Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Circulating-Water Mattress Enhances Thermal Comfort and Patient Satisfaction but Shows Non-Superiority in Temperature Maintenance in Ophthalmic Day-Case Surgery: A Randomized Controlled Trial
Authors Yan Y , Geng J , Xi C, Cui X, Wang G
Received 17 January 2025
Accepted for publication 5 May 2025
Published 15 May 2025 Volume 2025:21 Pages 669—680
DOI https://doi.org/10.2147/TCRM.S514218
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor De Yun Wang
Yanhong Yan,1,* Jiao Geng,2,* Chunhua Xi,1 Xu Cui,1 Guyan Wang1
1Department of Anesthesiology, Beijing Tongren Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guyan Wang, Department of Anesthesiology, Beijing Tongren Hospital, Capital Medical University, No. 1 Dongjiaominxiang, Beijing, 100730, People’s Republic of China, Tel +86 13910985139, Fax +86 01058268017, Email [email protected]
Purpose: Perioperative hypothermia, a common complication of general anesthesia, is associated with adverse outcomes. While active warming methods are recommended, the effectiveness of circulating-water mattresses during ophthalmic surgeries remains understudied. This randomized controlled trial assessed whether a circulating-water mattress combined with a cotton quilt (Group W) was superior to a cotton quilt alone (Group C) in maintaining patient body temperature during ophthalmic day-case surgery.
Patients and Methods: Group W patients (n=39) used a preheated circulating-water mattress (38°C) on the operating table and were covered with a cotton quilt (from their entry to the operating room until they returned to the ward). Group C patients (n=38) lay on an unheated table and were covered with a cotton quilt. The axillary temperature in the surgical waiting area served as the baseline. Axillary temperature, ambient temperature, heart rate, and mean arterial pressure were documented at anesthesia induction (T0), start of surgery (T1), every 5 min for the first hour (T2–T13), and end of surgery (T14). Satisfaction and thermal comfort scores were assessed at baseline in the waiting area, 5 min before anesthesia induction (t0), 15 min after entering the post-anesthesia care unit (PACU) (t1), and upon leaving the PACU (t2). The primary outcome measure was the axillary temperature at T14.
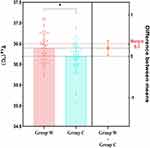
Results: At T14, Group W had a higher axillary temperature than Group C (36.40± 0.06°C vs 36.18± 0.06°C, P=0.011), with the mean difference 0.22°C, which did not exceed the predefined superiority threshold of 0.30°C, indicating that the warming effect in Group W was not superior to that in Group C. However, thermal comfort and satisfaction scores were significantly enhanced at t0 and t1 (P< 0.05).
Conclusion: During ophthalmic day-case surgeries, a circulating-water mattress plus a cotton quilt was not superior, regarding their warming effect, but they significantly enhanced patients’ thermal comfort and satisfaction.
Keywords: active warming, passive warming, ophthalmic day-case surgery, wireless axillary temperature monitoring
Introduction
Ophthalmic surgeries under general anesthesia are often indicated for patients with comorbidities, elderly patients unable to cooperate, and procedures with longer duration. These patients are at a higher risk of perioperative hypothermia, defined as a core temperature below 36.0°C, with an incidence rate ranging from 50% to 90%.1–4 Despite the small incisions and lack of significant fluid shifts in ophthalmic surgeries, hypothermia remains a concern, which may affect patient comfort and recovery. A study found that even with local anesthesia for vitreoretinal surgery, the hypothermia rate reached 44.6% without active warming measures.5
Perioperative hypothermia is associated with several adverse outcomes. It increases the risk of surgical site infections, as hypothermia impairs leukocyte function and reduces oxygen tension in tissues.6 Additionally, hypothermia can lead to shivering, which not only causes patient discomfort but may also increase metabolic demands and oxygen consumption.7 Furthermore, hypothermia is linked to increased blood product transfusion requirements due to its effects on coagulation and platelet function.8
Various warming methods have been employed to maintain normothermia during surgeries. Active warming techniques, such as forced-air warming, are commonly used and have been shown to reduce the incidence of perioperative hypothermia.9 However, the effectiveness of circulating-water mattresses, another form of active warming, in maintaining body temperature during ophthalmic surgeries remains unclear.
Accurate temperature monitoring is crucial for effective perioperative thermal management. In this study, we utilized the iThermonitor, a wireless axillary temperature monitoring device that offers several advantages over traditional methods, which include its accuracy and reliability in reflecting core body temperature, as well as its strong correlation with esophageal temperature measurements.10–13 Its non-invasive nature and real-time data transmission capabilities make iThermonitor particularly suitable for use in ophthalmic surgeries where a laryngeal mask airway (LMA) is employed for general anesthesia, prioritizing patient comfort and reducing local tissue damage.
In this study, we aim to investigate the effects of a circulating-water mattress combined with a cotton quilt on body temperature maintenance, thermal comfort, and patient satisfaction during ophthalmic day-case surgeries under general anesthesia. We hope to provide evidence-based recommendations for perioperative temperature management in this specific patient population.
Materials and Methods
Inclusion and Exclusion Criteria
This study was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University, and registered with the Chinese Clinical Trial Registration website (ChiCTR2400081934) on March 15, 2024. All patients provided informed consent. The research protocol complied with the Consolidated Standards of Reporting Trials (CONSORT) statement and the Helsinki Declaration.
The inclusion criteria comprised adult patients scheduled for ophthalmic day-case surgeries under general anesthesia at Beijing Tongren Hospital between March 16, 2024, and June 30, 2024. The exclusion criteria comprised refusal to participate, surgery with an anticipated duration <30 min, axillary area damage that would hinder probe placement, and allergy to the monitoring equipment.
Randomization and Blinding
A computer-generated random sequence was used to ensure equal probability of allocation of eligible participants to Group W (circulating-water mattress combined with cotton quilt) or Group C (cotton quilt alone). A nurse not involved in the study conducted the randomization process using SPSS 27.0 (IBM Corp., Armonk, NY, USA), ensuring unbiased allocation.
Allocation concealment was maintained using sequentially numbered (from 1 to 80), sealed, opaque envelopes. Before each surgery, an anesthesia nurse opened the envelope and followed the instructions, activating the circulating-water mattress (HICO-VARIOTHERM 550, Hirtz, Germany) if the patient was in Group W. The operation panel of the water mattress was covered to prevent anesthesiologists from knowing the group allocation. Anesthesiologists and data collectors were blinded to group allocation, while participants were not blinded due to the nature of the interventions. This could potentially influence their comfort ratings or satisfaction. However, the blinding of anesthesiologists and data collectors helped minimize bias in the measurement of primary outcomes. Additionally, the study design and analysis methods were robust enough to account for any potential subjective bias in patient-reported outcomes.
Warming Methods
In Group W, prior to patient entry, a dedicated nurse set the circulating-water mattress on the operating table to 38°C (which was maintained throughout the surgery), and each patient was covered with a cotton quilt for insulation. In Group C, each patient was covered with a cotton quilt during surgery; however, a warming mattress was not utilized. The laminar airflow temperature in the operating room was maintained at 22°C–25°C. All patients were positioned in the supine position, which remained constant throughout the procedure. The choice of anesthetics was at the discretion of the attending anesthesiologists. After surgery, patients were fully awakened and transferred to the post-anesthesia care unit (PACU) for continued observation.
Body Temperature Monitoring
A wireless axillary temperature monitoring device, the iThermonitor (Raiing Medical, Boston, MA, USA), continuously monitored axillary temperature. Prior to surgery, in the waiting area, the probe was placed in the axilla contralateral to the intravenous infusion site to minimize potential thermal interference. It was attached to the skin with hypoallergenic adhesive, trimming axillary hair as needed for secure placement. Patients were instructed to press their arm snugly against their body for at least 5 min to stabilize the initial temperature reading. The probe collected temperature data every 4 s and wirelessly transmitted it to a mobile app, providing real-time temperature monitoring. After surgery, once fully awake, patients had the probe removed before being transferred to the PACU.
Remedial Measures
If a patient’s temperature fell below 35.0°C, the circulating-water mattress’s temperature was immediately increased and other measures were implemented, including using a forced-air warming device (Bair Hugger Blanket, 3M Health Care, St. Paul, MN, USA) set to 42.0°C and increasing the operating room temperature until the patient’s body temperature returned to normal. The patient was excluded from the study. Similarly, if a patient’s temperature exceeded 37.5°C, the warming measures were temporarily halted, and the patient was also excluded. Data from excluded patients were still considered for analysis up to the point of exclusion to maintain data integrity and provide insights into the robustness of the warming methods under real-world conditions where temperature fluctuations may occur.
Data Recording
Patient age, gender, body mass index (BMI), and American Society of Anesthesiologists (ASA) physical status, along with surgery duration and fluid infusion volume, were recorded. In both groups, axillary temperature in the surgical waiting area was noted as a baseline value. Temperature, mean arterial pressure (MAP), and heart rate (HR) were recorded at anesthesia induction (T0), the start of surgery (T1), every 5 min for the first hour of surgery (T2–T13), and the end of surgery (T14).
Patient satisfaction (using the “discomfort” dimension14 of the Chinese version of the EVAN-G scale (EVAN-GC),15 where 0 indicates extremely dissatisfied, 100 indicates completely satisfied, and >80 indicates high satisfaction) and thermal comfort (using a verbal numerical scale,14 where 0 indicates extreme cold, 100 indicates extreme heat, and 50 indicates a comfortable warmth) were assessed at baseline in the surgical waiting area, 5 min before anesthesia induction (t0), 15 min after entering the PACU (t1), and upon leaving the PACU (t2).
Additionally, the occurrence of severe shivering (grade 3, with obvious tremors in the trunk and limbs) was assessed in the PACU using the Bedside Shivering Assessment Scale.16
Statistical Analysis
The primary outcome measure was axillary temperature in the two groups at T14. The secondary outcome measures were axillary temperature in the two groups in the surgical waiting area and at T0–T13, MAP and HR at the same time points, thermal comfort and satisfaction scores in the surgical waiting area and at t0–t2, and rate of severe shivering in the PACU.
Axillary temperatures at various time points (T0–T14) in Groups W and C were compared using a repeated-measures analysis of covariance (ANCOVA; with the baseline axillary temperature as a covariate). The assumption of sphericity was verified using Mauchly’s test, and if violated, the Greenhouse–Geisser correction was intended to be applied.
Thermal comfort and satisfaction scores at various time points (t0–t2) in Groups W and C were analyzed using generalized estimating equation (GEE) analysis with covariates (with the baseline thermal comfort and satisfaction as covariates). If there was a significant time×group interaction effect (P<0.05), separate effect analyses for group and time were intended to be conducted; otherwise, the main analysis was intended to focus on the overall effect of time and group.
Continuous data were tested for normality using the Shapiro–Wilk test and for homogeneity of variance using Levene’s test. Normally distributed data with equal variances are presented as mean ± SD and compared using independent-samples t-tests. Non-normally distributed data are presented as median (IQR) and compared using the Mann–Whitney U-test. Categorical data are presented as frequency (percentage) and compared using the Chi-Square test or Fisher’s exact test, as appropriate.
Statistical significance was set at P<0.05. All analyses were performed using SPSS 27.0 (IBM Corp., Armonk, NY, USA), GraphPad Prism 10.0 (GraphPad Software, San Diego, CA, USA), and Origin 2021 (OriginLab Corp., Northampton, MA, USA).
Sample Size Calculation
The sample size was calculated using PASS 15.0 (NCSS LLC, Kaysville, UT, USA) to ensure adequate power. The calculation was based on previous literature and preliminary experimental results, with a predefine superiority threshold of 0.3°C.17 The significance level (α) was set at 0.025 and the power (1-β) at 0.8, aiming to detect a between-group difference in axillary temperature at the end of the surgery. In a preliminary experiment, the difference in axillary temperature was 0.61°C, with SDs of 0.52°C and 0.38°C in Groups W and C, respectively. Accounting for an estimated dropout rate of approximately 10%, the sample size required to achieve significance was 80 patients, allocated equally across both groups (40 patients per group).
Results
Study Sample
Among the 85 screened patients, 5 were excluded (3 declined to participate and 2 did not fulfill the eligibility criteria as the anticipated duration was <30 min), leading 80 to be randomized. Among these 80, a further 3 were excluded (due to actual surgery duration <30 min), leading to a final analysis of 77 patients (39 in Group W and 38 in Group C) (Figure 1). There were no significant between-group differences in demographics, ASA physical status, surgery duration, operating room temperature, time-weighted average (TWA)-MAP, TWA-HR, or fluid infusion volume (P>0.05) (Table 1).
 |
Table 1 Patient Characteristics and Surgical Parameters of Ophthalmic Day-Case Surgery Patients in Group W (Circulating-Water Mattress Combined With Cotton Quilt) and Group C (Cotton Quilt) |
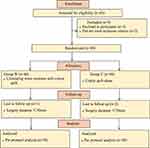 |
Figure 1 Flow chart. |
Axillary Temperature
Two-way repeated-measures ANCOVA was used to assess the impact of Group W versus Group C on axillary temperature. Based on Cook’s distance results and expert judgment, no data were identified as outliers requiring special handling. Normality testing of residuals (Shapiro–Wilk test) indicated that the data at each time point conformed to a normal distribution. Tests for homogeneity of variance (Levene’s test) suggested that between-group variances at each time point were equal. Sphericity testing revealed a violation of the sphericity assumption (P<0.001). There was a time×group interaction effect (Ftime×group=2.161, P=0.02), necessitating separate effect analyses.
Group W exhibited no significant changes in axillary temperature over time (F=1.157, P=0.331), whereas Group C exhibited significant decreases (F=4.633, P<0.001). Notably, these changes (compared to T0) first reached significance at T5 and persisted until the end of the surgery at T14.
There were significant between-group differences in axillary temperature from T7 to T14 (P<0.05). In particular, the mean temperature difference of 0.22°C (95% CI: 0.05°C–0.39°C) at T14 was statistically significant (P=0.011), but did not meet the predefined superiority threshold of 0.3°C, indicating that the warming effect of the circulating-water mattress was not clinically superior to the cotton quilt alone. (Table 2 and Figure 2).
 |
Table 2 Comparison of Axillary Temperature in Ophthalmic Day-Case Surgery Patients Between Group W (Circulating-Water Mattress Combined With Cotton Quilt) and Group C (Cotton Quilt) |
Thermal Comfort
A GEE analysis with covariates was used to investigate the impact of Group W versus Group C on the patients’ thermal comfort. There was a time×group interaction effect (Wald χ2time×group =267.004, P<0.001), necessitating separate effect analyses.
Within-Group Comparisons
Thermal comfort scores exhibited significant temporal variation in Group C (P < 0.001), but not in Group W (P = 0.08).
In Group W: Thermal comfort scores showed minor fluctuations over time, with no statistically significant changes.
In Group C: Thermal comfort scores increased by 8.82 points at t1 compared to t0 (P < 0.001), increased by 15.92 points at t2 compared to t0 (P < 0.001), and increased by 7.11 points at t2 compared to t1 (P < 0.001) (Figure 3A).
Between-Group Comparisons
Significant between-group differences in thermal comfort were observed at t0 and t1 (P < 0.001).
At t0: Group W reported 16.49 points higher thermal comfort than Group C (P < 0.001).
At t1: Group W reported 7.03 points higher thermal comfort than Group C (P < 0.001).
At t2: The between-group difference was not statistically significant (P = 0.678) (Figure 3B).
Patient Satisfaction
A GEE analysis with covariates was used to investigate the impact of Group W versus Group C on patient satisfaction. There was a time×group interaction effect (Wald χ2time×group =201.212, P<0.001) necessitating separate effect analyses.
Within-Group Findings
Both groups exhibited significant temporal variations in satisfaction scores (P < 0.05).
In Group W: Satisfaction scores decreased by 2.31 points at t1 compared to t0 (P < 0.001), by 1.54 points at t2 compared to t0 (P = 0.002), and increased by 0.77 points at t2 compared to t1 (P = 0.023).
In Group C: Satisfaction scores increased by 7.11 points at t1 compared to t0 (P < 0.001), by 8.82 points at t2 compared to t0 (P < 0.001), and by 1.71 points at t2 compared to t1 (P < 0.001) (Figure 3C).
Between-Group Findings
There were significant between-group differences in satisfaction at t0 and t1 (P<0.05). Specifically, satisfaction score was 11.09 points higher in Group W than Group C at t0 (P<0.001), 1.67 points higher at t1 (P=0.018), and not significant at t2 (P=0.232) (Figure 3D).
Discussion
Patients who underwent ophthalmic day-case surgery using a circulating-water mattress combined with a cotton quilt (Group W) maintained a higher body temperature at the end of the surgery (T14) compared to those using only a cotton quilt (Group C). Although the mean temperature difference of 0.22°C did not meet our predefined superiority threshold of 0.3°C,17 the combined use of these warming methods may still provide significant benefits. Notably, patients in Group W experienced less fluctuation in body temperature and reported higher comfort and satisfaction levels at both 5 min before anesthesia induction (t0) and 15 min after entering the PACU (t1). These results suggest potential advantages to using a circulating-water mattress in ophthalmic day-case surgeries.
Temperature Variations and Their Clinical Relevance
A circulating-water mattress, as an active warming intervention, is engineered to minimize the temperature gradient between the body’s core and periphery, thereby reducing peripheral heat loss and ensuring core temperature stability.18 Its aqueous medium, due to superior thermal conductivity, should theoretically offer enhanced insulation, effectively transferring heat across the contact area.19 A randomized controlled trial of healthy volunteers indicated that a circulating-water system doubled the speed of the warming process compared to a conventional forced-air warming blanket utilized in clinical settings.20 Additionally, a study involving patients undergoing abdominal surgery showed that this system promotes a more rapid restoration of normal body temperature, supporting its potential utility in surgery.21
Consistent with prior studies, our results highlight the benefits of using a circulating-water mattress in ophthalmic day-case surgeries. While patients using the mattress exhibited less variation in body temperature, the mean temperature difference at the end of surgery (T14) did not exceed the predefined superiority threshold of 0.3°C.17 This may be due to the minimally invasive nature of ophthalmic surgeries, which involve less tissue trauma and fluid volume changes.
Contrasting views exist regarding the clinical value of circulating-water mattresses. A randomized controlled trial in elective open abdominal surgery patients reported significantly higher temperature fluctuations with circulating-water mattresses compared to forced-air or carbon-fiber electric heating blankets.22 Similarly, a study on laparoscopic cholecystectomy highlighted the superior temperature maintenance related to a forced-air blanket or a carbon-fiber electric heating blanket compared to a circulating-water mattress.23 This discrepancy may stem from the fact that, compared to ophthalmic surgery, the anterior part of the body is more exposed in abdominal surgery, leading to greater heat dissipation, especially in open abdominal surgery. The circulating-water warming mattress only heats the back area, which is insufficient to compensate for the heat loss caused by radiation and convection on the anterior part of the body.19
Furthermore, under general anesthesia, the body primarily loses heat through the skin. A cotton quilt, acting as a thermal barrier between the skin and the external environment, reduces skin heat loss by approximately 30%,24 which might explain why the circulating water mattress is non-superior to the cotton quilt for ophthalmic day-case surgeries. However, there is insufficient evidence to conclusively support the efficacy of a cotton quilt at maintaining body temperature during ophthalmic procedures.
Clinical Significance and Generalizability
The mean temperature difference of 0.22°C between Group W (circulating-water mattress combined with cotton quilt) and Group C (cotton quilt alone) at T14, although statistically significant (P=0.011), did not surpass the superiority threshold. However, this difference may still hold clinical relevance. Previous studies have demonstrated that even small temperature fluctuations during surgery can impact patient outcomes, such as reducing the risk of surgical site infections and minimizing metabolic stress.8,25 In our study, while no significant hemodynamic instability was observed between groups, likely attributable to our study population of ASAIand IIpatients undergoing outpatient procedures. This finding assumes greater clinical relevance when extrapolated to more vulnerable populations, such as elderly patients or those with comorbidities, who are more susceptible to temperature fluctuations due to compromised thermoregulatory mechanisms. In such cases, the use of circulating-water mattresses could offer greater benefits in maintaining perioperative homeostasis.
The generalizability of our findings is limited by the study’s focus on a specific patient population (primarily ASA I and II patients) and the absence of long-term follow-up. While no significant differences in hemodynamic stability or shivering incidence were observed, these outcomes may differ in more complex surgical settings or in patients with compromised thermoregulatory function. Larger, multicenter studies with diverse patient populations and extended follow-up periods are needed to confirm the broader applicability of these results.
Enhancing Patient Experience through Thermal Comfort and Satisfaction
A key strength of this study is the assessment of thermal comfort and patient satisfaction, which are critical components of perioperative care. Compared to Group C patients, Group W patients reported higher thermal comfort and satisfaction levels 5 min before anesthesia induction (t0) and 15 min after entering the PACU (t1). Thermal comfort, which is approximately 50% influenced by skin surface temperature,26 is significantly enhanced by the use of surface warming devices.27,28 Consistent with the previous studies, our patients with the added circulating-water mattress experienced notably higher thermal comfort before anesthesia induction (t0). However, upon entering the PACU, the inability to use the circulating-water mattress on the transfer bed led to a minor non-significant decrease in thermal comfort at both t1 and t2 compared to t0 in group W.
The satisfaction score was measured using the EVAN-GC scale,15 which has high practicality and stability for assessing patients under general anesthesia.14,15,29 Our findings indicate that subjective warmth is positively associated with higher satisfaction, but thermal comfort alone does not fully dictate satisfaction levels. Notably, in Group W, satisfaction scores were higher at t0 (5 min before anesthesia induction) and t1 (15 min after entering the PACU) compared to Group C, but the differences were not statistically significant at t2 (upon leaving the PACU). Similarly, thermal comfort scores showed no statistically significant changes over time in Group W. This suggests that factors beyond thermal comfort, such as the Hawthorne effect, may have influenced satisfaction outcomes. The Hawthorne effect,14 where individuals may alter their behavior or perceptions due to awareness of being observed, could have led patients in Group W to report higher satisfaction at t0 and t1 due to the perceived additional care associated with the circulating-water mattress. However, this effect diminished over time, as evidenced by the lack of significant differences in satisfaction scores at t2. Additionally, other factors such as postoperative care or patient expectations may have contributed to the observed patterns in satisfaction.
No cases of severe shivering were observed in either group. Anesthetic agents dose-dependently depress the shivering threshold.7 Once the anesthetic effects begin to wane post-surgery, the inhibition of shivering lessens, potentially increasing the risk of shivering, particularly among hypothermic patients.25,30 In this study, the body temperatures of patients in both groups at the end of surgery were above the shivering threshold (35.5°C±0.5°C).7 Additionally, the use of a cotton quilt in the PACU likely mitigated the risk of shivering, contributing to the observed absence of severe episodes.
Limitations and Future Directions
This study has several limitations and areas for future research. First, the decision to use circulating-water mattresses instead of forced-air blankets was based on cost and staff comfort considerations; however, the relative benefits of forced-air blankets for patient warming require further validation. Second, the temperature management protocol was informed by the advice of Sessler et al that maintaining a core temperature >35.5°C is generally sufficient to avoid adverse patient outcomes, thereby potentially reducing material and labor costs.31 However, given the possible ±0.5°C variance between the iThermonitor readings and actual lower esophageal temperatures,10 remedial measures were proactively implemented at an axillary temperature of 35.0°C. The rationale for this threshold and its impact on prognosis should be corroborated with data from larger studies to ensure the generalizability of these findings. Third, the clinical significance of the 0.22°C temperature difference observed between groups, while statistically significant, remains unclear and warrants exploration in future research, particularly in vulnerable populations such as elderly patients or those with comorbidities. Long-term follow-up data would also provide valuable insights into the broader applicability of these findings.
Conclusion
This randomized controlled trial shows that using a circulating-water mattress during ophthalmic day-case surgeries offers benefits in enhancing patient thermal comfort and reducing perioperative temperature fluctuations, despite not surpassing the efficacy of a standard cotton quilt in temperature maintenance. The 0.22°C temperature difference, though statistically significant, did not meet the predefined superiority threshold. However, it may still be clinically relevant, especially for vulnerable populations. Our findings are limited to ASA I and II patients, and further research is needed for broader applicability. The circulating-water mattress is practical for improving patient experience and could be valuable in clinical practice. Future studies should explore its use in more diverse surgical settings and patient populations.
Data Sharing Statement
The dataset supporting the findings of this study is available upon reasonable request to the corresponding author, [Guyan Wang], at [[email protected]]. Requests for access to the data will be reviewed by the corresponding author and data access may be granted, subject to any necessary approvals and agreements to protect the privacy and confidentiality of participants.
Ethics Approval
This study was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University and registered with the Chinese Clinical Trial Registration website (ChiCTR2400081934) on March 15, 2024.
Acknowledgments
We thank XinXin Hao and Ying Liu for their assistance in preparing the envelopes for allocation concealment and for setting up the warming methods. We are also grateful to Yu Gong and Xiuhua Cui for their contributions to data collection and follow-up.
This study was previously posted as a preprint on SSRN. The preprint can be accessed at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5051144#:~:text=Conclusions,-This%20randomized%20controlled&text=While%20the%20circulating%2Dwater%20mattress,reducing%20perioperative%20body%20temperature%20fluctuations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Beijing Hospitals Authority’s Ascent Plan [grant number DFL20220203]. The funding organization had no role in the study design, data collection, or the decision to publish the results.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sari S, Aksoy SM, But A. The incidence of inadvertent perioperative hypothermia in patients undergoing general anesthesia and an examination of risk factors. Int J Clin Pract. 2021;75(6). doi:10.1111/ijcp.14103
2. Moola S, Lockwood C. Effectiveness of strategies for the management and/or prevention of hypothermia within the adult perioperative environment. Int J Evid Based Hea. 2011;9(4):337–345. doi:10.1111/j.1744-1609.2011.00227.x
3. Torossian A, Brauer A, Hocker J, Bein B, Wulf H, Horn EP. Preventing inadvertent perioperative hypothermia. Dtsch Arztebl Int. 2015;112(10):166–172. doi:10.3238/arztebl.2015.0166
4. Riley C, Andrzejowski J. Inadvertent perioperative hypothermia. Bja Educ. 2018;18(8):227–233. doi:10.1016/j.bjae.2018.05.003
5. Urfalioglu A, Urfalioglu S, Oksuz G, et al. The effects of active warming on perioperative inadvertent hypothermia in patients undergoing vitreoretinal surgery under local anesthesia. Indian J Ophthalmol. 2021;69(2):308–313. doi:10.4103/ijo.IJO_227_20
6. Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358(9285):876–880. doi:10.1016/S0140-6736(01)06071-8
7. De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002;96(2):467–484. doi:10.1097/00000542-200202000-00036
8. Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277(14):1127–1134. doi:10.1001/jama.1997.03540380041029
9. Pu Y, Cen G, Sun J, et al. Warming with an underbody warming system reduces intraoperative hypothermia in patients undergoing laparoscopic gastrointestinal surgery: a randomized controlled study. Int J Nurs Stud. 2014;51(2):181–189. doi:10.1016/j.ijnurstu.2013.05.013
10. Pei L, Huang Y, Mao G, Sessler DI. Axillary temperature, as recorded by the iThermonitor WT701, well represents core temperature in adults having noncardiac surgery. Anesth Analg. 2018;126(3):833–838. doi:10.1213/ANE.0000000000002706
11. Ji Y, Han D, Han L, Xie S, Pan S. The accuracy of a wireless axillary thermometer for core temperature monitoring in pediatric patients having noncardiac surgery: an observational study. J Perianesth Nurs. 2021;36(6):685–689. doi:10.1016/j.jopan.2021.02.008
12. Dai Y, Luo M, Liu F, Feng X, Nie H. Temperature measurements of a wearable and wireless axillary sensor iThermonitor but not a bladder probe represents the core temperature during laparoscopic rectal surgery. J Clin Monit Comput. 2023;37(1):303–309. doi:10.1007/s10877-022-00892-4
13. Yan Y, Geng J, Cui X, Lei G, Wu L, Wang G. Thoracic paravertebral block decreased body temperature in thoracoscopic lobectomy patients: a randomized controlled trial. Ther Clin Risk Manag. 2023;19:67–76. doi:10.2147/TCRM.S392961
14. Akhtar Z, Hesler BD, Fiffick AN, et al. A randomized trial of prewarming on patient satisfaction and thermal comfort in outpatient surgery. J Clin Anesth. 2016;33:376–385. doi:10.1016/j.jclinane.2016.04.041
15. Wang X, Lin W, Liu L, Wu Z, Wu Y, Yao Y. Validation of the translated version of the EVAN-G scale in a Chinese-speaking population. Bmc Anesthesiol. 2022;22(1). doi:10.1186/s12871-022-01909-w
16. Badjatia N, Strongilis E, Gordon E, et al. Metabolic impact of shivering during therapeutic temperature modulation. Stroke. 2008;39(12):3242–3247. doi:10.1161/STROKEAHA.108.523654
17. Fanelli A, Danelli G, Ghisi D, Ortu A, Moschini E, Fanelli G. The efficacy of a resistive heating under-patient blanket versus a forced-air warming system: a randomized controlled trial. Anesthesia Analg. 2009;108(1):199–201. doi:10.1213/ane.0b013e31818e6199
18. Cobb B, Cho Y, Hilton G, Ting V, Carvalho B. Active warming utilizing combined IV fluid and forced-air warming decreases hypothermia and improves maternal comfort during cesarean delivery: a randomized control trial. Anesthesia Analg. 2016;122(5):1490–1497. doi:10.1213/ANE.0000000000001181
19. John M, Ford J, Harper M. Peri-operative warming devices: performance and clinical application. Anaesthesia. 2014;69(6):623–638. doi:10.1111/anae.12626
20. Wadhwa A, Komatsu R, Orhan-Sungur M, et al. New circulating-water devices warm more quickly than forced-air in volunteers. Anesth Analg. 2007;105(6):1681–7,tableofcontents. doi:10.1213/01.ane.0000289534.65690.ce
21. Rein EB, Filtvedt M, Walløe L, Ræder JC. Hypothermia during laparotomy can be prevented by locally applied warm water and pulsating negative pressure. Brit J Anaesth. 2007;98(3):331–336. doi:10.1093/bja/ael369
22. Negishi C, Hasegawa K, Mukai S, Nakagawa F, Ozaki M, Sessler DI. Resistive-heating and forced-air warming are comparably effective. Anesth Analg. 2003;96(6):1683–1687. doi:10.1213/01.ANE.0000062770.73862.B7
23. Matsuzaki Y, Matsukawa T, Ohki K, Yamamoto Y, Nakamura M, Oshibuchi T. Warming by resistive heating maintains perioperative normothermia as well as forced air heating † †None of the authors has personal financial interests related to this study. Brit J Anaesth. 2003;90(5):689–691. doi:10.1093/bja/aeg106
24. Sessler DI, Mcguire J, Sessler AM. Perioperative thermal insulation. Anesthesiology. 1991;74(5):875–879. doi:10.1097/00000542-199105000-00012
25. Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387(10038):2655–2664. doi:10.1016/S0140-6736(15)00981-2
26. Frank SM, Raja SN, Bulcao CF, Goldstein DS. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J Appl Physiol. 1999;86(5):1588–1593. doi:10.1152/jappl.1999.86.5.1588
27. Kimberger O, Illievich U, Lenhardt R. The effect of skin surface warming on pre-operative anxiety in neurosurgery patients. Anaesthesia. 2007;62(2):140–145. doi:10.1111/j.1365-2044.2007.04934.x
28. Bozkurt Z, Akboğa Ö. The relationship of perioperative inadvertent hypothermia with anxiety and comfort. Ther Hypothermia Tem. 2025;15(1):31–39. doi:10.1089/ther.2023.0089
29. Maurice-Szamburski A, Auquier P, Viarre-Oreal V, et al. Effect of sedative premedication on patient experience after general anesthesia: a randomized clinical trial. JAMA. 2015;313(9):916–925. doi:10.1001/jama.2015.1108
30. Kurz A, Sessler DI, Narzt E, et al. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. J Clin Anesth. 1995;7(5):359–366. doi:10.1016/0952-8180(95)00028-g
31. Sessler DI, Pei L, Li K, et al. Aggressive intraoperative warming versus routine thermal management during non-cardiac surgery (PROTECT): a multicentre, parallel group, superiority trial. Lancet. 2022;399(10337):1799–1808. doi:10.1016/S0140-6736(22)00560-8
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.