Back to Journals » Cancer Management and Research » Volume 17
Ciltacabtagene Autoleucel for the Treatment of Relapsed/Refractory Multiple Myeloma: Efficacy, Safety, and Place in Therapy
Authors Goel U, Zanwar S, Cowan AJ, Banerjee R, Khouri J , Dima D
Received 4 December 2024
Accepted for publication 10 February 2025
Published 19 February 2025 Volume 2025:17 Pages 357—372
DOI https://doi.org/10.2147/CMAR.S510408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Utkarsh Goel,1 Saurabh Zanwar,2 Andrew John Cowan,3 Rahul Banerjee,3 Jack Khouri,4 Danai Dima3
1Department of Internal Medicine, Cleveland Clinic, Cleveland, OH, USA; 2Division of Hematology, Department of Medicine, Mayo Clinic, Rochester, MN, USA; 3University of Washington, Fred Hutchinson Cancer Center, Seattle, WA, USA; 4Cleveland Clinic Taussig Cancer Center, Cleveland Clinic, Cleveland, OH, USA
Correspondence: Utkarsh Goel, Department of Internal Medicine, Cleveland Clinic, Cleveland, OH, USA, Email [email protected]
Abstract: Idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) are two chimeric antigen receptor T cell (CAR T) therapies approved for use in patients with relapsed/refractory multiple myeloma (MM). Initially approved for late line MM (> 4 prior lines), these were recently approved for use in MM with 1– 2 prior lines of therapy in April 2024. As their use outside of the pivotal clinical trials continues to expand, it is important to critically evaluate the safety and efficacy of these therapies. Further, it is important to identify patients that would be most likely to benefit from the use of CAR T in earlier lines of therapy. Cilta-cel was initially studied in the phase-I LEGEND-2 study, followed by CARTITUDE-1 and CARTITUDE-4 trials, demonstrating remarkable efficacy. A recent large real-world study also demonstrated similar efficacy, in a mostly pivotal trial ineligible patient population. Based on these impressive results, cilta-cel is currently being studied in trials for newly diagnosed as well as smoldering multiple myeloma. Cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS) are known toxicities of cilta-cel (and other CAR Ts), however movement and cognitive disorders (delayed neurotoxicity) and second primary malignancies are an evolving concern. In this article we discuss safety and efficacy data from existing cilta-cel studies. We propose that all patients with MM who have received ≥ 4 prior lines of therapy should be considered for CAR T. Earlier line use of CAR T should be restricted to patients with a high-risk disease phenotype (eg, functional high-risk disease). This disease phenotype has historically shown poor outcomes with standard triplet regimens and would be most likely to benefit from earlier use of CAR T: considering the availability of other safe and highly effective therapies, and potential high-risk toxicities of CAR T.
Keywords: multiple myeloma, ciltacabtagene autoleucel, CAR T, chimeric antigen receptor T cell therapy, relapsed/refractory multiple myeloma
Introduction
Advances in the understanding of disease biology and development of novel therapeutic strategies over the past two decades have led to a dramatic and continued improvement in overall survival among patients with multiple myeloma (MM).1,2 Despite these advancements, MM remains incurable, highlighting the need for further novel and highly efficacious treatment strategies.3–6 The introduction of chimeric antigen receptor T-cell therapy (CAR T) transformed the treatment landscape of MM, demonstrating remarkable efficacy and a maintenance-free treatment option even among patients with heavily pre-treated disease. This is especially true for patients with high-risk diseases, where the median progression-free survival is close to 18–24 months and prior treatment options yielded suboptimal outcomes. Idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) are currently the only two CAR T products approved by the US Food and Drug Administration (FDA) for use in patients with relapsed/refractory MM (RRMM).7–9 Both of these target the B-cell maturation antigen (BCMA) on the surface of plasma cells and were initially approved for patients who had received at least 4 prior lines of therapy (LOT) including a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and an anti-CD 38 monoclonal antibody (mAb), based on the results from the KarMMa-1 and CARTITUDE-1 trials.7,8,10
These therapeutic indications were expanded in 2024, based on updated results from KarMMa-3 and CARTITUDE-4 trials.11,12 Currently, ide-cel is approved for patients with RRMM with at least two prior LOT including a PI, an IMiD, and an anti-CD 38 mAb, while cilta-cel is approved for use in RRMM patients with at least one prior LOT, including a PI and an IMiD, who are refractory to lenalidomide.13,14 With this approval and the increasing use of CAR T outside of clinical trials, there is a need to critically assess the role of these therapies in the current RRMM treatment paradigm. Further, it is important to identify the patients that are most likely to benefit from the use of CAR T in earlier lines, balancing the expected efficacy, toxicity, logistics, and costs of CAR T versus existing traditional therapies. In this review, we discuss data from clinical trials and real-world observational studies evaluating the role of cilta-cel for RRMM and provide insights on its ongoing and future use for the treatment of RRMM.
Background and Mechanism of Action
All chimeric antigen receptors (CARs) consist of an ectodomain, a transmembrane domain, and an endodomain on the finished CAR T-cell. Since the 1990s, several generations of CARs have been developed, which vary based on the presence of additional co-stimulatory endodomains. For example, the first-generation CAR T-cells only included the CD3ζ signaling domain as a part of their endodomain. Each successive generation of CAR has typically outperformed the previous one in terms of efficacy, and in-vivo persistence of CAR T.
Both ide-cel and cilta-cel are autologous second-generation CARs, with anti-BCMA antibodies in the ectodomain, along with CD3ζ and a costimulatory 4–1BB domain in the endodomain. However, there are important differences in their BCMA-binding ectodomains. While ide-cel consists of a murine-derived anti-BCMA binding domain to target only a single epitope of BCMA, cilta-cel consists of two llama-derived heavy monoclonal antibody chains to bind two distinct epitopes of BCMA. This biepitopic design confers a higher binding avidity to cilta-cel, leading to enhanced efficacy, and partly explaining the lower required CAR T dose for cilta-cel (100–450 × 106 total cells for ide-cel vs 0.5–1 × 106 cells/kg body weight for cilta-cel).7,8,15–19
This cilta-cel CAR construct, was initially referred to as LCAR-B38M, and was studied in humans first in the phase-I LEGEND-2 trial in China.20–22 Using an identical CAR construct as LCAR-B38M and an updated manufacturing process, this CAR T product was commercialized under the name of cilta-cel (brand name CARVYKTI).15,19
Manufacture and Administration in Clinical Practice
The manufacturing and administration process for cilta-cel, like most other CAR Ts, is time and labor-intensive.17 The process starts with the selection of appropriate patients with disease phenotype most likely to benefit from CAR T. These patients are then prepared for leukapheresis. A washout period of 14 days is typically used, and any myeloma therapy is stopped for at least 14 days (or 7 days for IMiDs) prior to leukapheresis.23 After the collection of peripheral blood mononuclear cells, patients can continue to undergo bridging therapy based on the existing disease burden and aggressiveness of disease relapse according to the discretion of the treating physician.24,25 In the meantime, the collected cells undergo T-cell enrichment and genetic modification via lentivirus transduction to incorporate the new CAR in autologous T cells.26 This is followed by extensive in-vitro testing to ensure appropriate product development and cytotoxic activity. Finally, the manufactured CAR T product is frozen and transported to the medical facility for infusion.
This entire process can take at least 3–6 weeks, with the median reported time from apheresis to product release of 44 days (range 25–127 days) in CARTITUDE-4.12 After the successful manufacturing of CAR T-cells, patients undergo lymphodepletion therapy with most commonly fludarabine and cyclophosphamide (standard of care) or other regimens based on institutional protocols. While the pivotal CARTITUDE trials used fludarabine and cyclophosphamide, the use of bendamustine in clinical practice has shown comparable outcomes in the setting of a worldwide fludarabine shortage in 2022.27,28 Once CAR T cells are administered, patients are monitored closely for unique toxicities including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). The infused CAR T cells proliferate rapidly after infusion and reach a peak expansion in a median of approximately 13 days (both in CARTITUDE-1 and CARTITUDE-4). Various models of inpatient and outpatient administration of commercial CAR T have been described, and monitoring and management of toxicities often vary based on respective institutional guidelines.29–33
Efficacy
Relapsed/Refractory Multiple Myeloma: Late Line
LCAR-B38M (subsequently marketed as cilta-cel) was initially studied in the LEGEND-2 study, which began enrollment in China in 2016.22 LEGEND-2 was a single-arm Phase I study that enrolled 74 patients with RRMM with a median of 3 prior LOT who were infused with LCAR-B38M. In the most recent 5-year follow-up analysis, the median progression-free survival (PFS) was 18 months, with a median overall survival (OS) of 55.8 months. Overall, 67.6% of patients achieved measurable residual disease (MRD) negativity at the 10−4 threshold (Table 1). Of note, the lymphodepletion regimen used in this initial study consisted of cyclophosphamide only, as compared to fludarabine plus cyclophosphamide subsequently used in the CARTITUDE studies.20–22
 |
Table 1 Clinical efficacy of Ciltacabtagene Autoleucel |
The results from the phase I LEGEND-2 study were confirmed in the phase Ib/II CARTITUDE-1 and the Phase II CARTIFAN-1 studies. CARTITUDE-1 was a single arm phase Ib/II study of cilta-cel in 97 (113 enrolled, 97 infused) patients with RRMM who had received at least 3 prior LOT. In the initial report with a median follow-up of 12.4 months, the median PFS and OS were not reached, with the 12-month PFS and OS rates being 77% and 89%, respectively. The overall response rate (ORR) was impressive at 97%. A total of 34% of all patients achieved MRD negativity at 10−5, and 93% of patients evaluable for MRD negativity had achieved an MRD negative status.7 Responses deepened over time, with MRD negativity (at 10−5) improving to 44.3% in the updated analyses. In the final report with a median follow-up of 27.7 months, the median PFS was 34.9 months, while the median OS was still not reached (36-month OS rate 62.9%).34,35 It is important to note that this was not an intention-to-treat analysis and the highest-risk patients, which were enrolled but not infused, were excluded from the analysis.
CARTIFAN-1 was a phase II study of cilta-cel conducted in China, which enrolled 64 patients with RRMM who had received at least 3 prior LOT (including a PI and IMiD). In contrast to CARTITUDE-1, prior exposure to an anti-CD 38 mAb was not a requirement for this study. Among the 48 patients that were infused with cilta-cel, the 18-month PFS and OS rates were 66.8% and 78.7% respectively (median PFS and OS not reached).36 A total of 77.1% of patients achieved a complete response (CR) or better with cilta-cel, and 72.9% achieved MRD negativity at the 10−5 threshold.
CARTITUDE-2 is an ongoing phase II multi-cohort study, with eight described cohorts (cohorts A-H).4,19,37–41 Interim data from cohorts A-D have been described thus far. CARTITUDE-2 cohort A included 20 patients with RRMM who had received 1–3 prior LOT including a PI and an IMiD and were lenalidomide refractory.44 In the most recent report with a median follow-up of 29 months, the 24-month PFS and OS rates were both 75%.37–39 Cohort B included patients with RRMM who had received one prior PI and IMiD-containing line, and experienced disease progression ≤12 months after the start of frontline therapy, or ≤12 months after frontline autologous stem cell transplantation (ASCT), if patients had had an ASCT. This cohort included a population that has been historically described as functional high-risk myeloma, which is less likely to respond to subsequent traditional therapies.45 At a median follow-up of 27.9 months, the 19 infused patients in cohort B had an ORR of 95%, and a 24-month PFS and OS of 73% and 84%, respectively.37–39
CARTITUDE-2 cohort C included 20 patients with heavily pre-treated RRMM (median of 8 prior LOT), who had previously been exposed to a PI, IMiD, an anti-CD38 mAb, and a non-CAR T BCMA-directed therapy (antibody drug conjugate or bispecific antibody).40 Cohort C also represents a difficult to treat disease phenotype, which has already been exposed to most available drugs and drug classes. At a median follow-up of 11.3 months, cilta-cel demonstrated an ORR of 60%, along with an MRD negativity rate of 35% among all patients. The median PFS was 9.1 months, and the median duration of response was 11.5 months in this heavily pre-treated cohort.
CARTITUDE-2 cohort D results were recently presented. Cohort D included 17 patients with newly diagnosed MM who received 4–8 cycles of induction therapy with or without ASCT and did not achieve a CR with this first-line therapy. The trial evaluated the use of cilta-cel with or without lenalidomide maintenance. Overall, 12 patients received cilta-cel in combination with lenalidomide, and five cilta-cel alone. At a median follow-up of 22 months, the ≥ CR rate was 94%, and 18-month PFS and OS were 94% each.41 Longer-term follow-up of this trial would help assess the role of CAR T as consolidation/salvage therapy after suboptimal response (<CR) to standard first-line induction and ASCT.
A recent large real-world study from the US MM Immunotherapy Consortium reported outcomes of 255 patients (255 apheresed, 236 infused) treated with commercial cilta-cel at 16 US academic centers.42 The ORR was 89% (≥ CR rate 70%), and of 98 patients with MRD data available, 93 (95%) were able to achieve MRD negativity at the 10−5 threshold. At a median follow-up of 13 months, the 12-month PFS was 68% (median PFS not reached) and the 12-month OS was 82%. These were somewhat lower than those reported in CARTITUDE-1; however, it is noteworthy that about half (54%) of patients in the study did not meet CARTITUDE-1 eligibility criteria (including 14% having prior exposure to a BCMA-directed therapy). Notably, the ORR and CR rates of the subgroup of patients treated with commercial in specification/conforming cilta-cel were 94% and 74%, while in the CARTITUDE-1 eligible patient subgroup, these were 93% and 74%, respectively, all of which are very similar to CARTITUDE-1, highlighting the overall efficacy of cilta-cel in a real-world setting as well.7,42
Several subgroups, however, did not derive an equal efficacy benefit with cilta-cel, including patients with extramedullary disease, prior BCMA-directed therapy exposure, and poor performance status at CAR T infusion. These populations remain an unmet need even with highly effective therapies. Notably, patients with extramedullary disease constitute a population with particularly dismal outcomes. Most recently, the RedirecTT-1 Phase 1b/2 study showed a relatively high response rate (59%) with the bispecific antibody combination of teclistamab and talquetamab across all dose levels in RRMM patients with extramedullary disease (61% with the recommended Phase 2 regimen).46 The likelihood of patients continuing in response at 18 months was 82% among those with extramedullary disease who received the recommended phase 2 regimen, and the 18-month PFS rate for these patients was 53%. Given these impressive outcomes, bispecific combination may become the preferred regimen in heavily pretreated patients with extramedullary disease soon, even among those who qualify for standard-of-care or experimental CAR T-cell therapies. More prospective and real-world data are eagerly awaited, particularly for these high-risk subgroups.
Relapsed/Refractory Multiple Myeloma: Early Line
CARTITUDE-4 was a pivotal Phase III randomized controlled trial that randomized 419 patients with lenalidomide refractory, early relapsed MM (1–3 prior LOT) to receive either cilta-cel or standard of care triplet regimens based on physician’s choice.12 The control arm regimens included bortezomib, pomalidomide, and dexamethasone (VPd) or daratumumab, pomalidomide, and dexamethasone (DPd). Patients in the cilta-cel arm received at least one cycle of bridging therapy with the same control arm regimens (VPd or DPd). In the most recent presentation with a median follow-up of 34 months, the median PFS was not reached for the cilta-cel arm (30-month PFS 59.4%) while the PFS for the control arm was 11.8 months (30-month PFS 25.7%), p < 0.001. Although the median OS was not reached in either arm, the cilta-cel arm had a significantly better 30-month OS rate (76.4% vs 63.8%, HR = 0.55, p = 0.0009).43 The observed improvement in OS marks an important milestone in the treatment of RRMM, demonstrating the clinical benefit of the use of cilta-cel in as early as the first relapse, and its use leading to improved outcomes as compared to standard of care daratumumab containing triplet regimens. Based on the impressive results of CARTITUDE-4, the FDA approved its use in earlier lines of therapy in April 2024.
CARTITUDE-4 is similar in design and interpretation to its counterpart KarMMa-3 trial that studied ide-cel.11 KarMMa-3 randomized 386 patients with early relapsed RRMM (2–4 prior LOT) to receive ide-cel vs standard of care triplet regimens. KarMMa-3 allowed a greater number of options for the control arm regimens – including DPd, DVd, ixazomib, lenalidomide, and dexamethasone; carfilzomib and dexamethasone; or elotuzumab, pomalidomide, and dexamethasone. At a median follow-up of 30.9 months, the PFS was significantly better with ide-cel vs standard of care (13.8 vs 4.4 months). However, there was no significant difference in the OS between the two arms (41.4 months for ide-cel vs 37.9 months for control, HR = 1.01, 95% CI: 0.73–1.40).47
With a higher efficacy observed in the pivotal trials and demonstrated improvement in OS, cilta-cel is often considered the preferred CAR T for use in patients with RRMM. This is further consolidated by a recent retrospective inverse probability of treatment weighting matched analysis of ide-cel vs cilta-cel among 586 patients.48 However, data from CARTITUDE-4 in the context of KarMMa-3 results should be interpreted with a few caveats. First, the allowed regimens in the control arm (DPd and VPd) of CARTITUDE-4 did not allow for potentially highly effective carfilzomib-based regimens, although around 80% of patients in the standard-of-care arm were not refractory to carfilzomib.12,49 This might partly account for the lower PFS seen in the control arm in this trial. Second, the trial population in CARTITUDE-4 represented a less pre-treated disease (median 2 versus 3 prior LOT, 14–16% versus 65–67% patients with triple class refractory disease, when compared to KarMMa-3), limiting comparisons of efficacy between the two CAR T products across these trials.50 Third, the cilta-cel arm in CARTITUDE-4 was required to have received at least one cycle of bridging therapy, as compared to a maximum of one cycle of bridging therapy in the ide-cel arm in KarMMa-3.47 Finally, while KarMMa-3 allowed patients in the standard regimens arm to cross over to ide-cel upon progression, this was not allowed in CARTITUDE-4. This is important to consider since CARTITUDE-4 did demonstrate an improvement in OS, and a lack of per protocol cross over does limit the interpretability of these results while considering the benefit of earlier vs later use of cilta-cel for RRMM.
Newly Diagnosed Multiple Myeloma
Several important CARTITUDE trials are ongoing, to assess the use cilta-cel in newly diagnosed MM (NDMM). The idea is based on the excellent CARTITUDE-4 results, demonstrating the utility of earlier use of cilta-cel. Since patients with MM undergo attrition with each successive line of therapy, there are ongoing clinical and research efforts to try to use the best available MM therapies as early as possible, to get the deepest possible response with first-line therapy,51,52 a concept that is particularly relevant in patients with high-risk cytogenetics or functional high-risk MM. While clinical data from these frontline cilta-cel trials are awaited, these would be crucial in exploring this important concept of frontline use of a highly efficacious CAR T product for MM. Brief designs of these ongoing trials are described below.
As previously described, CARTITUDE-2 (NCT04133636) is an ongoing multi-cohort study, with data from cohorts A-D discussed above. Cohort E is designed to evaluate cilta-cel in patients with NDMM, who are considered high risk due to the presence of: International Staging System (ISS) stage III disease or high-risk cytogenetic features del(17p), t(14; 16), t(14; 20), or 1q amplification.53 Cohort F will evaluate the role of cilta-cel as consolidation therapy in patients with NDMM who have received 4–8 cycles of induction therapy with daratumumab or carfilzomib-based triplet and quadruplet regimens and have at least a very good partial response to induction therapy. Cohort G will evaluate cilta-cel after ~4 cycles of induction with daratumumab, lenalidomide, and dexamethasone (DRd) in patients with NDMM who are not intended to undergo upfront ASCT. Finally, cohort H will evaluate cilta-cel infusion (similar to cohort G design) after ~4 cycles of daratumumab, bortezomib, lenalidomide, and dexamethasone (DVRd) in patients with NDMM who are considered a candidate for front line high-dose chemotherapy and ASCT.
CARTITUDE-5 plans to evaluate cilta-cel in patients with NDMM who are not considered candidates for ASCT, or in whom ASCT is not planned as part of first-line therapy. Patients will undergo induction with ~6 cycles of VRd, followed by 1:1 randomization to either 2 additional cycles of VRd followed by Rd maintenance; or 2 additional cycles of VRd followed by apheresis, VRd bridging, and cilta-cel infusion followed by no maintenance therapy. This trial would be important in assessing the utility of CAR T therapy in patients who otherwise would not have received ASCT as first-line therapy.54 CARTITUDE-6 plans to compare the upfront use of DVRd followed by cilta-cel versus DVRd followed by ASCT in transplant-eligible NDMM patients. Patients will be randomized to either 4 cycles of DVRd, ASCT, DVRd consolidation, and lenalidomide maintenance; or 6 cycles of DVRd, cilta-cel infusion, followed by lenalidomide maintenance.55
Smoldering Multiple Myeloma
The timing of treatment and choice of therapy for smoldering MM continues to be a point of debate.56,57 There are efforts towards using aggressive MM therapy, in patients with high-risk smoldering MM, as an effort to potentially cure some of these patients at an early stage and prevent progression to symptomatic MM. CAR-PRISM is a single-arm phase-II study evaluating the use of cilta-cel in patients with high-risk smoldering MM. Of a total planned enrollment of 20 patients, data from 6 patients in the safety run-in phase have been presented, with all (100%) patients achieving MRD negativity (10−6). At a median follow-up of 6 months, CRS events were low grade, along with no ICANS events.58 Long-term follow-up of this cohort will shed light on the durability of the observed responses, and one possible approach towards a cure of MM via early aggressive intervention.
Combination With Other Plasma Cell-Directed Therapies
The current use of standard-of-care cilta-cel employs a maintenance/consolidation-free treatment paradigm. However, the benefit of maintenance therapy in the post-CAR T setting remains under investigation, and data from most of these studies are not yet mature. CARTITUDE-2 cohort D evaluated 12 patients with NDMM with suboptimal response to first-line therapy, who received cilta-cel followed by lenalidomide maintenance.41 A phase II study (NCT06179888) is currently recruiting patients and plans to study the utility of iberdomide maintenance versus observation after cilta-cel administration. Two reports have previously demonstrated the possible benefits of using pomalidomide after BCMA-directed CAR T.59,60 Finally, the MonumenTAL-8 (NCT06550895) study is evaluating the use of the novel bispecific antibody, talquetamab, as consolidative therapy after cilta-cel and is currently recruiting.
Safety
CRS and ICANS are toxicities associated with most immune-directed therapies for hematologic malignancies, including CAR T and bispecific antibodies.61–64 Additionally, hematologic toxicities, including thrombocytopenia and high-grade neutropenia have been reported in clinical trials. A significant incidence of second primary malignancies (SPMs) has been noted, including myeloid neoplasms and T-cell lymphomas, although the direct causal association of cilta-cel with these SPMs is still controversial.
Cytokine Release Syndrome (CRS)
CRS is a syndrome characterized by endothelial activation and release of pro-inflammatory cytokines by the CAR T cells, which leads to activation of immune and other bystander cells, and causes clinical manifestations of fever, hypoxia, hypotension, and systemic inflammatory response.5,65 In early cilta-cel trials (LEGEND-2, CARTITUDE-1) that included heavily pre-treated RRMM, almost all patients (>90%) had CRS of any grade, with severe (≥ grade 3) CRS occurring in 4–7% of patients (Table 2). As cilta-cel has been studied in relatively earlier lines of therapy (CARTITUDE-4: 1–3 prior LOT, CARTITUDE-2 cohort B: 1 prior LOT, CARTITUDE-2 cohort D: NDMM), the rates of CRS have been lower (~70–80% for any grade CRS, 0–5% for ≥ grade 3 CRS).7,22,37–39,41,66 While cross-trial comparisons, like these, are always limited by the inherent differences in patient populations, these data could provide an indication of improving the tolerability of cilta-cel in RRMM with a lower prior treatment burden. Conversely, it is important to recognize that the lower rates of severe CRS are also a consequence of the lower treatment burden and more effective bridging therapy response in earlier lines of therapy.
 |
Table 2 Safety of Ciltacabtagene Autoleucel in Clinical Studies |
Neurotoxicity
The pathophysiology of CAR T-associated neurotoxicity remains poorly understood, although mechanisms of endothelial activation similar to CRS, causing cytokine release, systemic inflammation, and disruption of the blood–brain barrier have been proposed.67 ICANS (initially described as “CAR T-cell related encephalopathy syndrome”) is usually an early, short-duration syndrome of encephalopathy, characterized by confusion and depressed levels of consciousness; however, it can be associated with cerebral edema, increased intracranial pressure, seizures, and death in its most severe form.7,66 Several neurotoxicity symptoms (eg, peripheral motor and/or sensory neuropathy, such as Guillain Barre syndrome, nerve palsies, diplopia, sensory loss, gait disturbance, and parkinsonism) do not meet the definition of ICANS and have been described as “non-ICANS” or “other neurotoxicity” with both cilta-cel and ide-cel.7,68,69 A specific subset of non-ICANS neurotoxicity is a cluster of movement disorders (eg, tremors, micrographia, rigidity, and parkinsonism), cognitive disturbances (eg, memory impairment), and personality changes, which were observed in patients enrolled in CARTITUDE-1 and are referred to as delayed neurotoxicity, and specifically as movement and neurocognitive treatment-emergent adverse events (MNTs).68 It is important to note that ICANS, non-ICANS neurotoxicity including MNTs can have overlapping symptoms and presentations, and most patients that later developed MNTs (5 out of 6 in CARTITUDE-1), also had ICANS with the initial infusion.34,68
While MNTs including parkinsonism were initially thought to be mostly related to cilta-cel, a recent report has identified a case of movement disorder after ide-cell as well, although much less frequently.70–72 The occurrence of movement disorders with therapy is concerning, especially as cilta-cel (and other CAR T) trials study their use in the front-line setting. Further, data on the reversibility of these symptoms have been limited. Although there are not yet concrete predictors of MNTs after CAR T, the overall idea is that a lower disease burden while receiving CAR T might be able to offset some of these neurotoxic syndromes. For example, the incidence of ICANS and non-ICANS neurotoxicity was 21.6% (6.2% incidence of MNTs) in CARTITUDE-1, which was higher than that seen in CARTITUDE-4 (4.5% ICANS, 0.6% MNTs) with a similar follow-up period. To this point, effective bridging therapy prior to CAR T and close monitoring for neurotoxicity in the post CAR T period are crucial. The incidence of ICANS and MNTs seen with cilta-cel studies are summarized in Table 2.
Hematologic Toxicities
Hematologic toxicities (anemia, neutropenia, and thrombocytopenia) have been common adverse events associated with cilta-cel. Severe (≥ grade 3) thrombocytopenia was seen in 54.2–59.8% patients in late line CARTITUDE-1 and CARTIFAN-1 trials, as compared to 41.3% in CARTITUDE-4, following the overall trend of lower toxicities with earlier use of cilta-cel.7,36 Neutropenia was seen very frequently seen both in late line trials (85.3–97.9%), and in CARTITUDE-4 (89.9%).7,12,36 Of note, among all neutropenia events reported in these trials, most (>99%) were ≥ grade 3.73 Even when cilta-cel was used in earlier relapsed MM, the observed rates of neutropenia were mostly unchanged, suggesting that it might be related to cilta-cel administration itself, rather than the burden of disease or prior treatment history (Table 2).
Second Primary Malignancies
Second primary malignancies (SPMs), including non-melanoma skin cancers, have been observed with the use of cilta-cel in up to ~22% patients in CARTITUDE-1, and to a lesser extent ~4–5% in CARTITUDE-4 and real-world data.7,12,42 The occurrence of SPMs after cilta-cel and CAR T in general is under investigation, and it is not yet well known if SPMs are a consequence of CAR T or prior treatment-related factors. When considering all types of SPMs, the control arm of CARTITUDE-4 (with no or minimal crossover) also had a 6.7% incidence of SPMs versus the cilta-cel arm (4.3%).
There are several arguments against the association of CAR T with SPMs. First, the patients that currently receive CAR T in most cases, have disease that has already been exposed to most available therapies, many of which are also associated with SPMs (such as long-term lenalidomide maintenance, alkylating agent use, high-dose melphalan and ASCT).74,75 Despite this, the SPM incidence in CARTITUDE-4 (4.3%) was similar to that of many late-line cilta-cel trials (5.4% in LEGEND 2, 4.3% in the real-world study). Another point is that patients who receive CAR T are now living longer than before, which gives a greater opportunity for SPMs to develop and be detected.74 A recently published meta-analysis evaluating all CAR T therapies for hematologic malignancies found no increased risk of SPMs with CAR T as compared to conventional standard-of-care therapies.76
Nevertheless, secondary hematologic malignancies, especially difficult-to-treat secondary myeloid and aggressive T-cell malignancies, are the biggest concern.77–80 Although the control arm in CARTITUDE-4 had a higher incidence of all SPMs (6.7% vs 4.3% with cilta-cel), this was mostly driven by cutaneous and non-invasive malignancies (4.8% of 6.7% in the control arm). Overall, 1% (n = 2) in the cilta-cel arm developed acute myeloid leukemia and myelodysplastic syndrome, compared to 0 in the control arm. Ten out of ninety-seven patients (10.3%) treated with cilta-cel in CARTITUDE-1 developed secondary myeloid malignancies. In the real-world study, 1.3% of all patients developed myeloid malignancies/acute leukemia in a relatively short follow-up of 13 months. SPMs continue to be a concern, and as such, the risk of SPMs should be carefully discussed with the patient, similar to other MM treatments carrying a similar risk.81
Patient Selection, Place in Current Treatment Landscape
Overall, cilta-cel has demonstrated remarkable activity in both early and later lines of therapy for RRMM. Notably, it is a maintenance-free treatment option, which is important for patients’ quality of life, who often need to receive continuous therapy without breaks until progression. The CARTITUDE-4 trial showed significant improvements in PFS with earlier use of cilta-cel as compared to standard triplet regimens. However, the lack of cross-over limits the interpretation and does not completely answer the question of whether there is OS benefit with early vs later line use of cilta-cel.
The recent FDA approvals of cilta-cel and ide-cel into earlier lines, as well as the approvals of other novel BCMA-directed and non-BCMA-directed therapies, particularly bispecific antibodies (bsAb), for heavily pretreated patients after four or more prior lines have radically changed the treatment paradigm of RRMM. Both CAR T products and bsAbs share similar approval indications, and given the incurable nature of myeloma, most patients will likely receive both drug classes sequentially during their disease course. Optimal sequencing of these agents remains unclear, and treatment selection is mostly influenced by patient parameters, disease characteristics, patient and physician preference, and logistics.
None of these therapies have been compared head-to-head in randomized trials. Recent real-world data suggested higher efficacy but also higher toxicity with cilta-cel compared to ide-cel in heavily pretreated patients.48 Although cross-trial comparisons are challenging due to different patient populations included, it appears that, numerically, responses and progression-free survival with cilta-cel are better compared to BCMA-directed bispecific antibodies (teclistamab and elranatamab) in heavily pretreated patients with four or more lines of therapy (ORR: 97.9% cilta-cel, 63% teclistamab, 61% elranatamab).63,82,83 However, patients who are selected and ultimately infused with CAR T tend to have a slower disease tempo and otherwise favorable performance status, which limits direct comparisons of these trials.
Notably, the administration of CAR T is a time and resource-intensive process requiring apheresis, CAR T-cell manufacture, and lymphodepleting chemotherapy, as compared to bispecific antibodies, which are readily available off-the-shelf and can be used immediately for patients who are rapidly progressing requiring prompt treatment initiation. CAR T is not widely available across the globe and can only be given in accredited tertiary centers, thus there are limitations on access based on geographical location. Patients who are treated with CAR T need to either travel or temporarily relocate close to the CAR T center, as the early post-infusion period includes several restrictions and close monitoring, all of which may represent significant barriers.31,32,84
Another important aspect to consider is the safety profile of cilta-cel as compared to ide-cel and bsAb. Overall, CAR T administration requires a good performance status and adequate organ function to avoid excess toxicity. Cilta-cel appears to lead to higher rates of immune-mediated adverse events including severe CRS, and infections compared to ide-cel in heavily pretreated patients. In addition, cilta-cel demonstrated a higher incidence and severity of CRS and ICANS, compared to bsAb, making it a less appealing option for frail and unfit patients. Most importantly, cilta-cel has been linked with unique non-ICANS delayed MNTs such as nerve palsies, Guillain–Barré syndrome, and parkinsonism. Moreover, there are reports that both ide-cel and cilta-cel have been linked to secondary primary hematologic malignancies (SPMs). Although low in incidence, both delayed neurotoxicities and SPMs can be severely debilitating and even life-threatening and should be weighed against the benefits as cilta-cel is used in earlier lines. All the above should be taken into consideration and discussed with patients during the treatment selection process.
Data on sequencing BCMA-directed therapies are limited, and future emerging data will be crucial in shaping the treatment course of RRMM. The currently available literature suggests that the durability of responses with anti-BCMA CAR T seems to be inferior when given after a BCMA-directed bispecific antibody.40,85 Likewise, BCMA-directed bispecific antibodies may also have lower response rates and durability in patients with prior exposure to BCMA-directed CAR T.86–88 Longer time between BCMA-directed therapies may lead to better outcomes. Overall, for patients who are both CAR T and bsAb eligible, we believe that CAR T before vbsAb is the best approach, as this may lead to the best cumulative survival.89 One possible explanation for these findings could be that the treatment-free interval following the CAR T infusion may lead to less T-cell exhaustion.
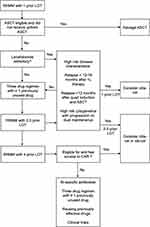
Overall, the goal of RRMM treatment is to select the best strategy based on patient parameters and disease characteristics for achieving a deep and durable response as early as possible in the disease course. Taken together, we believe that patients with RRMM who meet the FDA criteria for cilta-cel should be referred for an initial CAR T evaluation in a tertiary center. Given concerns for non-ICANS and delayed neurotoxicity, cilta-cel use at first relapse should be restricted to patients with a high-risk disease phenotype including patients with ultra high-risk disease (presence of 2 or more high-risk cytogenetic abnormalities), and functional high-risk myeloma, such as patients who are refractory to quadruplet frontline therapy or patients who relapse very quickly after front-line ASCT. This disease phenotype has historically shown poor outcomes with standard triplet regimens and would be most likely to benefit from earlier use of cilta-cel.90 For later relapses, if the patient has not received commercial CAR T, we prefer the use of cilta-cel or ide-cel first over bsAb, if possible and clinically appropriate. For later relapses, other options physicians should strongly consider include enrollment in clinical trials evaluating experimental BCMA and non-BCMA CAR Ts. A proposed treatment algorithm for RRMM incorporating cilta-cel is described in Figure 1.
Future Directions
At present, cilta-cel is an important cornerstone in the current treatment landscape of RRMM. However, the evaluation of cilta-cel in the upfront setting for NDMM as well as the development and future approval of other BCMA (eg, anitocabtagene autoleucel) and non-BCMA CAR Ts (eg, arlocabtagene autoleucel) will determine how the role of cilta-cel will continue to evolve as the choice of regimen at different time points during the disease course. Recently presented data reported an ORR of 97% with anitocabtagene autoleucel, which is very similar to the ORR of cilta cel, however, no delayed or non-ICANS neurotoxicities were observed after a median follow-up of almost 10 months. Lack of delayed neurotoxicity might have significant implications on CAR T product selection, as clinicians remain concerned with delayed and potentially irreversible neurotoxicity with cilta-cel. Arlocabtagene autoleucel is an anti-GPRC5D CAR T product and might also play a role in the future treatment paradigm of MM. Recent data showed that arlocabtagene autoleucel maintained its good response and PFS in patients previously exposed to BCMA-directed therapies, with a relatively manageable toxicity profile, including less than 20% rate of severe infections, a common treatment-emergent adverse event of anti-BCMA CAR Ts.91–93
Future research priorities for cilta-cel include safety optimization and earlier identification of patients likely to develop delayed and non-ICANS neurotoxicity. Long-term follow-up from trials evaluating cilta-cel in early relapse and frontline settings might help assess the role of prior disease and treatment burden in the development of these toxicities. Despite excellent efficacy, patients still experience disease progression after cilta-cel, and the role of maintenance therapy after CAR T infusion is under investigation. Apart from the well-known logistic and time burdens of pre-CAR T care, the management of patients post CAR T infusion is equally complicated, as patients can often experience long-term hematologic toxicities and recurrent infections. In this context, research efforts towards optimizing supportive care after cilta-cel are ongoing and essential.
Data Sharing Statement
Data sharing statement not applicable to this article as no new datasets were generated or analyzed.
Ethics and Patient Consent Statement
IRB approval not applicable as no new patient-related data were generated or analyzed.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
AC: Consulting or advisory role: Sanofi, HopeAI, Adaptive biotechnologies, Bristol-Myers Squibb, Janssen, Sebia. Research funding: Regeneron, IgM biosciences, Nektar, Adaptive Biotechnologies, Caelum, Juno/Celgene, Harpoon, AbbVie, Bristol-Myers Squibb, Janssen. Stock options in privately held company: HopeAI. RB: Consulting or advisory role: Adaptive Biotechnologies, Bristol-Myers Squibb/Celgene, Caribou Biosciences, Genentech, GlaxoSmithKline, Janssen, Karyopharm, Legend Biotech, Pfizer, Sanofi, SparkCures, Kite. Research funding: AbbVie, Bristol-Myers Squibb/Celgene, Janssen, Novartis, Pack Health/Quest Diagnostics, Prothena, Sanofi. JK: Consulting or advisory role: GPCR, Janssen, Prothena, Legend Biotech. Research funding: Prothena, Ascentage, Janssen, Karyopharm, GPCR. The other authors declare no conflicts of interest in this work.
References
1. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi:10.1038/leu.2013.313
2. Binder M, Nandakumar B, Rajkumar SV, et al. Mortality trends in multiple myeloma after the introduction of novel therapies in the United States. Leukemia. 2022;36(3):801–808. doi:10.1038/s41375-021-01453-5
3. Anderson LD. Idecabtagene vicleucel (ide-cel) CAR T-cell therapy for relapsed and refractory multiple myeloma. Future Oncol. 2022;18(3):277–289. doi:10.2217/fon-2021-1090
4. Jagannath S, Jackson CC, Schecter JM, et al. Cilta-cel, a BCMA-targeting CAR-T therapy for patients with multiple myeloma. Expert Opin Biol Ther. 2024;24(5):339–350. doi:10.1080/14712598.2024.2352591
5. Markouli M, Ullah F, Unlu S, et al. Toxicity profile of chimeric antigen receptor T-cell and bispecific antibody therapies in multiple myeloma: pathogenesis, prevention and management. Curr Oncol. 2023;30(7):6330–6352. doi:10.3390/curroncol30070467
6. Rao L, De Veirman K, Giannico D, et al. Targeting angiogenesis in multiple myeloma by the VEGF and HGF blocking DARPin® protein MP0250: a preclinical study. Oncotarget. 2018;9(17):13366–13381. doi:10.18632/oncotarget.24351
7. Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. doi:10.1016/S0140-6736(21)00933-8
8. Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. doi:10.1056/NEJMoa2024850
9. Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34(4):985–1005. doi:10.1038/s41375-020-0734-z
10. Rajkumar SV, Richardson P, San Miguel JF. Guidelines for determination of the number of prior lines of therapy in multiple myeloma. Blood. 2015;126(7):921–922. doi:10.1182/blood-2015-05-647636
11. Rodriguez-Otero P, Ailawadhi S, Arnulf B, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388(11):1002–1014. doi:10.1056/NEJMoa2213614
12. San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. 2023;389(4):335–347. doi:10.1056/NEJMoa2303379
13. US Food and Drug Administration. ABECMA (idecabtagene vicleucel) [Internet]. Available from: https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel.
14. US Food and Drug Administration. CARVYKTI.
15. Scheller L, Tebuka E, Rambau PF, et al. BCMA CAR-T cells in multiple myeloma–ready for take-off? Leuk Lymphoma. 2024;65(2):143–157. doi:10.1080/10428194.2023.2276676
16. Chekol Abebe E, Yibeltal Shiferaw M, Tadele Admasu F, Asmamaw Dejenie T. Ciltacabtagene autoleucel: the second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front Immunol. 2022;13:991092. doi:10.3389/fimmu.2022.991092
17. Teoh PJ, Chng WJ. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J. 2021;11(4):84. doi:10.1038/s41408-021-00469-5
18. Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci. 1993;90(2):720–724. doi:10.1073/pnas.90.2.720
19. Martin TG, Madduri D, Pacaud L, Usmani SZ. Cilta-cel, a BCMA-targeting CAR-T therapy for heavily pretreated patients with relapsed/refractory multiple myeloma. Future Oncol. 2023;19(34):2297–2311. doi:10.2217/fon-2022-1317
20. Xu J, Wang BY, Yu SH, et al. Long-term remission and survival in patients with relapsed or refractory multiple myeloma after treatment with LCAR-B38M CAR T cells: 5-year follow-up of the LEGEND-2 trial. J Hematol Oncol. 2024;17(1):23. doi:10.1186/s13045-024-01530-z
21. Zhao WH, Wang BY, Chen LJ, et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: a phase 1, single-arm, open-label, multicenter study in China (LEGEND-2). J Hematol Oncol. 2022;15(1):86. doi:10.1186/s13045-022-01301-8
22. Zhao WH, Liu J, Wang BY, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11(1):141. doi:10.1186/s13045-018-0681-6
23. Lin Y, Qiu L, Usmani S, et al. Consensus guidelines and recommendations for the management and response assessment of chimeric antigen receptor T-cell therapy in clinical practice for relapsed and refractory multiple myeloma: a report from the International Myeloma Working Group Immunotherapy Committee. Lancet Oncol. 2024;25(8):e374–87. doi:10.1016/S1470-2045(24)00094-9
24. Afrough A, Hashmi H, Hansen DK, et al. Real-world impact of bridging therapy on outcomes of ide-cel for myeloma in the U.S. Myeloma Immunotherapy Consortium. Blood Cancer J. 2024;14(1):63. doi:10.1038/s41408-024-00993-0
25. Dhakal B, Akhtar OS, Cowan AJ, Richard S, Friend R, Rees MJ. Talquetamab bridging: paving the way to B-Cell Maturation Antigen (BCMA) CAR-T cell therapy in Relapsed/Refractory Multiple Myeloma (RRMM). Blood. 2024;144:931. doi:10.1182/blood-2024-202017
26. Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. mol Ther Oncolytics. 2016;3:16015. doi:10.1038/mto.2016.15
27. Sidana S, Hosoya H, Jensen A, et al. Bendamustine vs. fludarabine/cyclophosphamide lymphodepletion prior to BCMA CAR-T cell therapy in multiple myeloma. Blood Cancer J. 2023;13(1):158. doi:10.1038/s41408-023-00929-0
28. Maziarz RT, Diaz A, Miklos DB, Shah NN. Perspective: an international fludarabine shortage: supply chain issues impacting transplantation and immune effector cell therapy delivery. Transplant Cell Ther. 2022;28(11):723–726. doi:10.1016/j.jtct.2022.08.002
29. Bansal R, Paludo J, De Menezes Silva Corraes A, et al. Outpatient practice utilization for CAR-T and T cell engager in patients with lymphoma and multiple myeloma. J Clin Oncol. 2023;41(16_suppl):1533. doi:10.1200/JCO.2023.41.16_suppl.1533
30. Furqan F, Bhatlapenumarthi V, Dhakal B, et al. Outpatient administration of CAR T-cell therapies using a strategy of no remote monitoring and early CRS intervention. Blood Adv. 2024;8(16):4320–4329. doi:10.1182/bloodadvances.2024013239
31. Wesson W, Dima D, Suleman N, et al. Timing of toxicities and non-relapse mortality following CAR T therapy in myeloma. Transplant Cell Ther. 2024;30(9):876–884. doi:10.1016/j.jtct.2024.06.012
32. Ahmed N, Wesson W, Lutfi F, et al. Optimizing the post-CAR T monitoring period in recipients of axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel. Blood Adv. 2024;8(20):5346–5354. doi:10.1182/bloodadvances.2023012549
33. Ailawadhi S, Shune L, Wong SW, Lin Y, Patel K, Jagannath S. Optimizing the CAR T-cell therapy experience in multiple myeloma: clinical pearls from an expert roundtable. Clin Lymphoma Myeloma Leuk. 2024;24(5):e217–25. doi:10.1016/j.clml.2024.01.014
34. Martin T, Usmani SZ, Berdeja JG, et al. Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2023;41(6):1265–1274. doi:10.1200/JCO.22.00842
35. Lin Y, Martin TG, Usmani SZ, et al. CARTITUDE-1 final results: phase 1b/2 study of ciltacabtagene autoleucel in heavily pretreated patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2023;41(16_suppl):8009. doi:10.1200/JCO.2023.41.16_suppl.8009
36. Mi JQ, Zhao W, Jing H, et al. Phase II, open-label study of ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor–T-cell therapy, in Chinese Patients With Relapsed/Refractory Multiple Myeloma (CARTIFAN-1). J Clin Oncol. 2023;41(6):1275–1284. doi:10.1200/JCO.22.00690
37. Hillengass J, Cohen AD, Agha ME, et al. The phase 2 CARTITUDE-2 trial: updated efficacy and safety of ciltacabtagene autoleucel in patients with multiple myeloma and 1–3 prior lines of therapy (Cohort A) and with early relapse after first line treatment (Cohort B). Blood. 2023;142(Supplement 1):1021. doi:10.1182/blood-2023-178882
38. Hillengass J, Cohen AD, Agha M, et al. The phase 2 Cartitude-2 trial: updated efficacy and safety of ciltacabtagene autoleucel in patients with multiple myeloma and 1–3 prior lines of therapy (Cohort A) and with early relapse after first line treatment (Cohort B). Transplant Cell Ther. 2024;30(2):S36–7. doi:10.1016/j.jtct.2023.12.054
39. Hillengass J, Cohen AD, Delforge M, et al. MM-183 CARTITUDE-2 Cohort A: updated clinical data and biological correlative analyses of ciltacabtagene autoleucel (cilta-cel) in lenalidomide-refractory patients with progressive multiple myeloma (MM) after 1–3 prior Lines of Therapy (LOT). Clin Lymphoma Myeloma Leuk. 2022;22:S411. doi:10.1016/S2152-2650(22)01600-7
40. Cohen AD, Mateos MV, Cohen YC, et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood. 2023;141(3):219–230. doi:10.1182/blood.2022015526
41. Arnulf B, Kerre T, Agha ME, et al. Efficacy and safety of ciltacabtagene autoleucel ± lenalidomide maintenance in newly diagnosed multiple myeloma with suboptimal response to frontline autologous stem cell transplant: CARTITUDE-2 cohort D. J Clin Oncol. 2024;42(16_suppl):7505. doi:10.1200/JCO.2024.42.16_suppl.7505
42. Sidana S, Patel KK, Peres LC, et al. Safety and efficacy of standard of care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma. Blood. 2024.
43. Worcester S. CARTITUDE-4 update: cilta-cel improves OS in R/R MM. Medscape; 2024.
44. Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the international myeloma workshop consensus panel 1. Blood. 2011;117(18):4691–4695. doi:10.1182/blood-2010-10-299487
45. Rees MJ, D’Agostino M, Leypoldt LB, Kumar S, Weisel KC, Gay F. Navigating high-risk and ultrahigh-risk multiple myeloma: challenges and emerging strategies. Am Soc Clin Oncol Educ Book. 2024;44(3):e433520. doi:10.1200/EDBK_433520
46. Cohen YC, Magen H, Gatt M, et al. Talquetamab plus Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2025;392(2):138–149.
47. Ailawadhi S, Arnulf B, Patel KK, et al. Ide-cel vs standard regimens in triple-class-exposed relapsed and refractory multiple myeloma: updated KarMMa-3 analyses. Blood. 2024;144(23):2389–2401. doi:10.1182/blood.2024024582
48. Hansen DK, Peres LC, Dima D, et al. Comparative safety and efficacy of ciltacabtagene autoleucel and idecabtagene vicleucel CAR T-cell therapies in relapsed or refractory multiple myeloma. Blood. 2024;144:936.
49. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, Phase 3 study. Lancet. 2020;396(10245):186–197. doi:10.1016/S0140-6736(20)30734-0
50. Mohan M, Van Oekelen O, Akhtar OS, Cohen A, Parekh S. Charting the course: sequencing immunotherapy for multiple myeloma. Am Soc Clin Oncol Educ Book. 2024;44(3):e432204. doi:10.1200/EDBK_432204
51. Fonseca R, Usmani SZ, Mehra M, et al. Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer. 2020;20(1):1087. doi:10.1186/s12885-020-07503-y
52. Goldman‐Mazur S, Kumar SK. Current approaches to management of high‐risk multiple myeloma. Am J Hematol. 2021;96(7):854–871. doi:10.1002/ajh.26161
53. Greipp PR, San Miguel J, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi:10.1200/JCO.2005.04.242
54. Dytfeld D, Dhakal B, Agha M, et al. Bortezomib, lenalidomide and dexamethasone (VRd) followed by ciltacabtagene autoleucel versus Vrd followed by lenalidomide and dexamethasone (Rd) maintenance in patients with newly diagnosed multiple myeloma not intended for transplant: a randomized, phase 3 study (CARTITUDE-5). Blood. 2021;138(Supplement 1):1835.
55. Boccadoro M, San-Miguel J, Suzuki K, et al. DVRd followed by ciltacabtagene autoleucel versus DVRd followed by ASCT in patients with newly diagnosed multiple myeloma who are transplant eligible: a randomized phase 3 study (EM agine/CARTITUDE-6). Blood. 2022;140(Supplement 1):4630–4632. doi:10.1182/blood-2022-157021
56. Gertz M. Smoldering multiple myeloma: reviewing the rationale for intervention. Leuk Lymphoma. 2022;63(9):2033–2040. doi:10.1080/10428194.2022.2068008
57. Rajkumar SV, Kumar S, Lonial S, Mateos MV. Smoldering multiple myeloma current treatment algorithms. Blood Cancer J. 2022;12(9):129. doi:10.1038/s41408-022-00719-0
58. Nadeem O, Nikiforow S, DeBraganca K, et al. Early safety and efficacy of CAR-T cell therapy in precursor myeloma: results of the CAR-PRISM study using ciltacabtagene autoleucel in high-risk smoldering myeloma. Blood. 2024;144:1027. doi:10.1182/blood-2024-202676
59. Zhao J, Yang H, Ge J, et al. Pomalidomide improves the effectiveness of CAR-T treatment in the relapsed and refractory multiple myeloma or B-cell leukemia/lymphoma with extramedullary disease. Blood Sci. 2024;6(2):e00184. doi:10.1097/BS9.0000000000000184
60. Yan Y, Tu Y, Wu DP, Li X. BCMA CAR-T-cell therapy in combination with long-term pomalidomide is a safe and effective treatment for relapsed/refractory multiple myeloma. Blood. 2023;142(Supplement 1):2116. doi:10.1182/blood-2023-181573
61. Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308.
62. Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell–redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232–2244. doi:10.1056/NEJMoa2204591
63. Moreau P, Garfall AL, Van De Donk NWCJ, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495–505. doi:10.1056/NEJMoa2203478
64. Westin JR, Oluwole OO, Kersten MJ, et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med. 2023;389(2):148–157. doi:10.1056/NEJMoa2301665
65. Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85–96. doi:10.1038/s41577-021-00547-6
66. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi:10.1016/j.bbmt.2018.12.758
67. Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. JNCI J Natl Cancer Inst. 2019;111(7):646–654. doi:10.1093/jnci/djz017
68. Cohen AD, Parekh S, Santomasso BD, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022;12(2):32. doi:10.1038/s41408-022-00629-1
69. Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. doi:10.1056/NEJMoa1817226
70. Gust J. BCMA-CAR T-cell treatment–associated parkinsonism. Blood. 2023;142(14):1181–1183. doi:10.1182/blood.2023021860
71. Graham CE, Lee WH, Wiggin HR, et al. Chemotherapy-induced reversal of ciltacabtagene autoleucel–associated movement and neurocognitive toxicity. Blood. 2023;142(14):1248–1252. doi:10.1182/blood.2023021429
72. Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–2221. doi:10.1182/blood-2018-12-893396
73. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. U.S. Department of Health and Human Services; 2017.
74. Banerjee R, Poh C, Hirayama AV, et al. Answering the “Doctor, can CAR-T therapy cause cancer?” question in clinic. Blood Adv. 2024;8(4):895–898. doi:10.1182/bloodadvances.2023012336
75. Zanwar S, Jacob EK, Greiner C, et al. The immunome of mobilized peripheral blood stem cells is predictive of long-term outcomes and therapy-related myeloid neoplasms in patients with multiple myeloma undergoing autologous stem cell transplant. Blood Cancer J. 2023;13(1):151. doi:10.1038/s41408-023-00920-9
76. Tix T, Alhomoud M, Shouval R, et al. Second primary malignancies after CAR T-cell therapy: a systematic review and meta-analysis of 5,517 lymphoma and myeloma patients. Clin Cancer Res. 2024;30(20):4690–4700. doi:10.1158/1078-0432.CCR-24-1798
77. Harrison SJ, Nguyen T, Rahman M, et al. CAR+ T-cell lymphoma post ciltacabtagene autoleucel therapy for relapsed refractory multiple myeloma. Blood. 2023;142(Supplement 1):6939. doi:10.1182/blood-2023-178806
78. Hamilton MP, Sugio T, Noordenbos T, et al. Risk of second tumors and T-cell lymphoma after CAR T-cell therapy. N Engl J Med. 2024;390(22):2047–2060. doi:10.1056/NEJMoa2401361
79. Elsallab M, Ellithi M, Lunning MA, et al. Second primary malignancies after commercial CAR T-cell therapy: analysis of the FDA adverse events reporting system. Blood. 2024;143(20):2099–2105. doi:10.1182/blood.2024024166
80. Kobbe G, Brüggemann M, Baermann BN, et al. Aggressive lymphoma after CD19 CAR T-cell therapy. N Engl J Med. 2024;391(13):1217–1226. doi:10.1056/NEJMoa2402730
81. Musto P, Anderson KC, Attal M, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol. 2017;28(2):228–245. doi:10.1093/annonc/mdw606
82. Lesokhin AM, Tomasson MH, Arnulf B, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29(9):2259–2267. doi:10.1038/s41591-023-02528-9
83. Hungria V, Robak P, Hus M, et al. Belantamab mafodotin, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2024;391(5):393–407. doi:10.1056/NEJMoa2405090
84. Banerjee R, Richards A, Khouri J, Janakiram M, Cicero KI, Dima D. Post-CAR-T driving restrictions after week 4 appear unnecessary: data from the United States myeloma immunotherapy consortium. Blood. 2024;144:3765. doi:10.1182/blood-2024-199609
85. Ferreri CJ, Hildebrandt MAT, Hashmi H, et al. Real-world experience of patients with multiple myeloma receiving ide-cel after a prior BCMA-targeted therapy. Blood Cancer J. 2023;13(1):117. doi:10.1038/s41408-023-00886-8
86. Riedhammer C, Bassermann F, Besemer B, et al. Real-world analysis of teclistamab in 123 RRMM patients from Germany. Leukemia. 2024;38(2):365–371. doi:10.1038/s41375-024-02154-5
87. Dima D, Vazquez-Martinez MA, Davis JA, et al. Outcomes of Teclistamab (Tec) in patients with Relapsed/Refractory Multiple Myeloma (RRMM) with prior exposure to BCMA-Directed Therapy (BCMA-DT): a multicenter study from the U.S. Multiple Myeloma Immunotherapy Consortium. Blood. 2024;144(Supplement 1):897.
88. Dima D, Davis JA, Ahmed N, et al. Safety and efficacy of teclistamab in patients with relapsed/refractory multiple myeloma: a real-world experience. Transplant Cell Ther. 2024;30(3):308.e1–308.e13. doi:10.1016/j.jtct.2023.12.016
89. Puertas B, Fernández-Sánchez A, Alejo E, et al. A research center’s experience of T-cell–redirecting therapies in triple-class refractory multiple myeloma. Blood Adv. 2024;8(13):3478–3487. doi:10.1182/bloodadvances.2024012773
90. Ravi G, Bal S, Joiner L, et al. Subsequent therapy and outcomes in patients with newly diagnosed multiple myeloma experiencing disease progression after quadruplet combinations. Br J Haematol. 2024;204(4):1300–1306. doi:10.1111/bjh.19303
91. Martin T, Raje NS, Miguel JS, et al. MM-382 iMMagine-3: a phase 3, randomized study to compare the efficacy and safety of anitocabtagene autoleucel (Anito-Cel) with standard of care in patients with Relapsed/Refractory Multiple Myeloma (RRMM). Clin Lymphoma Myeloma Leuk. 2024;24:S554–5. doi:10.1016/S2152-2650(24)01676-8
92. Freeman CL, Dhakal B, Kaur G, et al. Phase 2 registrational study of anitocabtagene autoleucel for the treatment of patients with relapsed and/or refractory multiple myeloma: preliminary results from the IMMagine-1 trial. Blood. 2024;144(Supplement 1):1031. doi:10.1182/blood-2024-198499
93. Bal S, Anderson LD, Nadeem O, et al. Efficacy and safety with extended follow-up in a phase 1 study of BMS-986393, a G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)-Targeted CAR T cell therapy, in patients (pts) with heavily pretreated Relapsed/Refractory (RR) Multiple Myeloma (MM). Blood. 2024;144(Supplement 1):922. doi:10.1182/blood.2024025628
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.