Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Changes in Oxidative Stress Markers in Pregnant Women of Advanced Maternal Age with Gestational Diabetes and Their Predictive Value for Neurodevelopmental Impact
Authors Wang Y , Fan Z, Ren J, Ma L
Received 11 July 2024
Accepted for publication 12 October 2024
Published 29 October 2024 Volume 2024:17 Pages 4003—4012
DOI https://doi.org/10.2147/DMSO.S475385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rebecca Conway
Yabing Wang,1 Zhenling Fan,2 Jianli Ren,3 Lin Ma4
1Department of Obstetrical, Shijiazhuang Obstetrics and Gynecology Hospital, Shijiazhuang, Hebei Province, People’s Republic of China; 2Department of Hemodialysis, Handan Mingren Hospital, Handan, Hebei Province, People’s Republic of China; 3Department of Supplies, Peking University Third Hospital Chongli, Beijing, People’s Republic of China; 4Department of Gynecology and Obstetrics, Zhengdingxian People’s Hospital, Shijiazhuang, Hebei Province, People’s Republic of China
Correspondence: Yabing Wang, Email [email protected]
Objective: To explore the relationship between changes in oxidative stress markers in pregnant women of advanced maternal age with gestational diabetes mellitus (GDM) and adverse pregnancy outcomes and neonatal neurodevelopment, as well as their predictive value.
Methods: Two hundred pregnant women of advanced maternal age were selected and divided into Group A (normal blood sugar) and Group B (GDM) based on the 75 g (Oral Glucose Tolerance Test) OGTT results. Oxidative stress markers were measured, and pregnancy outcomes and neonatal Neonatal Behavioral Assessment Scale (NABA) scores were recorded.
Results: Malondialdehyde (MDA) levels in Group B were higher than those in Group A, while Glutathione (GSH) and Superoxide Dismutase (SOD) levels were lower. Group B had higher rates of adverse pregnancy outcomes and neurological abnormalities than Group A. The Area Under the Curve (AUC) values for serum MDA, GSH, and SOD levels combined prediction were higher than those for individual predictions (P< 0.05).
Conclusion: Oxidative stress markers in pregnant women of advanced maternal age with GDM are associated with adverse pregnancy outcomes and neonatal abnormalities, and combined prediction has good predictive efficiency (AUC> 0.7).
Keywords: pregnant women of advanced maternal age with GDM, oxidative stress markers, pregnancy outcomes, neurodevelopment, correlation, predictive value
Introduction
With the continuous exacerbation of the aging population trend, the incidence of advanced maternal age pregnancies is gradually increasing.1 Gestational diabetes mellitus (GDM) as a common complication of pregnancy has attracted widespread clinical attention.2 GDM was first officially identified by O’Sullivan and Mahan in 19643 and is characterized by the onset of high blood sugar levels during pregnancy.4 As global obesity rates continue to soar, the prevalence of GDM among pregnant women is increasing, and this condition significantly heightens the risk of numerous pregnancy complications.5 Globally, statistics show that about 2% to 10% of pregnant women develop GDM,6 and the prevalence of GDM rises with gestational age from 25% in 23rd week of gestation,7 up to 33% in third trimester of pregnancy.8 Previous large-scale studies have reported obstetric risks related to diabetes, such as macrosomia, hypertension, and neonatal hypoglycemia.9–11 Another study by Ye et al identified a significant association of GDM with pregnancy complications when adjusted for confounders,12 and a significantly increased risk of developing chronic diseases such as diabetes, metabolic syndrome, and cardiovascular disease in GDM patients and their infants has also been found.13
Oxidative stress has been identified as part of the physiopathology of various diseases, with a particular emphasis on metabolic diseases like metabolic syndrome and diabetes, although it is also present in conditions such as obesity and pregnancy.14 Oxidative stress leads to the excessive generation of oxygen-free radicals in the body, damaging cell membranes, proteins, and nucleic acids, thereby accelerating the development of diabetes-related complications.15 Robust evidence has shown that GDM is marked by elevated oxidative stress, which may contribute to increased cardiovascular risk and postpartum insulin resistance.14,16,17 Oxidative stress plays a key role in the development and progression of GDM, creating a vicious cycle with inflammation.14 Therefore, monitoring the changes in oxidative stress marker levels in GDM women can help deepen the understanding of the pathogenesis of GDM. This study aimed to explore the relationship between the dynamic changes in oxidative stress marker levels in pregnant women of advanced maternal age with GDM and adverse pregnancy outcomes. It is hoped that the results of this study can provide more effective prediction and intervention strategies for clinical practice to help reduce the occurrence of adverse outcomes in pregnant women of advanced maternal age with GDM and their newborns, thereby safeguarding maternal and infant health.
Materials and Methods
Sample Source
Two hundred pregnant women of advanced maternal age admitted to our hospital from January 2021 to January 2024 were selected as the research subjects. (1) Inclusion criteria: Pregnant women aged ≥35 years who attended the obstetrics outpatient clinic of our hospital. (2) Exclusion criteria: ① Abnormal pre-pregnancy glucose tolerance, pre-pregnancy diabetes, hyperthyroidism or hypothyroidism, hypertension, obesity or overweight prior to pregnancy, or severe liver disease; ② Recent use of drugs affecting glucose and lipid metabolism; ③ Cardiovascular diseases, renal or hepatic insufficiency, and abnormal lipid metabolism; ④ Incomplete data, including missing key measurements such as glucose tolerance test results or oxidative stress markers (eg, MDA, GSH, SOD), lack of complete medical history (eg, age, pre-pregnancy BMI, previous pregnancy outcomes), insufficient follow-up information (eg, pregnancy outcomes, neonatal assessments like NABA scores), or inadequate documentation of treatments and interventions during the study period. (3) Dropout criteria: ① Poor compliance of the subjects; ② Natural dropout or loss to follow-up during the follow-up process, resulting in incomplete data; ③ Occurrence of severe adverse events or complications that are not suitable for continued participation in the study and are terminated.
This study was approved by the Clinical Experimental Ethics Committee of Shijiazhuang Obstetrics and Gynecology Hospital, and all patients were informed and consented to the study, signing informed consent forms. All the methods were carried out in accordance with the Declaration of Helsinki. Based on the results of the 75g OGTT, the pregnant women of advanced maternal age were divided into Group A (normal blood sugar levels) and Group B (pregnant women of advanced maternal age with GDM), with 100 cases in each group. The diagnostic criteria for gestational diabetes mellitus during pregnancy referred to the eighth edition of “Obstetrics and Gynecology” published in 2013: Perform a 75g OGTT at gestational weeks 24 to 28. Pregnant women with blood sugar meeting one of the following criteria are diagnosed with GDM: fasting plasma glucose ≥7.0 mmol/l (126 mg/dl)2-h plasma glucose ≥11.1 mmol/l (200 mg/dl) following a 75g oral glucose load random plasma glucose ≥11.1 mmol/l (200 mg/ dl) in the presence of diabetes symptoms.18
Determination of Oxidative Stress Markers
Venous blood samples were collected from pregnant women at gestational weeks 28, 32, 34, 36, and before delivery to measure the levels of serum Malondialdehyde (MDA), Glutathione (GSH), and Superoxide Dismutase (SOD) and observe the dynamic changes in oxidative stress levels. Detection methods: Venous blood samples (10 mL) were collected from all subjects and centrifuged (2500 rpm, 10 min) to obtain serum for measurement. The levels of MDA (thiobarbituric acid reactive substances method), GSH (dithiobisnitrobenzoic acid method), and SOD (pyrogallol autooxidation inhibition method) were detected using an automated biochemical analyzer, following the manufacturer’s instructions.
Follow-Up of Maternal Pregnancy Outcomes and Neonatal Neurodevelopment
A follow-up was conducted for the enrolled pregnant women to record maternal pregnancy outcomes, including: (1) Complications during delivery: preterm birth, premature rupture of membranes, chorioamnionitis, uterine rupture, postpartum hemorrhage; (2) Mode of delivery: cesarean section, vaginal delivery, assisted vaginal delivery; (3) Neonatal outcomes: hypoglycemia, macrosomia, neonatal asphyxia, admission to the Neonatal Intensive Care Unit (NICU), congenital defects. Neonatal neurodevelopment was assessed using the Neonatal Behavioral Assessment Scale (NABA) score, performed 5 days after birth. The scale ranges from 0 to 40, with higher scores indicating better neurological and motor development. Scores ≤35 were considered abnormal. The tests were conducted by the same healthcare professional who underwent specialized training and qualification assessment.
Data Analysis
Data were analyzed using SPSS 25.0 software. Normally distributed continuous data are presented as (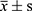 ), and between-group comparisons were made using independent samples t-tests, while comparisons at different time points within groups were performed using repeated measures analysis of variance. Non-normally distributed continuous data are presented as M (QR), and between-group comparisons were made using the Mann–Whitney U-test. Categorical data are presented as relative numbers, and between-group comparisons were made using the chi-square test. Pearson correlation analysis was conducted to examine the relationship between serum levels of MDA, GSH, and SOD and adverse pregnancy outcomes and neonatal neurodevelopment in pregnant women of advanced maternal age with GDM. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive efficacy of serum levels of MDA, GSH, and SOD for adverse pregnancy outcomes and neonatal neurodevelopment in pregnant women of advanced maternal age with GDM, and the area under the curve (AUC) and critical values were calculated. The AUCs were compared using non-parametric methods. To explore whether the observed associations between oxidative stress markers (MDA, GSH, SOD) and adverse pregnancy outcomes were independent of Body Mass Index (BMI), we conducted a multivariate logistic regression analysis. The regression model included oxidative stress markers (MDA, GSH, SOD) and BMI as independent variables, with the presence of adverse pregnancy outcomes as the dependent variable. A significance level of P<0.05 was considered statistically significant (Table 1).
), and between-group comparisons were made using independent samples t-tests, while comparisons at different time points within groups were performed using repeated measures analysis of variance. Non-normally distributed continuous data are presented as M (QR), and between-group comparisons were made using the Mann–Whitney U-test. Categorical data are presented as relative numbers, and between-group comparisons were made using the chi-square test. Pearson correlation analysis was conducted to examine the relationship between serum levels of MDA, GSH, and SOD and adverse pregnancy outcomes and neonatal neurodevelopment in pregnant women of advanced maternal age with GDM. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive efficacy of serum levels of MDA, GSH, and SOD for adverse pregnancy outcomes and neonatal neurodevelopment in pregnant women of advanced maternal age with GDM, and the area under the curve (AUC) and critical values were calculated. The AUCs were compared using non-parametric methods. To explore whether the observed associations between oxidative stress markers (MDA, GSH, SOD) and adverse pregnancy outcomes were independent of Body Mass Index (BMI), we conducted a multivariate logistic regression analysis. The regression model included oxidative stress markers (MDA, GSH, SOD) and BMI as independent variables, with the presence of adverse pregnancy outcomes as the dependent variable. A significance level of P<0.05 was considered statistically significant (Table 1).
 |
Table 1 Multivariate Logistic Regression Summary |
Results
Demographics of Pregnant Women of Advanced Maternal Age
In addition to 1-hour and 2-hour postprandial blood glucose which showed significant differences between the two groups (P<0.001), other baseline data of the two groups were comparable (P>0.05), as shown in Table 2.
 |
Table 2 Demographics of Pregnant Women of Advanced Maternal Age ( |
Comparison of Oxidative Stress Markers
The MDA levels in Group B were higher than those in Group A from gestational week 28 to before delivery, while GSH and SOD levels were lower than those in Group A (P<0.05). The gradual increase in MDA levels and the decrease in GSH and SOD levels observed from gestational week 28 to before delivery in both groups suggest that oxidative stress is more pronounced in elderly pregnant women with GDM. This statistical significance (P < 0.05) implies that elevated oxidative stress markers, particularly the lower antioxidant levels in Group B, may contribute to the higher risk of adverse pregnancy outcomes associated with GDM, as shown in Tables 3, 4 and 5.
 |
Table 3 Comparison of MDA Levels (Nmol/mL) Between Groups A and B at Different Gestational Weeks ( |
 |
Table 4 Comparison of GSH Levels (Mg/L) Between Groups A and B at Different Gestational Weeks ( |
 |
Table 5 Comparison of SOD Levels (U/L) Between Groups A and B at Different Gestational Weeks ( |
Comparison of Pregnancy Outcomes and Neonatal Neurodevelopment
Group B had higher rates of cesarean section, macrosomia, neonatal hypoglycemia, and neonatal abnormalities compared to Group A (P < 0.05). The higher rate of neonatal hypoglycemia in Group B (p = 0.001) indicates that infants born to mothers with GDM are at a greater risk of developing metabolic complications. The statistically significant differences between the two groups (P < 0.05) further support the hypothesis that oxidative stress plays a role in the adverse pregnancy outcomes and neurodevelopmental issues observed in GDM pregnancies, as shown in Table 6.
 |
Table 6 Comparison of Pregnancy Outcomes and Neonatal Neurodevelopment Between Groups A and Group B [n(%)] |
Relationship Between Serum MDA, GSH, and SOD Levels and Adverse Pregnancy Outcomes and Neonatal Neurodevelopment in Pregnant Women of Advanced Maternal Age with GDM
Spearman correlation analysis showed that MDA was positively correlated with the rates of cesarean section, macrosomia, neonatal hypoglycemia, and abnormalities (P < 0.05). Conversely, GSH and SOD were negatively correlated with these adverse outcomes (P < 0.05). These correlations suggest that higher oxidative stress, indicated by elevated MDA and reduced GSH and SOD levels, is associated with an increased risk of adverse outcomes in both mothers and newborns. The statistical significance (P < 0.05) indicates that the observed associations are unlikely to be due to chance, reinforcing the importance of oxidative stress as a contributing factor in GDM-related complications, as presented in Table 7.
 |
Table 7 Relationship Between Serum MDA, GSH, and SOD Levels and Adverse Pregnancy Outcomes and Neonatal Neurodevelopment in Pregnant Women of Advanced Maternal Age with GDM |
Predictive Efficacy of Serum MDA, GSH, and SOD Levels for Adverse Pregnancy Outcomes and Neonatal Neurodevelopment in Pregnant Women of Advanced Maternal Age with GDM
The ROC curves showed that the AUCs for predicting adverse pregnancy outcomes in older GDM women were 0.687, 0.646, 0.659, and 0.827 for serum MDA, GSH, SOD levels, and their combination, respectively (P<0.05), as shown in Table 8 and Figure 1. These values suggest that while individual markers like MDA, GSH, and SOD have moderate predictive power, their combination significantly improves the predictive accuracy for adverse pregnancy outcomes (AUC = 0.827), indicating a strong diagnostic ability. An AUC above 0.8 implies that the combined markers provide a reliable means to identify patients at higher risk for complications.
 |
Table 8 Predictive Efficacy of Serum MDA, GSH, and SOD Levels for Adverse Pregnancy Outcomes in Pregnant Women of Advanced Maternal Age with GDM |
 |
Figure 1 ROC curve of serum MDA, GSH, and SOD levels for predicting adverse pregnancy outcomes in pregnant women of advanced maternal age with GDM. |
Additionally, the AUCs for predicting neonatal abnormalities were 0.646, 0.605, 0.617, and 0.749 for serum MDA, GSH, SOD levels, and their combination, respectively (P<0.05), as presented in Table 9 and Figure 2. Although individual markers show a moderate level of prediction (AUCs slightly above 0.6), the combined AUC of 0.749 suggests a fairly good predictive ability for identifying neonates at risk of neurodevelopmental abnormalities. This finding implies that monitoring the combined levels of these oxidative stress markers in pregnant women with GDM could serve as an effective tool for early prediction and potential intervention.
 |
Table 9 Predictive Efficacy of Serum MDA, GSH, and SOD Levels for Neonatal Neurodevelopment |
 |
Figure 2 ROC curve of serum MDA, GSH, and SOD levels for predicting neonatal neurodevelopment. |
Multivariate Analysis of Oxidative Stress Markers and BMI
The multivariate analysis revealed that none of the oxidative stress markers—MDA, GSH, and SOD—were significantly associated with adverse pregnancy outcomes after adjusting for BMI. Specifically, the coefficients for MDA (β = 0.1006, p = 0.553), GSH (β = −0.3481, p = 0.250), and SOD (β = 0.1354, p = 0.784) did not reach statistical significance (p > 0.05). Similarly, BMI itself was not significantly associated with adverse pregnancy outcomes in this model (β = −0.0317, p = 0.543). These findings suggest that, within the context of this analysis, the relationship between oxidative stress markers and adverse pregnancy outcomes may not be independently significant when accounting for BMI. Further stratified analyses and interaction term evaluations are needed to clarify the potential interplay between GDM, oxidative stress, and BMI.
Discussion
This study investigated the relationship between oxidative stress markers and pregnancy outcomes in advanced maternal age women with GDM. We found that serum MDA levels were significantly higher, while GSH and SOD levels were lower in GDM patients compared to non-GDM controls. Elevated MDA levels were positively correlated with higher rates of cesarean section, macrosomia, neonatal hypoglycemia, and neurodevelopmental abnormalities, while reduced GSH and SOD levels were negatively correlated with these adverse outcomes. Our findings align with those of Nakshine and Jogdand,19 who reviewed the impact of GDM on maternal and fetal outcomes and found that oxidative stress plays a critical role in increasing risks such as preeclampsia and preterm birth. Similarly, Kinnunen et al20 demonstrated that GDM is associated with an increased risk of congenital anomalies, further linking oxidative stress to adverse neonatal outcomes. Furthermore, Jadhav et al21 highlighted the connection between oxidative stress, fatty acids, and neurotrophins in GDM, suggesting that elevated oxidative stress contributes to neurodevelopmental abnormalities in newborns. Mei et al22 also found that oxidative stress pathways can lead to neural tube defects, providing additional evidence for the role of oxidative stress in adverse neurodevelopmental outcomes. Previous studies have established the impact of GDM on adverse pregnancy outcomes and neonatal health, with a particular focus on insulin resistance, oxidative stress, and inflammation.14,23,24 Our study’s results are consistent with earlier research demonstrating that increased oxidative stress, indicated by elevated MDA and reduced antioxidant defenses like GSH and SOD, is prevalent in GDM patients. Studies by Zhang et al25 and Mei et al22 have similarly reported the association of oxidative stress with preterm birth and neural tube defects, corroborating our findings on the impact of oxidative stress on neurodevelopmental abnormalities. Furthermore, our study also delves into the potential mechanisms behind these observations. The observed oxidative stress in GDM patients could be attributed to an imbalance between reactive oxygen species production and antioxidant defenses, leading to cellular and tissue damage.26,27 Elevated oxidative stress may trigger inflammatory responses, promote uterine contractions, and induce placental dysfunction, contributing to adverse pregnancy outcomes such as preterm birth and macrosomia.28–30 The association of MDA, GSH, and SOD with pregnancy outcomes underscores the need for monitoring oxidative stress in GDM patients. Early intervention strategies targeting oxidative stress could mitigate adverse outcomes, promoting better maternal and neonatal health.31,32
Conclusion
Compared with pregnant women of advanced maternal age with normal blood glucose levels, pregnant women of advanced maternal age with GDM have higher serum MDA levels, which are positively correlated with adverse pregnancy outcomes and neonatal abnormalities; GSH and SOD levels are lower, and negatively correlated with adverse pregnancy outcomes and neonatal abnormalities. The combination of the three has good predictive efficacy for adverse pregnancy outcomes and neonatal abnormalities. However, several limitations of this study must be acknowledged. First, the relatively small sample size of 200 participants may affect the stability and reliability of the findings. Second, the retrospective design has inherent limitations in data acquisition and recording accuracy, potentially impacting the comprehensiveness of the results. Third, we did not control for factors such as maternal lifestyle, diet, and weight, which might influence oxidative stress markers and outcomes. Additionally, the study focused on MDA, GSH, and SOD, which may not fully represent the oxidative stress status, as other relevant indicators were not included. Lastly, the study’s findings are based on data from specific regions and populations, limiting generalizability. In summary, while this study provides initial insights into oxidative stress in GDM among advanced maternal age women, future research with larger samples, improved designs, and broader marker assessments is needed to strengthen these findings.
Future research should consider expanding the sample size and using a prospective design to enhance the reliability of findings. Investigating additional oxidative stress markers and controlling for other confounding factors like lifestyle and dietary habits will provide a more comprehensive understanding of the mechanisms involved. Further studies across diverse populations are recommended to improve the generalizability of the results and to develop targeted interventions for reducing adverse pregnancy outcomes in GDM patients.
Funding
Dynamic monitoring of oxidative stress state of high diabetic pregnancy in women of advanced maternal age and its predictive value for outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Attali E, Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2021;70:2–9. doi:10.1016/j.bpobgyn.2020.06.006
2. You H, Hu J, Liu Y, et al. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review & meta-analysis. Indian J Med Res. 2021;154(1):62–77. doi:10.4103/ijmr.IJMR_852_18
3. O’SULLIVAN JB, MAHAN CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285.
4. Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S preventive services task force and the national institutes of health office of medical applications of research. Ann Intern Med. 2013;159(2):123–129. doi:10.7326/0003-4819-159-2-201307160-00661
5. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. doi:10.1038/s41572-019-0098-8
6. Centers for Disease Control and Prevention. Gestational Diabetes. 2022. Available from: https://www.cdc.gov/diabetes/basics/gestational.html.
7. Perovic M, Gojnic M, Arsic B, et al.Relationship between mid-trimester ultrasound fetal liver length measurements and gestational diabetes mellitus. J Diabetes. 2015;7:497–505. doi:10.1111/1753-0407.12207
8. Perović M, Garalejić E, Gojnić M, et al. Sensitivity and specificity of ultrasonography as a screening tool for gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2012;25:1348–1353. doi:10.3109/14767058.2011.634458
9. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care. 2009;32(11):2005–2009. doi:10.2337/dc09-0656
10. Wu Y, Liu B, Sun Y, et al. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care. 2020;43(12):2983–2990. doi:10.2337/dc20-0261
11. Hildén K, Magnuson A, Hanson U, Simmons D, Fadl H. Trends in pregnancy outcomes for women with gestational diabetes mellitus in Sweden 1998-2012: a nationwide cohort study. Diabet Med. 2020;37(12):2050–2057. doi:10.1111/dme.14266
12. Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377(e067946). doi:10.1136/bmj-2021-067946
13. Zhuang W, Lv J, Liang Q, Chen W, Zhang S, Sun X. Adverse effects of gestational diabetes-related risk factors on pregnancy outcomes and intervention measures. Exp Ther Med. 2020;20(4):3361–3367. doi:10.3892/etm.2020.9050
14. Saucedo R, Ortega-Camarillo C, Ferreira-Hermosillo A, Díaz-Velázquez MF, Meixueiro-Calderón C, Valencia-Ortega J. Role of oxidative stress and inflammation in gestational diabetes mellitus. Antioxidants. 2023;12(10):1812. doi:10.3390/antiox12101812
15. Zhang P, Li T, Wu X, et al. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14(5):583–600. doi:10.1007/s11684-019-0729-1
16. Abell SK, De Courten B, Boyle JA, Teede HJ. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int J Mol Sci. 2015;16(6):13442–13473. doi:10.3390/ijms160613442
17. Poola-Kella S, Steinman RA, Mesmar B, Malek R. Gestational diabetes mellitus: post-partum risk and follow up. Rev Recent Clin Trials. 2018;13:5–14. doi:10.2174/1574887112666170911124806
18. World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva: World Health Organization; 2013. 4, Available from: https://www.ncbi.nlm.nih.gov/books/NBK169023/.
19. Nakshine VS, Jogdand SD. A comprehensive review of gestational diabetes mellitus: impacts on maternal health, fetal development, childhood outcomes, and long-term treatment strategies. Cureus. 2023;15(10):e47500. doi:10.7759/cureus.47500
20. Kinnunen J, Nikkinen H, Keikkala E, et al. Gestational diabetes is associated with the risk of offspring’s congenital anomalies: a register-based cohort study. BMC Preg Childbirth. 2023;23(1):708. doi:10.1186/s12884-023-05996-6
21. Jadhav A, Khaire A, Joshi S. Exploring the role of oxidative stress, fatty acids and neurotrophins in gestational diabetes mellitus. Growth Factors. 2020;38(3–4):226–234. doi:10.1080/08977194.2021.1895143
22. Mei X, Qi D, Zhang T, et al. Inhibiting MARSs reduces hyperhomocysteinemia-associated neural tube and congenital heart defects. EMBO Mol Med. 2020;12(3):e9469. doi:10.15252/emmm.201809469
23. Joo EH, Kim YR, Kim N, et al. Effect of endogenic and exogenic oxidative stress triggers on adverse pregnancy outcomes: preeclampsia, fetal growth restriction, gestational diabetes mellitus and preterm birth. Int J Mol Sci. 2021;22(18):10122. doi:10.3390/ijms221810122
24. Yousuf M, Shakir S, Khan I, Mammadova K, Ishrat U. Diabetes management through α-glucosidase inhibitors challenges and current perspectives. J Mod Biol Drug Discov. 2023;2:9. doi:10.53964/jmbdd.2023009
25. Zhang C, Yang Y, Chen R, et al. Aberrant expression of oxidative stress related proteins affects the pregnancy outcome of gestational diabetes mellitus patients. Am J Transl Res. 2019;11(1):269–279.
26. Hussain T, Murtaza G, Metwally E, et al. The role of oxidative stress and antioxidant balance in pregnancy. Mediators Inflamm. 2021;2021:9962860. doi:10.1155/2021/9962860
27. Cao F, Wang Y, Chu B, et al. Appetitive lifestyles and obesity with risk of senile cataract: an univariable and multivariable Mendelian randomization study. Clin Mol Epidemiol. 2024;1:6. doi:10.53964/cme.2024006
28. Phoswa WN, Khaliq OP. The role of oxidative stress in hypertensive disorders of pregnancy (preeclampsia, gestational hypertension) and metabolic disorder of pregnancy (gestational diabetes mellitus). Oxid Med Cell Longev. 2021;2021:5581570. doi:10.1155/2021/5581570
29. Chen S, Wan Y, Qian X, et al. Urinary metabolites of multiple volatile organic compounds, oxidative stress biomarkers, and gestational diabetes mellitus: association analyses. Sci Total Environ. 2023;875:162370. doi:10.1016/j.scitotenv.2023.162370
30. Huerta-Cervantes M, Peña-Montes DJ, López-Vázquez MÁ, et al. Effects of gestational diabetes in cognitive behavior, oxidative stress and metabolism on the second-generation off-spring of rats. Nutrients. 2021;13(5):1575. doi:10.3390/nu13051575
31. Ornoy A, Becker M, Weinstein-Fudim L, et al. Diabetes during pregnancy: a maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. a clinical review. Int J Mol Sci. 2021;22(6):2965. doi:10.3390/ijms22062965
32. Wang M, Chen Z, Hu Y, et al. The effects of vitamin D supplementation on glycemic control and maternal-neonatal outcomes in women with established gestational diabetes mellitus: a systematic review and meta-analysis. Clin Nutr. 2021;40(5):3148–3157. doi:10.1016/j.clnu.2020.12.016
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.