Back to Journals » International Journal of Nanomedicine » Volume 20
Chitosan Nanoparticles as an Alternative Therapeutic Approach for Knee Osteoarthritis Treatment: A Systematic Review
Authors Novy TCT , Joni IM, Lesmana R, Biben V, Setiawan
Received 30 October 2024
Accepted for publication 31 January 2025
Published 17 May 2025 Volume 2025:20 Pages 6187—6203
DOI https://doi.org/10.2147/IJN.S503829
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Theresia Chandra Tania Novy,1,* I Made Joni,2,3,* Ronny Lesmana,4 Vitriana Biben,5 Setiawan4
1Department of Biotechnology, Faculty of Graduate School, Universitas Padjadjaran, Bandung, Indonesia; 2Functional Nano Powder University Center of Excellence (Finder U-Coe), Universitas Padjadjaran, Bandung, Indonesia; 3Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Bandung, Indonesia; 4Physiology Division, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia; 5Department of Physical Medicine and Rehabilitation, Dr. Hasan Sadikin General Hospital Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
*These authors contributed equally to this work
Correspondence: Theresia Chandra Tania Novy, Department of Biotechnology, Faculty of Graduate School, Universitas Padjadjaran, Bandung, Indonesia, Email [email protected] I Made Joni, Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Bandung, Indonesia, Email [email protected]
Abstract: Osteoarthritis (OA) is a prevalent degenerative joint disease characterized by the progressive breakdown of cartilage, leading to pain, inflammation, and reduced joint function. There are many variations of conventional therapies that exist, however, none proven to halt or reverse cartilage degradation. Chitosan, a biocompatible and biodegradable polysaccharide, has emerged as a promising candidate in OA treatment due to its chondroprotective properties, and ability to enhance chondrocyte proliferation and suppress inflammatory mediators. Recent advancements in nanotechnology have led to the development of chitosan nanoparticles (NPs), which offer a novel and effective approach for addressing the limitations associated with standard chitosan formulations, such as poor solubility and limited tissue penetration. Chitosan NPs have demonstrated superior bioavailability, sustained drug release, and targeted delivery, leading to improved therapeutic outcomes in preclinical models. This review explores evidence-based the therapeutic potential of chitosan NPs in the management of knee osteoarthritis, focusing on their role in cartilage regeneration and drug delivery.
Keywords: chitosan NPs, cartilage regeneration, knee osteoarthritis, nanotechnology
Graphical Abstract:

Introduction
Osteoarthritis (OA) is the most common form of arthritis in adults, characterized by persistent pain and reduced mobility of the joints. It typically develops after age 40, with its incidence increasing sharply with age. In 2020, an estimated 595 million people worldwide (95% CI 535–656) were affected by osteoarthritis, representing 7.6% (95% CI 6.8–8.4) of the global population. Although the percentage seems small, OA is the seventh leading cause of years lived with disability.1 OA typically affects the joints such as knees, hands, hips, and spine.2 Projections suggest that by 2050, the number of osteoarthritis cases will rise by 74.9% (59.4–89.9) for the knee joint, 48.6% (35.9–67.1) for the hand, 78.6% (57.7–105.3) for the hip, and 95.1% (68.1–135.0) for other types.1
Cartilage degeneration in osteoarthritis is driven by both mechanical stress and inflammatory processes, leading to an imbalance between the breakdown and synthesis of extracellular matrix components3 The inflammatory process in OA is marked by elevated levels of pro-inflammatory cytokines like IL-1β and TNF-α. These cytokines trigger the production of molecules such as nitric oxide (NO) and prostaglandin E2 (PGE2), which result in pain and cartilage damage. They also increase the activity of enzymes that degrade cartilage and reduce the production of protective antioxidant enzymes, leading to further cartilage degeneration.3,4
The management of osteoarthritis includes non-pharmacological and pharmacological approaches, which focus on symptom management, slowing disease progression, and improving function. The choice of therapy depends on the severity of the disease, comorbidities, financial considerations, and patient preferences.5 Knee osteoarthritis, particularly in advanced stages, is challenging to manage completely without surgical intervention such as knee arthroplasty.6,7 The most recommended pharmacological treatment of OA is intraarticular injections, particularly with corticosteroids and hyaluronic acid. However, these agents have several limitations including the short duration of action and some adverse effects by reducing cartilage volume.8
The problems regarding the current treatments of OA indicate the need for a novel treatment of OA with better efficacy and minimal adverse effects, and a recent advancement in this matter is the use of chitosan. Chitosan is a natural copolymer of glucosamine and N-acetylglucosamine linked by β-1,4-glycosidic bonds which has been proven to have chondroprotective and anti-inflammatory effects in OA. Another strength that chitosan offers is its biocompatibility, progressive degradability, and non-toxicity, potentially causing very minimal side effects in humans.9
However, chitosan alone has a low water solubility, limiting its effects and penetration into the articular space.9 This problem can be overcome by several modifications through current advanced technology, including nanotechnology. With this technology, particles with large sizes can be modified into nano sizes, called nanoparticles.10 Chitosan nanoparticles (NPs) have better solubility and stability, enhancing their penetration into the intraarticular space before showing their therapeutic effects in OA. Chitosan NPs have also been used as a drug delivery system, which can control drug release, extend the duration of action, and enhance penetration into joint tissues.10–12 Thus, this systematic review aims to evaluate the advancements in osteoarthritis treatment utilizing chitosan NPs, focusing on their efficacy, mechanisms of action, and potential benefits.
Materials and Methods
This systematic review was undertaken about medical protocol and ethics, as well as Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Owing to the bibliographic nature of our study, the Ethics Review Board did not ask for ethical consent. This review has been registered on PROSPERO with registration number CRD42024566247 (Figure 1).
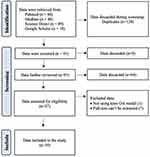 |
Figure 1 PRISMA Flow Diagram of the Systematic Review Process. Adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372. Creative Commons.13 |
Information Sources and Search Strategy
The following electronic databases will be searched independently for relevant material between 2000 and 2024 using predefined search engines such as PubMed; Cochrane Central Register of Controlled Trials (CENTRAL); ScienceDirect; ClinicalTrials.gov; MEDLINE Scopus Search Engine and limits applied to the databases included English language literature. Other studies that might be missed in the electronic database search will be identified using the following methods: reviewing the references of included studies; utilizing the related article functions on PubMed, MEDLINE, and other mentioned databases; checking websites for RCT registration to find recent trials or manuscripts available for publication on this topic; engaging in personal communications with authors of published trials to obtain additional data or clarification if needed.
Using Boolean search using keyword: (Chitosan OR Poly-(1,4-β-D-glucosamine) OR Deacetylated chitin OR Chitosan biopolymer OR Polyglucosamine OR Chitosan polymer) AND (Nanoparticle OR Nanocluster OR Nanocrystal OR Nanocapsule OR Nanomaterial OR Nano-sized particle) AND (Knee Osteoarthritis OR Osteoarthrosis of the knee OR Gonarthrosis OR Knee arthritis OR Arthritic knee OR Knee joint osteoarthropathy).
Study Risk of Bias Assessment
We utilized SYRCLE’s Risk of Bias Tool SYRCLE (Systematic Review Centre for Laboratory Animal Experimentation) assessment tools specific for pre-clinical animal studies, and Quality assessment of diagnostic accuracy studies-2 (QUADAS-2) in this study to assess bias in the included studies. The results of this assessment will be made explicit but will not affect data synthesis, as no quantitative data synthesis is planned. The risk of bias will be evaluated by one researcher.
Eligibility Criteria
The inclusion criteria for this review encompass studies that specifically examine the use of chitosan NPs in the treatment of OA. Eligible studies must involve human patients diagnosed with knee OA, knee OA animal models, or chondrocyte culture (in vitro study) along with detailed application of chitosan NPs as a therapeutic intervention. The outcomes measured in these studies should include radiologic evaluation, histopathological examination, or biochemical analysis. Additionally, studies that compare chitosan NPs with other knee OA treatments, such as traditional pharmacological therapies, physical therapy, or surgical options, will be included.
Conversely, the exclusion criteria will rule out studies not centered on osteoarthritis or those not involving chitosan or nanoparticles as a treatment. Studies that use chitosan or chitosan NPs in combination with other treatments without clearly distinguishing their effects, as well as studies that do not provide specific outcome measures related to osteoarthritis improvement or relevant clinical or biological markers, will not be considered.
Selection and Data Collection Process
Literature screening will be conducted independently by three reviewers, selecting studies according to the established search criteria. Data extraction will be a collaborative effort by a team of authors, gathering information on baseline characteristics. Any disagreements over study inclusion will be resolved through discussions among two additional authors.
Synthesis Methods
The synthesis method for this systematic review focuses on evaluating the efficacy of various chitosan-based nanoparticles (NPs) in modulating critical cellular and molecular mechanisms implicated in osteoarthritis (OA), specifically within in vitro and in vivo studies. Synthesis method is the preparation and a systematic approach to create something new by using several processes, elements and compounds. The nanoparticles studied incorporate diverse active compounds with varying sizes and formulations. Additionally, the synthesis explores the correlation between nanoparticle size and biological efficacy, along with an evaluation of the potential of chitosan-based NPs as a viable therapeutic option for OA based on radiographic evaluation, histopathological examination, and biochemical analysis.
The initial search and study selection were carried out by TCT. Two investigators (RL, S, and IMJ) subsequently reviewed the full-text articles for eligibility. Any discrepancies were resolved through discussion with all authors for the final selection. Data extraction for the included studies was conducted independently by VB. The authors collected and summarized study characteristics in Tables 1 and 2 which include author, publication year, country, study design, subjects, nanoparticle characteristics, and outcomes measured through radiographic evaluation, histopathological examination, and biochemical analysis.
 |
Table 1 Chitosan NPs in Knee Osteoarthritis in Vitro |
 |
Table 2 Chitosan NPs in Knee Osteoarthritis in Vivo |
Knee Osteoarthritis
The pathophysiology of OA. Bone erosion and cartilage damage are caused by inflammation and the cascade of proinflammatory cytokines, including IL-1β, TNF-α, and IL-6. These cytokines contribute through various mechanisms, such as the downregulation of anabolic processes, the upregulation of catabolic and inflammatory responses, and the decreased level of Type II collage which leads to structural damage in OA-affected joints. IL (interleukin), MMP (matrix metalloproteinase), and TNF (tumor necrosis factor) all play key roles in this process (Figure 2).
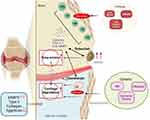 |
Figure 2 Pathophysiology and Risk Factors of Osteoarthritis. Created with BioRender.com. Chandra Tania Novy, T. (2025) https://BioRender.com/fl4jozc (Agreement Number: XR288ATXM5). Abbreviations: IL, interleukin; MMP, matrix metalloproteinase; TNF, tumor necrosis factor. |
Osteoarthritis (OA) is the 18th leading cause of disability worldwide.27 OA is characterized by anatomical and physiological abnormalities, such as cartilage degradation, bone remodeling, osteophyte formation, joint inflammation, joint pain, and loss of normal joint function, leading to impaired movement and a reduction in the patient’s physical and mental quality of life.28 Several factors can increase the risk of developing OA, such as genetics (age, hormonal factors, and ethnicity), and lifestyle (such as joint injuries, obesity, and high-impact activities29 as shown in Figure 2. There is a significant correlation between cartilage damage and inflammation in knee OA. The process of cartilage degeneration in knee OA involves the entire joint structure, including cartilage, subchondral bone, ligaments, meniscus, joint capsule, and synovial membrane, where changes in one structure trigger damage in others. Cartilage cells, particularly chondrocytes, produce catabolic factors such as cytokines (IL-6, IL-8), chemokines (RANTES, IP-10), metalloproteases (MMP1, MMP3), and heat-shock proteins (HSPA1A), which serve as biomarkers for the onset and progression of knee OA.30 Inflammation in the synovium and cartilage is also influenced by key proinflammatory cytokines, such as IL-1β, TNF-α, and IL-6, which contribute to cartilage damage and the inflammatory cascade. OA patients exhibit elevated levels of these cytokines in synovial fluid, synovial membrane, subchondral bone, and cartilage.4 This inflammation is often associated with increased pain and reduced joint function.31 Inflammation can exacerbate cartilage damage, creating a cycle where cartilage loss leads to more inflammation, which in turn accelerates cartilage degradation [18]. Thus, understanding this correlation is essential for developing treatments that target both cartilage preservation and inflammation control to manage knee OA effectively.
Management of Knee OA
The complex pathogenesis of knee OA makes the management of this disease challenging. In 2019, OARSI and ESCEO guidelines were introduced for the management of knee OA. OARSI and ESCEO both recommended patient education, structured exercise, and weight loss as core treatments, topical NSAIDs as first-line treatments, and oral NSAIDs and intra-articular injections for persistent pain. ESCEO recommended oral pharmaceutical-grade glucosamine and chondroitin sulfate supplements while OARSI strongly recommends against their use (including all glucosamine and chondroitin formulations).32 Despite this difference, the two guidelines are consistent in the majority of their recommendations and provide useful treatment recommendations for individuals with OA and healthcare providers.
Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee, Recommendation from the American College of Rheumatology (ACR), and American College of Rheumatology/Arthritis (ACR/A) gives several levels of recommendation for OA, such as strongly recommended, and conditionally recommended in the year of 2019. They strongly recommended therapy for knee OA including exercise, weight loss, tai chi, the use of a cane, tibiofemoral knee bracing, topical NSAIDs, oral NSAIDs, and Intraarticular glucocorticoid injection.7 In 2022, clinical practice guideline was introduced by the American Academy of Orthopaedics Surgeon (AAOS). AAOS provides guidelines for the management of knee OA without arthroplasty. AAOS gave recommendations for ton-pharmacology recommendations such as weight loss, supervised exercise, aquatic exercise, neuromuscular training, and patient education to improve the pain of knee OA. Meanwhile, in pharmacological management, AAOS recommends the routine use of oral NSAIDs, oral acetaminophen, Intra-articular corticosteroids injection, and intraarticular hyaluronic acid injection even though not for routine use.33
Based on these guidelines, we can conclude that the management of knee OA pain is complex and sometimes needs approach from different aspects. There are a lot of similarities between the guidelines, such as the recommendations for pharmacological therapies for osteoarthritis which include oral medications and injectable treatments. Oral medications generally have a slower onset of action due to systemic drug distribution. Oral medication such as acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids are frequently prescribed each offering different levels of pain relief. However, these treatments are accompanied by significant side effects and inconsistent clinical efficacy.34 NSAIDs, such as diclofenac and ibuprofen, are usually prescribed for short-term relief, while opioids like morphine and oxycodone are usually prescribed for more severe pain, with the risks of opioid dependency and other adverse effects.35 For patients who do not respond to oral treatments, viscosupplementation with hyaluronic acid (HA) injections is an option, though HA’s effect is often short-lived in the joint cavity.34,36,37
The limitations of these pharmacological options have led to the increasing use of intra-articular (IA) injections of corticosteroids (CS) and hyaluronic acid (HA), which offer localized and effective short-term relief.38 Intra-articular corticosteroid injections, particularly with triamcinolone acetonide, are frequently used to manage knee osteoarthritis pain, providing rapid pain relief and aligning with clinical guidelines.8,39 However, repeated and long-term use of corticosteroids may lead to reduced cartilage thickness and other side effects.8,40 This highlights the need for innovative treatments that are both effective and have minimal adverse effects on cartilage.
Recent research has highlighted the potential of chitosan as a novel treatment due to its potent anti-inflammatory properties and ability to inhibit cartilage degradation, indicating a promising avenue for further exploration in knee OA management.41
Chitosan NPs
Research on cartilage regeneration has found that chitosan, a natural copolymer of glucosamine and N-acetylglucosamine linked by β-1,4-glycosidic bonds, has demonstrated chondroprotective functions in animal models by increasing chondrocyte proliferation, increasing expression of cartilage matrix components, and inflammation suppresses and catabolic mediators. Chitosan has been proven to inhibit cartilage destruction and synovial membrane inflammation.9 It has been used in OA management as a therapeutic agent, drug carrier, and viscosupplementation.
Chitosan and its derivatives exhibit significant anti-inflammatory properties, influenced by structural elements rather than molecular weight (MW). Studies demonstrate their ability to modulate inflammatory and anti-inflammatory cytokines, such as increasing IL-10 and TGF-β1 while reducing TNF-α, IL-1β, and IL-6. Chitosan-alginate nanoparticles show dose-dependent inhibition of cytokines induced by P. acnes and high specificity for drug delivery. Low molecular weight chitosan oligosaccharides (COS) have potent anti-inflammatory effects, such as suppressing NF-κB and AP-1 activation, reducing nitric oxide production in macrophages, and alleviating allergic and osteoarthritis-induced inflammation. These findings highlight chitosan’s potential as a therapeutic agent for managing inflammation.42
The commercial use of chitosan in humans has been documented in a study by Emans et al, who demonstrated that a single intra-articular injection of KiOmedine®CM-Chitosan significantly reduced pain and improved joint function in patients with symptomatic knee OA, and can be sustained for up to 26 weeks.43 In another study by Lynen et al, the use of Carboxymethyl-chitosan (CM-chitosan) has been shown to significantly reduce pain and improve function in patients with knee osteoarthritis, with effects lasting up to 9 months following a single intra-articular injection.44 However, other than its advantages, a known limitation of chitosan is its limited solubility and cellular absorption, which can affect its penetration into human tissue.10 Advanced nanotechnology has been utilized to overcome this problem. Chitosan NPs alone have been used for their anti-inflammatory properties and ability to inhibit cartilage degradation of OA. On the other hand, chitosan NPs have also been used as nano-drug delivery systems in the formulation of pharmaceutical technology. Nanoparticle-based intra-articular delivery systems are particularly advantageous as they reduce drug distribution to reticuloendothelial organs and significantly increase drug half-lives, often tenfold compared to free drug formulations.45 Chitosan NPs are promising in drug delivery systems because of their significant advantages in the stabilization and controlled release of encapsulated drugs. These nanoparticles can extend drug retention time at the target site while minimizing toxicity and increasing therapeutic outcomes. Chitosan NPs can actively or passively target specific locations, which will improve drug efficacy and reduce the likelihood of adverse effects.10 Furthermore, chitosan NPs can be controlled to release their therapeutic payload in response to pathological conditions such as elevated reactive oxygen species (ROS), acidic pH, and the presence of overexpressed enzymes commonly associated with osteoarthritis (OA) and rheumatoid arthritis (RA).46
The capabilities of chitosan NPs depend on several factors, such as nanoparticle type and particle size. Polymeric nanoparticles (PNPs) are solid, biodegradable particles usually in size from 10 to 1000 nm and composed of natural or synthetic polymers such as chitosan. Polymeric NPs are particularly advantageous in prolonging drug circulation time and improving drug stability, and enhancing therapeutic efficacy while reducing systemic toxicity. Liposomes are spherical vesicles composed of one or more phospholipid bilayers surrounding an aqueous core. They can encapsulate both hydrophilic and hydrophobic drugs, offering a high flexibility for drug delivery. Micelles are structures formed of amphiphilic molecules in aqueous solutions, with a hydrophobic core and a hydrophilic shell. Micelles are primarily used to solubilize hydrophobic drugs, improving their bioavailability and enabling targeted delivery to desired sites of the disease. Exosomes are naturally occurring nanovesicles, secreted by various cell types, and contain a cargo of proteins, lipids, and nucleic acids. They play a crucial role in intercellular communication by transferring these bioactive molecules between cells. Exosomes can cross biological barriers and deliver therapeutic agents to specific tissues. Inorganic nanoparticles are known for their enzyme-like activities, particularly in scavenging reactive oxygen species (ROS). Dendrimers are highly branched, tree-like macromolecules that can be engineered to carry multiple therapeutic agents. Dendrimers are structured with a hydrophobic core and hydrophilic surface which allows for the encapsulation of both hydrophobic and hydrophilic drugs.43 These types of chitosan NPs are shown in Figure 3.
 |
Figure 3 Types of Chitosan Nanoparticle. Created with Biorender.com. Chandra Tania Novy, T. (2025) https:// BioRender.com/ksf2vh2 (Agreement Number: WK288AU6M4). Abbreviation: NPs, nanoparticles. |
The size of nanoparticles also affects cellular absorption and drug biodistribution. The interaction of nanoparticles with the avascular cartilage extracellular matrix (ECM), which varies in thickness and density across different zones, is heavily influenced by the particle size.10,47 Smaller nanoparticles, in the range of 50–1000 nm, have higher cell absorption efficacy compared to larger ones. Nanoparticles with a size of at least 50–60 nm can enter the superficial layers, while those smaller than 15 nm can penetrate the deepest intra-articular layers.10,47
Results
During the primary literature search using the Boolean method, we retrieved data from PubMed (n=80), Medline (n=40), ScienceDirect (n=89), and Google Scholar (n=10). After removing 128 duplicates, we screened a total of 91 articles. Additionally, 54 articles were discarded for not focusing on osteoarthritis, not involving chitosan or nanoparticles, including other conditions, lacking confirmed osteoarthritis diagnosis, not distinguishing treatment effects, or not providing specific outcome measures or comparative analysis. The data extraction and synthesis process yielded 14 articles for review. After thorough evaluation, we excluded 2 articles that did not use the knee OA model and full-text were unavailable to be extracted, resulting in a total of 12 studies included in the final analysis.
Tables 1 and 2 provides an overview of studies investigating the use of nanoparticles in treating osteoarthritis (OA) across both correspondingly in vitro and in vivo models, highlighting various nanoparticle types and their effects on bone and cartilage.
In vitro Studies
Table 1 presents various in vitro studies that explore the use of chitosan-based nanoparticles (PNPs) in treating osteoarthritis (OA). These studies consistently highlight the beneficial effects of chitosan NPs on cartilage protection and cell proliferation. For example, Wang et al demonstrated the ability of hyaluronic acid/chitosan NPs to promote chondrocyte proliferation while also enhancing collagen II expression and decreasing levels of matrix metalloproteinases (MMPs), particularly MMP-1 and MMP-13, both of which play a significant role in cartilage degradation.14 Similarly, Valentino et al showed that hydroxytyrosol-chitosan NPs offered antioxidative protection to chondrocytes by reducing inflammatory cytokine levels, such as TNF-α and IL-6, while boosting SOD2 activity, further contributing to matrix protection.16
The anti-inflammatory properties of chitosan NPs were a recurrent theme across several studies. Nabizadeh et al, for instance, reported that celecoxib-loaded chitosan NPs effectively reduced COX-2 and TNF-α expression, suggesting their potential as an anti-inflammatory agent in human chondrocyte OA models.15 Similarly, Li et al found that chitosan-fucoidan nanogels significantly lowered IL-6 and TNF-α levels while preserving >96% chondrocyte viability, further supporting their role in mitigating inflammation in OA.19
In addition to their anti-inflammatory effects, chitosan NPs demonstrated the ability to preserve the chondrogenic phenotype, essential for cartilage repair. This is proven by the increased expression of key chondrogenic markers, such as COL2A1, ACAN, and Sox9, in studies reported by Nabizadeh et al and Li et al.15,19 These markers are critical for maintaining cartilage structure and function, indicating that chitosan NPs could help regenerate damaged cartilage. Furthermore, Salama et al showed that etoricoxib-loaded chitosan NPs not only enhanced cell viability but also significantly increased alkaline phosphatase (ALP) levels, an early indicator of bone mineralization, suggesting that these NPs may also support osteogenesis.20
Overall, the in vitro findings suggest that chitosan NPs have a multifaceted role in OA treatment, offering cartilage protection, reducing inflammation, and supporting both chondrocyte proliferation and matrix preservation. This highlights their potential as a therapeutic tool for OA management.
In vivo Studies
Table 2 details the in vivo effects of chitosan NPs in animal models of osteoarthritis, showcasing their ability to protect cartilage, preserve bone structure, and reduce inflammatory responses. Multiple studies demonstrated that chitosan NPs can effectively regenerate cartilage and preserve joint integrity. For instance, Kang et al and Zhou et al reported significant cartilage regeneration and reduced cartilage degradation scores (OARSI scores) in rat OA models treated with chitosan NPs.21,22 Notably, Kang et al also highlighted the extended retention time of the nanoparticles in the joint—up to 24 days—allowing for effective treatment at lower doses [44]. This prolonged retention further contributed to better outcomes, with lesser cartilage loss and matrix damage observed.
In terms of bone preservation, Ramírez-Noguera et al and Wang et al show that chitosan NPs significantly improve subchondral bone health.11,14 Ramírez-Noguera et al found that chitosan NPs preserved bone structure, reduced matrix loss, and provided better protection against OA-induced damage.11 Similarly, Wang et al demonstrated that nanohydroxyapatite-chitosan micro-scaffolds improved bone volume, increased trabecular regeneration, and preserved cartilage integrity in rabbit OA models.14 These findings are critical as they suggest that chitosan NPs can contribute to both cartilage and bone tissue regeneration, which is essential for managing OA progression.
The anti-inflammatory effects of chitosan NPs are consistently observed across the studies. Zhou et al reported that hyaluronic acid-chitosan NPs containing CrmA plasmid DNA significantly inhibited the expression of pro-inflammatory mediators such as IL-1β, MMP-3, and MMP-13, which are central to cartilage degradation.12 Additionally, Gao et al found that chitosan NPs conjugated with superoxide dismutase (SOD) suppressed the production of pro-inflammatory cytokines like TNF-α, IL-6, and IL-1β, further emphasizing their anti-inflammatory potential.24 These findings are supported by Mohamed et al, who reported reductions in NO and MDA levels, along with a 74% decrease in COX-2 and a 50% decrease in TNF-α expression, further demonstrating the NPs’ ability to mitigate inflammation and oxidative stress.26
Discussion
Chitosan NPs have demonstrated significant potential as a treatment for knee osteoarthritis, with both in vitro and in vivo studies supporting their effectiveness.
In vitro Studies
The in vitro studies included in this review provide compelling evidence for the potential role of chitosan-based nanoparticles as therapeutic agents in osteoarthritis (OA). Several key findings consistently emerge across various experiments, underlining the versatile benefits these nanoparticles offer in the treatment of OA, particularly in protecting cartilage, reducing inflammation, and promoting chondrocyte proliferation and matrix preservation.
The in vitro studies used several markers to depict the effectiveness of chitosan NPs for cartilage protection and cell proliferation in OA. Wang et al demonstrated that hyaluronic acid/chitosan NPs not only promoted chondrocyte proliferation but also enhanced the expression of collagen II, a critical component of the extracellular matrix (ECM) in cartilage.14 This matrix-preserving property is particularly important given that OA is characterized by the degradation of cartilage, leading to joint pain and immobility.48 The suppression of matrix metalloproteinases (MMPs), particularly MMP-1 and MMP-13, further enhances the protective effect, as these enzymes are key players in cartilage breakdown. The reduction in MMP levels seen in these studies indicates that chitosan NPs can directly counteract cartilage degradation, which is crucial for preventing the progression of OA.12
As inflammation is another hallmark of OA pathophysiology, these studies also used inflammatory markers to highlight the use of chitosan NPs in OA, such as TNF-α and IL-6.4 Valentino et al demonstrated how hydroxytyrosol-chitosan NPs reduced inflammatory cytokines such as TNF-α and IL-6, while also increasing the activity of superoxide dismutase 2 (SOD2), an antioxidant enzyme.16 This combination of anti-inflammatory and antioxidative effects highlights the dual-action capability of chitosan NPs, which can address two critical aspects of OA pathology: inflammation and oxidative stress. Similarly, Nabizadeh et al showed that celecoxib-loaded chitosan NPs decreased the expression of COX-2 and TNF-α, further supporting their role as effective anti-inflammatory agents.15 Given that inflammation contributes to cartilage destruction and pain in OA, the anti-inflammatory action of these nanoparticles is highly relevant for clinical applications.4
Beyond their anti-inflammatory and antioxidative effects, chitosan NPs also contribute to maintaining the chondrogenic phenotype, which is essential for cartilage repair and regeneration. The studies by Nabizadeh et al and Li et al demonstrated the increased expression of key chondrogenic markers such as COL2A1, ACAN, and Sox9. These markers are critical for maintaining the structural integrity of cartilage, suggesting that chitosan NPs could play a role in cartilage regeneration—a highly desirable outcome in OA treatment.15,19 Additionally, Salama et al found that etoricoxib-loaded chitosan NPs enhanced alkaline phosphatase (ALP) levels, suggesting a potential role in osteogenesis.20 This finding could be particularly relevant in late-stage OA, where subchondral bone changes become prominent.30
In vivo Studies
The application of chitosan NPs in several in vivo studies has even proven their usage in OA even better. These studies provide further evidence of the nanoparticles’ ability to protect cartilage, enhance bone regeneration, and reduce inflammation, thereby offering a comprehensive approach to OA management. The in vivo findings build on the promising in vitro results and suggest that chitosan NPs could play a significant role in improving joint health and slowing OA progression.
One of the most notable advantages of chitosan NPs is their ability to promote cartilage regeneration and reduce degradation. The findings were assessed with OARSI score, which showed the severity of OA in the articular cartilage, resulting in a score of 0 to 6. A higher OARSI score means worse destruction of articular cartilage.49 Studies by Kang et al and Zhou et al demonstrated significant improvements in cartilage structure, as reflected in reduced OARSI scores, suggesting that these nanoparticles can effectively counteract the degenerative processes that define OA.21,22
Another advantage of chitosan NPs is the longer intraarticular retention time. In particular, Kang et al’s observation of the prolonged retention of chitosan NPs within the joint (up to 24 days) is a crucial finding. The extended residence time allows for more sustained therapeutic action, potentially reducing the frequency of treatments and the overall dosage required, thus minimizing potential side effects. This prolonged presence of the nanoparticles within the joint not only enhances cartilage protection but also reduces matrix damage, further preserving joint integrity.10
The ability of chitosan NPs to preserve and regenerate subchondral bone, as demonstrated by Ramírez-Noguera et al and Wang et a, is another key benefit.11,14 Subchondral bone changes are commonly observed in advanced stages of OA, and their preservation is essential for maintaining joint function and slowing disease progression.30 The studies reported that chitosan NPs significantly improved bone volume and trabecular structure, highlighting their role in bone health. Wang et al’s findings on the use of nanohydroxyapatite-chitosan micro-scaffolds for improving bone regeneration are particularly promising, as they suggest that chitosan NPs can support the repair of both cartilage and bone tissue. This dual action is critical because OA is not just a disease of cartilage; the underlying bone structure also plays a vital role in maintaining joint health. By addressing both aspects, chitosan NPs could offer a more holistic treatment approach compared to therapies that target only cartilage degradation.
The anti-inflammatory properties of chitosan NPs are also well-documented in these in vivo studies, with multiple models demonstrating significant reductions in pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β. Zhou et al and Gao et al both demonstrated that chitosan NPs not only reduced the levels of these cytokines but also suppressed matrix metalloproteinases (MMPs), including MMP-3 and MMP-13, which are directly responsible for cartilage breakdown.48 The inclusion of CrmA plasmid DNA and conjugation with superoxide dismutase (SOD) in these studies further enhanced the anti-inflammatory effects, pointing to the versatility of chitosan NPs as delivery vehicles for additional therapeutic agents. The reduction in oxidative stress, as indicated by lower levels of nitric oxide (NO) and malondialdehyde (MDA), further supports the nanoparticles’ ability to mitigate inflammatory damage, which is a key driver of OA progression.4
The findings from Mohamed et al, which showed substantial decreases in COX-2 and TNF-α expression, reinforce the anti-inflammatory potential of chitosan NPs. These reductions, along with the noted decrease in oxidative stress markers, suggest that chitosan NPs could play a role in both acute and chronic inflammatory responses in OA. This is particularly relevant for long-term OA management, where controlling inflammation is crucial for preventing further joint damage and improving patient outcomes.4
Chitosan NPs offer a multifaceted approach to OA treatment through their roles in cartilage protection, anti-inflammatory effects, enhanced cell proliferation, and antioxidant properties. Their potential is underscored by their ability to address several critical aspects of OA pathology. Further clinical research and development are warranted to explore their full potential and integrate them into effective OA management strategies.
Limitations of Chitosan NPs
Despite many developments in the applications of chitosan NPs in OA treatment in vitro and in vivo, however, several challenges must be overcome before further application. Some studies included in this review addressed the limitations of the current formulation of chitosan NPs. One significant issue is the inconsistency in size recommendations for intra-articular (IA) injections. Studies have not reached a consensus on the optimal size for drug formulations, with recommendations ranging from 51–85 μm to smaller than 60 nm.15 In this review, we also could not conclude the most ideal size with the best effects for knee OA. Additionally, studies have shown variability in the degradation rate and swelling behavior of chitosan NPs. Fattahpour et al observed degradation rates ranging from 28% to 42%,17 while Abpeikar et al found that the swelling ratios of nanoparticles could reach as high as 700% at 96 hours,25 affecting their stability over time. Furthermore, the retention time of chitosan NPs in joint cavities is still limited, lasting only up to 24 days, as reported by Kang et al.21 This short retention period necessitates frequent, repetitive injections, typically on a monthly basis, which may limit their practicality for long-term treatment.
Authors Prospective and Future Directions
Future research on chitosan NPs for knee OA should prioritize creating formulations that address current limitations in size, retention time, and stability. A crucial focus is determining the optimal nanoparticle size for intra-articular injections, as smaller particles might offer better penetration and efficacy, but further validation is needed. To extend retention time beyond the current 24 days, adjustments to the formulation are necessary to reduce the need for frequent, monthly injections. Moreover, enhancing drug stability by preventing rapid degradation and swelling is critical for sustained therapeutic effects.
These challenges could be overcome by improving the water solubility of chitosan NPs. One promising approach involves modifying chitosan NPs with oligosaccharides, which could enhance solubility. Chitosan oligosaccharide (COS) has a low molecular weight of 1 point 5 kDa, which causes its easy absorption through cells at physiological pH. The pH range from neutral to slightly alkaline is completely soluble for all heterogeneous COS with a degree of polymerization <10 and a degree of deacetylation (DD) between 50–100%. In contrast, commercially available chitosan with a higher DP precipitates out at such pH values. COS has intriguing biological qualities and is more soluble than the corresponding chitosan over a wider pH range and at comparatively higher concentrations. Neuronal, embryonic, and bone marrow-derived stem cells are among the many types of cells that can proliferate in COS, making it more conducive for cell growth than chitosan.50 Being the therapeutic agent itself, COS has shown its effects in suppressing synovial inflammation via AMPK activation in both rabbit and human synoviocytes.51 Another application of COS that has been studied is packaged COS in extracellular vesicles, which promotes the viability and migration of chondrocytes, suppresses cell apoptosis, and regulates chondroprotective pathways.52 These advancements in COS application in OA could lead to more consistent, durable, and effective treatments for knee OA.
Conclusion
This systematic review evaluated the advancements in osteoarthritis (OA) treatment using chitosan and chitosan NPs. The key findings indicate that chitosan NPs in vitro and in vivo studies shows that effectively promote cartilage regeneration, reduce inflammatory markers, and enhance drug retention within the joint. The stability of encapsulated drugs and the controlled release in response to OA’s pathological environment further highlight the therapeutic potential of chitosan NPs. Additionally, their biocompatibility and capacity for modification to improve targeting make chitosan NPs a versatile and promising tool in OA treatment.
The clinical relevance of using chitosan NPs for knee OA treatment lies in their ability to offer a more targeted and sustained therapeutic delivery. By enhancing drug retention in the joint and reducing systemic exposure, chitosan NPs can improve the efficacy of OA treatments while minimizing side effects. These nanoparticles also hold the potential to slow disease progression by promoting cartilage regeneration and reducing inflammation directly at the site of injury. Despite the challenges associated with their use, such as poor water solubility, potential toxicity, and variability in preparation methods, the advancements in chitosan nanoparticle technology represent a significant step forward in the management of knee OA.
One promising solution lies in improving the water solubility of chitosan NPs. Chitosan oligosaccharide (COS) is particularly advantageous due to its better solubility across a broader pH range and its ability to promote cell proliferation, making it more effective for therapeutic applications. It has shown potential in suppressing synovial inflammation and protecting chondrocytes, which are key to managing OA. Future COS-based formulations could provide more durable and effective treatments for knee OA.
Acknowledgment
Graphical abstract Created with BioRender.com. Chandra Tania Novy, T. (2025) https://BioRender.com/zvnqejw (Agreement Number: CL288AUDFP)
Funding
The authors would like to thank the Rector of Universitas Padjadjaran, via the Directorate of Research and Community Engagement for APC funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Steinmetz JD, Culbreth GT, Haile LM, et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the global burden of disease study 2021. Lancet Rheumatol. 2023;5(9):e508–e522. doi:10.1016/S2665-9913(23)00163-7
2. Xia B, Chen D, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95(6):495–505. doi:10.1007/s00223-014-9917-9
3. Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16(1). doi:10.1186/s13075-014-0470-8
4. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi:10.1038/nrrheum.2010.196
5. Grässel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Res. 2020;9:325. doi:10.12688/f1000research.22115.1
6. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29–30. doi:10.1016/j.eclinm.2020.100587
7. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–233. doi:10.1002/art.41142
8. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9(8). doi:10.1177/23259671211030272
9. Mou D, Yu Q, Zhang J, et al. Intra-articular injection of chitosan-based supramolecular hydrogel for osteoarthritis treatment. Tissue Eng Regen Med. 2021;18(1):113–125. doi:10.1007/s13770-020-00322-z
10. Wen J, Li H, Dai H, et al. Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater Today Bio. 2023;19:100597. doi:10.1016/j.mtbio.2023.100597
11. Ramírez-Noguera P, Zetina Marín I, Gómez Chavarin BM, Valderrama ME, López-Barrera LD, Díaz-Torres R. Study of the early effects of chitosan nanoparticles with glutathione in rats with osteoarthrosis. Pharmaceutics. 2023;15(8):2172. doi:10.3390/pharmaceutics15082172
12. Zhou PH, Qiu B, Deng RH, Li HJ, Xu XF, Shang XF. Chondroprotective effects of hyaluronic acid-chitosan nanoparticles containing plasmid DNA encoding cytokine response modifier A in a rat knee osteoarthritis model. Cell Physiol Biochem. 2018;47(3):1207–1216. doi:10.1159/000490217
13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;n71. doi:10.1136/bmj.n71
14. Wang J, Wang X, Cao Y, Huang T, Song D, Tao H. Therapeutic potential of hyaluronic acid/chitosan nanoparticles for the delivery of curcuminoid in knee osteoarthritis and an in vitro evaluation in chondrocytes. Int J Mol Med. 2018. doi:10.3892/ijmm.2018.3817
15. Nabizadeh Z, Nasrollahzadeh M, Kruppke B, Nasrabadi D. A combination of chitosan nanoparticles loaded with celecoxib and kartogenin has anti-inflammatory and chondroprotective effects: results from an in vitro model of osteoarthritis. Heliyon. 2024;10(10):e31058. doi:10.1016/j.heliyon.2024.e31058
16. Valentino A, Conte R, De Luca I, et al. Thermo-responsive gel containing hydroxytyrosol-chitosan nanoparticles (Hyt@tgel) counteracts the increase of osteoarthritis biomarkers in human chondrocytes. Antioxidants. 2022;11(6):1210. doi:10.3390/antiox11061210
17. Fattahpour S, Shamanian M, Tavakoli N, et al. An injectable carboxymethyl chitosan-methylcellulose-pluronic hydrogel for the encapsulation of meloxicam loaded nanoparticles. Int J Biol Macromol. 2020;151:220–229. doi:10.1016/j.ijbiomac.2020.02.002
18. Zeng J, Sun P, Zhao Y, Fang X, Wu Z, Qi X. Bone mesenchymal stem cell-derived exosomes involved co-delivery and synergism effect with icariin via mussel-inspired multifunctional hydrogel for cartilage protection. Asian J Pharm Sci. 2023;18(3):100799. doi:10.1016/j.ajps.2023.100799
19. Li T, Yang J, Weng C, et al. Intra-articular injection of anti-inflammatory peptide-loaded glycol chitosan/fucoidan nanogels to inhibit inflammation and attenuate osteoarthritis progression. Int J Biol Macromol. 2021;170:469–478. doi:10.1016/j.ijbiomac.2020.12.158
20. Salama AH, Abdelkhalek AA, Elkasabgy NA. Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int J Pharm. 2020;578:119081. doi:10.1016/j.ijpharm.2020.119081
21. Kang ML, Ko JY, Kim JE, Im GI. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35(37):9984–9994. doi:10.1016/j.biomaterials.2014.08.042
22. Zhou Y, Liu SQ, Peng H, Yu L, He B, Zhao Q. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int Immunopharmacol. 2015;28(1):34–43. doi:10.1016/j.intimp.2015.05.014
23. Li Y, Liu Y, Guo Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-β1 and BMP-2. Arthritis Res Ther. 2021;23(1). doi:10.1186/s13075-020-02382-x
24. Gao X, Ma Y, Zhang G, et al. Targeted elimination of intracellular reactive oxygen species using nanoparticle-like chitosan- superoxide dismutase conjugate for treatment of monoiodoacetate-induced osteoarthritis. Int J Pharm. 2020;590:119947. doi:10.1016/j.ijpharm.2020.119947
25. Abpeikar Z, Javdani M, Mirzaei SA, et al. Macroporous scaffold surface modified with biological macromolecules and piroxicam-loaded gelatin nanofibers toward meniscus cartilage repair. Int J Biol Macromol. 2021;183:1327–1345. doi:10.1016/j.ijbiomac.2021.04.151
26. Mohamed AL, Elmotasem H, Salama AAA. Colchicine mesoporous silica nanoparticles/hydrogel composite loaded cotton patches as a new encapsulator system for transdermal osteoarthritis management. Int J Biol Macromol. 2020;164:1149–1163. doi:10.1016/j.ijbiomac.2020.07.133
27. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. The Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
28. Primorac D, Molnar V, Rod E, et al. Knee osteoarthritis: a review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes. 2020;11(8):854. doi:10.3390/genes11080854
29. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138. doi:10.1016/j.rehab.2016.01.006
30. Berteau JP. Knee pain from osteoarthritis: pathogenesis, risk factors, and recent evidence on physical therapy interventions. J Clin Med. 2022;11(12):3252. doi:10.3390/jcm11123252
31. Salaffi F, Ciapetti A, Carotti M. The sources of pain in osteoarthritis: a pathophysiological review. Reumatismo. 2014;66(1):57–71. doi:10.4081/reumatismo.2014.766
32. Arden NK, Perry TA, Bannuru RR, et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol. 2021;17(1):59–66. doi:10.1038/s41584-020-00523-9
33. Brophy RH, Fillingham YA, Wright RW, Brophy RH. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (Nonarthroplasty), third edition. Am Acad Orthop Surg. 2018;26(19):678–687. doi:10.5435/JAAOS-D-21-01233
34. Primorac D, Molnar V, Matiši V, et al. Comprehensive review of knee osteoarthritis pharmacological treatment and the latest professional societies’ guidelines. Pharmaceuticals. 2021;14:205. doi:10.3390/ph14030205
35. Da Costa BR, V. PT, Saadat P, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. THE BMJ. 2021:375. doi:10.1136/bmj.n2321
36. Hermans J, Bierma-Zeinstra SMA, Bos PK, Niesten DD, Verhaar JAN, Reijman M. The effectiveness of high molecular weight hyaluronic acid for knee osteoarthritis in patients in the working age: a randomised controlled trial. BMC Musculoskelet Disord. 2019;20(1). doi:10.1186/s12891-019-2546-8
37. Koiri SP, Yang Y, Kui H. Hyaluronic acid in the treatment of knee osteoarthritis: review. Yangtze Medicine. 2018;02(02):62–72. doi:10.4236/ym.2018.22007
38. Nguyen C, Lefèvre-Colau MM, Poiraudeau S, Rannou F. Evidence and recommendations for use of intra-articular injections for knee osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):184–189. doi:10.1016/j.rehab.2016.02.008
39. Elksniņš-Finogejevs A, Vidal L, Peredistijs A. Intra-articular platelet-rich plasma vs corticosteroids in the treatment of moderate knee osteoarthritis: a single-center prospective randomized controlled study with a 1-year follow up. J Orthop Surg Res. 2020;15(1). doi:10.1186/s13018-020-01753-z
40. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis a randomized clinical trial. JAMA. 2017;317(19):1967–1975. doi:10.1001/jama.2017.5283
41. Zhang Z, Leong DJ, Xu L, et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res Ther. 2016;18(1). doi:10.1186/s13075-016-1025-y
42. Kim S. Competitive biological activities of chitosan and its derivatives: antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int J Polym Sci. 2018;2018:1–13. doi:10.1155/2018/1708172
43. Emans PJ, Skaliczki G, Haverkamp D, et al. KiOmedine® CM-chitosan is effective for treating advanced symptomatic knee osteoarthritis up to six months following a single intra-articular injection: a post hoc analysis of aproove clinical study. Open Rheumatol J. 2023;17(1). doi:10.2174/18743129-v16-e220206-2022-19
44. Lynen NA, Eichhorn C, Portelange N, Chausson M, Weyenberg W. Long-term efficacy following intra-articular injection of carboxymethyl-chitosan, a new product class for knee osteoarthritis: results from an observational study in Germany. Rheumatol Ther. 2024;11(3):649–662. doi:10.1007/s40744-024-00661-6
45. Li X, Dai B, Guo J, et al. Nanoparticle–cartilage interaction: pathology-based intra-articular drug delivery for osteoarthritis therapy. Nanomicro Lett. 2021;13(1). doi:10.1007/s40820-021-00670-y
46. Ul-Islam M, Alabbosh KF, Manan S, Khan S, Ahmad F, Ullah MW. Chitosan-based nanostructured biomaterials: synthesis, properties, and biomedical applications. Adv Ind Eng Polym Res. 2024;7(1):79–99. doi:10.1016/j.aiepr.2023.07.002
47. Zhao T, Li X, Li H, et al. Advancing drug delivery to articular cartilage: from single to multiple strategies. Acta Pharm Sin B. 2023;13(10):4127–4148. doi:10.1016/j.apsb.2022.11.021
48. Li H, Xie S, Qi Y, Li H, Zhang R, Lian Y. TNF-α increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp Ther Med. 2018. doi:10.3892/etm.2018.6770
49. Pritzker KPH, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi:10.1016/j.joca.2005.07.014
50. Anil S. Potential medical applications of chitooligosaccharides. Polymers. 2022;14(17):3558. doi:10.3390/polym14173558
51. Kunanusornchai W, Witoonpanich B, Tawonsawatruk T, Pichyangkura R, Chatsudthipong V, Muanprasat C. Chitosan oligosaccharide suppresses synovial inflammation via AMPK activation: an in vitro and in vivo study. Pharmacol Res. 2016;113:458–467. doi:10.1016/j.phrs.2016.09.016
52. Li S, Liu J, Liu S, Jiao W, Wang X. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J Nanobiotechnology. 2021;19(1):343. doi:10.1186/s12951-021-01086-x
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

