstream molecular mechanism.
Methods and Materials: GSE193861, containing healthy myocardial tissues and myocardial tissues with DOX-induced injury, was analyzed to screen for the involved proteins and pathways. Molecular docking was performed to identify candidate drugs. After H9c2 cells were treated with DOX and CBD, their viability, oxidative stress, and apoptosis were assessed. After YAP depletion, the role of the Hippo pathway in CBD function was investigated. C57BL/6 mice were treated with DOX to establish an in vivo model, and CBD and verteporfin (VP) were used to treat the mice. Histological analyses and immunofluorescence were used to evaluate myocardial tissue injury, and apoptosis and oxidative stress of the myocardial tissues were also analyzed. Western blotting was used to investigate the regulatory effects of CBD on the Hippo and apoptosis-related pathways.
Results: Bioinformatic analysis suggested that the Hippo pathway was a crucial pathway involved in DOX-induced myocardial injury. Molecular docking showed that CBD targeted multiple regulators of the Hippo pathway. CBD showed cardioprotective effects against DOX-induced myocardial injury both in vitro and in vivo and regulated Hippo pathway activity in cardiomyocytes. After inactivation of the Hippo pathway by YAP knockdown or VP intervention, the protective effects of CBD were reversed.
Conclusion: For the first time, we revealed that CBD is likely to reduce DOX-induced myocardial injury by regulating the Hippo signaling pathway.
Keywords: doxorubicin, heart injury, cannabidiol, Hippo pathway
Introduction
Doxorubicin (DOX) is one of the most commonly used chemotherapeutic agents for the treatment of malignancies.1 Although DOX is widely used, it exhibits a strong cardiotoxicity.2 The molecular mechanism of DOX-induced myocardial injury involves many aspects, including oxidative stress, calcium overload, energy metabolism disorder, DNA damage and so on.2,3 DOX can accumulate in heart muscle cells, causing their death or inhibiting their function, leading to heart failure or cardiomyopathy.2 On one hand, DOX cardiotoxicity limits its application; for example, patients with cardiac dysfunction are contraindicated with DOX; on the other hand, patients treated with DOX are at risk of heart damage and need to undergo regular electrocardiogram and echocardiogram tests.2,3
The incidence of late-onset complications is not reduced even when the cumulative dose of DOX was reduced, regardless of the antitumor effect.4 Slower infusion or liposome formulations may reduce the toxicity of DOX by reducing the peak concentration of DOX, but the implementation of these strategies is associated with higher medical costs.2 Although dexrazoxane is believed to reduce the cardiotoxicity of DOX, it has also been reported that dexrazoxane can reduce the effect of anti-tumor drugs and has potential carcinogenic effects.5,6 Therefore, it is important to develop new protective agents against DOX’s cardiotoxicity.
Natural active ingredients in plants are considered an important source for drug development, with many advantages such as low cost, few side effects, and easy access. Increasing evidence support that some natural drugs, such as phenolic acids, flavonoids, stilbenes, alkaloids and phenyl propanoids, have cardioprotective effects.7 Cannabidiol (CBD) is a natural compound derived from Cannabis Sativa L. Previous studies have shown that CBD has a variety of pharmacological effects, such as analgesia, anti-epilepsy, anti-anxiety, and inhibition of malignant phenotypes of tumor cells.8–12 Recent preclinical studies have reported that CBD has a positive effect on the treatment of cardiovascular disease.13–17 For example, in rat models, CBD can alleviate myocardial injury induced by ischemia-reperfusion by regulating the PI3K/AKT and MAPK/ERK signaling pathways.13 By regulating cardiac sodium channels, CBD can also relieve the inflammatory response of cardiomyocytes.14 CBD also has the potential to alleviate T cell-mediated autoimmune myocarditis.15 A recent study reports that CBD mitigates lipopolysaccharide-induced cardiovascular toxicity via repressing inflammatory signaling pathways.16 Importantly, CBD is undergoing a clinical trial to validate its beneficial role on myocardial recovery in patients with acute myocarditis.17 However, few studies have investigated whether CBD alleviates DOX-induced myocardial damage.
The Hippo signaling pathway inhibits cell growth. Phosphorylation of YAP/TAZ by MST1/2 and LAST1/2 in the upstream activates a series of kinase cascade phosphorylation reactions.18,19 Cytoskeletal proteins bind to phosphorylated YAP and TAZ, allowing them to remain in the cytoplasm and reduce their nuclear activity, thereby regulating organ size and volume.18,19 The Hippo signaling pathway is a key modulator of cardiomyocyte apoptosis.20 In this study, using bioinformatics, molecular docking, cell models, and animal models, for the first time, we revealed that CBD is likely to reduce DOX-induced myocardial injury by regulating the Hippo signaling pathway.
Methods and Materials
Analysis of Microarray Data
The Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/) was searched, and “GSE193861” microarray data were downloaded, including five cases of healthy controls and five cases of DOX-induced myocardial injury. R language was used to process and analyze the data and to screen the differentially expressed genes using the following screening criteria: |log2 fold change (FC)| > 0.7 and P value < 0.05. ggplot2 was used to visualize the results. These differentially expressed genes are considered potential drug targets for ameliorating myocardial injury induced by doxorubicin.
Enrichment Analysis
R software and clusterProfiler, enrichplot, GOplot packages were applied to perform GO enrichment analysis, and P value<0.05 was set as the criterion. R software, clusterProfiler, BiocManager, and enrichplot packages were used to perform Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis.
Molecular Docking
The TCMSP database (https://old.tcmsp-e.com/tcmsp.php) was searched to download the 3D structure of the CBD, and the file was saved in mol2 format. The Protein Data Bank (PDB) database (https://www.pdbus.org/) was searched to download the 3D structure of the protein targets, and the files were saved in the PDB format. Using AutoDock 4.2, the protein was dehydrated and hydrogenated, the charge was calculated, the atomic type was set, and the files were saved in PDBQT format. Using Autodock 4.2, the CBD file was saved as pdbqt format. Molecular docking was performed using AutoDock Vina software.
Cell Culture and Intervention
Rat cardiomyocyte H9c2 cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin, in a humidified incubator containing 5% CO2 at 37 °C. DOX (Sigma, Shanghai, China) was dissolved in normal saline and diluted with medium to treat cells. CBD (GlpBio, Montclair, CA, USA) was dissolved in dimethyl sulfoxide (DMSO), and diluted with medium to treat the cells at different concentrations for different times. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, Invitrogen) was applied for cell transfection. The siRNAs were obtained from GenePharma (Shanghai, China).
Animal Model
Animal experiments were approved by the Ethics Committee of the First Affiliated Hospital of Jiamusi University. The experimental procedures follow the guidelines of the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals. C57BL/6 mice (male, 20–30 g, SPF, Hibio, Hangzhou, China) were randomly divided into control, DOX treatment, DOX treatment + CBD treatment, and DOX treatment + CBD treatment + verteporfin (VP) (Sigma, Shanghai, China) treatment groups (10 mice in each group). DOX was administered on the first day and the fourth day (i.p.; 10 mg/kg). CBD and VP were administered daily (i.p.; 10 mg/kg). Two weeks later, the mice were sacrificed and heart tissues (left ventricular myocardial tissues) were collected for subsequent analysis.
Immunofluorescence
Immunofluorescence was performed to examine protein expression, reactive oxide species (ROS) production and apoptosis, according to the manufacturer’s instructions. The expression level of YAP1 was detected using YAP1 monoclonal antibody (Proteintech, Wuhan, China). A DCFH-DA probe kit (Beyotime, Shanghai, China) was used to detect ROS levels. The Terminal Deoxynucleotidyl Transferase (TUNEL) kit (Beyotime, Shanghai, China) was used to detect cardiomyocyte apoptosis. DAPI was used to stain the nuclei. The tissues and cells were observed under a fluorescence microscope (Olympus, Tokyo, Japan). For the in vitro assays, at least 6 parallel wells were set for each group, and the experiments were repeated independently for 3 times. For the in vivo assays, there were 10 mice in each group, and 6 images in each group were randomly captured under microscope and analyzed.
Western Blot
Myocardial tissue homogenate and H9c2 cells were lysed in 200 μL of RIPA lysis buffer (Sigma, St. Louis, MO, USA). The protein concentration was quantified using a BCA kit (Beyotime, Shanghai, China). Equivalent amounts of protein (40 μg) were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) after electrophoresis. The membrane was blocked with 5% skim milk at room temperature for 1 h and incubated with the primary antibody at 4 °C overnight. After washing with tris-buffered saline tween (TBST), the membranes were incubated with a secondary antibody coupled with horseradish peroxidase (HRP) at room temperature for 1 h. Finally, the bands were visualized using an enhanced chemiluminescence (ECL) Plus kit (Beyotime, Shanghai, China) and analyzed using Image Lab software. α-tubulin was used as an internal control. The antibodies used in this study are listed in Table 1. For the in vitro assays, at least 3 parallel wells were set for each group, and the experiments were repeated independently for 3 times. For the in vivo assays, there were 10 mice in each group, and the protein samples of 3 mice, randomly selected from each group, were used to perform the immunoblotting, and for each sample, the experiment was independently repeated for 3 times.
 |
Table 1 The Antibodies Used in the Present Work |
Flow Cytometry
To detect apoptosis in cardiomyocytes, for each sample, 1×106 H9c2 cells were collected, washed phosphate buffer saline (PBS), and resuspended in 100 μL binding buffer. Then 10 μL annexin V-Fluorescein Isothiocyanate and 10 μL propidium iodide (PI) (Beyotime, Shanghai, China) were added, and fully mixed with the binding buffer. Then the cells were placed in the dark at room temperature for 15 min for staining. After that, the cells were washed with binding buffer to remove the excessive dye. Subsequently, cell sorting was performed by flow cytometry. The percentage of Annexin V−/PI+ (necrosis), Annexin V+/PI− (early apoptosis), and Annexin V/PI+ (late apoptosis) cells was calculated. Gating strategy was based on forward scatter signal and side scatter signal, to exclude the influence of cell mass and cell debris on the result analysis. At least 6 parallel wells were set for each group, and the experiments were independently repeated for 3 times.
Evaluation of Marker of Myocardial Injury
The myocardial tissue was homogenized, and the supernatant was collected after centrifugation. For H9c2 cells, the cells and medium were centrifuged and the supernatant was collected. Additionally, the serum of the mice in different groups was collected. After that, the malondialdehyde (MDA) was detected for evaluation of lipid peroxidation with MDA Detection Kit (Beyotime, Shanghai, China), and superoxide dismutase (SOD) was detected for evaluation of oxidative stress with Total Superoxide Dismutase Assay Kit with WST-8 (Beyotime, Shanghai, China), and lactic dehydrogenase (LDH) and creatine kinase-MB (CK-MB) were detected with LDH Assay Kit (Beyotime, Shanghai, China) and CK-MB Detection kit (Elabscience, Wuhan, China), respectively, according to the manufacturer’s introductions. For the in vitro assays, at least 6 parallel wells were set for each group, and the experiments were repeated independently for 3 times. For the in vivo assays, there were 10 mice in each group, and at least 6 parallel wells were set for each sample, and the experiments were repeated independently for 3 times.
Hematoxylin-Eosin (HE) Staining
After dewaxing, the samples were stained with a hematoxylin solution for 5 min and washed with tap water. The sections were transferred to 1% hydrochloric acid-alcohol for 4 s and washed with tap water. The sections were then placed in anhydrous ethanol I for 5 minutes, anhydrous ethanol II for 5 minutes, xylene I for 5 minutes, and xylene II for 5 minutes. After air drying, the samples were sealed with neutral gum and visualized by microscopy. There were 10 mice in each group, and 6 images in each group were randomly captured under microscope and analyzed.
Wheat Germ Agglutinin (WGA) Staining
After dissection, the heart tissue was immersed in liquid nitrogen for snap-freezing, cut into 6-μm sections, and fixed with acetone. After washing with PBS, sections were blocked with 5% donkey serum and incubated with Alexa-488-conjugated WGA (1:500, W11261, Thermo Fisher Scientific). Finally, sections were observed under a fluorescence microscope (Olympus). There were 10 mice in each group, and 6 images in each group were randomly captured under microscope and analyzed.
Statistical Analysis
Data are expressed as the “mean ± standard deviation” and were analyzed using SPSS software (version 21.0; SPSS, Chicago, IL, USA). The normality of the data was tested with Shapiro–Wilk test, and the homogeneity of variances was tested with Levene’s test. Differences among multiple groups were analyzed using one-way analysis of variance (ANOVA) and Tukey’s post-hoc test or non-parametric test. P<0.05 was considered to be statistically significant.
Results
Multiple Genes are Dysregulated in DOX-Induced Myocardial Injury and Hippo Pathway May Function as a Crucial Mediator
First of all, bioinformatics analyses were performed to identify the crucial targets/pathways in DOX-myocardial injury. By analyzing the microarray data in GSE193861, a total of 1133 differential genes were obtained, including 583 upregulated genes and 550 downregulated genes (Supplementary Figure 1). Next, the 1133 genes were analyzed using KEGG enrichment analysis, and a total of five signal pathways/biological processes were obtained (Table 2). It mainly included the Hippo signaling pathway, the calcium signaling pathway, and human papillomavirus infection, etc. The Hippo signaling pathway may be the most important signaling pathway for DOX-induced myocardial injury because it had the highest number of dysregulated genes during DOX-induced myocardial injury.
 |
Table 2 The Results of KEGG Enrichment Analysis and Involved DEGs |
CBD Targets Multiple Regulators in Hippo Pathway
To preliminarily verify the inhibitory effects of CBD on Hippo pathway, molecular docking was performed based on the key regulators of the Hippo signaling pathway and CBD. The results of molecular docking are shown (Table 3 and Supplementary Figure 2). The results of molecular docking showed that the binding activity of CBD and key proteins was less than −5.0 kcal/mol, indicating a high binding affinity between them. These results suggested that CBD may regulates the pathogenesis of DOX-induced myocardial injury by targeting the Hippo pathway.
 |
Table 3 The Results of Molecular Docking |
CBD Shows Cardioprotective Effects Against DOX-Induced Myocardial Injury in vitro
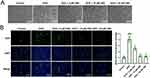
Then an in vitro model of DOX-induced myocardial injury was constructed. H9c2 cardiomyocytes were treated with DOX at concentrations of 0, 0.25, 0.5, 1.0, 2.5 and 5μM for 12 and 24h, respectively, and CCK-8 was used to detect cell viability. The results showed that the damaging effects of DOX on cardiomyocytes were dose- and time-dependent (Figure 1A). After the treatment of H9c2 cardiomyocytes with 0.5, 1.0, 2.5, 5, 10, 20μM CBD for 12h or 24h, the results showed that CBD treatment did not induce cardiomyocyte injury (Figure 1B), which validated its biosafety. In the following experiments, cells were divided into five groups: control group, DOX group, 5μM CBD group, 10μM CBD group, 20μM CBD group. As shown, under a microscope, shrinkage was observed in the cells of the DOX group, and CBD treatment reversed this change in a dose-dependent manner (Figure 2A). Immunofluorescence showed that DOX treatment upregulated ROS levels in H9c2 cells, suggesting that the DOX-induced oxidative stress response aggravates cardiomyocyte injury, while CBD treatment reduced ROS levels (Figure 2B). Consistently, the TUNEL assay showed that CBD reversed DOX-induced apoptosis in H9c2 cells (Figure 3A), and Western blotting showed that CBD treatment reversed the downregulation of the anti-apoptotic protein Bcl2, and upregulation of the pro-apoptotic proteins Bax and cleaved caspase-3 (Figure 3B). These data confirmed that CBD ameliorated DOX-induced cardiomyocyte injury.
CBD Modulates of Hippo Pathway in Cardiomyocytes Treated With DOX
Next, the effects of CBD on the protein expression levels of regulators of the Hippo pathway were investigated. Western blot showed that, DOX treatment increased the phosphorylation levels of MST1, LATS1 and YAP, and reduced the expression of TEAD4; after DOX treatment, the phosphorylation levels of MST1, LATS1 and YAP were reduced, and the expression of TEAD4 was up-regulated (Figure 3C). These data suggested that the Hippo pathway may be involved in the cardioprotective effects of CBD in cardiomyocytes against DOX.
Inhibition of YAP Reverses the Cardioprotective Effects of CBD on Cardiomyocytes
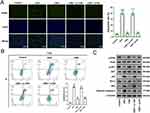
To validate whether CBD exerted cardioprotective effects on cardiomyocytes via Hippo signaling, siRNAs targeting YAP were transfected into H9c2 cells (Supplementary Figure 3) and then H9c2 cells were treated with DOX and CBD. Immunofluorescence showed that after DOX treatment, the level of nuclear YAP decreased, and CBD treatment reversed this, but after the depletion of YAP, the level of nuclear YAP decreased again (Figure 4A). After DOX treatment, the levels of MDA and SOD in H9c2 cells were markedly upregulated, and CBD treatment reversed these effects. If YAP was silenced, the cardioprotective effects of CBD were almost completely abolished (Figure 4B and C). Similarly, the TUNEL assay and flow cytometry showed that CBD reduced DOX-induced injury in H9c2 cells dependent on YAP (Figure 5A and B). Additionally, Western blot showed that DOX treatment increased the expression levels of p53, Bax, cleaved caspase 3, and reduced the expression levels of p-PI3K, p-AKT and Bcl2, which were reversed by CBD treatment; while after YAP was silenced, the effects of CBD were abrogated (Figure 5C). Collectively, these data suggested that CBD protected cardiomyocytes against DOX via the Hippo pathway.
 |
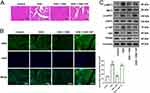
Figure 4 Depletion of YAP reverses the effects of CBD on the oxidative stress of cardiomyocytes in vitro. (A) H9c2 cells were divided into 5 groups: control group, DOX group, CBD treatment group, CBD treatment + YAP siRNA group, CBD treatment + control siRNA group. Immunofluorescence was applied to detect the expression and subcellular location of YAP (n=6 in each group). (B and C) The levels of SOD (B) and MDA (C) in H9c2 cells were detected to evaluate the oxidative stress of the cells (Figure 5B and C) (n=6 in each group). All of the experiments were repeated independently for 3 times. ## represents P<0.01 vs the control group. ** represents P<0.01, vs DOX group. ^^ represents P<0.01, vs CBD group. Abbreviations: DOX, doxorubicin; CBD, Cannabidiol; si-YAP, siRNA targeting YAP; si-NC, negative control siRNA. |
CBD Shows Cardioprotective Effects Against DOX-Induced Myocardial Injury in vivo via Hippo Pathway
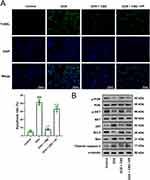
To further verify that CBD mitigated DOX-induced myocardial injury via the Hippo pathway, a mouse model of DOX-induced myocardial injury was established. HE staining showed that, in the control group, the myocardial tissue were normal, with clear edges and neatly arranged myocardial fibers; conversely, after DOX treatment, the swelling of cells were obvious, and the arrangement of myocardial fibers was dysregulated; after CBD treatment, the morphological changes of myocardial tissues were partly reversed, and the swelling of cardiomyocytes was significantly ameliorated; however, after VP treatment, the effects of CBD were markedly counteracted (Figure 6A). Consistently, WGA staining showed that compared to the control group, the cross-sectional area of cardiomyocytes was remarkably enlarged in the DOX treatment group, which was reversed by CBD treatment, and VP treatment reversed this role of CBD (Figure 6B). Western blotting was performed to detect the expression of Hippo pathway-related proteins. DOX treatment reduced the expression levels of YAP and TEAD4 but promoted the expression levels of p-YAP, p-MST1, and p-LATS1 (Figure 6C). Additionally, the TUNEL assay showed that the apoptosis level of myocardial tissues was significantly promoted, and CBD treatment reduced the apoptosis of cardiomyocytes, and VP counteracted CBD’s function of CBD (Figure 7A). Consistently, Western blotting showed that the PI3K/Akt pathway was inactivated in myocardial tissues, and the expression levels of pro-apoptotic proteins (Bax, p53, and cleaved caspase 3) were increased, while the expression level of anti-apoptotic protein Bcl2 was reduced; CBD treatment activated the PI3K/Akt pathway and reversed the dysregulation of apoptosis-related proteins, and VP counteracted CBD’s biological effects (Figure 7B). Biochemical tests showed that DOX induced upregulation of LDH, CK-MB, and MDA, and downregulation of SOD; CBD treatment reversed these effects, and after VP treatment, the effects of CBD were abrogated (Supplementary Figure 4). Take together, with in vivo data, these data suggest that CBD has certain cardioprotective effects against DOX-induced cardiomyocyte apoptosis and heart injury via Hippo pathway.
Discussion
DOX is an anti-tumor anthracycline antibiotic. The most prominent side effect of DOX is cardiotoxicity, which can manifest as acute ventricular dysfunction, dilated cardiomyopathy, and heart failure and the side effects may appear many years after the end of treatment.2,3 DOX cardiotoxicity is related to ultrastructural changes in cardiomyocytes, accompanied by myofibrillar disorders, and DOX increases the number of lysosomes, and induces contraction of chromatin and nucleoli.21 Mitochondrial damage during DOX treatment may lead to insufficient energy supply to the heart, causing an imbalance between ROS/reactive nitrogen species (RNS) and antioxidants, and oxidative stress which further aggravates damage to cardiomyocytes and eventually leads to apoptosis.22 In this study, we found that DOX treatment significantly altered the morphology of H9c2 cells, enhanced the level of oxidative stress, and increased apoptosis. In animal models, DOX treatment caused damage to myocardial tissues in mice. These results are consistent with previous reports,2,3,15,16 indicating that the DOX cardiotoxicity models were successfully constructed.
CBD was first isolated from plants in 1940 and its structure was determined in 1963.23 Nowadays, it can not only be extracted from the flowers and leaves of cannabis plants but also chemically synthesized, and it has the potential to be secreted by microorganisms after fermentation.24 These properties provide an industrial basis for their application in drugs. CBD has shown promise in treating chronic pain, can relieve a variety of body inflammation, and is a potential alternative to certain painkillers in some patients.8,25 CBD can also reduce autonomic nervous excitation, regulate heart rate and breathing, and relieve anxiety and panic in users.26,27 CBD has neuroprotective effects in preclinical models.28 In recent years, some studies have reported that CBD inhibits the malignant phenotypes of tumor cells, suggesting that it may be used as an adjuvant therapy for patients.10–12 In this study, in both cell and animal models, we found that CBD can reduce DOX-induced oxidative stress and apoptosis of cardiomyocytes, and reduce myocardial damage, which is consistent with the previously reported cardioprotective properties of CBD.13–17 As another kind of cardioprotective agent, dexrazoxane probably reduce the effect of DOX.29 On the contrary, increasing evidence supports CBD sensitize cancer cells to DOX.30–32 Our study suggests that CBD may not only play a synergistic role in killing tumor cells in patients receiving DOX treatment but may also reduce the side effects of this important chemotherapy drug, which has certain advantages compared with dexrazoxane. Additionally, as mentioned above, CBD-based pharmaceuticals hold promise in pain management, which may be helpful to control cancer pain.8,25 It is also worth noting that, some clinical trials have demonstrated the safety of CBD application in clinical practice.33–35 These previous studies suggest the versatility and safety of CBD in the management of cancer patients. Of course, the appropriate dose of CBD to prevent/ameliorate myocardial damage deserves further investigation in the following work.
The Hippo pathway is an important signaling pathway that regulates cell apoptosis and proliferation and is a key regulatory factor in cardiac development.20,36 Activation of the Hippo signaling pathway, with increased expression and phosphorylation levels of MST1, inhibits the expression of downstream genes related to cell proliferation and promotes the expression of genes related to apoptosis by promoting YAP/TAZ phosphorylation.18,19 Many studies have reported the function of the Hippo signaling pathway in myocardial injury and other cardiovascular diseases. Activation of the Hippo signaling pathway prevents heart muscle regeneration, whereas silencing its activity can reverse heart failure.37,38 Notably, several studies have reported that activation of the Hippo signaling pathway is associated with DOX-related heart injury. A recent study reported that doxorubicin treatment induced MST1 phosphorylation in both cellular and animal models.39 In human induced pluripotent stem cell-derived cardiomyocytes, the expression levels of YAP and TAZ are negatively correlated with the degree of myocardial injury.40 In this study, through bioinformatics analysis, we found that the Hippo signaling pathway is involved in DOX-induced myocardial injury, which is consistent with previous reports. Interestingly, some studies have reported that some natural drugs can protect cardiomyocytes by regulating the Hippo signaling pathway. For example, glycyrrhizic acid ameliorates ischemia reperfusion-induced cardiomyocyte injury by repressing Hippo/YAP signaling.41 This suggests that some natural drugs may reduce myocardial damage by regulating the Hippo signaling pathway. In this study, using molecular docking, we found that CBD can bind to multiple key mediators of the Hippo signaling pathway. Both in vitro and in vivo experiments confirmed that CBD could inhibit the phosphorylation of MST1 and LATS1 and that the effects of CBD on DOX-induced myocardial injury depend on its regulatory effects on the Hippo signaling pathway. A previous study has reported that CBD protects against DOX-induced myocardial damage by mitochondrial function and biogenesis in cardiomyocyte.42 Our work report a new mechanism by which CBD exerts cardioprotective effects against DOX.
The present study had some limitations. Although molecular docking shows good binding affinity between CBD and regulators of the Hippo pathway, there is still no direct evidence of binding between CBD and these proteins. In future work, technologies such as HPLC-MS will be helpful for validating this. Additionally, the downstream genes of the Hippo pathway were not detected, and this should be done in future studies, which will help fully explain the cardioprotective effect of CBD. It’s also worth noting that, in the present work, for the Western blotting assays based on mouse tissues, there were only 3 independent observations per experimental group, and this may be not enough for precise statistical analysis.43 In the following work, a more practical quantitative technique should be used to investigate the effects of CBD on Hippo pathway and apoptosis-related pathway in vivo, to make our conclusion more convincing. Additionally, only acute myocardial injury models were used in the present work, however, cardiac insufficiency due to DOX’s cardiotoxicity, in some cases, occur several months even several years after the chemotherapy.44 So it is impractical for us to observe the effects of CBD on cardiac function recovery or preventing late-onset cardiotoxicity in the long term, and epidemiological investigation and clinical trials with long follow-up may help answer this question in the future.
Conclusion
Overall, this study reports that CBD alleviates DOX-induced myocardial injury by regulating the Hippo pathway. Before its application in clinical practice, its efficiency and safety should be rigorously validated.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Statement
Animal experiments were approved by the Ethics Committee of the First Affiliated Hospital of Jiamusi University. The experimental procedures follow the guidelines of the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by the Heilongjiang Province Key Research and Development Plan Project (JD22A016), Cardiovascular Disease Basic and Clinical Application Research Team of the First Affiliated Hospital of Jiamusi University (202302), and Dongji Academic Team of Jiamusi University (DJXSTD202409).
Disclosure
The authors declare that they have no competing interests.
References
1. Mattioli R, Ilari A, Colotti B, Mosca L, Fazi F, Colotti G. Doxorubicin and other anthracyclines in cancers: activity, chemoresistance and its overcoming. mol Aspects Med. 2023;93:101205. doi:10.1016/j.mam.2023.101205
2. De Angelis A, Urbanek K, Cappetta D, et al. Doxorubicin cardiotoxicity and target cells: a broader perspective. Cardio-Oncology. 2016;2(1):2. doi:10.1186/s40959-016-0012-4
3. Ganz WI, Sridhar KS, Ganz SS, Gonzalez R, Chakko S, Serafini A. Review of tests for monitoring doxorubicin-induced cardiomyopathy. Oncology. 1996;53(6):461–470. doi:10.1159/000227621
4. Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(12):2629–2636. doi:10.1200/JCO.2005.12.121
5. Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol. 1997;15(4):1333–1340. doi:10.1200/JCO.1997.15.4.1333
6. Vrooman LM, Neuberg DS, Stevenson KE, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011;47(9):1373–1379. doi:10.1016/j.ejca.2011.03.022
7. Ullah A, Mostafa NM, Halim SA, et al. Phytoconstituents with cardioprotective properties: a pharmacological overview on their efficacy against myocardial infarction. Phytother Res. 2024;38(9):4467–4501. doi:10.1002/ptr.8292
8. de Almeida DL, Mendes Ferreira RC, Fonseca FC, et al. Cannabidiol induces systemic analgesia through activation of the PI3Kγ/nNOS/NO/KATP signaling pathway in neuropathic mice. A KATP channel S-nitrosylation-dependent mechanism. Nitric Oxide. 2024;28:
9. Sharma AA, Szaflarski JP. The longitudinal effects of cannabidiol on brain temperature in patients with treatment-resistant epilepsy. Epilepsy Behav. 2024;151:109606. doi:10.1016/j.yebeh.2023.109606
10. Crichton M, Dissanayaka T, Marx W, et al. Does medicinal cannabis affect depression, anxiety, and stress in people with cancer? A systematic review and meta-analysis of intervention studies. Maturitas. 2024;184:107941. doi:10.1016/j.maturitas.2024.107941
11. Mokoena D, George BP, Abrahamse H. Cannabidiol combination enhances photodynamic therapy effects on MCF-7 breast cancer cells. Cells. 2024;13(2):187. doi:10.3390/cells13020187
12. Śledziński P, Nowak-Terpiłowska A, Rzymski P, Słomski R, Zeyland J. In vitro evidence of selective pro-apoptotic action of the pure cannabidiol and cannabidiol-rich extract. Molecules. 2023;28(23):7887. doi:10.3390/molecules28237887
13. Franco-Vadillo A, Toledo-Blass M, Rivera-Herrera Z, et al. Cannabidiol-mediated RISK PI3K/AKT and MAPK/ERK pathways decreasing reperfusion myocardial damage. Pharmacol Res Perspect. 2021;9(4):e00784. doi:10.1002/prp2.784
14. Fouda MA, Fathy Mohamed Y, Fernandez R, Ruben PC. Anti-inflammatory effects of cannabidiol against lipopolysaccharides in cardiac sodium channels. Br J Pharmacol. 2022;179(24):5259–5272. doi:10.1111/bph.15936
15. Lee WS, Erdelyi K, Matyas C, et al. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: implications to autoimmune disorders and organ transplantation. Mol Med. 2016;22:136–146. doi:10.2119/molmed.2016.00007
16. Tepebaşı MY, Aşcı H, Özmen Ö, Taner R, Temel EN, Garlı S. Cannabidiol ameliorates lipopolysaccharide-induced cardiovascular toxicity by its antioxidant and anti-inflammatory activity via regulating IL-6, Hif1α, STAT3, eNOS pathway. Mol Biol Rep. 2024;51(1):825. doi:10.1007/s11033-024-09772-3
17. McNamara DM, Cooper LT, Arbel Y, et al.; ARCHER Study Group. Impact of cannabidiol on myocardial recovery in patients with acute myocarditis: rationale & design of the ARCHER trial. ESC Heart Fail. 2024;11(5):3416–3424. doi:10.1002/ehf2.14889
18. Zhou J, Li L, Wu B, Feng Z, Lu Y, Wang Z. MST1/2: important regulators of Hippo pathway in immune system associated diseases. Cancer Lett. 2024;587:216736. doi:10.1016/j.canlet.2024.216736
19. Zhong Z, Jiao Z, Yu FX. The Hippo signaling pathway in development and regeneration. Cell Rep. 2024;43(3):113926. doi:10.1016/j.celrep.2024.113926
20. Zheng A, Chen Q, Zhang L. The Hippo-YAP pathway in various cardiovascular diseases: focusing on the inflammatory response. Front Immunol. 2022;13:971416. doi:10.3389/fimmu.2022.971416
21. Santin Y, Formoso K, Haidar F, et al. Inhalation of acidic nanoparticles prevents doxorubicin cardiotoxicity through improvement of lysosomal function. Theranostics. 2023;13(15):5435–5451. doi:10.7150/thno.86310
22. Erfu C, Li L, Weiting Q, et al. Matrine attenuating cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity through improved mitochondrial membrane potential and activation of mitochondrial respiratory chain Complex I pathway. Biomed Pharmacother. 2024;173:116464. doi:10.1016/j.biopha.2024.116464
23. Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23(7):1377–1385. doi:10.1016/j.bmc.2015.01.059
24. Carvalho Â, Hansen EH, Kayser O, Carlsen S, Stehle F. Designing microorganisms for heterologous biosynthesis of cannabinoids. FEMS Yeast Res. 2017;17(4):fox037. doi:10.1093/femsyr/fox037
25. Bimonte S, Nocerino D, Schiavo D, Crisci M, Cascella M, Cuomo A. Cannabinoids for cancer-related pain management: an update on therapeutic applications and future perspectives. Anticancer Res. 2024;44(3):895–900. doi:10.21873/anticanres.16883
26. Kwee CMB, van der Flier FE, Duits P, van Balkom AJLM, Cath DC, Baas JMP. Effects of cannabidiol on fear conditioning in anxiety disorders: decreased threat expectation during retention, but no enhanced fear re-extinction. Psychopharmacology. 2024;241(4):833–847. doi:10.1007/s00213-023-06512-6
27. Kwee CM, Baas JM, van der Flier FE, et al. Cannabidiol enhancement of exposure therapy in treatment refractory patients with social anxiety disorder and panic disorder with agoraphobia: a randomised controlled trial. Eur Neuropsychopharmacol. 2022;59:58–67. doi:10.1016/j.euroneuro.2022.04.003
28. Al-Khazaleh AK, Zhou X, Bhuyan DJ, et al. The neurotherapeutic arsenal in cannabis sativa: insights into anti-neuroinflammatory and neuroprotective activity and potential entourage effects. Molecules. 2024;29(2):410. doi:10.3390/molecules29020410
29. Palvia AR, Damera AR, Nandi AR, et al. Cardio-oncology’s modern approaches to prevent doxorubicin-induced cardiotoxicity: a systematic review. Cureus. 2024;16(8):e66215. doi:10.7759/cureus.66215
30. Tabatabaei K, Moazzezi S, Emamgholizadeh M, Vaez H, Baradaran B, Shokouhi B. Improved therapeutic efficacy of doxorubicin chemotherapy with cannabidiol in 4T1 mice breast cancer model. Cancer Med. 2024;13(21):e70395. doi:10.1002/cam4.70395
31. Kalvala AK, Nimma R, Bagde A, et al. The role of Cannabidiol and tetrahydrocannabivarin to overcome doxorubicin resistance in MDA-MB-231 xenografts in athymic nude mice. Biochimie. 2023;208:19–30. doi:10.1016/j.biochi.2022.12.008
32. Patel N, Kommineni N, Surapaneni SK, et al. Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in-vitro and in vivo models. Int J Pharm. 2021;607:120943. doi:10.1016/j.ijpharm.2021.120943
33. Nayak MM, Chai P, Catalano PJ, et al. Cannabidiol for scan-related anxiety in women with advanced breast cancer: a randomized clinical trial. JAMA Netw Open. 2024;7(12):e2450391. doi:10.1001/jamanetworkopen.2024.50391
34. Gundugurti PR, Banda N, Yadlapalli SSR, et al. Evaluation of the efficacy, safety, and pharmacokinetics of nanodispersible cannabidiol oral solution (150 mg/mL) versus placebo in mild to moderate anxiety subjects: a double blind multicenter randomized clinical trial. Asian J Psychiatr. 2024;97:104073. doi:10.1016/j.ajp.2024.104073
35. Jirasek P, Jusku A, Frankova J, et al. Phytocannabinoids and gingival inflammation: preclinical findings and a placebo-controlled double-blind randomized clinical trial with cannabidiol. J Periodontal Res. 2024;59(3):468–479. doi:10.1111/jre.13234
36. Rao K, Rochon E, Singh A, et al. Myoglobin modulates the Hippo pathway to promote cardiomyocyte differentiation. iScience. 2024;27(3):109146. doi:10.1016/j.isci.2024.109146
37. Heallen TR, Martin JF. Hippo pathway knockdown gene therapy in the heart. Tex Heart Inst J. 2023;50(5):e238272. doi:10.14503/THIJ-23-8272
38. Ren Y, Wu Y, He W, Tian Y, Zhao X. Exosomes secreted from bone marrow mesenchymal stem cells suppress cardiomyocyte hypertrophy through Hippo-YAP pathway in heart failure. Genet Mol Biol. 2023;46(1):e20220221. doi:10.1590/1678-4685-gmb-2022-0221
39. Schirone L, Vecchio D, Valenti V, et al. MST1 mediates doxorubicin-induced cardiomyopathy by SIRT3 downregulation. Cell mol Life Sci. 2023;80(9):245. doi:10.1007/s00018-023-04877-7
40. Berecz T, Yiu A, Vittay O, et al. Transcriptional co-activators YAP1-TAZ of Hippo signalling in doxorubicin-induced cardiomyopathy. ESC Heart Fail. 2022;9(1):224–235. doi:10.1002/ehf2.13756
41. Cheng X, Liu Y, Qi B, et al. Glycyrrhizic acid alleviated MI/R-induced injuries by inhibiting Hippo/YAP signaling pathways. Cell Signal. 2024;115:111036. doi:10.1016/j.cellsig.2024.111036
42. Hao E, Mukhopadhyay P, Cao Z, et al. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol Med. 2015;21(1):38–45. doi:10.2119/molmed.2014.00261
43. Panos GD, Boeckler FM. Statistical analysis in clinical and experimental medical research: simplified guidance for authors and reviewers. Drug Des Devel Ther. 2023;17:1959–1961. doi:10.2147/DDDT.S427470
44. Mitry MA, Laurent D, Keith BL, et al. Accelerated cardiomyocyte senescence contributes to late-onset doxorubicin-induced cardiotoxicity. Am J Physiol Cell Physiol. 2020;318(2):C380–C391. doi:10.1152/ajpcell.00073.2019
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.