Back to Journals » International Journal of Nanomedicine » Volume 19
Characteristics of Ultrasound-Driven Barium Titanate Nanoparticles and the Mechanism of Action on Solid Tumors
Authors Li S, He N , Wu X, Chen F, Xue Q, Li S, Zhao C
Received 16 August 2024
Accepted for publication 4 November 2024
Published 28 November 2024 Volume 2024:19 Pages 12769—12791
DOI https://doi.org/10.2147/IJN.S491816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Shuao Li,1,* Ningning He,2,* Xiaoyu Wu,2 Fang Chen,1 Qingwen Xue,1 Shangyong Li,1,2 Cheng Zhao1
1Department of Abdominal Ultrasound, The Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 2School of Basic Medicine, Qingdao University, Qingdao, People’s Republic of China
*These authors have contributed equally to this work
Correspondence: Cheng Zhao; Shangyong Li, Email [email protected]; [email protected]
Abstract: Sonodynamic therapy (SDT) utilizes specific sound waves to activate sonosensitizers, generating localized biological effects to eliminate tumor cells. With advancements in nanomedicine, the application of nano-acoustic sensitizers has significantly advanced the development of SDT. BaTiO3 (BTO), an inorganic nano-acoustic sensitizer, possesses light refraction characteristics and a high dielectric constant, and can generate an electric field under ultrasound (US) stimulation. With continuous progress in multidisciplinary fields of US research, scientists have developed various types of barium titanate nanoparticles (BTNPs) to further advance SDT research and applications in tumor therapy. In this review, we present recently proposed and representative BTNPs, including their pathways of action, such as the induction of tumor cell senescence, ferroptosis, and glutathione depletion to reshape the tumor microenvironment, as well as their surface modifications. Research indicates that the mechanisms of action of ultrasound-driven BTNPs in tumor therapy are multifaceted. These mechanisms, whether utilized individually or synergistically, offer a potent and targeted strategy for cancer treatment. Furthermore, we discuss the application of BTNPs in various tumor types. Finally, we summarize the current challenges and future prospects for the clinical translation of BTNPs.
Keywords: ultrasound, BaTiO3, tumor, piezoelectric effect, nanotechnology
Introduction
The world is currently facing the challenge of an aging population, and non-communicable diseases, particularly cancer, pose a significant threat to human health.1 According to the World Health Organization, in 2022, cancer accounted for 19.97 million new cases and 9.43 million deaths worldwide.2 Cancer is now a primary factor affecting human longevity. Conventional treatments, such as chemotherapy, radiation, and surgery, frequently have severe side effects and inherent limitations, especially in their ability to target cancer cells precisely.3 This challenge has spurred a continual search for more effective treatments with fewer side effects. In this context, nanotechnology has emerged as a revolutionary approach, offering innovative dimensions to both the diagnosis and treatment of cancer.4
Among the diverse nanomaterials studied to date, barium titanate (BaTiO3, BTO) nanoparticles (BTNPs) are perovskite ceramic materials with unique properties, including a high dielectric constant and notable ferroelectric, piezoelectric, and thermoelectric properties.5 BTNPs are widely used to manufacture multilayer ceramic capacitors, thermistors, sensors, and optoelectronic devices. With the advancement of nanotechnology, the application of BTNPs and their heterostructures has been significantly broadened across various fields. For instance, barium titanate-based heterostructured nanoparticles exhibit remarkable properties in energy storage and capacitors, positioning them as strong candidates for efficient energy storage materials.6,7 Additionally, BTNPs, including certain heterostructural varieties, have demonstrated potential in photodynamic therapy (PDT). A notable example is the Au@BTO core-shell structure, which is capable of generating oxygen-independent reactive oxygen species (ROS) under near-infrared (NIR) laser irradiation. Similarly, zinc-doped BTNPs, synthesized through green methods involving banana peel extract,8 not only exhibit significant photocatalytic and anticancer activities but also efficiently degrade organic pollutants under light, making them valuable for environmental remediation.9 They have also shown promise in tumor immunotherapy, where genetically engineered cell membranes coated with ultra-small BTO nanoparticles enhance the immune response.10 Furthermore, multi-enzyme-piezoelectric synergistic tumor therapies, such as the modifications of black BTO nanoparticles through PD-catalysis, which produced (ROS) and improved the hypoxic tumor microenvironment under ultrasonic stimulation, have yielded encouraging results.11 These diverse applications underscored the extensive potential and versatility of BTNPs. In recent years, researchers have recognized the unique advantages of these materials in tissue engineering, drug delivery, and cancer treatment.12–15
Among these, the piezoelectric catalytic properties of barium titanate render it an excellent candidate for use as an acoustic sensitizer in sonodynamic therapy SDT, a promising strategy for cancer treatment.16 SDT employs specific sound waves to activate acoustic sensitizers, thereby inducing the destruction of tumor cells through localized biological effects. As an alternative to PDT, this treatment method capitalizes on the non-invasive and controllable nature of ultrasound (US), enabling precise targeting of tumor tissue while minimizing damage to surrounding healthy tissues.17 Furthermore, when combined with chemotherapy or radiotherapy, SDT can enhance therapeutic efficacy, offering patients a more comprehensive treatment approach. For instance, BTNPs can facilitate the cellular uptake of the anti-cancer drug doxorubicin (DOX),3 thereby improving its therapeutic potency. In addition, due to their unique acoustoelectric effect, BTNPs show great potential in ultrasound-driven tumor therapy.18 The acoustoelectric effect refers to the polarization of BTNPs into dipoles under ultrasonic field stimulation, thus generating a local electric field. Marino’s simulation study demonstrated that piezoelectric BTNPs could generate an oscillating voltage in the range of 1–10 mV under ultrasonic stimulation. Experimental evidence suggests that BTNPs could remotely activate nerve cells via nanosensors.19 In vitro experiments with HER2-positive breast cancer cells have shown that ultrasound-driven BTNPs can induce significant anti-proliferative effects. By comparing the biological effects under different conditions-US alone, US combined with non-piezoelectric BTNPs, and US combined with piezoelectric BTNPs, it has been confirmed that these effects are due to the electrical stimulation (ES) generated by the acoustoelectric effect of BTNPs.20 Furthermore, BTNPs can be selectively activated by US at tumor sites to perform specific biological functions, including the production of (ROS) and the induction of immunogenic death of tumor cells.10 Ultrasonic waves not only activate piezoelectric nanoparticles but also enhance their ability to penetrate into deep tissues. Studies have shown that US can increase the permeability of biofilms and promote the penetration of nanoparticles into tumor tissues.21 This effect, known as the acoustic hole effect, can increase cellular absorption of the therapeutic agent and promote more effective tumor regression.22
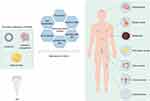
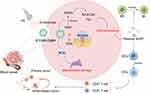
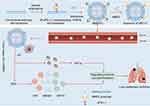
In recent years, significant advances in the synthesis and functionalization of ultrasound-driven BTNPs have greatly expanded their applications in the treatment of malignant tumors. Theses developments further underscored their promising potential in oncology. Through surface modification and coating with biocompatible materials, the biocompatibility and circulation time of BTNPs have been significantly enhanced. This has led to a reduction in potential toxicity and immune responses. Additionally, functionalizing BTNPs with specific ligands or antibodies can more effectively target cancer cells by exploiting the overexpression of certain receptors present on tumor cells.23 In this review, we discussed various ultrasound-driven BTNPs for cancer therapy and their mechanisms of action, such as the improvement of the tumor microenvironment, the normalization of tumor blood vessels, the enhancement of piezoelectric catalytic activity through ion doping, the induction of cell senescence and ferroptosis, and their combination with other therapeutic modalities (Figure 1). Finally, we summarized the current progress and challenges in the clinical translation of ultrasound-responsive BTNPs and anticipated the future development of piezoelectric catalytic therapy.
Advancements in the Design and Functionalization of Ultrasound-Driven BTNPs
The Morphology, Size, and Surface Modification of BTNPs Significantly Influence Their Biological Effects
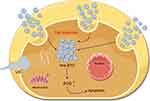
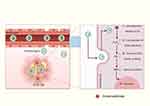
The synthesis of BTNPs is meticulously designed to precisely control their size, shape, and surface characteristics to optimize their biological interactions and therapeutic effects. The size and shape of nanoparticles significantly influence biodistribution, cellular uptake, and excretion.24 Larger particles (around 200 nm) exhibit higher piezoelectric catalytic activity but are easily phagocytosed by macrophages and have difficulties accumulating within tumor tissues. Conversely, smaller particles are readily internalized by cells but are quickly excreted, affecting their specific accumulation.25,26 To overcome these challenges as well as achieve precise and efficient treatment, Xiang et al developed tumor microenvironment-responsive BTNPs (tma-BTO NPs).27 These nanoparticles self-assemble into submicron-sized aggregates in the acidic tumor environment through Coulomb forces (Figure 2). The self-assembled aggregates not only mechanically damage tumor cells but also exhibit stronger piezoelectric catalytic efficiency and higher ROS production than dispersed nanoparticles. The enhanced piezoelectric catalytic effect is primarily due to the mutual extrusion of adjacent particles within the confined space of the aggregates. This mechanism allows the self-assembled tma-BTO NPs to significantly increase ROS generation under US stimulation, thereby potentiating the apoptosis of tumor cells. Subsequent animal experiments further validated the practical applicability of these nanoparticles.
BTNPs produced by conventional solid-state methods often exhibit broad size distributions and poor dispersion in aqueous media. Additionally, uncoated BTNPs may exhibit cytotoxicity due to the leaching of heavy metal ions (Ba2+).28 Jordan reported a surface hydroxylation-based modification method, covalently attaching hydrophilic polyethylene glycol (PEG) polymers.29 This polymer coating enables further modifications, such as fluorescence labeling, surface charge adjustment, or directed linkage of IgG antibodies. The binding of anti-EGFR antibodies to BTNP surfaces demonstrated their effective molecular targeting of A431 cells. Huang used citrate-coated BTNPs, synthesized by the gel collection method, which achieved high water dispersibility (up to 50 mg/mL) and a small hydrodynamic size (11 nm).30 They also applied modified Stober and reverse microemulsion methods to coat BTNPs with silica, producing larger nanoparticle aggregates (100–200 nm) and single BTNP encapsulates (29 nm), respectively. The -OH groups present on the silica surface easily facilitated surface PEGylation, which helped maintain high stability of the nanoparticles in saline solutions. Taheri studied the stability of PEGylated BTNPs in water and ethylene glycol using three different surfactants: sodium dodecylbenzene sulfonate, cetyltrimethylammonium bromide, and sorbitan monooleate, indicating the anionic surfactant (sodium dodecylbenzene sulfonate) as the optimal stabilizer for BTO nanofluids.31
Enhancement of Piezoelectric Properties
The efficiency of SDT primarily depends on the generation of ROS by sensitizers under US stimulation. Due to the low separation efficiency of electron-hole pairs, ROS generation is generally low.32 Researchers aim to enhance the piezoelectric response of BTO by narrowing its bandgap and promoting carrier separation, for example, by decreasing oxygen vacancies,33 doping elements, and constructing heterojunctions.34 Deng et al developed a palladium-catalyzed hydrogenation strategy to reduce the bandgap of BTO and enhance the synergistic multi-enzyme piezoelectric therapy effect.11 This Pd-catalyzed hydrogenation reaction significantly reduces the BTO bandgap, improving the separation efficiency of electron-hole pairs and thereby increasing ROS production. Under US stimulation, the piezoelectric potential-induced multiple redox processes that significantly enhance the dual enzyme activity of palladium (Pd). The dual enzyme activity of Pd refers to its catalytic properties resembling those of two natural enzymes: POD and catalase (CAT). Pd can mimic the function of POD, catalyzing the generation of ·OH from H2O2. Additionally, Pd can act like CAT, decomposing H2O2 into water and oxygen. This reaction helps alleviate hypoxia in the tumor microenvironment (TME), and the generated oxygen further promotes ROS production, enhancing the overall therapeutic effect. The synergistic effect of these enzyme activities significantly improves the multi-enzyme piezoelectric synergistic therapy. Both in vitro and in vivo experiments have confirmed that this material effectively inhibited the growth of mouse breast cancer cells. Furthermore, strontium doping and oxygen vacancy defect engineering (BST@LA) can significantly enhance the performance of piezoelectric sonosensitizers. This strategy triggers the release of NO from L-arginine (LA) through the piezoelectric effect, thereby achieving controlled NO release at the tumor site.32 Additionally, Bismuth-doped BTO, with a smaller bandgap and enhanced ROS generation, has been proven effective in treating ovarian cancer.
Improving the TME
The TME consists of cells, molecules, and blood vessels surrounding tumor cells, influencing tumor development, metastasis, and therapeutic response.35 High concentrations of glutathione (GSH) in the TME help tumor cells counteract oxidative stress, leading to chemotherapy resistance.36 Deng et al developed Pd-BTO NPs with catalase-like activity that react with H2O2 in the TME to produce oxygen, reversing the hypoxic TME and enhancing oxygen-dependent therapy.37 GSH helps tumor cells survive and proliferate by reducing ROS production and increasing antioxidant defense capabilities, thereby supporting tumor growth and survival. Chen et al developed a novel hybrid nanocomposite (BTO-Pd-MnO2-HA) with a BTO core coated with hyaluronic acid, Pd, and manganese dioxide. BTO-Pd-MnO2-HA continuously consumes GSH and produces O2, enhancing the efficiency of SDT and chemodynamic therapy (CDT).
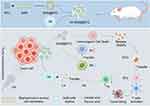
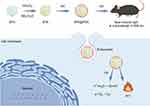
The accumulation of lactic acid in TME not only promotes tumor resistance,38 but also poses a major challenge to the effectiveness of immunotherapy. Lactic acid not only inhibits the proliferation and activation of immune cells, but also promotes the differentiation of regulatory T cells, thus weakening the anti-tumor immune response.39 Veillonella atypica (VA), a beneficial microorganism widely present in the human body, is primarily distributed in the oral cavity, respiratory tract, and digestive tract. VA’s main function is to degrade acidic substances produced by other microorganisms or cells, particularly lactate.40 Due to its ability to utilize lactate as a food source and produce propionate, VA holds potential application value in regulating lactate levels within the TME. Fan et al designed a microrobot (VA-SAM@BTO) by loading BTO and Staphylococcus aureus cell membranes (SAM) onto the surface of VA for targeted catalytic and immunotherapy against colorectal cancer (Figure 3).41 The study found that VA-SAM@BTO could accurately target cancer sites through oral administration. Under US stimulation, it triggered various oxidative and reductive reactions, effectively inducing immunogenic cell death of tumor cells. Furthermore, the VA-SAM@BTO+US combination treatment significantly reduced lactate levels in tumors, improved the immune environment. It enhanced dendritic cell maturation and macrophage M1 polarization, increased the proportion of effector T cells, and reduced the number of regulatory T cells. By doing so, it achieved synergistic catalytic and immunotherapeutic effects.
Mechanisms of Ultrasound-Driven BTNPs in Cancer Treatment
The therapeutic potential of BTNPs derives from the elevated piezoelectric coefficient of the tetragonal phase of BTO. The piezoelectric properties of these nanoparticles are particularly attractive for tissue engineering applications. On the one hand, the piezoelectric effect promotes bone growth and nerve tissue repair. Additionally, incorporating supergravity into piezoelectric nanoparticles enhances bone regeneration by promoting the differentiation of mesenchymal stem cells into osteoblasts. On the other hand, ultrasound-driven BTNPs can induce calcium and sodium flux in neuron-like cells and reversibly enhance the electrical activity of in vitro neural networks.42 Furthermore, recent studies suggest that long-term US stimulation of BTNPs can disrupt cellular calcium balance and potentially induce the reorganization of the mitotic spindle, resulting in antiproliferative effects on HER2-positive breast cancer cells and glioblastoma cells. This section details the various mechanisms by which US interacts with and influences BTNPs to treat tumors.
Disruption of Cells Through the Sonoelectric Effect
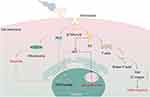
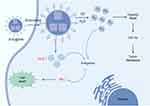
When US waves act on BTNPs, they induce mechanical vibrations that, due to the piezoelectric effect, are converted into electrical charges.23 This phenomenon is crucial in cancer treatment because it can directly convert US energy into electrical energy, producing a series of biological effects. The combination of mechanical stress and electric fields generated around BTNPs can disrupt cellular homeostasis, resulting in apoptosis, or programmed cell death. Studies have demonstrated that internal ES can significantly inhibit the growth and migration of cancer cells by inducing the overexpression of the p53 gene, which causes cell cycle arrest and mitochondria-dependent apoptosis.43 Zhan et al observed that internal ES significantly inhibited the growth and migration of triple-negative breast cancer (TNBC) cells. Experimental data suggested that internal ES induced overexpression of the p53 gene, leading to cell cycle arrest and mitochondria-dependent apoptosis, cytoskeletal disruption, and down-regulation of FAK and β1-integrin gene expression, thereby inhibiting TNBC cell migration (Figure 4). Additionally, the anti-cancer effect was more pronounced with higher intensities of ES. Despite its potential, further research is needed to assess the long-term biosafety and potential side effects of internalES. Notably, Zhan et al’s study systematically varied the intensity of internal electrical stimuli by using BTNPs with different crystalline phases and piezoelectric properties. Future research should focus on addressing the challenge of precisely controlling the intensity and frequency of ES to maximize therapeutic efficacy, while exploring the optimal intensity for different types of malignancies.
Generation of ROS
Under the stimulation of US, BTNPs produce a piezoelectric effect, which means that during mechanical vibration, charge recombination occurs on their surface, leading to the generation of electron-hole pairs.44 These electron-hole pairs react with oxygen and water molecules in the surrounding medium to produce various types of ROS, primarily superoxide anions, hydroxyl radicals, and singlet oxygen.45 ROS are chemically reactive molecules containing oxygen, including well-known free radicals capable of damaging cellular components such as DNA, proteins, and lipids.46 The overproduction of ROS and oxidative damage to cellular macromolecules are considered as key mechanisms of the nanoparticles’ biotoxicity. ROS can directly oxidize the unsaturated fatty acids in the cell membrane, leading to lipid peroxidation,47 which destroys the integrity of the membrane and increases its permeability.48 This membrane damage not only causes abnormal exchange of materials both inside and outside the cell, but also affects membrane proteins and signal transduction pathways, ultimately culminating in apoptosis or necrosis.48 Furthermore, the accumulation of ROS can cause DNA base damage and impair the function of DNA polymerase and repair proteins.49 DNA damage activates the expression of the p53 gene, which subsequently upregulates p21 expression and inhibits cyclin-CDK complex activity, leading to cell cycle arrest in the G0/G1 phase.43 (Figure 4) Recent studies have shown that nanoparticles can induce cytotoxicity through ROS production and oxidative damage to cellular components, and that regulating the homeostasis between intracellular ROS levels and oxidative stress can be used as a tool for cancer therapy. Ahamed’s experimental studies demonstrated that BTNPs effectively inhibit the growth of human lung cancer cells (A549).50 BTNPs decreased cell viability in a dose- and time-dependent manner. After exposure to BTNPs, a decrease in mitochondrial membrane potential was observed, along with the induction of Caspase-3 and −9 activity in lung cancer cells. BTNPs also induced additional oxidative stress, manifested by the induction of pro-oxidants (ROS and H2O2) and the reduction of antioxidants (GSH and several antioxidant enzymes). Furthermore, BTNP-induced cytotoxicity and oxidative stress were effectively mitigated by N-acetylcysteine (NAC), a ROS scavenger, indicating that BTNP-induced cytotoxicity is a result of oxidative stress.
Studies also found that BTNPs did not affect non-cancerous human lung fibroblasts (IMR-90), highlighting the selective cytotoxicity of BTNPs against different types of cancer cells and their normal counterparts.50 The reason is that ordinary cells typically maintain a highly regulated REDOX balance, which is essential for normal cell function.51 A variety of antioxidant defense systems exist within cells, including enzymes (eg, superoxide dismutase (SOD), CAT, GSH peroxidase (GPx), etc) and non-enzymatic antioxidants (eg, glutathione, vitamin C, vitamin E).52 These antioxidant mechanisms play a critical role in clearing excess ROS. When ordinary cells are stimulated by ROS, the antioxidant system activates rapidly, reducing damage to cell structures by removing or neutralizing ROS. In contrast, the REDOX balance in cancer cells is frequently disrupted due to their high metabolic activity, resulting in significantly elevated endogenous ROS levels.53 While these ROS promote cancer cell proliferation and survival, cancer cells also rely on enhanced antioxidant mechanisms to sustain their survival. However, cancer cells exist in a state of “high oxidative stress”, and any further increase in ROS levels by external factors may cause their antioxidant system to collapse, leading to uncontrolled oxidative stress.54–57 This sensitivity to ROS makes ultrasound-driven BTNPs producing ROS an effective anti-tumor therapy. Therefore, the cancer cell-selective toxicity mechanism of these semiconductor nanoparticles may be related to the severe oxidative stress environment of cancer cells. During ultrasound-driven BTNPs treatment, some cancer cells are already in a severely compromised oxidative stress state, making them more susceptible to further ROS damage, while normal cells under relatively less stress are less affected. Fakhar observed that BTNPs exhibit strong, time- and dose-dependent cytotoxicity against breast cancer cell lines (MCF-7).24 In a BTNP dispersion at 200 μg/mL, the maximum percentage of cell viability loss was 57%, while the minimum cell viability loss percentage was 19% at 50 μg/mL of BTNP concentration.
While many studies have shown that tumor cells are more sensitive to ROS damage, there is still a need to maximize the benefits of US drive to minimize systemic toxicity. Therefore, it can be seen that BTNPs should be committed to studying the local biological effects at the tumor site. This ensures not only the drug concentration in the tumor but also minimizes systemic toxicity, offering significant advantages over traditional, non-selective ROS-generating therapies. To better confine drugs to the tumor area, Zhu et al combined T-BTO nanoparticles with chitosan (CS)/carboxymethyl cellulose (CMC)/beta-glycerophosphate (BGP) hydrogels to create a therapeutic biologic injection.16 This hydrogel is renowned for its excellent injectability and thermal sensitivity as well as designed to control spills during hydatid imbibition.58 Its thermal sensitivity enables the hydrogel to transition from a liquid to a gel at physiological temperatures (approximately 37°C) after injection, thus confining the T-BTO nanoparticles to the tumor area and minimizing damage to normal cells. Additionally, Li et al discovered that oleic acid-coated BTNPs can generate carbon-centered free radicals under ultrasonic stimulation.59 The high-energy reactivity and oxygen-independent properties of these free radicals offer significant advantages, particularly in treating hypoxic tumors. This discovery provides new insights for developing more effective carbon free radical inducers for tumor therapy.
Induction of Tumor Cell Senescence
Nicotinamide adenine dinucleotide (NAD+) is a critical molecule for cellular function and metabolism and is closely associated with cellular senescence.60 Insufficient NAD+ levels lead to inadequate cellular energy, thereby promoting cellular senescence and weakening tumor cell resistance.61 Hao et al encapsulated BaTiO3/(Cp*RhCl2)2 (BTO/Rh) and DOX inside the tumor cell membrane to construct a biocompatible nanomedicine (BTO/Rh-D@M).62 The study demonstrated that the negative charges generated by the catalytic activity of the piezoelectric material can reduce intracellular NAD+ to NADH, inducing cellular senescence and rendering tumor cells more susceptible to chemotherapeutic drugs (Figure 5). Additionally, senescent tumor cells secrete senescence-associated secretory phenotype (SASP) factors, which include pro-inflammatory cytokines, chemokines, and growth factors. On the one hand, SASP factors attract immature dendritic cells to the tumor microenvironment and promote their maturation. The mature dendritic cells then internalize antigens released by the senescent tumor cells and process these antigens intracellularly. The processed antigens are presented in the form of antigen peptides bound to major histocompatibility complex (MHC) molecules on the surface of dendritic cells. These mature dendritic cells migrate to lymph nodes, where they present the MHC-antigen peptide complexes to T cells, including CD4+ T cells and CD8+ T cells. CD4+ T cells assist in the immune response, while CD8+ T cells directly kill tumor cells. Activated T cells proliferate and differentiate before departing from the lymph nodes to infiltrate the tumor site and enter systemic circulation, where they identify and eliminate tumor cells. On the other hand, SASP factors can induce the polarization of macrophages into the M1 phenotype, which is characterized by pro-inflammatory and anti-tumor activities. Simultaneously, the positive charges generated by piezoelectric catalysis can oxidize H2O into ROS, causing mitochondrial damage in tumor cells, thereby further enhancing the effects of chemotherapy and immunotherapy.
Inducing Ferroptosis in Tumor Cells
Ferroptosis is a type of programmed cell death characterized by iron homeostasis disruption, GSH metabolism disorder, and lipid peroxidation.63 GSH and GPX4 play crucial roles in ferroptosis. As a cofactor of GPX4, GSH supports its function in reducing lipid peroxides to protect cells from oxidative damage.64 During ferroptosis, the depletion of GSH and the inactivation of GPX4 lead to the accumulation of lipid peroxides, triggering cell death.
Wang et al grew molybdenum disulfide (MoS2) nanosheets on the surface of BTO nanoparticles and combined them with pH-responsive cinnamaldehyde (CA) to form a nanocatalyst capable of self-feeding H2O2 in the acidic TME (BTO/MoS2@CA).65 The catalyst induced ferroptosis in tumor cells through a catalytic cascade mediated by US (Figure 6). The aldehyde group (-CHO) on the CA molecule binds to H+ in TME and hydrolyzes to produce H2O2. This process is facilitated by an acidic environment, resulting in spontaneous production of H2O2 at the tumor site. Under ultrasonic irradiation, positive and negative charges are generated on the surface of BTO, which significantly reduces the dissociation energy barrier of H2O2. H2O2 is a key substrate in the peroxidase-catalyzed reaction, thus enhancing the peroxidase (POD) activity of MoS2. This leads to the production of more hydroxyl free radicals (·OH), the consumption of GSH, the induction of oxidative stress imbalance, and ultimately, the realization of efficient ferroptosis. This procedure has demonstrated significant anti-tumor effects and low side effects. In vitro cell experiments showed that BTO/MoS2@CA significantly inhibited the proliferation and induced cell death of CT-26 cells under ultrasonic irradiation. Experiments on ROS production and GSH consumption demonstrated that BTO/MoS2@CA effectively increased intracellular ROS levels, decreased GSH concentration, decreased GPX4 activity, induced lipid peroxidation, and promoted ferroptosis. In vivo experiments showed that the BTO/MoS2@CA combined with US treatment group significantly inhibited tumor growth and reduced tumor volume and weight in a CT-26 tumor mouse model.
Normalization of Tumor Vasculature
Changes in tumor vasculature play a crucial role in tumor development and progression. Tumor vasculature not only provides necessary oxygen and nutrients to tumor cells but also affects the TME, promoting tumor growth and metastasis.66 Therefore, targeting tumor vasculature has become an important strategy. In recent years, the concept of tumor vasculature normalization has gained attention. Restoring the normal structure and function of tumor vasculature can enhance the effectiveness of tumor treatments, reduce drug side effects, and increase the sensitivity of tumors to radiotherapy and chemotherapy.67 Li et al utilized a solvothermal method to synthesize polarized barium titanate (P-BTO) nanoparticles and investigated the effects of ES on endothelial cells in vitro.68 In vitro experiments demonstrated that P-BTO nanoparticles generated pulse voltages under low-intensity focused ultrasound (LIPUS), disrupting the intracellular bioelectrical balance and significantly inhibiting the migration and differentiation of human umbilical vein endothelial cells (HUVECs). This inhibition was mediated through interference with the eNOS/NO pathway and the intracellular distribution of calcium ions. Furthermore, in vivo experiments revealed that LIPUS, when combined with polarized BTO nanoparticles, optimized tumor vascular structure, improved blood perfusion, reduced vascular leakage, and restored local oxygenation. This combination significantly enhanced the anti-tumor efficacy of the chemotherapy drug DOX. These findings indicate that this method holds potential for normalizing tumor vasculature.
Controlled Drug Release and Combination with Other Therapies
One of the primary applications of ultrasound-driven BTNPs is the controlled drug release facilitated by this nanoparticle platform. The nanoparticle platform can encapsulate drugs, protecting them from premature degradation during circulation and targeting delivery to tumor sites through specific surface modifications. Once at the target site.69,70 BTNPs can precisely release drugs under US influence. By adjusting US parameters such as frequency, power, and duration, drug release can be fine-tuned.71,72 The versatility of ultrasound-driven BTNPs allows for combination with other therapies to achieve synergistic effects. The mechanical stress generated by BTNPs creates temporary pores in the cell membrane, allowing more chemotherapeutic drugs to enter, thereby enhancing cytotoxic effects. Additionally, the local thermal effects generated by US can increase blood flow to the tumor area, improving drug delivery and efficacy. This section further discusses combining ultrasound-driven BTNPs with other therapeutic approaches.
Synergistic Photothermal Therapy
Photothermal therapy (PTT) utilizes the conversion of light energy into heat to treat diseases. This therapy typically employs photosensitizers, such as nanoparticles, to absorb light of specific wavelengths and convert it into heat.73 The resulting high local temperature can kill or damage diseased cells, such as tumor cells. Xu et al introduced ultrafine gold nanoparticles on the surface of BTO nanoparticles and encapsulated them with polydopamine to develop a novel type of nanoparticle (BaTiO3-Au@PDA, BTAP).74 These nanoparticles not only combine the synergistic effects of piezocatalysis and PTT but also possess potential for CT imaging. The CT imaging potential of these new nanoparticles is due to the gold nanoparticles on their surface. Gold, with its high atomic number and high X-ray attenuation coefficient, can significantly enhance the contrast in CT imaging, making tumors or other pathological tissues more distinct. With this design, the nanoparticles can generate ROS under US irradiation and effectively convert light energy into heat under NIR light irradiation, enhancing the cytotoxic effect on tumor cells (Figure 7). Additionally, the CT imaging capability provides real-time imaging guidance during the treatment process, further improving the precision and safety of the therapy.
Synergistic Gas Therapy
Gas therapy (GT) has been extensively studied as a green and promising therapeutic approach.75 Notably, nitric oxide (NO) has shown significant potential in cancer treatment because it can damage mitochondrial cells at high concentrations, disrupt energy supply, and inhibit cell proliferation.76 However, due to its high reactivity and short half-life, effectively delivering NO to target tissues remains a major challenge. Chen et al developed BTO@DPA nanoparticles by coating BTO with amphiphilic polyarginine (DPA) (Figure 8).77 The study found that BTO@DPA nanoparticles could efficiently generate NO under US irradiation, which reacted with ROS to form more effective peroxynitrite (ONOO−), causing severe apoptosis in tumor cells under both hypoxic and normoxic conditions. After intravenous injection, BTO@DPA accumulated in tumor tissues within 4 hours, and subsequent US irradiation resulted in approximately 70% tumor inhibition and complete prevention of lung metastasis. Specifically, BTO generates H2O2, singlet oxygen (1O2), and oxygen under US, thereby oxidizing surface arginine to produce NO. This method can function in hypoxic environments and allows for controlled generation of NO and ROS by adjusting US intensity and duration, achieving more efficient tumor treatment.
Synergistic Immune Checkpoint Blockade (ICB) Therapy
Immune checkpoint inhibitors are a class of immunotherapy drugs designed to enhance the body’s ability to attack tumors by removing the “brakes” on the immune system.78 These drugs primarily target proteins known as immune checkpoints, such as PD-1, PD-L1, and CTLA-4. Under normal circumstances, these proteins help maintain immune system balance and self-tolerance, but tumor cells often exploit these mechanisms to avoid immune attacks.79 However, the clinical application of ICBs still faces challenges such as low host response rates and non-specific distribution, which significantly affect therapeutic efficacy. Tang et al designed cell membrane-coated BTO nanoparticles that can activate ICB therapy in response to matrix metalloproteinase-2 (MMP2).10 The MMP2-activated PD-L1 inhibitor, M-αPD-L1 protein, is genetically engineered to be expressed on wild-type cell membranes, which are then extracted and coated onto the BTO nanoparticles. When these nanoparticles enter the protease-rich TME, the shielding domain is cleaved from the MMP2-sensitive peptide, exposing the antibody-binding domain, thus blocking the PD-L1 receptors (Figure 9). Additionally, this design allows the nanoparticles to specifically release ROS and oxygen within the TME, significantly enhancing the infiltration of cytotoxic T lymphocytes, inducing immunogenic cell death, and alleviating hypoxia, thereby improving the precision and efficiency of the treatment.
In summary, the mechanisms of ultrasound-driven BTNPs in tumor therapy are multifaceted. They convert US energy into mechanical, electrical, and thermal energy through their piezoelectric properties, inducing cell disruption, ROS generation, and localized drug release at tumor sites. These mechanisms, either individually or in combination, provide an effective and targeted approach to cancer treatment, which minimize side effects and simultaneously enhance therapeutic efficacy.
Applications of Ultrasound-Driven BTNPs in Treating Tumors Across Various Tissues
Breast Cancer
Researchers have focused on applying the piezoelectric effect of BTNPs under US stimulation for breast cancer treatment, developing various BTNPs for this purpose (Table 1). The challenges encountered by SDT in breast cancer treatment can be primarily categorized into two main areas: enhancing catalytic efficiency and improving TME. Hypoxia is a significant feature of the TME and is closely related to the overexpression of hypoxia-inducing factor-1α (HIF-1α), which directly contributes to tumor metastasis.80 Studies have shown that alleviating hypoxia can reduce HIF-1α levels, thereby inhibiting tumor metastasis. To address hypoxia in the TME, Wang et al developed ultrasonically triggered, piezoelectric catalytic, water-cracking DSPE-PEG2000 coated polarized barium titanate nanoparticles (P-BTO).45 These nanoparticles not only produce ROS but also generate oxygen, significantly improving the hypoxic environment, killing tumor cells, and inhibiting tumor metastasis. In addition, Yue developed manganese oxide (MnO2)-coated barium titanate nano-acoustic sensitized agent (BTO@M NPs),34 which promoted carrier separation by the piezoelectric effect and p-n junction effect, thereby improving ROS generation efficiency and reshaping the TME. In PDT, CDT, and SDT, tumor cells are suppressed by producing an excess of toxic ROS. However, the ROS generation efficiency of these monotherapies requires enhancement. Zhao et al synthesized Cu2-xO nanotubes (Cu2-xO-BTO NCs) to improve the therapeutic efficacy of cancer by integrating SDT and CDT, but further research on its specific molecular mechanisms is warranted.81
 |
Table 1 Summary of the Application of Ultrasound-Driven BTNPs in Breast Cancer Treatment |
Melanoma
Melanoma, a malignant tumor originating from melanocytes, is the most lethal skin cancer due to its high invasiveness and rapid metastasis, despite accounting for a smaller proportion of all skin cancers. For advanced or refractory melanoma, current treatments have limited efficacy, highlighting the urgent need for new strategies and technologies to improve outcomes and patient quality of life.82 SDT offers new insights for melanoma treatment (Table 2). Research on ultrasound-driven BTNPs in melanoma, the advancement of multi-enzyme catalysis, and the integration of collaborative ICB therapy represent the primary research directions. Wang et al developed novel nanoparticles (Met@BF) combining Fe3O4 and BTO, surface-modified with metformin (Met), which can block the PD-1/PD-L1 axis, mobilizing the immune system against cancer. Local metformin release further downregulated PD-L1, enhancing antitumor effects.83 As detailed in Tang et al, the research team developed an immune checkpoint-blocking nanoplatform (M@BTO NPs) activated by MMP2.10 Zhu et al developed a ruthenium nanocluster enzyme (RuNC/BTO) nanocatalyst supported by barium titanate to enhance its biocatalytic activity for tumor therapy. Studies have shown that RuNC/BTO has excellent POD and halogen peroxidase (HPO) activities; specifically, it can produce a large amount of superoxide anion (·O2−) and hypochlorous acid (HClO) with H2O2 as the substrate under US, thereby inhibiting tumor cells.84
 |
Table 2 Summary of the Application of Ultrasound-Driven BTNPs in Melanoma Treatment |
Other Tumors
In addition to breast cancer and melanoma, BTO materials have also shown a wide application potential in the treatment of a variety of other tumor types. In recent years, researchers have been exploring its application in treating various tumors such as lung cancer, cervical cancer, and colon cancer to achieve more efficient therapies. Especially for refractory diseases such as pancreatic cancer, glioblastoma multiforme, and osteosarcoma, BTNPs show great potential (Table 3).
 |
Table 3 Summary of the Application of Ultrasound-Driven BTNPs in the Treatment of Other Tumors |
Pancreatic Ductal Adenocarcinoma
The treatment of pancreatic ductal adenocarcinoma (PDAC) is particularly challenging due to its dense fibrotic stroma, which not only protects cancer cells from immune system attacks but also impedes drug penetration and distribution, rendering standard chemotherapy regimens less effective.87 Additionally, the fibrotic stroma promotes hypoxia within the TME, further enhancing cancer cell survival and drug resistance. Current treatment options include surgical resection, chemotherapy, and radiotherapy, but only a few patients are eligible for surgery as most are diagnosed at an advanced stage.88 In this context, researchers are exploring new therapeutic strategies to improve the prognosis of PDAC. By modulating the dense fibrotic stroma of the TME, NO can enhance drug penetration. Wang et al developed an ultrasound-activated piezoelectric catalytic platform that generates ROS in a hypoxic tumor environment through an ultrasound-triggered piezoelectric catalytic process.85 This process breaks the thioether bond to release the chemotherapeutic drug camptothecin (CPT) and oxidizes LA to release NO, thereby delivering both CPT and NO simultaneously at the tumor site. In in vivo and in vitro experiments, the controlled release of CPT and NO under ultrasonic stimulation was observed, contributing to the depletion of the dense fibrotic matrix and subsequently enhancing the delivery and efficacy of CPT.
Glioblastoma Multiforme
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor in adults.89 GBM cells are highly heterogeneous, grow rapidly, and infiltrate normal brain tissue quickly, making complete surgical removal extremely difficult.90 Although radiotherapy is a standard treatment, the radiation dose is limited by the sensitivity of brain tissue to radiation. Chemotherapy effectiveness is also limited by the blood-brain barrier (BBB), which prevents many drugs from penetrating the brain, further complicating treatment.91 To address these challenges, Marino et al present an innovative treatment strategy for GBM utilizing the ultrasonic activation of piezoelectric BTNPs.23 These functionalized BTNPs, equipped with anti-transferrin receptor antibodies, specifically target the BBB and GBM cells. Upon ultrasound-mediated piezoelectric stimulation, BTNPs notably reduce GBM cell proliferation in vitro. Furthermore, when combined with low doses of temozolomide (TMZ), this stimulation enhances the cells’ sensitivity to chemotherapy, exhibiting significant anti-proliferative and pro-apoptotic effects. BTNPs effectively traverse the in vitro BBB model and selectively target GBM cells. This novel approach holds promising potential for the remote treatment of brain cancer and neurodegenerative diseases. However, due to the absence of experimental data from animal models, future research must validate the therapeutic efficacy of BTNPs on GBM using more complex in vivo models and preclinical studies.
Osteosarcoma
Osteosarcoma is a highly malignant tumor that occurs in the bones, primarily affecting children and adolescents.92 This tumor is known for its rapid growth and high metastatic rate, often involving long bones such as the femur, tibia, and humerus. The main treatment methods for osteosarcoma include surgical tumor resection and chemotherapy.93 Surgery typically aims to preserve limb function but often requires the removal of a significant amount of bone tissue, leading to substantial bone defects. Bone reconstruction is a major challenge for patients, as it is essential to ensure good integration of bone implants with surrounding healthy bone. To improve the bone integration of implants, researchers have explored various biomaterials and surface modification techniques.94 Xiao et al have developed a novel one-dimensional ferroelectric nanoarray (PNBTO) that can selectively modulate osteogenesis and anti-osteosarcoma therapy by wirelessly switching between static and dynamic ES.95 The study demonstrated that under ultrasonic stimulation, PNBTO generates high-intensity dynamic electrical signals, effectively inhibiting tumor cell growth, leading to cell cycle arrest and cell death. Static ES promoted the early osteogenic differentiation of bone marrow mesenchymal stem cells (mBMSCs) and the formation of calcified nodules. Specifically, dynamic ES blocks the G2/M phase of mitosis by interfering with the orientation of tumor cell spindle filaments, ultimately leading to tumor cell death with minimal cytotoxic to normal bone cells. In contrast, static ES state utilizes the high surface potential of PNBTO to provide continuous electrostatic stimulation, which promotes the osteogenic differentiation of mBMSCs and improves bone regeneration quality. This method has been validated both in vitro and in vivo. In vivo experiments showed that dynamic ES significantly inhibited tumor growth and prolonged the survival time of mice. Static ES, on the other hand, accelerated bone regeneration and improved the fusion of the implant with bone tissue.
In summary, SDT based on BTNPs and certain barium titanate heterostructured nanoparticles shows significant therapeutic potential in various tumor models. Its unique piezoelectric catalysis can generate a substantial amount of ROS through ultrasonic stimulation, achieving targeted destruction of tumor cells while minimizing damage to surrounding healthy tissues. Additionally, the therapeutic effect is further amplified through various surface functionalization approaches, such as inducing ferroptosis and cellular senescence, synergistic chemotherapy and GT. While optimization of US parameters remains a crucial challenge, previous studies have shown that in different types of tumors, such as breast cancer, melanoma, and osteosarcoma, appropriate adjustments in US power and treatment duration can effectively improve treatment outcomes. These findings lay a solid foundation for the clinical translation of BTNPs in cancer therapy and provide an essential basis for further optimization of treatment strategies in the future.
Conclusion and Outlook
Conclusions of Ultrasound-Driven BTNPs in Tumor Therapy
US is a non-invasive, precise, and low-energy technology with the advantages of deep tissue penetration and high biosecurity. Building on these advantages, BTNPs show great potential in tumor therapy due to their excellent piezoelectric properties. These BTNPs can efficiently generate ROS and an endogenous electric field under ultrasonic activation, thus inducing tumor cell death and minimizing damage to surrounding healthy tissues compared to traditional chemoradiotherapy. By optimizing their synthesis and surface modification, the biocompatibility of BTNPs has been significantly improved, while endowing them with multiple therapeutic mechanisms, making them highly advantageous in targeted tumor therapy. This review highlights recent advances in the field of ultrasound-responsive BTNPs and certain barium titanate heterostructured nanoparticles, focusing on the use of nanotechnology to develop various ultrasound-responsive barium titanate nanoplatforms for tumor therapy, including induced tumor cell senescence, ferroptosis, and GSH depletion to reshape the tumor microenvironment. BTNPs offer a promising solution for future tumor therapy. However, several significant challenges still need to be solved in their clinical translation.
Challenges for Clinical Translation
(i) In promoting the clinical application of BTNPs in SDT, the optimization of ultrasonic parameters remains a significant challenge that needs to be addressed. Due to the diffraction and scattering effects of sound waves, ultrasonic therapy not only affects the target tumor but may also cause damage to surrounding healthy tissue, thereby compromising therapeutic efficacy. Therefore, the precise regulations of US intensity, frequency, pulse duration, and treatment cycles are essential to ensuring both the effectiveness and safety of treatment, making this one of the core issues in clinical translation. At present, significant variation exists in the ultrasonic parameters used in different experiments, and no unified standard has been established. For example, in breast cancer research (Table 1), the commonly used US frequency is 1.0 MHz, with a power density of 1.0 W/cm2, a 50% duty cycle, and treatment durations in vitro varying from 1 to 10 min. In SDT, these parameters harness the piezoelectric effect of BTNPs to effectively generate ROS, thereby inducing tumor cell apoptosis. However, translating these laboratory results into clinical practice remains a pressing issue. In the treatment of melanoma (Table 2), the commonly used US intensity is high, with in vitro experiments typically employing a frequency of 1.0 MHz and a power density between 1.0 and 2.5 W/cm2, and treatment durations of 2 min in vitro, extending to 5 min in vivo to ensure adequate ROS generation. These higher parameter settings may respond to the aggressiveness of melanoma but also demand greater attention to the safety of healthy tissues. Other tumors, such as colon and liver cancer (Table 3), typically employ a 1.0 MHz frequency and a 1.0 W/cm2 power density, with similar parameters used in vitro and in vivo, though treatment durations and cycles differ. For more complex tumors such as osteosarcoma, higher intensity US is used to ensure effective treatment in deep tissues, which simultaneously increases the potential risk to healthy tissues. Optimizing and standardizing US parameters is one of the primary challenges in the clinical application of BTNPs in SDT. Although SDT shows significant potential in cancer treatment, the selection and regulation of US parameters are crucial to determining its clinical feasibility and safety. Therefore, future studies should further explore the biological effects of these parameters on tissues, optimize treatment regimens, and ensure the safety and efficacy of SDT in clinical applications.
(ii) The biosafety of nanosystems needs to be adequately addressed, particularly the potential for toxicity that may arise from long-term use or accumulation within the body. The high reactivity of nanoparticles and their wide range of biological interactions can have unpredictable effects on the immune system and organ function. In addition, although US has strong penetration ability, its efficacy is limited in tissues with high bone or gas content, especially in the treatment of deep tumors. At present, most studies focus on the treatment of subcutaneous tumors in mice, while further research on orthotopic, primary, and patient-derived xenograft tumor models is still required. This will enable a better understanding of the potential synergy between US’s deep penetration and nano-barium titanate in cancer treatment. Large-scale production is also a challenge, as the bulk preparation of nanoparticles requires high yields and consistency, which often involves high costs and technical difficulties. Furthermore, the complexity of the production process and the uncertainty of storage conditions further complicate its clinical application.
Future Developments
Owing to the aging global population and the increasing prevalence of non-communicable diseases, such as cancer, there is an escalating need for highly effective treatments with minimal side effects. With future advances in nanotechnology and US technology, the application of BTNPs in cancer treatment is expected to become more extensive and precise. However, despite significant advancements, the clinical translation of these ultrasound-responsive materials remains limited. It is important to note that, while preliminary studies indicate an ideal safety profile for nanomaterials, their long-term use, especially with reuse, may present unforeseen health risks. These potential risks, particularly those related to the accumulation of barium ions in the body, demand comprehensive research. Therefore, a thorough understanding of their safety is a prerequisite for widespread clinical application. Additionally, experimental recordings of ultrasonic-driven piezoelectric stimulation on different cell types reveal varied biological effects. For example, piezoelectric stimulation inhibited cell cycle progression in breast cancer and glioblastoma cells but had no effect on lung cancer cells.20 However, in fibroblasts96 and macrophages,97 ultrasonic piezoelectric stimulation promotes cell proliferation. The molecular mechanisms underlying these varied biological effects have yet to be well understood.
Thus, multidisciplinary cooperation and exchange are essential for developing and synthesising more biocompatible and versatile ultrasound-responsive BTNPs for future clinical translation. The primary future research direction is to elucidate the molecular mechanisms through which ultrasound-driven BTNPs generate endogenous electric fields in the treatment of malignant tumors, focusing on three main aspects. These aspects include: (i) Mechanism exploration: Investigate the molecular mechanisms through which ultrasound-driven BTNPs kill tumor cells and determine how nanoparticle size and morphology affect anti-cancer effects, such as inhibiting proliferation, inducing apoptosis, and increasing sensitivity to chemotherapy and immunotherapy. (ii) Quantification of piezoelectric effects: The accurate measurement of the piezoelectric effects of ultrasound-driven piezoelectric nanoparticles, including the generated electric field, is primarily conducted through in vitro experiments. This provides a tool for studying cell behavior and the molecular mechanisms underlying ultrasound-driven ES of piezoelectric nanoparticles. (iii) Optimization of driving parameters: Study the optimal driving parameters for different types of cancer cells and their organelles, including nanoparticle size, concentration, US excitation power, frequency, and ultrasound dose. Given the current challenges, elucidating the molecular mechanisms by which ultrasound-driven BTNPs induce endogenous electric fields to treat tumors, and further innovating ultrasound-responsive materials, are crucial steps. These advancements are expected to promote the application of BTNPs in cancer treatment, offering patients more effective and safer treatment options.
Data Sharing Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
All figures were edited using the FIGDRAW 2.0 software. We would like to express our sincere gratitude to the online scientific illustration platform Figdraw. This work was supported by the Natural Science Foundation of Shandong Province under Grant [ZR2021MH403].
Disclosure
Shuao Lia and Ningning Heb have contributed equally to this work and share first authorship. The authors declare that there are no conflicts of interest.
References
1. Dizon DS, Kamal AH. Cancer statistics 2024: all hands on deck. Ca-a Cancer J Clin. 2024;74(1):8–9. doi:10.3322/caac.21824
2. WHO. Available from: https://gco.iarc.fr/today/home.
3. Lin JH, Chen L, Cho EC, Lee KC. Influence of bonding variance on electron affinity in graphene quantum dot-barium titanate nanocomposites for drug delivery system. Flatchem. 2024;47:9. doi:10.1016/j.flatc.2024.100713
4. Khan MI, Batool F, Ali R, et al. Tailoring radiotherapies and nanotechnology for targeted treatment of solid tumors. Coord. Chem. Rev. 2022;472:20. doi:10.1016/j.ccr.2022.214757
5. Jiang BB, Iocozzia J, Zhao L, et al. Barium titanate at the nanoscale: controlled synthesis and dielectric and ferroelectric properties. Chem Soc Rev. 2019;48(4):1194–1228. doi:10.1039/C8CS00583D
6. Alkathy MS, Rajesh Y, Kassim HA, et al. Enhancing energy storage performance in barium titanate ceramics through mg-doping via creation of defect dipoles engineering. J Aust Ceram Soc. 2024;2024:1–13.
7. Liu JL, Qu SN, Chen ZW, et al. Significantly enhanced electrostatic energy storage performances of polyetherimide nanocomposites with ultralow loadings of barium titanate nanoparticles. Appl Phys Lett. 2024;124(23):7. doi:10.1063/5.0190323
8. Feng YL, Wang JL, Ning X, et al. BaTiO3@Au nanoheterostructure suppresses triple-negative breast cancer by persistently disrupting mitochondrial energy metabolism. Nano Res. 2023;16(2):2775–2785. doi:10.1007/s12274-022-4927-9
9. Ahamed M, Khan MAM. Enhanced photocatalytic and anticancer activity of Zn-Doped BaTiO3 Nanoparticles Prepared Through A Green Approach Using Banana Peel Extract. Catalysts. 2023;13(6):15. doi:10.3390/catal13060985
10. Tang QS, Sun SH, Wang P, et al. Genetically engineering cell membrane-coated BTO nanoparticles for MMP2-activated piezocatalysis-immunotherapy. Adv Mater. 2023;35(18):19.
11. Deng RX, Zhou H, Qin QX, et al. Palladium-catalyzed hydrogenation of black barium titanate for multienzyme-piezoelectric synergetic tumor therapy. Adv Mater. 2024;36(9):13.
12. Beak K, Choi M, Kim DH, et al. Silane-treated BaTiO3 ceramic powders for multilayer ceramic capacitor with enhanced dielectric properties. Chemosphere. 2022;286:6. doi:10.1016/j.chemosphere.2021.131734
13. Bell JG, Graule T, Stuer M. Barium titanate-based thermistors: past achievements, state of the art, and future perspectives. Appl Phys Rev. 2021;8(3):42. doi:10.1063/5.0048697
14. Abel S, Stöferle T, Marchiori C, et al. A strong electro-optically active lead-free ferroelectric integrated on silicon. Nat Commun. 2013;4:6. doi:10.1038/ncomms2695
15. Reynolds AJ, Conboy JC. Barium titanate nanoparticle based nonlinear optical humidity sensor. Sens Actuator B-Chem. 2018;273:921–926. doi:10.1016/j.snb.2018.07.004
16. Zhu P, Chen Y, Shi JL. Piezocatalytic tumor therapy by ultrasound-triggered and BaTiO3-mediated piezoelectricity. Adv Mater. 2020;32(29):8.
17. Sun LH, Wang P, Zhang JX, et al. Design and application of inorganic nanoparticles for sonodynamic cancer therapy. Biomater Sci. 2021;9(6):1945–1960. doi:10.1039/D0BM01875A
18. Huang YX, Ouyang WH, Lai ZJ, et al. Nanotechnology-enabled sonodynamic therapy against malignant tumors. Nanoscale Adv. 2024;6(8):1974–1991. doi:10.1039/D3NA00738C
19. Marino A, Arai S, Hou YY, et al. Piezoelectric nanoparticle-assisted wireless neuronal stimulation. Acs Nano. 2015;9(7):7678–7689. doi:10.1021/acsnano.5b03162
20. Marino A, Battaglini M, De Pasquale D, Degl’Innocenti A, Ciofani G. Ultrasound-activated piezoelectric nanoparticles inhibit proliferation of breast cancer cells. Sci Rep. 2018;8:13.
21. Jia L, Li X, Liu H, Xia JD, Shi XY, Shen MW. Ultrasound-enhanced precision tumor theranostics using cell membrane-coated and pH-responsive nanoclusters assembled from ultrasmall iron oxide nanoparticles. Nano Today. 2021;36:9. doi:10.1016/j.nantod.2020.101022
22. Xu F, Zhu JZ, Lin LZ, et al. Multifunctional PVCL nanogels with redox-responsiveness enable enhanced MR imaging and ultrasound-promoted tumor chemotherapy. Theranostics. 2020;10(10):4349–4358. doi:10.7150/thno.43402
23. Marino A, Almici E, Migliorin S, et al. Piezoelectric barium titanate nanostimulators for the treatment of glioblastoma multiforme. J Colloid Interface Sci. 2019;538:449–461.
24. Fakhar-E-Alam M, Saddique S, Hossain N, et al. Synthesis, characterization, and application of BaTiO3 Nanoparticles for anti-cancer activity. J Clust Sci. 2023;34(4):1745–1755. doi:10.1007/s10876-022-02346-y
25. Genchi GG, Marino A, Rocca A, Mattoli V, Ciofani G. Barium titanate nanoparticles: promising multitasking vectors in nanomedicine. Nanotechnology. 2016;27(23):19. doi:10.1088/0957-4484/27/23/232001
26. Sher F, Boskailo E, Smjecanin N, Nemtanu MR, Sher EK, Lima EC. Magnetic multifunctional nanomaterials for enhanced transverse chemical and bioanalytical applications - A review. Trac-Trends Anal Chem. 2024;173:19. doi:10.1016/j.trac.2024.117622
27. Xiang Z, Xu L, Shan Y, et al. Tumor microenviroment-responsive self-assembly of barium titanate nanoparticles with enhanced piezoelectric catalysis capabilities for efficient tumor therapy. Bioact Mater. 2024;33:251–261. doi:10.1016/j.bioactmat.2023.11.004
28. Taheri M, Zanca B, Dolgos M, Bryant S, Trudel S. Water-dispersible and ferroelectric PEGylated barium titanate nanoparticles††Electronic supplementary information (ESI) available: synthetic details, X-ray diffraction results, scanning electron micrographs, HRTEM of sample. Mater Adv. 2021;2(15):5089–5095. doi:10.1039/d1ma00317h
29. Jordan T, O’Brien MA, Spatarelu CP, Luke GP. Antibody-conjugated barium titanate nanoparticles for cell-specific targeting. ACS Appl Nano Mater. 2020;3(3):2636–2646. doi:10.1021/acsanm.0c00019
30. Huang RH, Sobol NB, Younes A, et al. Comparison of methods for surface modification of barium titanate nanoparticles for aqueous dispersibility: toward biomedical utilization of perovskite oxides. ACS Appl Mater Interfaces. 2020;12(46):51135–51147. doi:10.1021/acsami.0c10063
31. Hedayati M, Taheri-Nassaj E, Yourdkhani A, et al. BaTiO3 nanotubes by co-axial electrospinning: rheological and microstructural investigations. J Eur Ceram Soc. 2020;40(4):1269–1279. doi:10.1016/j.jeurceramsoc.2019.11.078
32. Zhao YC, Wang SB, Yao SC, et al. Piezoelectric Ba0.85Sr0.15TiO3 nanosonosensitizer with nitric oxide delivery for boosting cancer therapy. Small Methods. 2024;8(1):11.
33. Qi FW, Li HX, Gao XW, et al. Oxygen vacancy healing boosts the piezoelectricity of bone scaffolds. Biomater Sci. 2024;12(2):495–506. doi:10.1039/D3BM01283B
34. Yue Z, Zhao Q, Wang S, et al. Manganese dioxide coated piezoelectric nanosonosensitizer for cancer therapy with tumor microenvironment remodeling and multienzyme-like catalysis. Small Methods;2024. e2400018. doi:10.1002/smtd.202400018
35. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30(16):R921–R925. doi:10.1016/j.cub.2020.06.081
36. Zhu JW, Wang XR, Su Y, et al. Multifunctional nanolocks with GSH as the key for synergistic ferroptosis and anti-chemotherapeutic resistance. Biomaterials. 2022;288:12. doi:10.1016/j.biomaterials.2022.121704
37. Chen C, Yu DH, Wang WD, et al. Hyaluronic acid-covered piezoelectric nanocomposites as tumor microenvironment modulators for piezoelectric catalytic therapy of melanoma. Int J Biol Macromol. 2023;236:13.
38. Chen HX, Li Y, Li HF, et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature. 2024;631(8021)):27. doi:10.1038/s41586-024-07620-9
39. Tuomela K, Levings MK. Acidity promotes the differentiation of immunosuppressive regulatory T cells. Eur J Immunol. 2023;53(6):3. doi:10.1002/eji.202350511
40. Scheiman J, Luber JM, Chavkin TA, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25(7):1104–+. doi:10.1038/s41591-019-0485-4
41. Fan Y, Ye J, Kang Y, et al. Biomimetic piezoelectric nanomaterial-modified oral microrobots for targeted catalytic and immunotherapy of colorectal cancer. Sci Adv. 2024;10(19):eadm9561. doi:10.1126/sciadv.adm9561
42. Rojas C, Tedesco M, Massobrio P, et al. Acoustic stimulation can induce a selective neural network response mediated by piezoelectric nanoparticles. J Neural Eng. 2018;15(3):036016. doi:10.1088/1741-2552/aaa140
43. Zhan L, Xiao C, Li C, et al. Internal wireless electrical stimulation from piezoelectric barium titanate nanoparticles as a new strategy for the treatment of triple-negative breast cancer. ACS Appl Mater Interfaces. 2022;14(39):45032–45041. doi:10.1021/acsami.2c12668
44. Yang SY, Wang Y, Liang XL. Piezoelectric nanomaterials activated by ultrasound in disease treatment. Pharmaceutics. 2023;15(5):35. doi:10.3390/pharmaceutics15051338
45. Wang P, Tang QS, Zhang LL, et al. Ultrasmall barium titanate nanoparticles for highly efficient hypoxic tumor therapy via ultrasound triggered piezocatalysis and water splitting. Acs Nano. 2021;15(7):11326–11340.
46. Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Update. 2004;7(2):97–110. doi:10.1016/j.drup.2004.01.004
47. Endale HT, Tesfaye W, Mengstie TA. ROS induced lipid peroxidation and their role in ferroptosis. Front Cell Dev Biol. 2023;11:7. doi:10.3389/fcell.2023.1226044
48. Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482(3):419–425. doi:10.1016/j.bbrc.2016.10.086
49. Wang LH, Chen XW, Du ZY, et al. Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase γ depletion disrupting cellular bioenergetics. J Exp Clin Cancer Res. 2017;36:14. doi:10.1186/s13046-017-0513-5
50. Ahamed M, Akhtar MJ, Khan MAM, Alhadlaq HA, Alshamsan A. Barium titanate (BaTiO(3)) nanoparticles exert cytotoxicity through oxidative stress in human lung carcinoma (A549) cells. Nanomaterials. 2020;10(11):2309.
51. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363–383. doi:10.1038/s41580-020-0230-3
52. Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi:10.1016/j.biocel.2006.07.001
53. Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. doi:10.3109/10715761003667554
54. Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–591. doi:10.1038/nrd2803
55. Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27(2):156–157. doi:10.1016/j.ccell.2015.01.007
56. Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38(2):167–197. doi:10.1016/j.ccell.2020.06.001
57. Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10(3):241–252. doi:10.1016/j.ccr.2006.08.009
58. Azadi MDA, Hassanajili S, Zarrabi K, Sarkari B. Solidification of hydatid cyst fluid with an injectable chitosan/carboxymethylcellulose/β-glycerophosphate hydrogel for effective control of spillage during aspiration of hydatid cysts. Progress Biomater. 2018;7(1):35–54. doi:10.1007/s40204-018-0082-5
59. Li Y, Zhang QQ, Sun Z, Rong MJ, Jiang CH, Lu LH. Unexpected emergence of carbon-centered radicals from piezoelectric effect in oleic acid-capped BaTiO3. Acs Nano. 2024;18(13):9645–9655.
60. Guarente L. The resurgence of NAD (+) Restoring a mitochondrial metabolite slows stem cell loss and aging. Science. 2016;352(6292):1396–1397. doi:10.1126/science.aag1718
61. Li YM, Tian XT, Luo JY, Bao TT, Wang SJ, Wu X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun Signal. 2024;22(1):24. doi:10.1186/s12964-023-01364-1
62. Hao Z, Guo S, Tu W, et al. Piezoelectric catalysis induces tumor cell senescence to boost chemo-immunotherapy. Small. 2024;20(25):e2309487. doi:10.1002/smll.202309487
63. Zhou C, Zhao YH, Yang M, et al. Diselenide-containing polymer based on new antitumor mechanism as efficient GSH depletion agent for ferroptosis therapy. Adv Healthc Mater. 2024;13(17):11. doi:10.1002/adhm.202303896
64. Li K, Lin CC, Li MH, et al. Multienzyme-like reactivity cooperatively impairs glutathione peroxidase 4 and ferroptosis suppressor protein 1 pathways in triple-negative breast cancer for sensitized ferroptosis therapy. Acs Nano. 2022;16(2):2381–2398. doi:10.1021/acsnano.1c08664
65. Wang L, Zhang X, You Z, et al. A molybdenum disulfide nanozyme with charge-enhanced activity for ultrasound-mediated cascade-catalytic tumor ferroptosis. Angewandte Chemie. 2023;62(11):e202217448. doi:10.1002/anie.202217448
66. Jiang XJ, Wang J, Deng XY, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39(1):19. doi:10.1186/s13046-020-01709-5
67. Zhao ZX, Shuang T, Gao Y, et al. Targeted delivery of exosomal miR-484 reprograms tumor vasculature for chemotherapy sensitization. Cancer Lett. 2022;530:45–58. doi:10.1016/j.canlet.2022.01.011
68. Li C, Xiao C, Zhan L, et al. Wireless electrical stimulation at the nanoscale interface induces tumor vascular normalization. Bioact Mater. 2022;18:399–408. doi:10.1016/j.bioactmat.2022.03.027
69. Zhao YD, Shi DD, Guo L, et al. Ultrasound targeted microbubble destruction-triggered nitric oxide release via nanoscale ultrasound contrast agent for sensitizing chemoimmunotherapy. J Nanobiotechnol. 2023;21(1):17. doi:10.1186/s12951-023-01776-8
70. Chen ZG, Yang LZ, Yang ZM, Wang ZH, He W, Zhang W. Ultrasonic-responsive piezoelectric stimulation enhances sonodynamic therapy for HER2-positive breast cancer. J Nanobiotechnol. 2024;22(1):17. doi:10.1186/s12951-024-02639-6
71. Hamdy NM, Eskander G, Basalious EB. Insights on the dynamic innovative tumor targeted-nanoparticles-based drug delivery systems activation techniques. Int J Nanomed. 2022;17:6131–6155. doi:10.2147/IJN.S386037
72. Yang N, Li JM, Yu SJ, et al. Application of nanomaterial-based sonodynamic therapy in tumor therapy. Pharmaceutics. 2024;16(5):30. doi:10.3390/ph16010030
73. Pu XQ, Ju XJ, Zhang L, et al. Novel multifunctional stimuli-responsive nanoparticles for synergetic chemo-photothermal therapy of tumors. ACS Appl Mater Interfaces. 2021;13(24):28802–28817. doi:10.1021/acsami.1c05330
74. Xu Q, Chen G, Hu H, et al. Multifunctional nanoplatform based on tetragonal BaTiO(3)-Au@polydopamine for computed tomography imaging-guided photothermal synergistic and enhanced piezocatalytic cancer therapy. J Colloid Interface Sci. 2024;658:597–609. doi:10.1016/j.jcis.2023.12.055
75. Wang YS, Yang T, He QJ. Strategies for engineering advanced nanomedicines for gas therapy of cancer. Natl Sci Rev. 2020;7(9):1485–1512. doi:10.1093/nsr/nwaa034
76. Wang F, Zhang R, Xia T, et al. Inhibitory effects of nitric oxide on invasion of human cancer cells. Cancer Lett. 2007;257(2):274–282. doi:10.1016/j.canlet.2007.08.001
77. Chen J, Tang Q, Wang Y, et al. Ultrasound-induced piezocatalysis triggered NO generation for enhanced hypoxic tumor therapy. ACS Appl Mater Interfaces. 2023;15(12):15220–15234. doi:10.1021/acsami.3c00603
78. Gaynor N, Crown J, Collins DM. Immune checkpoint inhibitors: key trials and an emerging role in breast cancer. Semi Cancer Biol. 2022;79:44–57. doi:10.1016/j.semcancer.2020.06.016
79. Darvin P, Toor SM, Nair VS, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:11. doi:10.1038/s12276-018-0191-1
80. Feng WM, Xue T, Huang SX, et al. HIF-1α promotes the migration and invasion of hepatocellular carcinoma cells via the IL-8-NF-κB axis. Cell Mol Biol Lett. 2018;23:8. doi:10.1186/s11658-018-0077-1
81. Zhao Y, Wang S, Ding Y, et al. Piezotronic effect-augmented Cu(2-x)O-BaTiO(3) sonosensitizers for multifunctional cancer dynamic therapy. ACS Nano. 2022;16(6):9304–9316. doi:10.1021/acsnano.2c01968
82. Long GV, Swetter SM, Menzies AM, Gershenwald JE, Scolyer RA. Cutaneous melanoma. Lancet. 2023;402(10400):485–502. doi:10.1016/S0140-6736(23)00821-8
83. Wang Y, Tang QS, Wu RQ, et al. Metformin-mediated fast charge-reversal nanohybrid for deep penetration piezocatalysis-augmented chemodynamic immunotherapy of cancer. Acs Nano. 2024;18(8):6314–6332 doi:10.1021/acsnano.3c11174.
84. Zhu H, Deng JH, Yuan MJ, et al. Semiconducting titanate supported ruthenium clusterzymes for ultrasound-amplified biocatalytic tumor nanotherapies. Small. 2023;19(18):11 doi:10.1002/smll.202206911.
85. Wang Y, Tang Q, Wu R, et al. Ultrasound-triggered piezocatalysis for selectively controlled NO gas and chemodrug release to enhance drug penetration in pancreatic cancer. ACS Nano. 2023;17(4):3557–3573. doi:10.1021/acsnano.2c09948
86. Xiao C, Fan L, Zhou S, et al. One-dimensional ferroelectric nanoarrays with wireless switchable static and dynamic electrical stimulation for selective regulating osteogenesis and antiosteosarcoma. ACS Nano. 2022;16(12):20770–20785.
87. Kota J, Hancock J, Kwon J, Korc M. Pancreatic cancer: stroma and its current and emerging targeted therapies. Cancer Lett. 2017;391:38–49. doi:10.1016/j.canlet.2016.12.035
88. Baker H. Neoadjuvant therapy for pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1(3):180. doi:10.1016/S2468-1253(16)30123-6
89. Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:11. doi:10.3389/fonc.2019.00963
90. Yabo YA, Heiland DH. Understanding glioblastoma at the single-cell level: recent advances and future challenges. PLoS Biol. 2024;22(5):18. doi:10.1371/journal.pbio.3002640
91. Rabah N, Mohand FEA, Kravchenko-Balasha N. Understanding glioblastoma signaling, heterogeneity, invasiveness, and drug delivery barriers. Int J Mol Sci. 2023;24(18):24. doi:10.3390/ijms241814256
92. Lee JA, Lim J, Jin HY, et al. Osteosarcoma in adolescents and young adults. Cells. 2021;10(10):9. doi:10.3390/cells10102684
93. Hu ZG, Wen S, Huo ZJ, et al. Current status and prospects of targeted therapy for osteosarcoma. Cells. 2022;11(21):19. doi:10.3390/cells11213507
94. Oliveira TM, Berti FCB, Gasoto SC, Schneider B Jr, Stimamiglio MA, Berti LF. Calcium phosphate-based bioceramics in the treatment of osteosarcoma: drug delivery composites and magnetic hyperthermia agents. Front med techn. 2021;3:700266. doi:10.3389/fmedt.2021.700266
95. Xiao CR, Fan L, Zhou SQ, et al. One-dimensional ferroelectric nanoarrays with wireless switchable static and dynamic electrical stimulation for selective regulating osteogenesis and antiosteosarcoma. Acs Nano. 2022;16(12):20770–20785.
96. Du S, Zhou NY, Gao YJ, et al. Bioinspired hybrid patches with self-adhesive hydrogel and piezoelectric nanogenerator for promoting skin wound healing. Nano Res. 2020;13(9):2525–2533. doi:10.1007/s12274-020-2891-9
97. Murillo G, Blanquer A, Vargas-Estevez C, et al. Electromechanical nanogenerator-cell interaction modulates cell activity. Adv Mater. 2017;29(24):7. doi:10.1002/adma.201605048
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.