Back to Journals » International Journal of Nanomedicine » Volume 19
Cathepsin B-Activatable Bioactive Peptide Nanocarrier for High-Efficiency Immunotherapy of Asthma
Authors Song T, Yao L, Zhu A, Liu G, Zhu B , Zhao Q, Zhao Y, Wang J
Received 19 December 2023
Accepted for publication 23 July 2024
Published 7 August 2024 Volume 2024:19 Pages 8059—8070
DOI https://doi.org/10.2147/IJN.S455633
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Taiyu Song,* Lulu Yao,* Angang Zhu,* Guangling Liu, Beibei Zhu, Qian Zhao, Yue Zhao, Jinya Wang
Department of Pediatrics, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinya Wang, Email [email protected]
Introduction: Asthma, a chronic respiratory disease closely associated with inflammation, presents ongoing treatment challenges. IALLIPF (le-Ala-Leu-Leu-Ile-Pro-Phe) is one of millet prolamins peptides (MPP) which shows anti-oxidant bioactivity by reducing the production of reactive oxygen species (ROS). Tryptophan (Trp, W) is an amino acid that has been demonstrated to possess anti-inflammatory effects. We introduce a novel cathepsin B-activatable bioactive peptides nanocarrier, PEG-IALLIPF-GFLG-W (MPP-Trp), designed for immunotherapy of asthma.
Methods: MPP-Trp is synthesized, purified, and its characteristics are investigated by dynamic light scattering (DLS) and transmission electron microscopy (TEM). The yield of nitric oxide (NO) and pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) are examined to evaluate anti-inflammatory effects of IALLIPF, Trp and MPP-Trp. The immunomodulatory effects of IALLIPF, Trp and MPP-Trp on Th1/Th2 cell populations and cytokines are investigated by flow cytometry, qRT-PCR and ELISA assays. We explore the therapeutic effect of MPP-Trp in the mouse model of asthma by the analysis of lung histology and ELISA. It is necessary to study the biocompatibility of MPP-Trp by CCK8 assay and histopathologic analysis using hematoxylin and eosin (HE) staining.
Results: In asthmatic peripheral blood mononuclear cells (PBMCs), IALLIPF, Trp and MPP-Trp are able to significantly alleviate inflammation by inhibiting the yield of nitric oxide (NO) and pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β), especially MPP-Trp. MPP-Trp significantly upregulates Th1 cell levels while notably reducing Th2 cell levels. Furthermore, MPP-Trp effectively elevates the expression and production of interferon-gamma (IFN-γ), an essential cytokine from Th1 cells. Additionally, MPP-Trp markedly diminishes the mRNA expression and levels of key asthma pathogenesis cytokines, such as interleukin-4 (IL-4), interleukin-13 (IL-13), and interleukin-5 (IL-5), in asthma PBMCs. MPP-Trp ameliorates pulmonary pathological alterations and significantly inhibits OVA-induced inflammation in mice with asthma. It has little influence on the cell viability in Asthma-PBMCs treated with various concentrations or durations of MPP-Trp. No pathological changes, including in the heart, liver, spleen, lung, and kidney tissues, are observed in non-sensitized and non-challenged mice treated with MPP-Trp (20 mg/kg).
Discussion: Our research demonstrates that MPP-Trp has immunomodulatory effects on Th1/Th2 cell populations, essential in managing asthma. It considerably alleviates OVA-induced asthma by shifting the immune response towards a Th1-dominant profile, thereby reducing Th2-driven inflammation. Therefore, this novel bioactive peptide nanocarrier, MPP-Trp, holds promise as a candidate for asthma immunotherapy.
Keywords: asthma, cathepsin B, bioactive peptide nanocarrier, MPP-Trp, immunomodulatory
Graphical Abstract:

Introduction
Asthma is indeed a significant global health concern, characterized by chronic airway inflammation and increased airway reactivity (AHR).1,2 This condition involves a complex interplay of various cells and cytokines in the respiratory system.2,3 Unfortunately, in recent years, the prevalence and mortality rates associated with asthma have been steadily increasing, highlighting the need for improved treatment strategies.4 Currently, the primary approach to managing asthma relies on symptomatic and supportive drug therapies.1,4–6 These include inhaled glucocorticoids (ICS), long-acting beta2 receptor agonists, leukotriene receptor antagonists, theophylline, anticholinergics, mesulfonist, bio-targeted drugs, and second-generation antihistamines.1,4,5,7,8 Although these medications effectively control asthma symptoms, it is common for asthma symptoms to recur once treatment is stopped.9,10 Hence, it is essential to discover more effective and sustainable treatment strategies.
Immunotherapy has emerged as a promising approach for the treatment of asthma.3 Unlike traditional drug therapies, immunotherapy offers continuous benefits and can maintain its effectiveness even after the completion of the treatment course.3,11 This long-term treatment effect helps improve symptoms, reduce the need for medications, enhance the quality of life for patients, and alleviate the economic burden on affected families. As diseases become increasingly complex, there is a growing recognition of the limitations of single-drug delivery systems.4 Challenges such as drug resistance, high toxicity, and limited clinical applications have prompted the exploration of combination therapies involving two or more drugs.12–14 This strategy aims to maximize therapeutic effects, reduce drug dosages, achieve controlled drug release, and minimize toxic side effects. While this approach has shown great promise in various conditions, concerns about potential side effects arising from drug interactions have led to cautious research in this field.15
In recent years, bioactive peptides have gained significant attention due to their physiological regulatory functions, which include immune modulation and anti-inflammatory effects.16 These peptides are highly regarded in the field of biology for their rapid absorption and safety profiles. Consequently, the development of a novel nanocarrier system for delivering plant-derived anti-inflammatory peptides and anti-inflammatory drugs represents an exciting avenue of research with the potential to address the challenges associated with asthma treatment and other inflammatory conditions.17 This innovative approach may offer safer and more effective ways to manage these complex diseases.
It has been reported that peptides derived from natural plant proteins have demonstrated various bioactivities in many studies. IALLIPF (le-Ala-Leu-Leu-Ile-Pro-Phe) is one of millet prolamins peptides (MPP) that exhibits anti-oxidant bioactivity by reducing the production of reactive oxygen species (ROS) and malondialdehyde (MDA), while increasing the level of glutathione (GSH) in H2O2-induced cells.18,19 Bioactive peptides have been widely used in drug development due to their good biological activity, biocompatibility, and low toxicity and side effects.
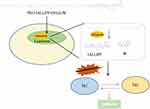
Herein, we present a novel cathepsin B-activatable nanocarrier called MPP-Trp (PEG-IALLIPF-GFLG-W) for asthma immunotherapy. PEG possesses great flexibility due to the absence of bulky substituents along the chain, high hydration of the polymeric backbone, and a high degree of safety.20 It is reported that PEG can be covalently or noncovalently conjugated to proteins, peptides, and nanoparticles without compromising their bioactivity, to limit their clearance by the reticuloendothelial system, and reduce their immunogenicity.21 Tryptophan (Trp, W) is an amino acid known for its anti-inflammatory effects. GFLG (Gly-Phe-Leu-Gly) is a specific cleavage site of cathepsin B, which is abundant in the lysosome.22,23 Upon the carrier’s (MPP-Trp) entry into the lysosome, PEG and IALLIPF-tryptophan are first dissociated because of that the semicarbazide bond is stable under slightly basic conditions but breaks under acidic conditions, which is formed by aldehyde group on PEG and the amino group on the peptide chain. The anti-inflammatory peptide IALLIPF and the anti-inflammatory drug also get cleaved by cathepsin B, forming free anti-inflammatory peptide IALLIPF and the anti-inflammatory drug separately. They exhibit their respective pharmacological effects, achieving a synergistic effect. Our study demonstrates that MPP-Trp exhibits exceptional sensitivity and selectivity towards cathepsin B, a crucial enzyme in asthma-related inflammation. Furthermore, our research highlights the immunomodulatory effects of MPP-Trp on Th1/Th2 cell populations. Compared to the asthma-PBMCs group, the addition of MPP-Trp led to a significant upregulation of Th1 cells while causing a substantial downregulation of Th2 cells. This shift towards a Th1-dominant response holds great promise for asthma treatment, as Th2-driven inflammation is a hallmark of the disease. Furthermore, MPP-Trp effectively increased the expression levels and content of IFN-γ, an important Th1 cytokine. Additionally, MPP-Trp significantly reduced the mRNA expression levels and contents of IL-4, IL-13, and IL-5, which are key Th2 cytokines associated with asthma pathogenesis. This downregulation of Th2 cytokines further supports the potential of MPP-Trp as a high-efficiency immunotherapy approach for asthma. Our findings suggest that MPP-Trp has the potential to be a promising immunotherapy candidate for asthma, given its ability to modulate the immune response towards a Th1-dominant profile and reduce Th2-driven inflammation. Further research and clinical studies are warranted to explore the full therapeutic potential of MPP-Trp in the management of asthma.
Materials and Methods
Peptide Synthesis
Solid-phase peptide synthesis is a prevalent technique for peptide assembly, employedexpertly by Nanjing Jiepeptide Biotechnology Co, Ltd. Beginning from the C-terminal amino acid and progressing to the N-terminus, an activated resin anchors the initial Fmoc-protected amino acid. Once attached, the Fmoc protection is removed to reveal a free amino end. The process is repeated, coupling each subsequent amino acid to the prior one. The final product is Fmoc-IALLIPF-GFLG-W. After completing the peptide sequence, protective groups are removed, and the resultant crude peptide is purified to the desired purity using HPLC.
MPP-Trp Synthesis
Synthesis of NH2-IALLIPFGFLG-W: Fmoc-IALLIPF-GFLG-W is dissolved in a pyridine-based DMF solution and reacts at room temperature. Upon completion, a product is precipitated by adding methyl tert-butyl ether, filtered, and the resulting filter cake washes three times with methyl tert-butyl ether to yield the product.
Synthesis of PEG-IALLIPFGFLG-W: NH2-IALLIPFGFLG-W and CH3O-PEG-CHO are dissolved in DMSO, and DIPEA is added. The reaction proceeds at 50°C for 24 h. After completion, 20 mL of ether is added, the mixture is filtered, and the filter cake washes three times with 10 mL of ether. The crude product was purified by high-performance liquid chromatography to yield the final product.
Animals
All procedures with animals were approved by the Institutional Ethics Committee of Nanjing Drum Tower Hospital ([2021]-KY-035-01) and performed in accordance with the “Guidelines for the Ethical Review of Laboratory Animal Welfare” (GB/T 35892-2018). 6–8 week-old male C57BL/6 mice (Charles River Lab, China) were housed with sufficient food, and drinking water under 24–26 °C before the experiments.
Establishment of Mouse Model of OVA-Induced Asthma
The mice were divided into two groups: the control group (n=6) and Asthma group (n=6). Alum sensitization adjuvant was used to establish the Asthma model in vivo. For alum sensitization adjuvant: Mix 2 mL of 10% alum solution (soluble in double distilled water) with 2 mL of 500 μg/mL ovalbumin (OVA, soluble in PBS) in equal amounts, adjust the pH value to 6.5 with NaOH, incubate at room temperature for 60 min, centrifuge for 5 min at 750 r/min, remove the supernatant, and dissolve again in 2 mL PBS. Next, for sensitization: On day 0 and 14, mice were intraperitoneally injected with 0.2 mL of alum sensitization adjuvant, while the control group was injected with the same dose of PBS and fed on a normal diet. The following excerpt was stimulating by nebulization inhalation. On the 21st day, mice were then challenged. Specifically, the mice were placed in an organic glass box and nebulized with 5% OVA for 30 min per day for 7 days, and tested within 24 h after the last sensitization. Positive reactions such as restlessness, shortness of breath, and abdominal muscle spasms in model mice were regarded as the criteria for successful modeling. The control group was treated with PBS nebulization inhalation, and all other procedures were the same. Finally, the mice were anesthetized for 24 h after the last challenge, and their eyeballs were removed for blood collection.
Cell Isolation, Culture and Treatment
The total peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood samples of control group and asthma group by mouse peripheral blood mononuclear cell isolation kit (P6340; Solarbio, Beijing, China) according to the manufacturer's protocol. Briefly, the separation liquid was added into the anticoagulated whole blood and centrifuged. After that, the second layer of the stratified liquid was isolated and rinsed with PBS twice for use. Control-PBMCs and Asthma-PBMCs were completely cultured in RPMI1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in 5% CO2. Asthma-PBMCs were treated with PEG-Liker, Trp, or MPP-Trp, respectively, to observe the effect of Th1/Th2 balance. Next, to select the optimal treatment concentration and duration of MPP-Trp, Asthma-PBMCs were incubated with different concentrations of MPP-Trp (10, 50, 100 and 200 μg/mL) for 12h, or 100 μg/mL MPP-Trp for 6, 12, 24 and 48h.
The Route of Administration and Biocompatibility Evaluation of MPP-Trp
The mice were divided into five groups: control, Asthma, Asthma+MPP-Trp (5 mg/kg), Asthma+MPP-Trp (10 mg/kg) and Asthma+MPP-Trp (20 mg/kg). MPP-Trp was dissolved in PBS and given by tail vein injection once a day 10 min before OVA challenge for 7 days (from day 21 to day 27). The experimental design is illustrated (Figure S3). All the data for the administration with MPP-Trp (20 mg/kg) alone in non-sensitized and non-challenged mice are presented to evaluate the biocompatibility and biosafety of MPP-Trp in vivo (Figure S4C and D).
For cell experiment, Control-PBMCs and Asthma-PBMCs were plated in 96-well plates, and incubated overnight. Then, the Asthma-PBMCs were washed with PBS, and treated with the medium containing different concentrations of MPP-Trp (10, 50, 100 and 200 μg/mL) for 12h, or 100 μg/mL MPP-Trp for 6, 12, 24 and 48h. Enhanced Cell Counting Kit-8 (C0046) was purchased from the Beyotime Biotechnology (Shanghai, China). 10 µL CCK8 solution was added to each well, and the cells were further incubated for 1 h. The absorbance was measured at 450 nm with a SpectraMax M2e Microplate System (Molecular Devices, CA, USA).
Detection of the Production of NO and Pro-Inflammatory Cytokines (TNF-α, IL-1β, and IL-6)
The NO assay kit (A012-1) was purchased from the Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). Pro-inflammatory cytokines kits, including TNF-α (SEKM-0034), IL-1β (SEKM-0002), and IL-6 (SEKM-0007) were purchased from Solarbio Biotechnology Co., Ltd (Beijing, China). The cells were cultured in 6-well plates at a density of 5×105 cells per well overnight. Subsequently, the cells were treated with medium containing 100 μg/mL PEG-Liker, Trp, or MPP-Trp for 24h. For detection of NO, the OD values were measured at 550 nm. TNF-α, IL-1β, and IL-6 levels in the collected supernatants were quantified by measuring the absorbance at 450 nm. The concentrations of these cytokines were determined using standard curves.
Total RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated using Trizol reagent (9108, Takara, Otsu, Japan). qRT-PCR was performed using SYBR Green Mix (Q311, Vazyme, Nanjing, China) on ABI 7500 FAST Real-time PCR system (Applied Biosystems, Darmstadt, Germany). β-actin was employed as an endogenous control to normalize the relative expression of mRNA. The primer sequences of IFN-γ, IL-4, IL-13, IL-5, and β-actin were as followed: IFN-γ forward 5’-GTATTGCCAAGTTTGAGGTCAAC-3’ and reverse 5’-GCTTCCTGAGGCTGGATTC-3’; IL-4 forward 5’-TCCTCACAGCAACGAAGAAC-3’ and reverse 5’-CAAGCATGGAGTTTTCCCATG-3’; IL-13 forward 5’-TCTTGCTTGCCTTGGTGG-3’ and reverse 5’-GGCGAAACAGTTGCTTTGTG-3’; IL-5 forward 5’-AGGCTTCCTGTCCCTACT-3’ and reverse 5’- TTGGCGGTCAATGTATTT-3’; β-actin forward 5’-CTGTCCCTGTATGCCTCTG-3’ and reverse 5’-ATGTCACGCACGATTTCC-3’.
The Measure of IFN-γ, IL-4, IL-13, and IL-5
On the basis of the protocols, high-sensitive mouse IFN-γ (PI507), IL-4 (PI613), IL-13 (PI539), and IL-5 (PI620) ELISA kits were purchased from Beyotime Biotechnology (Shanghai, China) and used to detect their contents after the Asthma-PBMCs were treated with PEG-Liker, Trp, or MPP-Trp. Absorbance in each well was read at 450 nm. The contents of IFN-γ, IL-4, IL-13, and IL-5 in PEG-Liker, Trp, or MPP-Trp group were counted by the standard curves.
Flow Cytometry Assay
Firstly, we used RPMI1640 medium containing 10% fetal bovine serum to resuspend and precipitate PBMCs at a concentration of 1×107/mL. Then, we transfered 250 μL PBMCs to the flow tube, added 1 μL PMA/Ionomycin Mixture (250×) and 1 μL BFA/Monensin Mixture (250×). They were incubated at 37 °C for 4–6 h, removed and shaked well every 1–2 h. We transfered the cell suspension to a new flow tube, and added 5 μL anti-mouse CD3 and 5 μL anti-mouse CD4. For detecting Th1, 100 μL cell suspension was incubated with anti-mouse IFN-γ antibodies for 30 min under dark and fixed with Fixation/Permeabilization buffer. After incubation, the cells were rinsed twice with PBS. To detect Th2, equivalent amount of cell suspension was incubated with anti-mouse IL-4 antibodies for 30 min under dark then rinsed with PBS. After that, the cells were incubated with Fixation/Permeabilization buffer for another 30 min, rinsed and centrifuged to discard the supernatant. Finally, flow cytometry assay was performed on CytoFLEX SRT Cell Sorter (C71885; Beckman Coulter).
Hematoxylin and Eosin (HE) Staining
Left lung, heart, liver, spleen, and kidney tissues were removed, fixed in 4% paraformaldehyde for 48h to preserve tissue architecture, embedded in paraffin, and sectioned at 5 μm. Tissue sections were subjected to haematoxylin and eosin staining. Images were captured using Olympus BX51 microscope (Olympus, Tokyo, Japan).
Statistical Analysis
Using Prism 6 (GraphPad), the unpaired two-tailed Student’s t-test was utilized to analyze differences between the experimental groups. When appropriate, one-way ANOVA was employed, followed by the Tukey’s post hoc comparison test. A P-value <0.05 was considered to be significant.
Results and Discussion
Design of cathepsin B-Activatable Nanocarrier for Immunotherapy of Asthma
We have successfully synthesized cathepsin B-responsive polypeptide segments by solid-phase synthesis (Figure S1), and then we linked them to anti-inflammatory drug tryptophan (Trp), and then the aldehyde group on CH3O-PEG-CHO reacts with the amino group on the polypeptide chain to form hydrazone bonds, and successfully synthesized cathepsin B activatable nanocarrier (MPP-Trp, PEG-IALLIPF-GFLG-W) (Figure S2). Dynamic light scattering (DLS) analysis of MPP-Trp demonstrated a good mono-dispersity in aqueous solution with a mean hydrodynamic diameter of ∼69 nm (Figure 1A). Transmission electron microscopy (TEM) showed MPP-Trp was spherical morphology (Figure 1B). We subsequently examined the sensitivity and specificity of MPP-Trp toward cathepsin B (Figure 1C). Absorption of released Trp became enhanced with cathepsin B concentration, showing a linear correlation between absorption of released Trp and cathepsin B concentrations from 0 to 25 U/L, with limit of detection (LOD) of ~0.53 U/L (3σ/k) (Figure 1D). We then evaluated the selectivity of the nanocarrier MPP-Trp. MPP-Trp showed that only cathepsin B was able to trigger the release of tryptophan (Trp), and therefore, the nanocarrier had excellent specific to cathepsin B (Figure 1E and F).
PEG-Liker (PEG-IALLIPF), Trp, or MPP-Trp Treatment Influenced the Production of NO and Pro-Inflammatory Cytokines
Many existing researches reported that the inhibition of NO synthesis and pro-inflammatory cytokines played a significant role in the therapeutic intervention of inflammatory disorders.24 The levels of NO showed a significant increase in Asthma-PBMCs compared with the Control-PBMCs group, leading to a 2.43-fold increase. PEG-Liker (PEG-IALLIPF), Trp, or MPP-Trp reduced the levels of NO compared with Asthma-PBMCs group. It is noteworthy that MPP-Trp had the highest NO inhibitory activity (Figure 2A). Asthma-PBMCs group had a increased TNF-α, IL-6, and IL-1β productions compared with the Control-PBMCs group. However, after the treatment with PEG-Liker (PEG-IALLIPF), Trp, or MPP-Trp, the secretions of pro-inflammatory cytokines were significantly reduced compared with Asthma-PBMCs group. MPP-Trp exhibited a superior anti-inflammatory effect as the cytokine levels (TNF-α, IL-6, and IL-1β) were the lowest in PEG-Liker (PEG-IALLIPF), Trp, or MPP-Trp group (Figure 2B–D). The anti-inflammatory effect of IALLIPF was reported in some reasearches. In HaCaT cells and RAW264.7 murine macrophages, IALLIPF had been reported to show high anti-inflammatory activities in terms of the inhibition the production of NO and pro-inflammatory cytokines.19 The findings mentioned above were consistent with our results. The potent inhibitory effects of MPP-Trp on the inflammatory factor activity may be attributed to its combination of IALLIPF and Trp.
PEG-Liker (PEG- IALLIPF), Trp, or MPP-Trp Treatment Stimulated Asthma-PBMCs to Regulate Th1/Th2 Population and Their Cytokine Productions
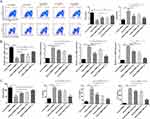
CD4+ T cells are subdivided into Th1 and Th2 cells. Th1 cells or proinflammatory T cells are known to produce cytokines with proinflammatory activities. Th2 or helper T cells are known to produce cytokines that help B cells to become activated and to switch their class of antibody. Some of the cytokines produced by Th2 cells (IL-4, IL-5, and IL-13) also have immune regulatory qualities. The abnormal and excessive activation of Th2 cells causes the imbalance of the Th1/Th2 immune response, which further leads to the vicious circle of disease, even seriously affecting the immune homeostasis of patients.25 It was reported that inducing an increase in Th1 activity could be considered as a treatment strategy for asthma.26 According to the result of flow cytometry assay, we found that Th2 cells were significantly increased but Th1 cells were markedly decreased in Asthma-PBMCs group compared with Control-PBMCs group. To explore the role of PEG-Liker, Trp, or MPP-Trp in asthma, we examined the Th1/Th2 population, and the expression levels and contents of IFN-γ, IL-4, IL-13, and IL-5 in Asthma-PBMCs treated with PEG-Liker, Trp, or MPP-Trp. Compared to Asthma-PBMCs group, Th1 cells were significantly upregulated in Asthma-PBMCs+PEG-Liker group, Asthma-PBMCs+Trp group, and Asthma-PBMCs+MPP-Trp group, especially in Asthma-PBMCs+MPP-Trp group. Th2 cells level showed the most significantly down-regulated in Asthma-PBMCs+MPP-Trp group compared to Asthma-PBMCs group (Figure 3A). Further analysis by qRT-PCR and ELISA assays, the mRNA expression level and content of IFN-γ were dramatically reduced in Asthma-PBMCs group compared with Control-PBMCs group. The treatment of PEG-Liker, Trp, or MPP-Trp effectively increased the expression level and content of IFN-γ. Contrary to the results of IFN-γ, the mRNA expression levels and contents of IL-4, IL-13 and IL-5 were dramatically down-regulated in Asthma-PBMCs+PEG-Liker group, Asthma-PBMCs+Trp group, and Asthma-PBMCs+MPP-Trp group (Figure 3B and C). On the whole, the treatment with MPP-Trp resulted in significantly higher expression levels of IFN-γ and lower levels of IL-4, IL-13 and IL-5. Therefore, MPP-Trp was selected for further investigation as a potential treatment strategy for Asthma-PBMCs.
MPP-Trp Regulated Th1/Th2 Cytokine Production in a Concentration-Dependent Manner
The modulatory effect of MPP-Trp on the Th1/Th2 cytokine-producing ability in Asthma-PBMCs was investigated. To explore the effective concentration range for MPP-Trp treatment, Asthma-PBMCs were performed in 10, 50, 100, or 200 μg/mL MPP-Trp for 12 h. The treatment of 100 μg/mL MPP-Trp significantly increased the content and mRNA expression levels of IFN-γ relative to the Asthma-PBMCs group and other concentration treatments (Figure 4A and B). In contrast, we found that the transcription expressions and contents of IL-4, IL-13 and IL-5 were obviously decreased in a dose-dependent manner. And there was the lowest mRNA expression levels and contents of IL-4, IL-13 and IL-5 for Asthma-PBMCs group after the stimulation of 100 μg/mL MPP-Trp (Figure 4). In the present study, we showed that MPP-Trp concentration-dependently modulated Th1/Th2 cytokine production in Asthma-PBMCs. In addition, compared with Asthma-PBMCs, it has little influence on the cell viability in Asthma-PBMCs treated with different concentrations of MPP-Trp (Figure S4A).
MPP-Trp Regulated Th1/Th2 Cytokine Production in a Time-Dependent Manner
Based on the results of the effective concentration, we chosen the stimulation condition (100 μg/mL MPP-Trp) for further time-point screening experiments. Asthma-PBMCs were treated with 100 μg/mL MPP-Trp for 6, 12, 24, and 48 h. The elevated IFN-γ-producing capacity and transcription levels were notable following the 100 μg/mL MPP-Trp treatment for 6 h, 12 h, 24 h, and 48 h. It was worth noting that 100 μg/mL MPP-Trp for 24 h treatment had a highest expression level and content of IFN-γ among these time-points (Figure 5A and B). Compared with the results of IFN-γ, Th2-type cytokines (IL-4, IL-5, and IL-13) showed the opposite effect. Specifically, the mRNA levels and productions of IL-4, IL-5, and IL-13 were lowest when Asthma-PBMCs were treated with 100 μg/mL MPP-Trp for 24 h. Therefore, the optimum treatment condition (100 μg/mL MPP-Trp for 24 h) for Asthma-PBMCs was verified. In addition, compared with Asthma-PBMCs, it has little influence on the cell viability in Asthma-PBMCs treated with different durations of 100 μg/mL MPP-Trp (Figure S4B). The no cell cytotoxicity of the MPP-Trp reflected its excellent biocompatibility.
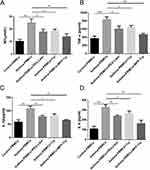
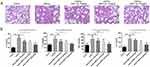
MPP-Trp Improved Pulmonary Pathological Alterations in OVA-Induced Asthma
To observe the pathological alterations of the lungs, HE staining was performed in our study. The lung tissue from OVA-induced mice showed inflammatory cell infiltration and epithelial cell hyperplasia in the peribronchial and perivascular areas, and the pretreatment with MPP-Trp reduced pulmonary lesions, dose-dependently, suggesting that MPP-Trp (20 mg/kg) had a good therapeutic effect for the asthma (Figure 6A). And no pathological changes, including heart, liver, spleen, lung, and kidney, were found in the PBS group and non-sensitized and non-challenged mice treated with MPP-Trp (20 mg/kg) (Figure S4D). We examined the effect of the MPP-Trp on the levels of some cytokines (IL-4, IL-5, IL-13 and TNF-α). MPP-Trp (20 mg/kg) significantly inhibited the production of these cytokines for OVA-induced mice (Figure 6B). However, MPP-Trp (20 mg/kg) had no effect on the production of these cytokines in non-sensitized and non-challenged mice compared with PBS group (Figure S4C). The no influence of the MPP-Trp in mice reflected its excellent biocompatibility and biosafety in vivo.
Conclusion
In summary, our study introduces an innovative cathepsin B-activatable nanocarrier, MPP-Trp, as a potential breakthrough in asthma immunotherapy. We demonstrate that MPP-Trp possesses remarkable sensitivity and selectivity towards cathepsin B. The potent inhibitory effects of MPP-Trp on the inflammatory factor activity may be attributed to its combination of IALLIPF and Trp. Moreover, MPP-Trp effectively modulates the Th1/Th2 cell populations (Figure 7). Compared to untreated asthma peripheral blood mononuclear cells (PBMCs), MPP-Trp significantly boosts Th1 cell levels while notably suppressing Th2 cells, which are key drivers of asthma. Additionally, MPP-Trp enhances the expression and production of IFN-γ, and substantially reduces the mRNA expression and levels of IL-4, IL-13, and IL-5 in asthma PBMCs. MPP-Trp improved pulmonary pathological alterations for OVA-induced asthma. The no influence of the MPP-Trp on mice and cell viability reflected its excellent biocompatibility. These findings underscore the potential of MPP-Trp as a highly efficient approach for asthma immunotherapy.
Ethical Approval
The study involving animals were reviewed and approved by Nanjing Drum Tower Hospital, Affiliated Hospital of Nanjing University ([2021]-KY-035-01).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Science Foundation of China (No.82100035).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Miller RL, Grayson MH, Strothman K. Advances in asthma: new understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J Allergy Clin Immunol. 2021;148(6):1430–1441. doi:10.1016/j.jaci.2021.10.001
2. Sockrider M, Fussner L. What is asthma? Am J Respir Crit Care Med. 2020;202(9):P25–P26. doi:10.1164/rccm.2029P25
3. Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(9):2521–2522. doi:10.1016/j.cell.2021.04.019
4. Cevhertas L, Ogulur I, Maurer DJ, et al. Advances and recent developments in asthma in 2020. Allergy. 2020;75(12):3124–3146. doi:10.1111/all.14607
5. Gans MD, Gavrilova T. Understanding the immunology of asthma: pathophysiology, biomarkers, and treatments for asthma endotypes. Paediatr Respir Rev. 2020;36:118–127. doi:10.1016/j.prrv.2019.08.002
6. Reddel HK, Bacharier LB, Bateman ED, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. J Allergy Clin Immunol Pract. 2022;10(1S):S1–S18. doi:10.1016/j.jaip.2021.10.001
7. O’Byrne P, Fabbri LM, Pavord ID, Papi A, Petruzzelli S, Lange P. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J. 2019;54(1):1900491. doi:10.1183/13993003.00491-2019
8. Katial RK, Bensch GW, Busse WW, et al. Changing paradigms in the treatment of severe asthma: the role of biologic therapies. J Allergy Clin Immunol Pract. 2017;5(2S):S1–S14. doi:10.1016/j.jaip.2016.11.029
9. Thomas D, McDonald VM, Pavord ID, Gibson PG. Asthma remission: what is it and how can it be achieved? Eur Respir J. 2022;60(5):2102583. doi:10.1183/13993003.02583-2021
10. Kritikos V, Harvey ES, Stevens S, et al. Comorbidities modify the phenotype but not the treatment effectiveness to mepolizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2023;11(3):885–895e13. doi:10.1016/j.jaip.2022.12.004
11. Nakagome K, Nagata M. Allergen immunotherapy in asthma. Pathogens. 2021;10(11):1406. doi:10.3390/pathogens10111406
12. Caramori G, Nucera F, Mumby S, Lo Bello F, Adcock IM. Corticosteroid resistance in asthma: cellular and molecular mechanisms. Mol Aspects Med. 2022;85:100969. doi:10.1016/j.mam.2021.100969
13. Palumbo ML, Prochnik A, Wald MR, Genaro AM. Chronic stress and glucocorticoid receptor resistance in asthma. Clin Ther. 2020;42(6):993–1006. doi:10.1016/j.clinthera.2020.03.002
14. Mei D, Tan WSD, Wong WSF. Pharmacological strategies to regain steroid sensitivity in severe asthma and COPD. Curr Opin Pharmacol. 2019;46:73–81. doi:10.1016/j.coph.2019.04.010
15. Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020;29(155):190151. doi:10.1183/16000617.0151-2019
16. Akbarian M, Khani A, Eghbalpour S, Uversky VN. Bioactive peptides: synthesis, sources, applications, and proposed mechanisms of action. Int J Mol Sci. 2022;23(3). doi:10.3390/ijms23031445
17. Guha S, Majumder K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: a brief review. J Food Biochem. 2019;43(1):e12531. doi:10.1111/jfbc.12531
18. Ji Z, Feng R, Mao J. Separation and identification of antioxidant peptides from foxtail millet (Setaria italica) prolamins enzymatic hydrolysate. Cereal Chem. 2019;96(6):981–993. doi:10.1002/cche.10202
19. Ji Z, Mao J, Chen S, Mao J. Antioxidant and anti-inflammatory activity of peptides from foxtail millet (Setaria italica) prolamins in HaCaT cells and RAW264.7 murine macrophages. Food Bioscience. 2020;36:100636. doi:10.1016/j.fbio.2020.100636
20. Pasut G, Veronese FM. State of the art in PEGylation: the great versatility achieved after forty years of research. J Control Release. 2012;161(2):461–472. doi:10.1016/j.jconrel.2011.10.037
21. Lu X, Perera TH, Aria AB, Callahan LAS. Polyethylene glycol in spinal cord injury repair: a critical review. J Exp Pharmacol. 2018;10:37–49. doi:10.2147/JEP.S148944
22. Dheer D, Nicolas J, Shankar R. Cathepsin-sensitive nanoscale drug delivery systems for cancer therapy and other diseases. Adv Drug Delivery Rev. 2019;151:130–151. doi:10.1016/j.addr.2019.01.010
23. Li Y, Mei T, Han S, et al. Cathepsin B-responsive nanodrug delivery systems for precise diagnosis and targeted therapy of malignant tumors. Chin Chem Lett. 2020;31(12):3027–3040. doi:10.1016/j.cclet.2020.05.027
24. He R, Liu M, Zou Z, et al. Anti-inflammatory activity of peptides derived from millet bran in vitro and in vivo. Food Funct. 2022;13(4):1881–1889. doi:10.1039/d1fo03711k
25. Zheng H, Kang Q, Zhang C, Yang L, Liu X. rMBP-NAP suppresses OXA-induced allergic dermatitis by regulating the Th1/Th2 balance. Iran J Immunol. 2023;20(1):36–44. doi:10.22034/iji.2023.92594.2157
26. Kianmehr M, Haghmorad D, Nosratabadi R, Rezaei A, Alavinezhad A, Boskabady MH. The effect of Zataria multiflora on Th1/Th2 and Th17/T regulatory in a mouse model of allergic asthma. Front Pharmacol. 2017;8:458. doi:10.3389/fphar.2017.00458
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.