Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Cerebrovascular Autoregulation-Based Optimal Mean Arterial Pressure During Prostate Surgery – A Secondary Analysis of a Prospective Cohort Study
Authors Mewes C, Wei P , Yang Y, Kainz E, Kahl U , Beck S, Graefen M, Zöllner C, Fischer M
Received 6 December 2024
Accepted for publication 21 April 2025
Published 27 May 2025 Volume 2025:21 Pages 757—767
DOI https://doi.org/10.2147/TCRM.S505676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Caspar Mewes,1 Peipei Wei,1 Yi Yang,1 Elena Kainz,1 Ursula Kahl,1 Stefanie Beck,1 Markus Graefen,2 Christian Zöllner,1 Marlene Fischer1,3
1Department of Anesthesiology, University Medical Center Hamburg-Eppendorf, Hamburg, D-20251, Germany; 2Martini-Klinik, Prostate Cancer Center, University Medical Center Hamburg-Eppendorf, Hamburg, D-20251, Germany; 3Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, D-20251, Germany
Correspondence: Marlene Fischer, Department of Intensive Care Medicine, University Medical Center-Hamburg-Eppendorf, Martinistrasse 52, Hamburg, 20246, Germany, Email [email protected]
Purpose: Cerebrovascular autoregulation (CVA) is a homoeostatic regulatory function to maintain constant cerebral blood flow (CBF) despite changes in systemic blood pressure. The CVA-based optimal mean arterial pressure (MAPopt) refers to the MAP level at which the CVA mechanism reaches its lowest degree of pressure passiveness, allowing for optimal autoregulation. This study aimed to determine MAPopt by analyzing existing CVA data from patients undergoing non-cardiac surgery.
Methods: This single-center investigation is a secondary analysis of prospectively recorded CVA data of patients undergoing oncologic prostate surgery. Intraoperative CVA was assessed using the cerebral oxygenation index (COx) derived from the simultaneous measurement of MAP and regional cerebral oxygen saturation (rSO2). Patient-specific MAPopt values were calculated using a second-order polynomial formula, in which the MAP related to the lowest COx was considered to be the intraoperative MAPopt.
Results: A total of 180 patients were enrolled into the study. The average age was 63 years, 83.9% of patients had no or mild systemic disease. MAPopt determination was feasible in 128 patients, while 52 patients exhibited no U-shaped correlation between MAP and COx. The average intraoperative MAPopt was 81.7 mmHg ranging from 60.2 to 101.4 mmHg. The mean duration of intraoperative CVA measurement was 178 min.
Conclusion: This study demonstrates a wide range of individual intraoperative MAPopt values and underscores that CVA-based MAPopt during non-cardiac surgery may differ from commonly accepted intraoperative MAP thresholds in clinical practice (ie 65 mmHg).
Keywords: cerebral autoregulation, optimal mean arterial pressure, limits of cerebral autoregulation, non-cardiac surgery, personalized arterial pressure management
Introduction
Cerebrovascular autoregulation (CVA) is a physiological regulatory system that maintains stable cerebral blood flow (CBF) and tissue perfusion by balancing fluctuations in cerebrovascular dynamics and mean arterial pressure (MAP).1 CVA plays a critical role in various clinical scenarios, including perioperative and critical care settings. Autoregulatory dysfunction may lead to cerebral ischemia (hypoperfusion) during episodes of low CBF or vasogenic edema and hemorrhagic insults (hyperperfusion) during periods of elevated CBF.1 In addition, impaired CVA may aggravate primary and secondary brain injury.2
The CVA-based optimal mean arterial pressure (MAPopt) represents the blood pressure threshold at which the CVA mechanism achieves its minimal degree of pressure responsiveness.3,4 In other words, it is the ideal blood pressure level that enables optimal CBF regulation through the cerebral vessels.5 One widely used approach to calculate MAPopt is employing a second-order polynomial formula, also referred to as a U-shaped curve.5 This formula generates a parabolic curve that effectively accommodates all the data points, with MAP on the X-axis and an index of CVA on the Y-axis. Consequently, the MAPopt is defined as the value on the X-axis corresponding to the lowest point of this parabolic curve.6
The lower (LLA) and upper limits of autoregulation (ULA) can be estimated from CVA monitoring:7 The LLA is defined as the minimum MAP value below which CBF decreases monotonically with decreases in MAP. It is the baseline threshold to maintain sufficient cerebral perfusion and effective autoregulatory mechanisms. On the other hand, the ULA refers to the maximum MAP value above which CBF increases in response to increases in MAP.7 The ULA serves as the ceiling threshold below which the cerebral blood vessels retain the ability to constrict and regulate CBF.7,8 MAP values below the LLA or above the ULA have been linked with adverse outcomes, such as delirium and acute kidney injury.9–11
Utilizing CVA-based MAPopt, ULA and LLA as individual target variables during surgical procedures holds promise in potentially improving patient outcomes. The impact of deviations below the CVA-based MAPopt on patient-centered outcomes, including mortality, acute kidney injury, and delirium, is currently being investigated and remains a subject of ongoing discussion.6,9,12,13 However, the body of evidence on this topic is limited and of varying quality due to the paucity of data and heterogeneous methodology, especially in non-cardiac surgery patients.14,15
The objective of this study is to provide additional evidence on the concept of MAPopt and the ULA and LLA in non-cardiac surgery. Our primary aim is to describe MAPopt and limits of CVA by analyzing prospectively collected CVA data from a well-defined and homogenous cohort of patients undergoing non-cardiac surgery. This study further sought to identify demographic and clinical variables associated with the magnitude of MAPopt.
Methods
Ethical Concerns
The original cohort study was performed at a high-volume prostate cancer clinic at the University Medical Center Hamburg-Eppendorf, Germany from January 2015 to March 2016. All investigations were approved under the ethical project identification code PV4782 by the local ethics committee at the Hamburg Chamber of Physicians. Written informed consent was obtained from all patients before participating in the study. The study was performed in accordance with the provisions and regulations of the Declaration of Helsinki.
Study Design and Population
This single-center investigation is a secondary analysis of data from a prospective observational study, performed by our research group, that intended to compare CVA between robot-assisted radical prostatectomy in Trendelenburg position and open retropubic radical prostatectomy in supine position.16 Together with data from two other cohorts, data from the original study had been used in a previous secondary analysis to investigate the association between impaired CVA and delayed neurocognitive recovery after non-cardiac surgery.17,18
Patients were chosen by convenience and included into the study based on the following inclusion criteria:
- Age ≥18 years,
- Scheduled for radical prostatectomy for prostate cancer,
- Fluent in German language.
Exclusion criteria were defined as:
- History of cerebrovascular or neurodegenerative disease,
- Other intracranial pathology,
- Inability or contraindication to measure invasive arterial pressure in the radial arteries (ie due to severe atherosclerosis or vascular anomalies).
Anesthetic Procedures
The anesthesiologic management was performed as follows: Patients received 7.5 mg midazolam p. o. for premedication, provided there were no contraindications. Sufentanil (0.3–0.7 mg/kg), propofol (2–3 mg/kg), and rocuronium (0.5–0.6 mg/kg) were used for the induction of general anesthesia. The maintenance of anesthesia included either a continuous infusion of propofol at a rate of 5–7 mg/kg/h or sevoflurane at a concentration of 0.8–1.2 minimum alveolar concentration, along with sufentanil (0.1–0.2 mg/kg) and intermittent muscle relaxation with 0.15 mg/kg rocuronium at the discretion of the attending physician. Monitoring of anesthesia depth was performed by targeting a bispectral index of 30–40.
Invasive continuous arterial blood pressure monitoring was performed in all patients using an arterial catheter (Leader-Cath, VYGON GmbH & Co KG, Aachen, Germany or BD ™ FloSwitch, Becton Dickinson AG, Allschwil, Switzerland) in either left- or right-side radial artery.
Intraoperative hypotension (MAP < 65 mmHg or a decrease in MAP > 20% of baseline for more than 5 min) was managed by administering crystalloid fluids and/or a bolus of norepinephrine (5–10 μg), followed by a continuous infusion of norepinephrine in incremental doses. End-tidal carbon dioxide (CO2) levels were continuously monitored and maintained between 32 and 42 mmHg. The postoperative pain regimen involved the administration of piritramide, tramadol and metamizole at specific predefined time intervals, as previously described in detail.19
Cerebrovascular Autoregulation Monitoring
In our study, intraoperative CVA was continuously assessed using the time correlation method.20,21 CVA cannot be directly measured but can be calculated based on the moving Pearson correlation between rSO2 and MAP, resulting in a surrogate index, namely the cerebral oximetry index (COx). COx values range from −1 to 1 with values higher than 0 indicating impaired CVA. Negative values suggest that CBF surrogates change independently from MAP, reflecting intact CVA.21
In the present study, continuous non-invasive measurement of rSO2 was performed by near-infrared spectroscopy using the INVOS 5100c monitor with a resolution of 1 hz (INVOS™ 5100C Cerebral Oximeter, Medtronic GmbH, Meerbusch, Germany). Continuous arterial pressure signals were recorded at a higher sampling rate (100 hz) using a GE patient monitor (Patient Monitor B40, GE Healthcare Technologies Inc., Chicago, Illinois, USA) and were subsequently downsampled to ensure temporal alignment with the NIRS data. Signal synchronization was performed based on recorded timestamps. A moving linear correlation between MAP and rSO2 was computed using a sliding 300-second window updated every 10 seconds (ICM +, Cambridge Enterprise Ltd, Cambridge, UK).22,23 This technique allowed for a real-time, non-invasive assessment of cerebral oxygenation, reducing the need for complex equipment or specialized skills required for methodologies such as transcranial Doppler (TCD). While TCD represents an alternative, well-established method for assessing cerebral blood flow velocity, it demands specific technical expertise and may not be applicable to all patients, particularly those with insufficient temporal bone windows. Additionally, the use of intraoperative electrosurgical devices and various low-frequency signals can introduce background noise, potentially compromising the accuracy of TCD measurements.
Optimal Mean Arterial Pressure Calculation
Individual MAPopt, LLA, and ULA values were determined utilizing the ICM+ software (ICM +, Cambridge Enterprise Ltd, Cambridge, UK). In order to ensure data quality, artifacts were eliminated by excluding MAP values that exceeded 150 mmHg or fell below 30 mmHg, along with their corresponding COx values.24
A second-order polynomial formula was applied to determine the MAPopt based on COx values.5 In this approach, the MAP values were allocated to the X-axis in bins of 2 mmHg intervals, while the corresponding COx values were categorized and averaged for each MAP bin and subsequently placed on the Y-axis. The lowest point of this U-shaped curve, representing the MAP related to the best autoregulatory function, was considered to be the intraoperative MAPopt. The exclusion of cases without a U-shaped correlation was based on visual inspection within the ICM+ software. A figure illustrating the computation of MAPopt has been included in Supplementary Figure 1.
The corresponding MAP values at which the U-shaped COx curve intersected the defined threshold of 0.3 were identified as the LLA and ULA, respectively. These autoregulatory limits correspond to the range of the autoregulatory curve where CBF passively responds to changes in systemic blood pressure.25
Statistical Analysis
The software used for all statistical analyses in this study was STATISTICA 13 (version 13.4.0, StatSoft, Tulsa, OK, USA).
Categorical values are presented as absolute numbers or percentages, while continuous variables are reported as mean ± standard deviation. Alternatively, if applicable, median and interquartile ranges are used to describe continuous variables.
The General Regression Model (GRM) in STATISTICA 13 software was employed to analyze the relationship between the dependent variable, intraoperative MAPopt, and a combination of continuous and categorical independent variables. The categorical variables were transformed into binary values (0 and 1) to accommodate the model. The GRM utilized a sigma-restricted parameterization, allowing for a comprehensive analysis of the association between the independent variables and MAPopt. The model estimated the ß (beta) coefficients, which represent the magnitude and direction of the relationship between each independent variable and MAPopt.
Results
Characteristics of the Study Population
Of 189 patients enrolled, six individuals were excluded due to unavailability of CVA data, while another three patients were excluded due to incomplete CVA datasets (Figure 1). A total of 180 patients, for which CVA data were available, were included for analysis in the present study.
 |
Figure 1 Flow diagram of study exclusion and successful determination of MAPopt and LLA/ULA. Abbreviations: RP, radical prostatectomy; RRP, radical retropubic prostatectomy. *16. |
The mean age of the study population was 63 ± 6.4 years. Their mean body mass index (BMI) was 27.1, and the majority of participants were classified as “healthy (I)” or with “mild systemic disease (II)” according to the American Society of Anesthesiologists physical status classification (83.9%) (Table 1). Preexisting conditions such as arterial hypertension (50.6%), dyslipoproteinemia (27.8%), and obesity (24.4%) were prevalent among the participants. The majority of patients underwent robot-assisted radical prostatectomy (56.6%), while the remaining 43.4% underwent open retropubic radical prostatectomy. Detailed demographic and clinical characteristics are presented in Table 1.
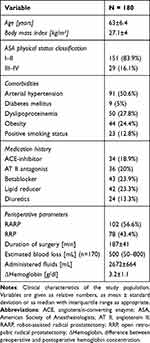 |
Table 1 Demographic and Clinical Characteristics |
Optimal Mean Arterial Pressure and Limits of Cerebrovascular Autoregulation
The duration of intraoperative CVA measurement was 178.3 min. The mean MAPopt was 81.7 mmHg, ranging from 60.2 to 101.4 mmHg (Table 2). The median MAPopt value was calculated as 80.8 mmHg (interquartile range 76.1–87.1 mmHg). The analysis of LLA and ULA values revealed a mean LLA of 71 mmHg and a mean ULA of 93 mmHg (Table 2).
 |
Table 2 Optimal Mean Arterial Pressure and Limits of Cerebrovascular Autoregulation |
The distribution of all individually calculated MAPopt values for the 128 patients in whom MAPopt determination was feasible is displayed in Figures 2 (histogram) and Figure 3 (error bar plot with median and IQR). Intraoperative MAPopt values followed a normal distribution, as confirmed by visual inspection of the histogram and supported by the Kolmogorov–Smirnov test; a corresponding histogram has been added to Supplementary Figure 2.
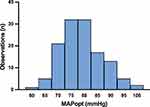 |
Figure 2 Optimal mean arterial pressure based on the continuous assessment of cerebrovascular autoregulation (MAPopt) distribution for the entire cohort. |
Multivariable Linear Regression
None of the independent variables, age, BMI, American Society of Anesthesiologists physical status classification, arterial hypertension, obesity, smoking status, or preexisting medication with angiotensin-converting enzyme inhibitors, angiotensin II antagonists, betablockers or diuretics were independently associated with intraoperative MAPopt in our study population (Table 3).
 |
Table 3 General Linear Model for the Association with Optimal Mean Arterial Pressure |
Discussion
The primary objective of this study was to investigate CVA-based MAPopt values and lower and upper limits of CVA during non-cardiac surgery. The main findings were (1) the average intraoperative MAPopt was 81.7 mmHg, (2) the mean LLA was 71 mmHg and the mean ULA was 93 mmHg. Clinically relevant baseline variables were not associated with MAPopt in the general linear regression model.
Substantial inter-individual variability was observed in autoregulatory metrics among patients undergoing non-cardiac surgery. The distribution of MAPopt values ranged from 60.2 to 101.4 mmHg with a standard deviation of 7.8 mmHg, while LLA values varied from 46.5 mmHg to 89.2 mmHg and ULA values ranged from 63.2 to 126.7 mmHg. These results emphasize that a patient-tailored approach to arterial pressure management might be beneficial during non-cardiac surgical procedures to optimize cerebral perfusion and to minimize the risk of neurological complications.
In clinical practice, a MAP of 65 mmHg has been commonly accepted as the lower threshold for intervention.26 A recent consensus statement, however, recommended a minimum arterial pressure of 60 mmHg for at-risk patients.27 Yet, a “one-size-fits-all” approach may not be appropriate for each patient. Therefore, individual pressure targets have been suggested to improve organ protection and postoperative outcomes.28–30 One approach to determine individualized pressure targets relies on the concept of CVA-based MAPopt.5 Here, the brain is considered as the index organ representing sufficient organ perfusion due to its high metabolic demand.31
Several studies have investigated intraoperative CVA-based MAPopt in patients undergoing cardiac surgery.3,6,13,32 These studies consistently demonstrated a wide range of intraoperative MAPopt values between 71 and 87.3 mmHg that differed substantially from the commonly accepted intervention threshold of 65 mmHg and even more from the newly proposed value of 60 mmHg.26,27 In line with previous investigations, the present results underscore that the CVA-based MAPopt during non-cardiac surgery may differ from the clinically accepted intraoperative MAP thresholds.
The ULA and LLA values provide crucial information regarding the upper and lower bounds within which CVA remains effective. Under normal physiological circumstances, CBF remains sufficient within a prefusion pressure between 60 and 150 mmHg.1 A perfusion pressure outside the autoregulatory plateau results in impaired CVA.20 As a consequence, CBF becomes pressure passive and ultimately, correlates with MAP in a linear pressure-flow relationship. This may result in cerebral ischemia due to hypoperfusion or cerebral edema resulting from hyperperfusion.20 Compared with previous reports of ULA and LLA, this study identified narrower autoregulation limits of 71 to 93 mmHg.3,33 This discrepancy might be attributable to several factors: first, the original concept of CVA with the sinusoid Lassen curve has been challenged repeatedly, since the autoregulatory plateau and its limits underlie a high inter- and intraindividual variation.34,35 Second, anesthetics may have a differential impact on CVA.33,36,37 While a left shift of autoregulatory limits has been described under the influence of propofol, sevoflurane has been reported to narrow the autoregulatory plateau, potentially rendering the cerebral perfusion more vulnerable to fluctuations in systemic blood pressure.33,36 Our results are consistent with previous reports in patients undergoing surgery with general anesthesia.33 Thus, our findings corroborate the assumption that the traditional limits of CVA are subject to substantial interindividual and intraindividual variation.38 As a consequence, CBF may be more susceptible to fluctuations of the cerebral perfusion pressure under the influence of anesthetic medication.37 It is crucial to acknowledge the inherent limitations of correlation-based indices of cerebrovascular autoregulation, particularly within the context of general anesthesia. Anesthetic agents can significantly alter autonomic control mechanisms, including the baroreflex and Cushing reflex, which may lead to changes in cerebrovascular dynamics that could compromise the validity of correlation-based autoregulatory markers.39 Moreover, the reliability of correlation-based indices has been debated, as their variable accuracy and precision may undermine their utility in classifying impaired cerebral autoregulation in healthy adults.40 These considerations should be taken into account when interpreting the results of our study.
Targeting patient-specific CVA-based MAPopt values and autoregulation limits during non-cardiac surgery may offer advantages in the perioperative care of older adults. By maintaining MAP within the optimal range, the incidence of postoperative neurological complications, such as delirium and cognitive decline, as well as end-organ damage, such as acute kidney injury, may potentially be reduced.3,10,13,41 Of note, individualized CVA-guided hemodynamic management may be particularly valuable in older adults with increased vascular stiffness, a right shift of the autoregulation curve, and impaired autoregulatory capacity. However, the monitoring of individual CVA metrics requires specialized equipment and technical expertise.
In this study, cerebrovascular autoregulation was assessed using NIRS, a non-invasive technique that provides real-time monitoring of regional cerebral oxygen saturation (rSO2). NIRS-based indices have been demonstrated to correlate with cerebral perfusion and have been used to assess autoregulation in various settings.42,43 While TCD remains a widely used technique for cerebral autoregulation assessment by measuring cerebral blood flow velocity (CBFV), its intraoperative application is limited due to technical challenges such as the need for specialized expertise, susceptibility to signal interference from electrosurgical devices, and difficulties in obtaining adequate insonation windows.44,45 However, given the differences in the physiological parameters measured by TCD and NIRS, future studies comparing both modalities in order to determine CVA-based MAPopt in surgical patients would help to further clarify their respective advantages and limitations.
Limitations
Despite valuable insights into individual CVA patterns during non-cardiac surgery provided by this study, several limitations should be acknowledged. First, this study was not primarily designed for the analysis of MAPopt and limits of CVA, but represents a secondary analysis of existing data, which may have introduced potential constraints related to data availability, completeness, and the inability to control for certain confounding variables during data collection. It may have also contributed to the large number of patients, in which MAPopt and LLA/ULA calculation was not feasible. Second, we did not investigate patient-centered outcomes, such as perioperative neurocognitive disorders or mortality. In order to conclude a potentially beneficial effect of targeting MAP based on autoregulation variables during non-cardiac surgery, further investigations are necessary. Future studies should aim to assess the long-term implications of targeting individualized MAPopt values and/or MAP within the auto-regulatory range during non-cardiac surgery. Evaluating patient-centered outcomes, such as postoperative cognitive function and neurological recovery, may underscore the clinical significance of CVA-guided MAP management. Third, we used the polynomial derivation method to determine MAPopt, which is considered as more objective compared with using the nadir, ie the MAP at the lowest cerebral autoregulation index.5 Yet, the latter allows for MAPopt determination in a higher number of samples. Additionally, the Trendelenburg positioning used in a subset of patients may have influenced cerebrovascular dynamics as head-down tilt can increase intracranial pressure and alter baroreceptor function. Specifically, Trendelenburg positioning has been suggested to unload the baroreflex, which could, in turn, modify cerebral autoregulation.46 However, previous analyses in the same cohort did not indicate a significant impact on cerebrovascular autoregulation, suggesting that this effect may be limited in this specific population.16 Finally, our study cohort consisted exclusively of male individuals undergoing prostate surgery, which may limit the generalizability of our findings to broader surgical populations, particularly female patients who may exhibit different autoregulatory responses. Future studies should aim to validate these findings in more diverse cohorts.
Conclusion
The novelty of our findings lies in the simultaneous determination of MAPopt and CVA limits in a homogenous cohort of elderly male patients undergoing prostatectomy. Additionally, this is the first study to identify factors associated with the magnitude of MAPopt. In contrast to previous studies of our research group, we did not focus on CVA impairment, but rather aimed to determine CVA-based, critical thresholds of cerebral blood flow.16,17
In conclusion, the current study contributes to the body of evidence on CVA-based optimal MAP values and autoregulation limits in non-cardiac surgery patients. The observed interindividual variability underscores the potential importance of individualized arterial pressure management to optimize CBF and to reduce the risk of perioperative neurocognitive disorders. Implementing patient-specific CVA-based MAP targets may hold promise in improving patient outcomes and merits further investigation in larger prospective studies.
Data Sharing Statement
The data that support the findings of this study are available from the authors but restrictions apply to the availability of these data. Data are, however, available from the authors upon reasonable request and with permission from the Data Protection Officer at the University Medical Center Hamburg-Eppendorf.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Hamburg Chamber of Physicians on September 2, 2014 (protocol number PV4782).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
This manuscript does not contain individual person’s data.
Acknowledgments
Parts findings of his paper were presented as a poster presentation at the German Capital Congress of Anesthesiology in October 2023 (‘HAI 2023 - 25. Hauptstadtkongress der DGAI für Anästhesiologie und Intensivtherapie’) with interim findings. The poster’s abstract was published in ‘A&I, Supplement 13; Anästh Intensivmed 2023;64:S346–S371: https://www.ai-online.info/images/ai-ausgabe/2023/10-2023/HAI_Abstracts_2023_online.pdf.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by a research support grant of the Else Kröner Fresenius-Stiftung (grant number 2015_A33).
Disclosure
M.F. and UK receive research support from the Medtronic External Research Program (Medtronic GmbH, Minneapolis, MN, USA). M.F. also reports grants from Pfizer Pharma. The authors report no other conflicts of interest in this work.
References
1. Donnelly J, Budohoski KP, Smielewski P, Czosnyka M. Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit Care. 2016;20(1):129. doi:10.1186/s13054-016-1293-6
2. Sarwal A, Robba C, Venegas C, Ziai W, Czosnyka M, Sharma D. Are we ready for clinical therapy based on cerebral autoregulation? A pro-con debate. Neurocrit Care. 2023;s12028. doi:10.1007/s12028-023-01741-1
3. Hori D, Nomura Y, Ono M, et al. Optimal blood pressure during cardiopulmonary bypass defined by cerebral autoregulation monitoring. J Thorac Cardiovasc Surg. 2017;154(5):1590–1598.e2. doi:10.1016/j.jtcvs.2017.04.091
4. Sekhon MS, Smielewski P, Bhate TD, et al. Using the relationship between brain tissue regional saturation of oxygen and mean arterial pressure to determine the optimal mean arterial pressure in patients following cardiac arrest: a pilot proof-of-concept study. Resuscitation. 2016;106:120–125. doi:10.1016/j.resuscitation.2016.05.019
5. Rivera-Lara L, Zorrilla-Vaca A, Geocadin R, Healy R, Ziai W, Mirski M. Cerebral autoregulation-oriented therapy at the bedside: a comprehensive review. Anesthesiology. 2017;126(6):1187–1199. doi:10.1097/ALN.0000000000001625
6. Hori D, Max L, Laflam A, et al. Blood pressure deviations from optimal mean arterial pressure during cardiac surgery measured with a novel monitor of cerebral blood flow and risk for perioperative delirium: a pilot study. J Cardiothorac Vasc Anesth. 2016;30(3):606–612. doi:10.1053/j.jvca.2016.01.012
7. Donnelly J, Czosnyka M, Adams H, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017;45(9):1464–1471. doi:10.1097/CCM.0000000000002575
8. Joshi B, Ono M, Brown C, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012;114(3):503–510. doi:10.1213/ANE.0b013e31823d292a
9. Hori D, Brown C, Ono M, et al. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br J Anaesth. 2014;113(6):1009–1017. doi:10.1093/bja/aeu319
10. Liu X, Donnelly J, Brady KM, et al. Comparison of different metrics of cerebral autoregulation in association with major morbidity and mortality after cardiac surgery. Br J Anaesth. 2022;129(1):22–32. doi:10.1016/j.bja.2022.03.029
11. Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147(1):483–489. doi:10.1016/j.jtcvs.2013.07.069
12. Brown CH, Neufeld KJ, Tian J, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial. JAMA Surg. 2019;154(9):819. doi:10.1001/jamasurg.2019.1163
13. Hori D, Hogue C, Adachi H, et al. Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interact CardioVasc Thorac Surg. 2016;22(4):445–451. doi:10.1093/icvts/ivv371
14. Chuan A, Short TG, Peng AZ, et al. Is cerebrovascular autoregulation associated with outcomes after major noncardiac surgery? A prospective observational pilot study. Acta Anaesthesiol Scand. 2019;63(1):8–17. doi:10.1111/aas.13223
15. Zhang Y, Tan J, Li P, et al. The perioperative application of continuous cerebral autoregulation monitoring for cerebral protection in elderly patients. Ann Palliat Med. 2021;10(4):4582–4592. doi:10.21037/apm-21-707
16. Beck S, Ragab H, Hoop D, et al. Comparing the effect of positioning on cerebral autoregulation during radical prostatectomy: a prospective observational study. J Clin Monit Comput. 2021;35(4):891–901. doi:10.1007/s10877-020-00549-0
17. Kahl U, Rademacher C, Harler U, et al. Intraoperative impaired cerebrovascular autoregulation and delayed neurocognitive recovery after major oncologic surgery: a secondary analysis of pooled data. J Clin Monit Comput. 2022;36(3):765–773. doi:10.1007/s10877-021-00706-z
18. Lendner JD, Harler U, Daume J, et al. Oscillatory and aperiodic neuronal activity in working memory following anesthesia. Clin Neurophysiol. 2023;150:79–88. doi:10.1016/j.clinph.2023.03.005
19. Knipper S, Hagedorn M, Sadat-Khonsari M, et al. Comparison of intra- and postoperative analgesia and pain perception in robot-assisted vs. open radical prostatectomy. World J Urol. 2020;38(6):1451–1457. doi:10.1007/s00345-019-02938-w
20. Xiong L, Liu X, Shang T, et al. Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry. 2017;88(6):520–531. doi:10.1136/jnnp-2016-314385
21. Zweifel C, Dias C, Smielewski P, Czosnyka M. Continuous time-domain monitoring of cerebral autoregulation in neurocritical care. Med Eng Phys. 2014;36(5):638–645. doi:10.1016/j.medengphy.2014.03.002
22. Ono M, Zheng Y, Joshi B, Sigl JC, Hogue CW. Validation of a stand-alone near-infrared spectroscopy system for monitoring cerebral autoregulation during cardiac surgery. Anesth Analg. 2013;116(1):198–204. doi:10.1213/ANE.0b013e318271fb10
23. Rivera-Lara L, Geocadin R, Zorrilla-Vaca A, et al. Validation of near-infrared spectroscopy for monitoring cerebral autoregulation in comatose patients. Neurocrit Care. 2017;27(3):362–369. doi:10.1007/s12028-017-0421-8
24. Hirsch J, DePalma G, Tsai T, Sands L, Leung J. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br J Anaesth. 2015;115(3):418–426. doi:10.1093/bja/aeu458
25. Petersen NH, Silverman A, Strander SM, et al. Fixed compared with autoregulation-oriented blood pressure thresholds after mechanical thrombectomy for ischemic stroke. Stroke. 2020;51(3):914–921. doi:10.1161/STROKEAHA.119.026596
26. Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563–574. doi:10.1016/j.bja.2019.01.013
27. Saugel B, Fletcher N, Gan TJ, et al. PeriOperative quality initiative (POQI) international consensus statement on perioperative arterial pressure management. Br J Anaesth. 2024;133(2):264–276. doi:10.1016/j.bja.2024.04.046
28. Bergholz A, Meidert AS, Flick M, et al. Effect of personalized perioperative blood pressure management on postoperative complications and mortality in high-risk patients having major abdominal surgery: protocol for a multicenter randomized trial (IMPROVE-multi). Trials. 2022;23(1):946. doi:10.1186/s13063-022-06854-0
29. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346. doi:10.1001/jama.2017.14172
30. Meng L, Yu W, Wang T, Zhang L, Heerdt PM, Gelb AW. Blood pressure targets in perioperative care: provisional considerations based on a comprehensive literature review. Hypertension. 2018;72(4):806–817. doi:10.1161/HYPERTENSIONAHA.118.11688
31. Murkin J. Cerebral oximetry: monitoring the brain as the index organ. Anesthesiology. 2011;114(1):12–13. doi:10.1097/ALN.0b013e3181fef5d2
32. Hori D, Ono M, Rappold TE, et al. Hypotension after cardiac operations based on autoregulation monitoring leads to brain cellular injury. Ann Thorac Surg. 2015;100(2):487–493. doi:10.1016/j.athoracsur.2015.03.036
33. Goettel N, Patet C, Rossi A, et al. Monitoring of cerebral blood flow autoregulation in adults undergoing sevoflurane anesthesia: a prospective cohort study of two age groups. J Clin Monit Comput. 2016;30(3):255–264. doi:10.1007/s10877-015-9754-z
34. Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39(2):183–238. doi:10.1152/physrev.1959.39.2.183
35. Meng L, Wang Y, Zhang L, McDonagh DL. Heterogeneity and variability in pressure autoregulation of organ blood flow: lessons learned over 100+ years. Crit Care Med. 2019;47(3):436–448. doi:10.1097/CCM.0000000000003569
36. Meng L, Gelb AW, McDonagh DL. Changes in cerebral tissue oxygen saturation during anaesthetic‐induced hypotension: an interpretation based on neurovascular coupling and cerebral autoregulation. Anaesthesia. 2013;68(7):736–741. doi:10.1111/anae.12254
37. Slupe AM, Kirsch JR. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J Cereb Blood Flow Metab. 2018;38(12):2192–2208. doi:10.1177/0271678X18789273
38. Jones‐Muhammad M, Warrington JP. Redefining the cerebral autoregulatory range of blood pressures: not as wide as previously reported. Physiol Rep. 2021;9(17). doi:10.14814/phy2.15006
39. Bari V, Fantinato A, Vaini E, et al. Impact of propofol general anesthesia on cardiovascular and cerebrovascular closed loop variability interactions. Biomed Signal Process Control. 2021;68:102735. doi:10.1016/j.bspc.2021.102735
40. Olsen MH, Riberholt CG, Mehlsen J, Berg RM, Møller K. Reliability and validity of the mean flow index (mx) for assessing cerebral autoregulation in humans: a systematic review of the methodology. J Cereb Blood Flow Metab. 2022;42(1):27–38. doi:10.1177/0271678X211052588
41. Hogue CW, Brown CH, Hori D, et al. Personalized blood pressure management during cardiac surgery with cerebral autoregulation monitoring: a randomized trial. Semin Thorac Cardiovasc Surg. 2021;33(2):429–438. doi:10.1053/j.semtcvs.2020.09.032
42. Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38(10):2818–2825. doi:10.1161/STROKEAHA.107.485706
43. Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10(1):122–128. doi:10.1007/s12028-008-9140-5
44. Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res. 2009;19(4):197–211. doi:10.1007/s10286-009-0011-8
45. Arnolds BJ, Von Reutern GM. Transcranial Dopplersonography. Examination technique and normal reference values. Ultrasound Med Biol. 1986;12(2):115–123. doi:10.1016/0301-5629(86)90016-5
46. Porta A, Cairo B, Bari V, Gelpi F, De Maria B, Colombo R. Model-based spectral causality of cardiovascular variability interactions during head-down tilt. Physiol Meas. 2023;44(5):54001. doi:10.1088/1361-6579/acce1f
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


