Back to Journals » Cancer Management and Research » Volume 16
Case Report: Efficacy of Multiparameter MRI in Diagnosis of Chronic Breast Inflammation Complicated with Invasive Ductal Carcinoma and Ductal Carcinoma in situ
Authors Zhao X , Guo H, Shi G, Li B, Wang N
Received 15 July 2024
Accepted for publication 18 October 2024
Published 25 October 2024 Volume 2024:16 Pages 1517—1521
DOI https://doi.org/10.2147/CMAR.S481987
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bilikere Dwarakanath
Xia Zhao,1,* Huimin Guo,2,* Guangxi Shi,3 Bingying Li,1 Ning Wang2
1Department of Radiology, Shandong University of Traditional Chinese Medicine Affiliated Hospital, Jinan, 250014, People’s Republic of China; 2Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, 250021, People’s Republic of China; 3Department of Breast and Thyroid Surgery, Shandong University of Traditional Chinese Medicine Affiliated Hospital, Jinan, 250014, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ning Wang, Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, No. 9677 Jingshi Road, Jinan, Shandong, 250021, People’s Republic of China, Email [email protected]
Introduction: Incidental Enhancement Lesions (IELs) complicate patient management but may be detected through multiparameter MRI including dynamic contrast enhancement magnetic resonance imaging (DCE-MRI) and synthetic magnetic resonance imaging (syMRI). The multiparameter MRI model gave greater objectivity to avoid unnecessary biopsy.
Case Presentation: A 60 year-old woman had a history of occasional right breast pain and a mass was identified in the right breast. A thickening in the upper quadrant of the right outer breast was found during physical examination but no mass was palpable. Breast dynamic contrast enhancement MRI and synthetic MRI were performed prior to ultrasound-guided biopsy of the right breast lesion. Resection of the right breast lesion and sentinel lymph node was performed 2 days later. Chronic inflammation, locally invasive ductal carcinoma and high-grade ductal carcinoma in situ were found by pathological examination.
Discussion: Differentiation between benign and malignant breast IELs was facilitated by use of a multiparameter MRI model with DCE-MRI and syMRI, giving greater objectivity in differentiating between benign and malignant lesions.
Keywords: breast cancer, intraductal carcinoma, magnetic resonance imaging
Background
Prognosis and management of malignant breast tumors are greatly facilitated by an early diagnosis which differentiates between benign and malignant lesions. Dynamic contrast enhancement magnetic resonance imaging and synthetic MRI were performed on a 3.0T magnetic resonance imaging device (Pioneer, GE Healthcare) and morphological and metabolic parameters were evaluated at a AW4.7 workstation (Table 1)1 from conventional MRI, DCE-MRI and syMRI clinical features.
 |
Table 1 The Information Provided by the Conventional MRI, DCE-MRI and syMRI |
Incidental Enhancing Lesions (IELs) revealed by MRI may give quantitative information to facilitate patient management and allay patient anxiety. The current case report describes chronic breast inflammation combined with ductal cancer and ductal carcinoma in situ which was confirmed by pathology.
Case History
Written informed consent for publication was given by the patient. A 60-year-old woman presented to the Department of Breast and Thyroid Surgery with a two-week history of breast mass and an unremarkable past medical history apart from occasional pain in the right breast. No medication had been given. Patchy thickening of the outer upper quadrant of the right breast with no palpable mass was revealed by physical examination. No discharge from or depression of either nipple or enlarged lymph nodes in the bilateral armpits and supraclavicular fossa were found. Breast ultrasound examination showed low echogenicity in the right breast (9–10 o’clock position) with an estimated breast imaging reporting and data system (BI-RADS) classification of 4A. Mammography was not performed. MRI was performed in the prone position using a 3.0T whole-body scanner (Pioneer, GE Healthcare, USA) with an eight-channel phased-array breast surface coil. Conventional MRI (OAx T1WI, FS-T2WI and DWI) and the syMRI sequence (OAx MAGiC) were performed first, followed by DCE-MRI sequences obtained with consistent scan parameters and a rapid bolus of 0.2mL/kg gadodiamide contrast agent (GE Healthcare, Ireland) injected intravenously at an injection rate of 2.5mL/s, followed by flushing with 20mL normal saline at a rate of 3mL/s. Two radiologists with 11 and 6 years of breast imaging experience and who were blinded to the pathology result reviewed all images via Advantage Workstation (AW 4.7, GE Healthcare) and reached a consensus.
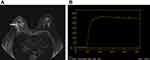
Glandular tissue in the outer upper quadrant of the right breast, presenting slightly longer T1 and T2 signals, was observed to be locally dense and irregularly shaped and mixed with normal breast tissue and adipose tissue. The DWI sequence had no diffusion limitation and significant uneven enhancement of the enhanced DISCO sequence with a plateau in the TIC curve was seen (Figure 1A and B). Angiogenesis and microvascular density within the lesion were evaluated by the DISCO sequence and several small focal areas with significantly increased Ktrans and Kep were visible (Figure 2A and B). Mean Ktrans value within the lesion was 0.990±0.121min−1 and mean Kep was 0.883±0.143min−1. Maximal Ktrans was 1.605±1.417min−1 and maximal Kep was 0.996±0.023min−1. BI-RADS 3–4A was considered based on the stated morphological and hemodynamic characteristics. A quantitative evaluation of lesion histological parameters was produced using the syMRI sequence (OAx MAGiC) (Figure 3A–C). Values of T1=1375ms, T2=84ms and PD= 75.5pu (pu: proton density per unit voxel) were found within the lesion compared with T1=661ms, T2=119ms and PD=99.1pu for normal glandular tissue distant from the lesion. Values of T1=657ms, T2=111ms and PD=104.3pu were found for the outer upper quadrant gland tissue of the healthy breast. Thus, the T1 value was higher, with the T2 and PD values lower within the lesion than in healthy breast gland tissue. The lesion was classified as BI-RADS 4B with reference to relevant literature.
 |
Figure 2 (A and B) The Ktrans and Kep maps obtained from the lesion area show small focal high perfusion areas. |
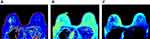 |
Figure 3 (A–C) T1, T2 and PD map images obtained by MAGiC sequence. Higher T1 and lower T2 and PD map values are seen compared with surrounding normal glandular tissue. |
Ultrasound guided biopsy of the right breast lesion was performed and chronic inflammation, possibly accompanied by ductal carcinoma in situ, shown by pathological examination. A modified radical mastectomy with nipple and areola preservation and regional lymph node resection was performed two days later. The lesion had a 3cm diameter with white coloration, tough texture, unclear boundary and no capsule and was seen to be located in the outer upper quadrant of the right breast during surgery. Rapid pathology revealed an invasive carcinoma and high-grade ductal carcinoma in situ located in the lateral quadrant of the right breast. The right breast gland was completely resected and level I, II, III and lateral triangular lymph fat tissue removed. Postoperative pathology showed an invasive ductal carcinoma of a non-specific type with a high-grade intraductal carcinoma in situ in the lateral quadrant of the right breast. Microscopic inspection of the tumor showed a 0.6cm×0.4cm maximal diameter with a histological score of 8 points and low differentiation (G3). Focal chronic inflammation was found in the remaining breast tissue. No intravascular tumor thrombus or nerve invasion was found. Immunohistochemical results were as follows: ER (-), PR (-), AR (-), Her2 (0), CK5/6 (+), Ki-67 (+, 15% −30%), P63 (-), E-Cadherin (+) and P120 (membrane+).
Discussion
The performance of syMRI and DCE-MRI with DISCO enhance sequence in diagnosing IELs of breast tissue was investigated in the current study. Breast ductal carcinoma in situ (DCIS) is a malignant tumor where cancer cells invade the entire epithelial layer but remain confined within the duct, having not yet broken through the basement membrane.2 DCIS is defined as a precancerous lesion by the WHO pathological classification of breast tumors and has varying clinical manifestation and significant biological heterogeneity.3 It is a carcinoma in situ but is usually treated in a similar manner to invasive cancer. Early detection and diagnosis are crucial for patient survival.4 Puncture biopsy may be used to diagnose breast cancer, but it is invasive and gives little information on lesion heterogeneity.
Few studies of multi-modal imaging methods in the diagnosis of breast lesions have been done. Multi-modal imaging technology overcomes the shortcomings of a single imaging mode to improve diagnostic efficiency.5 DCE‑MRI offers superior discrimination of benign and malignant breast lesions for the screening of high-risk breast cancer patients by comparison with mammography and ultrasound approaches. T1-weighted imaging (T1WI), T2WI, DW, and syMRI were performed at 3.0T MRI device. Relaxation time (T1, T2 and PD), TIC Curve, apparent diffusion coefficient (ADC), conventional MRI features and clinical features were assessed.6 Morphological and hemodynamic characteristics may be inferred from DCE-MRI scans and angiogenesis and micro-vessel density evaluated. Enhanced spatial and temporal resolution MRI scans allow the detection of small lesions,7 identifying early breast cancer that is undetectable by mammography or breast ultrasound.
IELs detected during MRI examination are not uncommon and complicate management, causing patient anxiety.1 The difficulty in distinguishing between IELs of benign or malignant origin arises from the limited literature available, although previous scrutiny has concluded that IELs may represent the probability of cancer. A meta-analysis investigating the cancer likelihood ratio of MRI, mammography or breast physical examination indicated that IELs were highly unlikely to represent invasive breast cancer among patients with benign breast lesions detected by MRI and had a positive predictive value of 0.44%.8 In addition, IELs found in patients with tumors known to be malignant may typically represent multifocal or multicenter lesions.9 The diagnostic sensitivity of DCE⁃MRI is high at 80% −95%7 but may be limited by its low specificity.10 Enhancement features may simulate the appearance of malignant disease, leading to unnecessary biopsy.11
Breast lesion classification by BI-RADS has a degree of subjectivity and depends on the level of experience of the interpreting radiologist. Quantitative diagnostic methods are required to impose objectivity on the process and quantitative data from Ktrans, Kep, relaxation time of T1, T2, PD, and other MRI parameters have much to offer. Relaxation time of T1, T2 and PD are inherent properties of matter12 and differences in MRI relaxation time are related to the free water content, with a higher free water content giving a longer relaxation time.6,13 Synthetic MRI technology collates T1, T2 and PD quantitative maps by modulating TE, TR and TI, reflecting signal differences in different tissues. The pathological information obtained may be usefully applied to breast cancer diagnosis and assessment, allowing differentiation between benign and malignant lesions. Synthetic MRI technology has been shown to represent an advance in the sensitivity and specificity of diagnostic imaging with T2 relaxation time being especially meaningful for differential diagnosis.14,15 Continuous cell proliferation and release of necrotic material by malignant lesions reduces the extracellular space, decreasing free water and accounting for shorter T2 relaxation times.
The current case report includes a breast lesion with morphological characteristics that are non-mass enhancement (NME) mixed with normal gland and adipose tissue, giving a hemodynamic TIC curve with a plateau. Localized adenosis, chronic inflammation or breast cancer were thus difficult to identify. This situation may have arisen due to the small size of the cancerous lesion so that the ROI delineation included normal glands, fat and chronic inflammatory lesions around the cancerous lesion. The BI-RADS grading was also ambiguous. Perfusion analysis showed a focal increase in Ktrans and Kep within the lesion, raising suspicions of cancerous components. Synthetic MRI analysis revealed lower T2 values in the lesion relative to the area of chronic inflammation and normal glandular tissue, increasing the likelihood of malignancy.
Conclusion
In summary, use of multiparameter MRI for breast Incident Enhancement Lesions (IELs) is recommended. DCE-MRI with multiparameter MRI and syMRI may enhance diagnostic specificity, such as Ktrans, Kep and the relaxation time of T1, T2, PD. Different imaging parameters may be used to give information about different lesion characteristics. In conclusion, the multiparameter MRI model represents an advance over existing approaches for differentiation between benign and malignant lesions due to greater objectivity. Further studies of multi-modal imaging methods in the diagnosis of breast lesions are required to confirm the current findings.
Ethical Approval
Ethical approval was granted by the ethics committee of Shandong University of Traditional Chinese Medicine Affiliated Hospital.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The authors declare that they have no conflicts of interest in this study.
References
1. Hun KS, Lee HS, Kang BJ, et al. Dynamic contrast-enhanced MRI perfusion parameters as imaging biomarkers of angiogenesis. PLoS One. 2016;11(12):e0168632. doi:10.1371/journal.pone.0168632
2. Zujewski JA, Harlan LC, Morrell DM, et al. Ductal carcinoma in situ: trends in treatment over time in the US. Breast Cancer Res Treat. 2011;127(1):251–257. doi:10.1007/s10549-010-1198-z
3. Böcker W. [WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics]. Verh Dtsch Ges Pathol. 2002;86:116–119.
4. Bray F, Ferlay J, Soerjomataram I, Rebecca L, Lindsey S, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2018;68:394–424. doi:10.3322/caac.21492
5. Bai D, Zhou N, Liu X, et al. The diagnostic value of multimodal imaging based on MR combined with ultrasound in benign and malignant breast diseases. Clin Exp Med. 2024;24(1). doi:10.1007/s10238-024-01377-1
6. Y SS, Ding Y, Li Z, et al. Multiparameter MRI Model With DCE-MRI, DWI, and Synthetic MRI improves the diagnostic performance of BI-RADS 4 Lesions. Front Oncol. 2021;11. doi:10.3389/fonc.2021.699127
7. Ikeda DM, Baker DR, Daniel BL. Magnetic resonance imaging of breast cancer: clinical indications and breast MRI reporting system. J Magn Reson Imaging. 2000;12(6):975–983. doi:10.1002/1522-2586(200012)12:6<975::AID-JMRI24>3.0.CO;2-Y
8. Lawrence WF, Liang W, Mandelblatt JS, et al. Serendipity in diagnostic imaging: magnetic resonance imaging of the breast. J National Cancer Inst. 1998;23:23.
9. Schmelzel L, Ikeda D, Daniel B, et al. Incidental Enhancing Lesions (IEL) detected during contrast-enhanced breast magnetic resonance (MR) imaging scans: prevalence and clinical impact. In:
10. Padia SA, Freyvogel M, Dietz J, et al. False-positive extra-mammary findings in breast MRI: another cause for concern. Breast J. 2016;22: 90.
11. Millet I, Pages E, Hoa D, et al. Pearls and pitfalls in breast MRI. Br J Radiol. 2012;1011:85.
12. Xin G, Wang M, Ma H, et al. Investigated diagnostic value of synthetic relaxometry, three-dimensional pseudo-continuous arterial spin labelling and diffusion-weighted imaging in the grading of glioma. Magnet Reson Imag. 2022;86:20–27. doi:10.1016/j.mri.2021.11.006
13. Lüssea a S, Claassen H, Gehrke T, et al. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magnet Reson Imag. 2000;18:423–430. doi:10.1016/S0730-725X(99)00144-7
14. Weibo G, Shuqun Z, Jin G, et al. Investigation of synthetic relaxometry and diffusion measures in the differentiation of benign and malignant breast lesions as compared to BI-RADS. J Magn Reson Imaging. 2021;53(4):1118–1127. doi:10.1002/jmri.27435
15. Liu L, Yin B, Shek K, et al. Role of quantitative analysis of T2 relaxation time in differentiating benign from malignant breast lesions. J Int Med Res. 2018;46(5):1928.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.