Back to Journals » International Journal of Nanomedicine » Volume 20
Classification of Nanomaterial Drug Delivery Systems for Inflammatory Bowel Disease
Authors Wang H, Zhou F, Shen M , Ma R , Yu Q
Received 23 October 2024
Accepted for publication 16 January 2025
Published 3 February 2025 Volume 2025:20 Pages 1383—1399
DOI https://doi.org/10.2147/IJN.S502546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sachin Mali
Haichen Wang,1,* Feifei Zhou,2,* Mengdan Shen,1 Ronglin Ma,1 Qiang Yu1
1Department of Gastroenterology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School, Nanjing Medical University, Suzhou, Jiangsu, 215002, People’s Republic of China; 2Department of Gastroenterology, Suzhou City Wuzhong District Chengnan Street Community Health Service Center, Suzhou, Jiangsu, 215002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Yu; Ronglin Ma, Email [email protected]; [email protected]
Abstract: Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, primarily arises from defects in the colonic barrier, imbalances of the gut microbiota, and immune response issues. These complex causes make it difficult to achieve a complete cure. Patients with IBD frequently experience recurrent abdominal pain and bloody diarrhea, while severe cases may result in intestinal obstruction, perforation, and cancer. Lifelong maintenance therapy may thus be needed to manage these symptoms; however, traditional IBD drugs, such as 5-aminosalicylic acid, glucocorticoids, immunosuppressants, and biological agents, are often associated with problems including poor solubility, instability, and ineffective targeting, as well as causing serious side effects in non-target tissues. Nanomaterial drug delivery systems (NDDS) have recently shown great promise in optimizing drug distribution, solubility through biocompatible coatings, enhancing bioavailability via PEGylation and reducing side effects. These formulations can enhance a drug’s pharmacokinetics by modifying its properties, improve its ability to cross barriers, and boost bioavailability. In addition, NDDS can enable targeted delivery, increase local drug concentrations, improve efficacy, and reduce side effects, as well as protecting active drug molecules from immune recognition and protease degradation. The clinical use of these systems for treating IBD, however, requires further research. This review summarizes the classification of NDDS for IBD, and concludes that, despite ongoing challenges, NDDS may represent an effective treatment approach for IBD. In summary, NDDS enhance the targeted delivery of therapeutic agents to specific cells or tissues, thereby improving drug bioavailability and therapeutic efficacy. These systems effectively surmount biological barriers, facilitating efficient drug delivery to targeted sites, which is crucial for attaining optimal therapeutic outcomes. This review contributes to a deeper understanding of how the physicochemical properties of NDDS influence pharmacological behavior in vivo and can expedite their clinical translation.
Keywords: inflammatory bowel disease, nanomaterials, drug delivery, nano-delivery systems, nanomedicine applications
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by chronic gastrointestinal inflammation.1 CD can affect the entire digestive tract, while UC is confined to the colon; however, both conditions increase the risk of colorectal cancer.2 The incidence of IBD in developing regions like Asia, Eastern Europe, and Africa has risen rapidly in recent years.3 Although the causes of IBD are not fully understood, research suggests they are multifactorial, involving genetic susceptibility, immune dysfunction, environmental factors (diet, chemicals, stress), and microbial exposure,4–7 with an imbalance in gut microbiota adding further complexity to the study of IBD etiology.5,8–10
The pathophysiology of IBD involves two main aspects: impaired intestinal epithelial integrity due to increased pro-inflammatory mediators, and intestinal immune system dysfunction leading to chronic inflammation caused by immune cell aggregation and microbiota imbalance.11 Based on the pathological and physiological symptoms, traditional IBD treatments aim to reduce inflammation by lowering the immune response using drugs such as 5-aminosalicylic acid (5-ASA), corticosteroids, immunosuppressants, antibiotics, or anti-tumor necrosis factor (TNF) biological preparations.12–14 The initial standard treatment for IBD is aminosalicylates, followed by corticosteroids and then immunosuppressants, while antibiotics (eg ciprofloxacin, rifampicin) and probiotics (eg bifidobacteria, lactobacilli) are also used to address gut microbiota imbalances. If these treatments fail to control inflammation, surgery, such as colorectal resection, may be necessary.15
Regarding the above treatment methods however, clinical studies have indicated that prolonged use of drugs such as 5-ASA can cause severe side effects, including cytotoxic effects in healthy cells.16 Although sustained-release devices like nanoparticles (NPs), capsules, or tablets have been developed, their effectiveness is limited and they have only shown benefit in some patients with IBD.17–19 For example, Sandborn et al found that anti-TNF-α monoclonal antibody therapy was effective in many patients, but required a high dose.20 Additionally, up to 50% of patients experienced reduced effectiveness over time, with increasing risks of adverse reactions such as lymphoma, infections (notably tuberculosis recurrence), and lupus-like syndrome.21 In addition, traditional medicines can frequently lead to side effects like allergic reactions, nausea, and pancreatitis.21 It is therefore crucial to ensure the efficacy and safety of drugs used to treat colorectal inflammation. Advanced targeted nanomaterial drug delivery systems (NDDS) have been developed to address this issue, allowing drugs to be delivered to inflamed areas, while minimizing absorption by healthy tissues and improving drug effectiveness.
NDDS utilize nanotechnology to create nanomaterials of 1–1000 nm, similar in scale to biomolecules and viruses, which are thus ideal for cellular recognition and uptake. Nano-sized aluminum adjuvants have been used in vaccines since 1930, and lipid NPs have also recently been explored for mRNA delivery. Over 80 nanomedicines had been globally approved by 2022, including over 60 by the US Food and Drug Administration (FDA) and 30 by the European Medicines Agency (EMA).22 NDDS have been shown to enhance IBD treatment by improving drug bioavailability, protecting drugs from gastrointestinal acidity, and concentrating them at inflammation sites, thus boosting their therapeutic efficacy and minimizing drug exposure to healthy tissues.23–26 In addition, targeted NDDS can concentrate drugs at inflammation sites, thus reducing the required dosage and enhancing efficacy, by using inflammatory tissue-specific ligands to actively target specific cells.27 These findings demonstrate that targeted NDDS can improve IBD treatment by delivering drugs directly to inflamed tissues, minimizing exposure to healthy tissues and reducing side effects.28–30
Conventional novel NDDS for IBD encounter numerous challenges in effectively targeting the intestinal region. These challenges include physiological and pathological barriers, genetic variability, disease severity, and nutritional status, often resulting in non-specific tissue distribution and uncontrolled drug release. To address these challenges and clinical barriers in IBD treatment, NDDS have implemented various strategies. Among these, stimulus-responsive NDDS have been developed, which exploit stimuli such as pH, reactive oxygen species (ROS), enzymes, and redox materials to facilitate targeted drug delivery. There also exist some biologically barrier-overcoming NDDS, such as those modified with polyethylene glycol (PEG) or zwitterionic polymers, which effectively mitigate drug degradation during circulation and enhance drug stability. Furthermore, there are multimodal stimulus-responsive NDDS capable of utilizing a variety of stimuli-physical (temperature, light, ultrasound), chemical (organic compounds), biological (enzymes and glucose), and multimodal environmental stimuli—to facilitate the controlled release of drugs.
Given the rapid advancements in NDDS, we provide a comprehensive overview of NDDS classification, to support the advancement of NDDS targeting IBD. Expanding upon our research group’s previous studies on the utilization of alginate hydrogel and nano dietary fiber as drug carriers for targeting colorectal inflammation, we have allocated more attention to the discussion of polysaccharide-targeted NDDS among all the NDDS explored in this review.31
Classification of IBD NDDS
NDDS can integrate drugs via encapsulation, adsorption, and coupling, and are administered via oral, intramuscular, or intravenous routes. They can enhance efficacy and reduce side effects by influencing drug absorption, distribution, metabolism, and excretion without altering the drug’s properties or effects. Regarding IBD, targeted NDDS are categorized as inorganic or organic drug carriers, based on their physical properties: inorganic NDDS mainly include metallic and non-metallic NDDS and nanoenzymes, while organic NDDS are divided into liposomes, proteins, and polysaccharides (Figure 1).
 |
Figure 1 Classification of nanomaterial drug delivery systems in inflammatory bowel disease research. |
Inorganic NDDS
Inorganic NDDS show uniform size, stability, a large surface area, easy functionalization, and unique properties, and offer potential for synergistic therapy and targeted imaging. Inorganic NDDS are currently being explored for IBD treatment. Inorganic NDDS under investigation for IBD are summarized in Table 1, including details of their surface modifications, charge, size, and drug-carrying components.
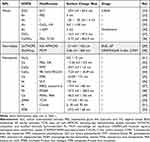 |
Table 1 Studies of Inorganic Nanomaterial Drug Delivery Systems for Inflammatory Bowel Disease |
Metallic and Non-Metallic NDDS
Metallic and non-metallic NDDS are popular drug carriers for treating colorectal inflammation, due to their easy preparation and size control. Regarding metallic NDDS, Ma et al developed a pH-responsive ZIF-8 metal-organic framework carrier that effectively cleared ROS by loading carbon nanodot-superoxide dismutase (SOD) nanozyme and the CRISPR/Cas9 system, which significantly alleviated colitis symptoms in mice (Figure 2A).40 Abdelmegid et al discovered that 5 nm naked gold NPs effectively targeted colon tissue and were absorbed into intestinal cells in mice, with no adverse effects,34 while Li et al found that gold NPs embedded in ceria with a core-shell and porous structure outperformed commercial ceria NPs in terms of enzymatic catalytic activity, possibly due to the effective exposure to catalytic sites promoting enzyme reduction.35 Lu et al created glutathione-protected gold nanoclusters that polarized macrophages toward an M2-like pro-tumoral phenotype and reduced IBD via Nrf2. These nanoclusters also significantly inhibited azoxymethane/dextran sodium sulfate (DSS)-induced colitis-associated colorectal cancer, with no major tissue damage.36 Li et al developed a synergistic treatment for DSS-induced UC in mice using ZnO nanocarriers and 5-ASA, and found that ZnO nanocarriers were more effective than ZnO microcarriers, because the nanocarriers persistently released Zn2+, effectively alleviating UC in mice by activating the cellular antioxidant defense system.32,53 Tungsten-based NPs showed promise as therapeutic agents by specifically inhibiting facultative anaerobic Escherichia coli in the intestine, maintaining intestinal barrier function, and regulating the gut microbiota.37 Qin et al studied the impact of tungsten oxide (WO3) NPs on DSS-induced acute colitis in mice, and showed that WO3NPs (47.9 nm) significantly reduced intestinal inflammation and bacterial translocation, and restored the colonic epithelial barrier and microbiota balance, compared with free tungsten (sodium tungstate). WO3 NPs primarily accumulated in the mucus layer, minimizing systemic toxicity and enhancing the therapeutic effect of tungsten on acute colitis, thus aiding IBD intervention.37 Yang et al used bionic yeast cell wall to encapsulate CaWO4, which kills harmful bacteria and provides prebiotics to reprogram the gut microbiome.38 In addition, new oral zero valent molybdenum NPs,54 PEG-modified Mo3Se4 nano flakes,55 two-dimensional Ti3C2 nanosheets,56 and tannic acid(TA)-coated hafnium disulfide nanosheets57 have all been explored in relation to IBD treatment. The Fe3+/TA network has been shown to exert good antimicrobial and antioxidant effects and also exhibit adhesion encapsulation behavior owing to physicochemical interactions. Jin et al designed a metal−polyphenol network with Fe3+ and TA to encapsulate nano@Curcumin, which exhibit good biocompatibility and can be used to treat IBD by regulating the oxidative and inflammatory response of the gastrointestinal tract.58 In addition, nanomaterials synthesized from a variety of metals are also used in the treatment of IBD. Layered double hydroxides (LDHs) are a class of inorganic materials with a layered structure, usually composed of metal cations and hydroxide ions. They are easy to synthesize, low-cost, and have good biocompatibility. Recently, Niu et al demonstrated that Mg–Al LDH-NO3- promoted an increase in CD206+CX3CR1+ lamina propria (LP) macrophages, restricted T helper 17 cells, significantly inhibited the activation of the IL-17 signaling pathway, and enhanced immune tolerance.59
 |
Figure 2 Applications of inorganic nanomaterial drug delivery systems in inflammatory bowel disease (IBD). (A) Synthesis of biomimetic MOFs. Adapted with permission from Ma Y, Gao W, Zhang Y, et al. Biomimetic MOF Nanoparticles Delivery of C-Dot Nanozyme and CRISPR/Cas9 System for Site-Specific Treatment of Ulcerative Colitis. ACS Appl Mater Interfaces. Feb 9 2022;14(5):6358–6369. Copyright 2022 American Chemical Society.40 (B) Proposed mechanism for drug delivery by hierarchical structured and programed vehicles (AP@PSi-HA@HPMCAS) through gastrointestinal tract with IBD. Adapted from Li W, Li Y, Liu Z et al. Hierarchical structured and programmed vehicles deliver drugs locally to inflamed sites of intestine. Biomaterials. Dec 2018;185:322–332. Under a Creative Commons license.39 (C) Mechanism of IBD nanozyme therapy and nine-tier high-throughput screening framework for predicting multifeatured nanozymes in IBD therapy. Adapted from Yu Y, Zhao X, Xu X et al. Rational Design of Orally Administered Cascade Nanozyme for Inflammatory Bowel Disease Therapy. Adv Mater. Nov 2023;35(44):e2304967. Copyright 2023, John Wiley and Sons.41 (D) Design and synthesis of orally administered CeO2@MMT to target inflamed colon for treating IBD. Adapted from Zhao S, Li Y, Liu Q, et al. An Orally Administered CeO2@Montmorillonite Nanozyme Targets Inflammation for Inflammatory Bowel Disease Therapy. Advanced Functional Materials. 2020;30(45). Copyright 20230 John Wiley and Sons.43 (E) Schematic of inflamed colon-targeted antioxidant nanotherapeutics and proposed mechanism for modulation of oxidative stress in colitis. Adapted with permission from Min DK, Kim YE, Kim MK, Choi SW, Park N, Kim J. Orally Administrated Inflamed Colon-Targeted Nanotherapeutics for Inflammatory Bowel Disease Treatment by Oxidative Stress Level Modulation in Colitis. ACS Nano. Dec 12 2023;17(23):24,404–24416. Copyright 2023 American Chemical Society.49 Abbreviations: MOFs, metal organic framework; HA, hyaluronic acid; AP, acorbyl palmitate; HPMCAS, hydroxypropyl methylcellulose acetate succinate; Psi, porous silicon; AP@PSi-HA@HPMCAS, a hierarchical structured vehicle based on HA functionalized PSi nano-particles, AP and HPMCAS with different solubility profiles depending on pH for efficient local drug delivery to inflamed intestine via oral administration; MMT, montmorillonite. |
Non-metallic nanomaterials, such as mesoporous silica NDDS, have a high surface area and are popular as IBD drug carriers due to their effective H2O2 removal and drug delivery capabilities.60 Li et al utilized hyaluronic acid-functionalized porous silicon nanocarriers to deliver drugs to inflamed sites to alleviate colorectal inflammation (Figure 2B).39
Nanoenzyme NDDS
Nanoenzymes with enzyme-like activity have recently shifted the view of nanomaterials from biologically inert to highly catalytic, stable, and cost-effective systems, which are now considered as promising candidates for managing IBD redox balance. For example, Yu et al demonstrated that Ni3S4 had strong anti-inflammatory properties both in vitro and in vivo because of its dual SOD and catalase (CAT) activities (Figure 2C).41 Similarly, cerium materials exhibit SOD- and CAT-like activities due to the presence of trivalent Ce III and tetravalent Ce IV on their surface: Ce III primarily mimics SOD activity by removing O2•- and oxidizing to Ce IV, while Ce IV mimics CAT activity by decomposing H2O2 and reducing to Ce III.61,62 Traditional CeO2 nanomaterials tend to aggregate and precipitate in solution, which can hinder electron transfer and oxygen vacancy availability. Biocompatible coatings are therefore needed to enhance dispersibility and stability in water.63,64 Zhao et al addressed this issue by combining cerium oxide nanoenzymes with negatively charged montmorillonite, resulting in improved efficacy for treating UC in mice (Figure 2D).43 Min et al developed a dual NDDS using mesoporous silica and cerium oxide to manage colorectal ROS oxidative stress. This system targeted inflamed colorectal tissue and modulated the intestinal microenvironment by balancing redox levels and reducing inflammatory cell infiltration to effectively inhibit colitis (Figure 2E).49 Zhang et al utilized polydopamine-coated, PEG-modified CeO2 nanorods to shift intestinal macrophages from the M1 to M2 phenotype, to restore the immune balance and alleviate colitis.44 In addition, Wang et al found that mesoporous polydopamine NPs captured Ce4+ ions from Ce3+ oxidation in weakly alkaline solutions and reduced them, effectively enhancing catalysis and repairing the intestinal mucosal barrier.45 Selenium compounds also exhibit glutathione peroxidase enzyme activity by reducing peroxides and balancing ROS.65 Zhu et al used selenium nanomaterials with Ulva lactuca polysaccharides to inhibit nuclear factor-κB nuclear translocation and macrophage activation, thereby reducing IBD inflammation. Chen et al developed porous Se NPs coated with hyaluronic acid using the “hard template” method to enhance reactivity, and showed that enhancing the reactivity of Se NPs through structural modifications improved ROS elimination, significantly impacting local inflammation and sepsis.46,60
Cheng et al created a biomimetic nanoformulation with a cubic palladium core and macrophage-derived extracellular vesicle shell, effectively scavenging ROS and regulating macrophage polarization by inhibiting glycolysis and reducing neutrophil infiltration. By reshaping colitis and the immune microenvironment, it prevented cell apoptosis, improved colonic mucosal barrier function, and alleviated colitis in mouse models.48 Zhu et al evaluated platinum NPs mimicking SOD and CAT in terms of their protective potential against DSS-induced colitis in C57BL/6 mice, and found that platinum NPs inhibited lipopolysaccharide-induced inflammation in RAW264.7 cells, depending on their size (5, 30, and 70 nm), but caused intestinal dysbiosis by altering the alpha diversity and Firmicutes/Bacteroidetes ratios, and the abundance of certain specific bacteria.51 Manganese Prussian Blue nanoenzymes, made from Mn and Fe, exhibit multi-enzyme activity and were prepared using a straightforward method. Zhao et al discovered that these nanoenzymes effectively treated mouse IBD with no side effects, primarily by acting on the Toll-like receptor signaling pathway.52 Most anti-inflammatory nanoenzymes target ROS elimination, and nanoenzymes scavenging active nitrogen (RNS) are uncommon. Miao et al discovered that PEG-coated ultrasmall rhodium nanodots acted as metal nanoenzymes scavenging both ROS and RNS, and also possessed photothermal properties, showing effective anti-inflammatory action against dextran sulfate sodium-induced colitis.33 Zhang et al pioneered the application of NDDS combined with zinc ions and tannic acids for the treatment of IBD that are capable of clearing RONS without significant adverse effects.66 Li et al also found that Cu2O@His exhibit outstanding RONS-clearing capability, demonstrating good efficacy in treating mouse IBD.67
Inorganic NPs show good promise for treating IBD because of their exceptional catalytic and enzyme-like activities, and their ability to change metal valence. They can directly neutralize ROS/RNS, effectively mitigating oxidative stress-induced damage. Despite their promise for treating IBD, however, the biocidal properties of inorganic nanomaterials might disrupt the gut microbiota, potentially impacting immune function.68 Given the aggregation, cytotoxicity, and instability of nanomaterials in biological environments, along with the biodegradability of inorganic NDDS, further research is needed to monitor their safety and biological distribution while ensuring their therapeutic efficacy.26
Organic NDDS
Organic NDDS are molecules that attach drugs via chemical bonds or physical embedding, such as liposomes, nanoemulsions, and dendritic and polysaccharide polymers. These NDDS are simple in design and allow controlled drug release through self-deformation or degradation. Details of the modification methods, charge properties, size, and drug types of various organic NDDS used for IBD are summarized in Table 2.
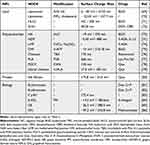 |
Table 2 Studies of Organic Nanomaterial Drug Delivery Systems for Inflammatory Bowel Disease |
Lipid-Containing Organic NDDS
Lipid nanomaterials are favored as NDDS because of their low toxicity, prolonged drug release, high cell absorption, and large surface area. Liposomes are spherical carriers with a phospholipid bilayer, ranging from 20–100 nm in diameter. They feature a lipid shell and a hydrophilic core, and are capable of transporting hydrophilic drugs in the core and hydrophobic drugs in the bilayers.87,88 Solid lipid NPs and nanostructured lipid carriers, collectively known as lipid NPs, differ from traditional liposomes by containing solid lipids or a mix of solid and liquid lipids, respectively. These NDDS offer superior physical stability, making them prominent in drug delivery research.
Lipid NDDS have been extensively researched in relation to IBD treatment. Aib et al encapsulated mesalazine and curcumin in lipid NDDS, resulting in reduced side effects and doses while effectively treating UC (Figure 3A).89 Xian et al used cationic lipid NDDS to deliver a prodrug, alleviating DSS-induced UC in mice (Figure 3B).90 Kim et al discovered that krill liposomes with budesonide significantly reduced pro-inflammatory cytokine production and improved membrane barrier function in an intestinal model.69 Shabana et al used marine phospholipids in nanoliposomes to bind phosphatidylcholine with polyunsaturated fatty acids, enhancing drug stability and reducing ROS.69,70 Liposome suspensions, however, also present challenges including limited stability, drug leakage, low targeting, and nonspecific macrophage clearance, which limit their effectiveness in treating IBD. Researchers are currently modifying liposomes to overcome these issues. For instance, Vong et al discovered that PEG-modified nanoliposomes protected drugs from the acidic gastrointestinal environment to achieve drug accumulation in the colorectal region, avoiding drug-mucus interactions, and increasing the rate of drug diffusion.91 In addition to PEGs, liposomes can also be modified by polysaccharides, peptides, aptamers, proteins, and small molecules.73
 |
Figure 3 Lipid-containing nanomaterial drug delivery systems in inflammatory bowel disease (IBD). (A) A pH-sensitive liposomes for colonic co-delivery of mesalazine and curcumin for the treatment of ulcerative colitis. Reprinted from Journal of Drug Delivery Science and Technology, Aib S, Iqbal K, Khan Net al, pH-sensitive liposomes for colonic co-delivery of mesalazine and curcumin for the treatment of ulcerative colitis. 2022,72, 103335. Copyright (2022), with permission from Elsevier.89 (B) Budsome synthesis and formulation for IBD therapy. When administered orally, budsomes remain stable under the harsh conditions of the stomach, preventing the burst release of prodrug payloads. After preferential accumulation at the inflamed intestinal tissue in DSS-induced colitis mice, budsomes release biologically active budesonide. Adapted from Xian S, Zhu J, Wang Y, Song H, Wang H. Oral liposomal delivery of an activatable budesonide prodrug reduces colitis in experimental mice. Drug Deliv. Dec 2023;30(1):2183821. Creative Commons Attribution-NonCommercial License (http://creativecommons.org/licenses/by-nc/4.0/).90 |
Lipid NDDS, made from biodegradable lipids with high melting points, provide a solid core for sustained drug release. Studies by Dianzani et al and Beloqui et al demonstrated that solid lipid NPs loaded with drugs such as dexamethasone, butyrate, and budesonide significantly improved colorectal histology and IBD remission while reducing neutrophil infiltration and the production of the pro-inflammatory cytokines interleukin (IL)-1β and TNF-a.71,72
Organic NDDS Containing Polysaccharide Components
Polysaccharides are used in various NDDS because of their biocompatibility, anti-inflammatory, antioxidant, and controlled-release properties, as well as their ability to regulate the intestinal microbiota.92–95 Kanwal et al and Prabaharan et al discovered that polysaccharide nanomaterial drug carriers regulated the intestinal microbiota, promoted beneficial bacterial growth, repaired the intestinal barrier, modulated inflammation pathways, and were converted into beneficial short-chain fatty acids after enzymatic hydrolysis.96,97 Polysaccharides such as alginic acid,98 chitosan,99,100 and hyaluronic acid101 can be made into hydrophilic nanopharmaceutical carrier hydrogels, ideal for targeted drug delivery to colorectal inflammation sites and for overcoming gastric pH and enzyme degradation,102,103 making them a research focus in nanomedicine carrier design.
Foodborne polysaccharides, particularly those high in soluble fiber, have been shown to alleviate IBD by modulating the immune system, strengthening the intestinal barrier, and restoring the gut microbiota. For example, polysaccharides from apples, Gracilaria lemaneiformis, Blidingia minima, Arctium lappa L., Astragalus membranaceus, and Codonopsis pilosula improved the balance of inflammatory factors and reduced colonic mucosal damage by strengthening the intestinal barrier.104–108 This restoration of intestinal flora structure effectively prevented and alleviated colitis in mice (Figure 4A).105 In a previous study, we derived composite micron dietary fiber from oat flour and converted it into nano-sized fibers, which were then encapsulated together with probiotics and 5-ASA into alginate hydrogels to form gel microspheres. Following oral administration, these microspheres were broken down by probiotics and intestinal flora, enabling controlled drug release and ultimately reshaping the intestinal microbiota (Figure 4B).31 In addition, food-derived natural polysaccharides such as pectin, xanthan gum, and guar gum are used as NDDS for IBD treatment. These polysaccharides resist digestive enzymes in the upper digestive tract and are broken down by intestinal flora in the colon, enabling targeted drug release.109,110 Polysaccharide nanocarriers are thus ideal for colon-targeted drug delivery.111
 |
Figure 4 Nanopharmaceutical materials containing polysaccharide components. (A) Brief sketch of mechanism by which SP protects mice against colitis. Reprinted from Han R, Wang L, Zhao Z, et al. Polysaccharide from Gracilaria Lemaneiformis prevents colitis in Balb/c mice via enhancing intestinal barrier function and attenuating intestinal inflammation. Food Hydrocolloids. 2020;109., Copyright (2020), with permission from Elsevier.105 (B) Sketch showing 5-aminosalicylic acid (5-ASA) release process induced by Bac fermentation after NDF-M oral administration. First, gel microspheres were used to overcome the barriers of gastrointestinal digestible enzymes and pH causing NDFs and Bac within the microspheres to leak into the colorectum after oral administration. NDF-Pro/5-ASA can then target the inflamed colon through interleukin (IL)-1β antibodies due to the in situ accumulation of IL-1β inflammatory factors at the site of inflammation. Finally, fermentation of Bac, feeding on NDFs and proteins as carbon and nitrogen sources, respectively, will overcome the interspace diffuse resistance from the mucus layer to promote the release of 5-ASA. Meanwhile, improvement of the gut microbiota will further alleviate inflammation. Adapted from Qiu L, Shen R, Wei L, et al. Designing a microbial fermentation-functionalized alginate microsphere for targeted release of 5-ASA using nano dietary fiber carrier for inflammatory bowel disease treatment. J Nanobiotechnology. Sep 23 2023;21(1):344. Creative Commons Attribution 4.0 International License.31 (C) Brief sketch of mechanism by which SP protected mice against colitis. Adapted with permission from Shen C, Zhao L, Du X, et al. Smart Responsive Quercetin-Conjugated Glycol Chitosan Prodrug Micelles for Treatment of Inflammatory Bowel Diseases. Mol Pharm. 2021;18(3):1419–1430. Copyright 2021 American Chemical Society.79 (D) Therapeutic effects of HA-KPV-NPs against ulcerative colitis. Oral administration of HA-KPV-NPs embedded in hydrogel (chitosan/alginate) conferred combined therapeutic effects against UC by accelerating mucosa healing and alleviating inflammation. Reprinted from Xiao B, Xu Z, Viennois E, et al. Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis. Molecular therapy: the journal of the American Society of Gene Therapy. 2017;25(7):1628–1640., Copyright (2017), with permission from Elsevier.112 Abbreviations: SP, sulfated polysaccharide; NDFs, nanoscale dietary fibers; NDF-M, an alginate hydrogel microsphere encapsulating Bac and drug-modified NDFs; KPV, lysine-proline-valine; HA, hyaluronic acid; NPs, nanoparticles; the HA-KPV-NPs, HA-functionalized polymericNPs loaded with KPV. |
Chitosan is a cationic natural polysaccharide and an effective mucin-binding polymer with several benefits. Its positive charge attracts the negatively charged intestinal mucosa, thus enhancing drug release and absorption into the bloodstream and increasing drug bioavailability. In addition, chitosan easily self-assembles with a core-shell structure, improving drug encapsulation and protection.113,114 Finally, chitosan dissolves in the stomach’s acidic environment but remains insoluble at pH 5.5 or higher in the small intestine, allowing it to reach the colorectum for degradation by the intestinal microbiota. Researchers have accordingly developed hydrogel NDDS made of chitosan to encapsulate various anti-inflammatory drugs, including resveratrol, phycocyanin, and 6-shogaol. Chitosan NDDS can encapsulate drugs to protect and delay their release, ensuring their selective delivery to the colorectum to effectively target colonic inflammation and alleviate DSS-induced proctitis in mice, by regulating pro- and anti-inflammatory factors (Figure 4C).73,76–79,115
Hyaluronic acid, a natural anionic proteoglycan found in the extracellular matrix and connective tissues, is highly hydrophilic because of its many carboxyl groups. It also acts as a ligand for cell surface receptors such as CD44, which are overexpressed in inflamed intestinal mucosal epithelial cells.116 Vafaei et al discovered that hyaluronic acid could self-assemble into NDDS in water and target drug delivery to colorectal inflammation sites via CD44 receptors on inflamed epithelial cells. Similarly, Xiao et al showed that hyaluronic acid-functionalized NDDS could deliver tripeptides to these inflammation sites, effectively alleviating UC (Figure 4D).112 Hyaluronic acid’s low toxicity, cost, and degradability make it an ideal material for NDDS.
Despite their benefits, however, some polysaccharides have high water solubility and poor film-forming properties, leading to premature drug release before they reach the colorectum. Researchers have mitigated this issue through chemical modifications or combining polysaccharides.93,117,118 For instance, Gunter et al demonstrated that incorporating Na2SiO3 into a pectin-based gel formulation from duckweed callus effectively controlled the slow release of the drug in the colorectum.74 Sun et al synthesized carboxymethyl inulin from inulin and chloroacetic acid, and then modified it with 4-aminothiophenol to create an amphiphilic nano-inulin, which effectively controlled the release of the encapsulated drug, helping to reduce inflammation in IBD.75
In addition to carbohydrate- and lipid-related NNDS, protein-based NNDS have also been investigated in relation to IBD. Non-toxic silk fibroin nanocarriers, made from biocompatible and biodegradable proteins, enhanced drug stability and bioavailability and reduced inflammation in colorectitis.80,119
Other IBD NDDS
Polymer-Based IBD NDDS
Similar to polysaccharides, organic compound polymers are long-chain molecules formed by repeating chemical units, including poly lactic-co-glycolic acid(PLGA), polylactic acid, polyglycolic acid copolymers, and polycaprolactones. These polymers can be synthesized to be water-soluble and can have both hydrophilic and hydrophobic groups, making them suitable for carrying various drugs. Organic polymer cores with sub-nanometer pores can load drug molecules, and surface branches with functional groups like amines and carboxyls also allow for modification with drug molecules. Additionally, their water solubility and ability to form polymer micelles facilitate drug solubilization and encapsulation. Moreover, these organic polymers are typically purer and more uniform than natural ones and can break down into lactic and glycolic acid in vivo.
These abovementioned qualities enhance the research potential for polymer-based IBD NDDS. For instance, Zhao et al utilized the numerous amino groups in generation 4 polyamidoamine to create G4-ASA NPs with 5-ASA. They then crosslinked these with a dextran’s aldehyde group via a Schiff base reaction to form injectable hydrogels with a high 5-ASA content for slow local release in the colon, for the treatment of DSS-induced UC.120 Bhavsar et al demonstrated that NDDS made with gelatin and encapsulated in poly(epsilon-caprolactone) microspheres efficiently loaded IL-10 plasmid DNA and controlled its release in the gut via lipase-induced hydrolysis, leading to effective transfection and transgene expression in lumen macrophages to reduce inflammation in an acute colitis model.121 Similarly, Mishra et al developed nanosized micelles prepared from stearic acid and caffeic acid, which successfully normalized the colitis activity index and colorectal length changes when loaded with budesonide.122 Moreover, Wang et al found that when DNase-I was immobilized on the surface of a dopamine-coated PLGA core, it preserved maximal enzymatic activity while increasing in vivo stability. This eventually decreased neutrophil infiltration and NETosis in the colon compared to free DNase-I or mesalamine.123
Bio-Nanomaterial-Related IBD NDDS
Bio-nanomaterial-related IBD NDDS, derived from animal and plant cells or exosomes, such as red and white blood cells and exosomes derived from plants or bacteria, offer high biocompatibility and easy availability, making them advantageous for drug delivery.
Autologous red blood cell membranes can be extracted, chemically modified in vitro, and re-infused into patients with IBD as NDDS. This system has shown good biocompatibility and prolonged circulation. For instance, Annese et al demonstrated that an inactive drug encapsulated in erythrocyte membranes was gradually converted by resident enzymes into an active drug, which then entered the bloodstream.81 Castro et al discovered that this strategy enabled patients to sustain minimal drug levels in their blood, leading to prolonged remission in children with steroid-dependent mild-to-moderate CD.82
Exosomes are nano-sized extracellular vesicles released by cells in various organisms. Their stability, low immunogenicity, biocompatibility, and effective biofilm penetration make them ideal natural NDDS for delivering drugs to IBD inflammation sites.124 For example, Ye et al discovered that extracellular vesicles from the bacterium Faecalibacterium prausnitzii could reduce DSS-induced proctitis in mice by improving intestinal mucosal barrier function and the immune response.83 Zhou et al demonstrated that injecting a synthetic plasmid expressing multiple small interfering RNAs (siRNAs) into mice resulted in liver-produced exosomes containing these siRNAs for IBD treatment.125
In addition, edible plant cell-derived nanovesicles can effectively be used to treat IBD. For instance, Yang et al demonstrated that ginger-derived nanovesicles loaded with the drug 6-shogaol showed strong anti-inflammatory and antioxidant effects to improve IBD symptoms.84 Ju et al discovered that grape-derived nanovesicles could inhibit the β-catenin signaling pathway in intestinal cells, boost stem cell proliferation, and reduce inflammation,85 while grapefruit nanovesicles enhanced the anti-inflammatory abilities of intestinal macrophages, and broccoli-derived nanovesicles showed anti-inflammatory effects on IBD.86,126 These findings suggest promising potential for plant-derived nanovesicles.
Engineered Microbe-Based IBD NDDS
Engineered microbe-based therapeutics are an emerging class of pharmaceutical agents aimed at achieving a controlled and prolonged retention time of engineered microbes within the GI tract and further targeting them to specific disease sites.127–130 They show promise as a local treatment strategy, potentially reducing the systemic side effects associated with systemic administration of therapeutic agents. Compared to injection or infusion methods, these engineered microorganisms and probiotics are more suitable for formulation into oral dosage forms, which are preferred by patients. Additionally, engineered microorganisms can exert therapeutic effects through complementary mechanisms of action.131 Heavey et al engineered a targeted Saccharomyces boulardii probiotic yeast platform, showing it exhibits high adherence to extracellular matrix proteins, resulting in longer gut residence, higher colon concentrations, and enhanced recovery in murine colitis.132 Additionally, ActoGenix have developed two engineered live biotherapeutic products to treat these conditions that have entered clinical trials. The first, AG011, was an engineered L. lactis strain that secreted hIL-10 and was shown to be safe and well-tolerated in an UC patient cohort (NCT00729872). AG014, based on the same L. lactis platform as AG011, was engineered to produce an anti-TNFα antibody fragment of certolizumab.131,133
In summary, inorganic, organic, and polymeric nanocarriers can be used as NDDS to deliver drugs for IBD treatment, with their biological effects being influenced by their shape, size, and physicochemical properties.134 Inorganic nanocarriers, noted for their easy preparation, good stability, and high drug-loading capacity, show promise but face clinical safety challenges,135 while organic nanomaterials derived from animals and plants can also serve as effective drug carriers, with high biocompatibility, low toxicity, controlled release, and the ability to regulate the gut microbiota. Despite their clinical potential however, they face challenges including unstable preservation and decomposition by gastric acid.
In addition, synthetic polymers, such as PEG, poly-L-lactic acid, polyvinyl alcohol, and poly(lactic acid-glycolic acid copolymer), are also commonly used as NDDS due to their ease of modification; however, concerns about their clinical safety have limited their use in medical applications.136 In summary, nanomaterial drug carriers offer benefits including targeted delivery, better bioavailability, lower doses, controlled release, and higher dissolution rates; notably however, significant challenges remain to be resolved to enable their widespread clinical use.137 In addition to being used as a therapeutic drug, nanoparticles can also be used as contrast agents. It has been found that cerium oxide nanoparticles can not only produce strong CT contrast, but also scavenge oxygen free radicals generated in cells and tissues under X-ray radiation. The dextran coated on the surface can accumulate in the inflammatory sites of the intestine, which has a certain targeting. However, IBD patients receive a relatively higher number of tests due to recurrent episodes of the disease, which also makes the toxicity of nanoparticles accumulating in vivo more uncertain. Therefore, nanoparticles has potential as a CT contrast agent for GIT imaging as well as imaging IBD.138
Concluding Remarks
To date, numerous NDDS have been studied and offer an efficient and safe treatment for IBD. Unlike traditional treatments, NDDS extend the drug residence time at inflammation sites, reduce the administration frequency, and precisely target colorectal inflammation using specific ligands or antibodies. This ensures localized drug release at inflammation sites and minimizes exposure and side effects in healthy tissues. This article thus reviews recent advancements in NDDS for IBD treatment, focusing on carrier classification.
Our research on IBD treatment shows that polysaccharide NDDS, such as dietary fiber and cellulose, regulate the gut microbiota and immunity. They can induce significant changes in the gut microbiota via microbial fermentation, which mainly manifests as an increase in beneficial bacteria and reduction in harmful bacteria, and promotion of system normalization. Natural polysaccharides are ideal for drug delivery in the colon due to the high bacterial concentration in the colon, which allows bacteria to release drugs by metabolizing the polysaccharide drug coatings or conjugates such as gum, guar gum, inulin, and chitosan, which also offer mucosal adhesion, high bioavailability, biocompatibility, nontoxicity, and low cost. These polysaccharides can be obtained from a variety of natural plants/animals and have been approved by the FDA for clinical use,104,109 allowing further exploration of the roles of NDDS with natural polysaccharide components in the gut microbiota and inflammation in IBD. Polysaccharide NDDS however, also have some shortcomings, such as instability, complex processes, and difficulty in realizing industrialization. It is therefore necessary to develop a comprehensive understanding of the physicochemical properties of natural polysaccharides, including their hydrophilicity, stability, and reproducibility. Further studies are also needed to confirm their effectiveness in treating IBD, and to determine their synergistic effects in relation to treatment outcomes and mechanisms.
This review of NDDS research highlights the application prospects of NDDS in relation to IBD. Although the trend of using multifunctional, multi-targeted NDDS for diagnosis and treatment is growing, they still have some shortcomings in terms of IBD. Further studies are therefore needed to investigate ways of simplifying the delivery system for scalable and reliable manufacturing, and developing effective formulations and dosage forms for human administration to achieve personalized IBD treatment. In addition, current research is limited to chemically-induced acute colitis models, such as trinitrobenzene sulfonic acid and DSS, which mimic intestinal damage and microbiota reduction but fail to replicate the pathology’s cause and immune system dysfunctions. Targeting drugs specifically to inflamed areas while avoiding healthy intestinal absorption is another major challenge in IBD treatment. Importantly, for NDDS to be clinically viable, they must meet stringent histocompatibility and safety standards.
Acknowledgments
The authors thank Professor Meilin Wang from the School of Public Health, Nanjing Medical University, China, for helpful guidance and the Medical Science and Technology Innovation Center, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School of Nanjing Medical University, China, for providing equipment to conduct the experiment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Key Health Talents in Gusu (GSKY20210220), the Scientific Research Project of Jiangsu Provincial Health Commission (No.H2023146), and the Suzhou Municipal Bureau of Science and Technology (No. SKY2023211).
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi:10.1038/nrgastro.2015.150
2. Francescone R, Hou V, Grivennikov SI. Cytokines IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21(2):409–418. doi:10.1097/MIB.0000000000000236
3. Li CJ, Wang YK, Zhang SM, Ren MD, He SX. Global burden of inflammatory bowel disease 1990-2019: a systematic examination of the disease burden and twenty-year forecast. World J Gastroenterol. 2023;29(42):5751–5767. doi:10.3748/wjg.v29.i42.5751
4. Venkataraman GR, Rivas MA. Rare and common variant discovery in complex disease: the IBD case study. Hum Mol Genet. 2019;28(R2):R162–R169. doi:10.1093/hmg/ddz189
5. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205–217. doi:10.1038/nrgastro.2015.34
6. Hammer T, Lophaven SN, Nielsen KR, et al. Dietary risk factors for inflammatory bowel diseases in a high-risk population: results from the Faroese IBD study. United Eur Gastroenterol J. 2019;7(7):924–932. doi:10.1177/2050640619852244
7. Kaplan GG. IBD: global variations in environmental risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2014;11(12):708–709. doi:10.1038/nrgastro.2014.182
8. van der SlootKWJ, Weersma RK, Dijkstra G, Alizadeh BZ. Development and validation of a web-based questionnaire to identify environmental risk factors for inflammatory bowel disease: the Groningen IBD environmental questionnaire (GIEQ). J Gastroenterol. 2019;54(3):238–248. doi:10.1007/s00535-018-1501-z
9. Lim JS, Lim MY, Choi Y, Ko G. Modeling environmental risk factors of autism in mice induces IBD-related gut microbial dysbiosis and hyperserotonemia. Mol Brain. 2017;10(1):14. doi:10.1186/s13041-017-0292-0
10. Putignani L, Del Chierico F, Vernocchi P, et al. Gut microbiota dysbiosis as risk and premorbid factors of IBD and IBS along the childhood-adulthood transition. Inflamm Bowel Dis. 2016;22(2):487–504. doi:10.1097/MIB.0000000000000602
11. Vezza T, Rodriguez-Nogales A, Algieri F, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Flavonoids in inflammatory bowel disease: a review. Nutrients. 2016;8(4):211. doi:10.3390/nu8040211
12. Han W, Xie B, Li Y, et al. Orally deliverable nanotherapeutics for the synergistic treatment of colitis-associated colorectal cancer. Theranostics. 2019;9(24):7458–7473. doi:10.7150/thno.38081
13. Zhang Z, Dong L, Jia A, et al. Glucocorticoids promote the onset of acute experimental colitis and cancer by upregulating mTOR signaling in intestinal epithelial cells. Cancers. 2020;12(4). doi:10.3390/cancers12040945
14. Pu J, Zhou X, Liu J, Hou P, Ji M. Therapeutic potential and deleterious effect of glucocorticoids on azoxymethane/dextran sulfate sodium-induced colorectal cancer in mice. Am J Cancer Res. 2021;11(10):4866–4883.
15. Ma HQ, Yu TT, Zhao XJ, Zhang Y, Zhang HJ. Fecal microbial dysbiosis in Chinese patients with inflammatory bowel disease. World J Gastroenterol. 2018;24(13):1464–1477. doi:10.3748/wjg.v24.i13.1464
16. Raza H, John A, Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur J Pharmacol. 2011;668(1–2):15–24. doi:10.1016/j.ejphar.2011.06.016
17. Wang D, DuBois RN. The role of anti-inflammatory drugs in colorectal cancer. Annu Rev Med. 2013;64:131–144. doi:10.1146/annurev-med-112211-154330
18. Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–570. doi:10.1016/j.addr.2011.12.009
19. Dulai PS, Siegel CA, Colombel JF, Sandborn WJ, Peyrin-Biroulet L. Systematic review: monotherapy with antitumour necrosis factor alpha agents versus combination therapy with an immunosuppressive for IBD. Gut. 2014;63(12):1843–1853. doi:10.1136/gutjnl-2014-307126
20. Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5(2):119–133. doi:10.1097/00054725-199905000-00008
21. Navaneethan U, Lashner BA. Effects of immunosuppression and liver transplantation on inflammatory bowel disease in patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11(5):524–525. doi:10.1016/j.cgh.2013.01.020
22. Halwani AA. Development of pharmaceutical nanomedicines: from the bench to the market. Pharmaceutics. 2022;14(1). doi:10.3390/pharmaceutics14010106
23. Miao L, Guo S, Lin CM, Liu Q, Huang L. Nanoformulations for combination or cascade anticancer therapy. Adv Drug Deliv Rev. 2017;115:3–22. doi:10.1016/j.addr.2017.06.003
24. Qiu N, Gao J, Liu Q, Wang J, Shen Y. Enzyme-responsive charge-reversal polymer-mediated effective gene therapy for intraperitoneal tumors. Biomacromolecules. 2018;19(6):2308–2319. doi:10.1021/acs.biomac.8b00440
25. Chen Y, Song W, Shen L, et al. Vasodilator hydralazine promotes nanoparticle penetration in advanced desmoplastic tumors. ACS Nano. 2019;13(2):1751–1763. doi:10.1021/acsnano.8b07830
26. Li X, Lu C, Yang Y, Yu C, Rao Y. Site-specific targeted drug delivery systems for the treatment of inflammatory bowel disease. Biomed Pharmacothe. 2020;129:110486. doi:10.1016/j.biopha.2020.110486
27. Di Sabatino A, Biancheri P, Rovedatti L, Macdonald TT, Corazza GR. Recent advances in understanding ulcerative colitis. Intern Emerg Med. 2012;7(2):103–111. doi:10.1007/s11739-011-0719-z
28. Xiao B, Merlin D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin Drug Deliv. 2012;9(11):1393–1407. doi:10.1517/17425247.2012.730517
29. Sun T, Kwong CHT, Gao C, et al. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics. 2020;10(22):10106–10119. doi:10.7150/thno.48448
30. Chung CH, Jung W, Keum H, Kim TW, Jon S. Nanoparticles derived from the natural antioxidant rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano. 2020;14(6):6887–6896. doi:10.1021/acsnano.0c01018
31. Qiu L, Shen R, Wei L, et al. Designing a microbial fermentation-functionalized alginate microsphere for targeted release of 5-ASA using nano dietary fiber carrier for inflammatory bowel disease treatment. J Nanobiotechnol. 2023;21(1):344. doi:10.1186/s12951-023-02097-6
32. Li J, Chen H, Wang B, et al. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci Rep. 2017;(7):43126. doi:10.1038/srep43126
33. Miao Z, Jiang S, Ding M, et al. Ultrasmall rhodium nanozyme with RONS scavenging and photothermal activities for anti-inflammation and antitumor theranostics of colon diseases. Nano Lett. 2020;20(5):3079–3089. doi:10.1021/acs.nanolett.9b05035
34. Abdelmegid AM, Abdo FK, Ahmed FE, Kattaia AAA. Therapeutic effect of gold nanoparticles on DSS-induced ulcerative colitis in mice with reference to interleukin-17 expression. Sci Rep. 2019;9(1):10176. doi:10.1038/s41598-019-46671-1
35. Li M, Liu J, Shi L, et al. Gold nanoparticles-embedded ceria with enhanced antioxidant activities for treating inflammatory bowel disease. Bioact Mater. 2023;25:95–106. doi:10.1016/j.bioactmat.2023.01.015
36. Lu C, Xue L, Luo K, et al. Colon-accumulated gold nanoclusters alleviate intestinal inflammation and prevent secondary colorectal carcinogenesis via Nrf2-dependent macrophage reprogramming. ACS Nano. 2023;17(18):18421–18432. doi:10.1021/acsnano.3c06025
37. Qin Y, Zhao R, Qin H, et al. Colonic mucus-accumulating tungsten oxide nanoparticles improve the colitis therapy by targeting Enterobacteriaceae. Nano Today. 2021;39:101234. doi:10.1016/j.nantod.2021.101234
38. Yang J, Zhang G, Peng M, et al. Bionic regulators break the ecological niche of pathogenic bacteria for modulating dysregulated microbiome in colitis. Adv Mater. 2022;34(39):e2204650. doi:10.1002/adma.202204650
39. Li W, Li Y, Liu Z, et al. Hierarchical structured and programmed vehicles deliver drugs locally to inflamed sites of intestine. Biomaterials. 2018;185:322–332. doi:10.1016/j.biomaterials.2018.09.024
40. Ma Y, Gao W, Zhang Y, et al. Biomimetic MOF nanoparticles delivery of C-Dot nanozyme and CRISPR/Cas9 system for site-specific treatment of ulcerative colitis. ACS Appl Mater Interfaces. 2022;14(5):6358–6369. doi:10.1021/acsami.1c21700
41. Yu Y, Zhao X, Xu X, et al. Rational design of orally administered cascade nanozyme for inflammatory bowel disease therapy. Adv Mater. 2023;35(44):e2304967. doi:10.1002/adma.202304967
42. Zeng F, Shi Y, Wu C, et al. A drug-free nanozyme for mitigating oxidative stress and inflammatory bowel disease. J Nanobiotechnol. 2022;20(1):107. doi:10.1186/s12951-022-01319-7
43. Zhao S, Li Y, Liu Q, et al. An orally administered CeO2@Montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv Funct Mater. 2020;30(45). doi:10.1002/adfm.202004692
44. Zhang B, Li Q, Xu Q, Li B, Dong H, Mou Y. Polydopamine modified ceria nanorods alleviate inflammation in colitis by scavenging ROS and regulating macrophage M2 polarization. Int J Nanomed. 2023;18:4601–4616. doi:10.2147/IJN.S416049
45. Wang H, Wang L, Chen Y, et al. Catalytically proficient ceria nanodots supported on redox-active mesoporous hosts for treatment of inflammatory bowel disease via efficient ROS scavenging. J Mater Chem B. 2023;11(43):10369–10382. doi:10.1039/d3tb01602a
46. Zhu C, Zhang S, Song C, et al. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-kappaB mediated hyper inflammation. J Nanobiotechnol. 2017;15(1):20. doi:10.1186/s12951-017-0252-y
47. Yang H, Zhu C, Yuan W, et al. Mannose-rich Oligosaccharides-functionalized selenium nanoparticles mediates Macrophage reprogramming and inflammation resolution in ulcerative colitis. Chem Eng J. 2022;435:131715. doi:10.1016/j.cej.2021.131715
48. Cheng J, Zhang Y, Ma L, et al. Macrophage-derived extracellular vesicles-coated palladium nanoformulations modulate inflammatory and immune homeostasis for targeting therapy of ulcerative colitis. Adv Sci. 2023;10(33):e2304002. doi:10.1002/advs.202304002
49. Min DK, Kim YE, Kim MK, Choi SW, Park N, Kim J. Orally administrated inflamed colon-targeted nanotherapeutics for inflammatory bowel disease treatment by oxidative stress level modulation in colitis. ACS Nano. 2023;17(23):24404–24416. doi:10.1021/acsnano.3c11089
50. Shi Z, Li X, Chen J, et al. Enzyme-like biomimetic oral-agent enabling modulating gut microbiota and restoring redox homeostasis to treat inflammatory bowel disease. Bioact Mater. 2024;35:167–180. doi:10.1016/j.bioactmat.2024.01.016
51. Zhu S, Zeng M, Feng G, Wu H. Platinum nanoparticles as a therapeutic agent against dextran sodium sulfate-induced colitis in mice. Int J Nanomed. 2019;14:8361–8378. doi:10.2147/IJN.S210655
52. Zhao J, Gao W, Cai X, et al. Nanozyme-mediated catalytic nanotherapy for inflammatory bowel disease. Theranostics. 2019;9(10):2843–2855. doi:10.7150/thno.33727
53. Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690(1–2):12–23. doi:10.1016/j.mrfmmm.2009.09.007
54. Zhang C, Wang H, Yang X, et al. Oral zero-valent-molybdenum nanodots for inflammatory bowel disease therapy. Sci Adv. 2022;8(37):eabp9882. doi:10.1126/sciadv.abp9882
55. Guo H, Guo H, Xie Y, et al. Mo(3)Se(4) nanoparticle with ROS scavenging and multi-enzyme activity for the treatment of DSS-induced colitis in mice. Redox Biol. 2022;56:102441. doi:10.1016/j.redox.2022.102441
56. Zhao X, Wang LY, Li JM, et al. Redox-mediated artificial non-enzymatic antioxidant mxene nanoplatforms for acute kidney injury alleviation. Adv Sci. 2021;8(18):e2101498. doi:10.1002/advs.202101498
57. Hou L, Gong F, Liu B, et al. Orally administered titanium carbide nanosheets as anti-inflammatory therapy for colitis. Theranostics. 2022;12(8):3834–3846. doi:10.7150/thno.70668
58. Jin J, Ye X, Huang Z, Jiang S, Lin D. Curcumin@Fe/tannic acid complex nanoparticles for inflammatory bowel disease treatment. ACS Omega. 2024;9(12):14316–14322. doi:10.1021/acsomega.3c10214
59. Niu J, Guo Y, Jing G, et al. Anion‐dependent layered double hydroxide nanoparticles regulate differentiation of CD206+ CX3CR1+ macrophages by inhibiting the IL‐17 signaling pathway contributing to inflammatory bowel disease. Adv Funct Mater. 2023;34(14). doi:10.1002/adfm.202305042
60. Chen X, Zhu X, Gong Y, et al. Porous selenium nanozymes targeted scavenging ROS synchronize therapy local inflammation and sepsis injury. Appl Mater Today. 2021;22:100929. doi:10.1016/j.apmt.2020.100929
61. Lord MS, Berret JF, Singh S, Vinu A, Karakoti AS. Redox active cerium oxide nanoparticles: current status and burning issues. Small. 2021;17(51):e2102342. doi:10.1002/smll.202102342
62. Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3(4):1411–1420. doi:10.1039/c0nr00875c
63. Caputo F, Mameli M, Sienkiewicz A, et al. A novel synthetic approach of cerium oxide nanoparticles with improved biomedical activity. Sci Rep. 2017;7(1):4636. doi:10.1038/s41598-017-04098-6
64. Wang M, He H, Liu D, Ma M, Preparation ZY. Characterization and multiple biological properties of peptide-modified cerium oxide nanoparticles. Biomolecules. 2022;12(9). doi:10.3390/biom12091277
65. Huang X, Liu X, Luo Q, Liu J, Shen J. Artificial selenoenzymes: designed and redesigned. Chem Soc Rev. 2011;40(3):1171–1184. doi:10.1039/c0cs00046a
66. Zhang C, Li Q, Xing J, et al. Tannic acid and zinc ion coordination of nanase for the treatment of inflammatory bowel disease by promoting mucosal repair and removing reactive oxygen and nitrogen species. Acta Biomater. 2024;177:347–360. doi:10.1016/j.actbio.2024.02.015
67. Li S, Chen Z, Wang M, et al. Ultrasmall Cu(2)O@His nanozymes with RONS scavenging capability for anti-inflammatory therapy. ACS Appl Mater Interfaces. 2024;16(3):3116–3125. doi:10.1021/acsami.3c15083
68. Lamas B, Martins Breyner N, Houdeau E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: potential consequences for host health. Part Fibre Toxicol. 2020;17(1):19. doi:10.1186/s12989-020-00349-z
69. Kim JH, Hong SS, Lee M, et al. Krill oil-incorporated liposomes as an effective nanovehicle to ameliorate the inflammatory responses of DSS-induced colitis. Int J Nanomed. 2019;14:8305–8320. doi:10.2147/IJN.S220053
70. Shabana S, Hamouda HI, Hamadou AH, Ahmed B, Chi Z, Liu C. Marine phospholipid nanoliposomes: a promising therapeutic approach for inflammatory bowel disease: preparation, safety, and efficacy evaluation. Colloids Surf B Biointerfaces. 2023;234:113702. doi:10.1016/j.colsurfb.2023.113702
71. Beloqui A, Coco R, Alhouayek M, et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int J Pharm. 2013;454(2):775–783. doi:10.1016/j.ijpharm.2013.05.017
72. Dianzani C, Foglietta F, Ferrara B, et al. Solid lipid nanoparticles delivering anti-inflammatory drugs to treat inflammatory bowel disease: effects in an in vivo model. World J Gastroenterol. 2017;23(23):4200–4210. doi:10.3748/wjg.v23.i23.4200
73. Manconi M, Mura S, Manca ML, et al. Chitosomes as drug delivery systems for C-phycocyanin: preparation and characterization. Int J Pharm. 2010;392(1–2):92–100. doi:10.1016/j.ijpharm.2010.03.038
74. Gunter EA, Markov PA, Melekhin AK, et al. Preparation and release characteristics of mesalazine loaded calcium pectin-silica gel beads based on callus cultures pectins for colon-targeted drug delivery. Int J Biol Macromol. 2018;120(Pt B):2225–2233. doi:10.1016/j.ijbiomac.2018.07.078
75. Sun Q, Luan L, Arif M, et al. Redox-sensitive nanoparticles based on 4-aminothiophenol-carboxymethyl inulin conjugate for budesonide delivery in inflammatory bowel diseases. Carbohydr Polym. 2018;189:352–359. doi:10.1016/j.carbpol.2017.12.021
76. Iglesias N, Galbis E, Diaz-Blanco MJ, Lucas R, Benito E, de-Paz MV. Nanostructured chitosan-based biomaterials for sustained and colon-specific resveratrol release. Int J mol Sci. 2019;20(2). doi:10.3390/ijms20020398
77. Laroui H, Dalmasso G, Nguyen HT, Yan Y, Sitaraman SV, Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010;138(3):843–53e1–2. doi:10.1053/j.gastro.2009.11.003
78. Castangia I, Nacher A, Caddeo C, et al. Therapeutic efficacy of quercetin enzyme-responsive nanovesicles for the treatment of experimental colitis in rats. Acta Biomater. 2015;13:216–227. doi:10.1016/j.actbio.2014.11.017
79. Shen C, Zhao L, Du X, et al. Smart responsive quercetin-conjugated glycol chitosan prodrug micelles for treatment of inflammatory bowel diseases. Mol Pharm. 2021;18(3):1419–1430. doi:10.1021/acs.molpharmaceut.0c01245
80. Diez-Echave P, Ruiz-Malagon AJ, Molina-Tijeras JA, et al. Silk fibroin nanoparticles enhance quercetin immunomodulatory properties in DSS-induced mouse colitis. Int J Pharm. 2021;606:120935. doi:10.1016/j.ijpharm.2021.120935
81. Annese V, Latiano A, Rossi L, et al. Erythrocytes-mediated delivery of dexamethasone in steroid-dependent IBD patients-a pilot uncontrolled study. Am J Gastroenterol. 2005;100(6):1370–1375. doi:10.1111/j.1572-0241.2005.41412.x
82. Castro M, Rossi L, Papadatou B, et al. Long-term treatment with autologous red blood cells loaded with dexamethasone 21-phosphate in pediatric patients affected by steroid-dependent Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44(4):423–426. doi:10.1097/MPG.0b013e3180320667
83. Ye L, Wang Y, Xiao F, et al. F. prausnitzii-derived extracellular vesicles attenuate experimental colitis by regulating intestinal homeostasis in mice. Microb Cell Fact. 2023;22(1):235. doi:10.1186/s12934-023-02243-7
84. Yang C, Zhang M, Lama S, Wang L, Merlin D. Natural-lipid nanoparticle-based therapeutic approach to deliver 6-shogaol and its metabolites M2 and M13 to the colon to treat ulcerative colitis. J Control Rel. 2020;323:293–310. doi:10.1016/j.jconrel.2020.04.032
85. Ju S, Mu J, Dokland T, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol ther. 2013;21(7):1345–1357. doi:10.1038/mt.2013.64
86. Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol ther. 2014;22(3):522–534. doi:10.1038/mt.2013.190
87. Mishra V, Bansal KK, Verma A, et al. Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics. 2018;10(4). doi:10.3390/pharmaceutics10040191
88. Khalid S, Salman S, Iqbal K, et al. Surfactant free synthesis of cationic nano-vesicles: a safe triple drug loaded vehicle for the topical treatment of cutaneous leishmaniasis. Nanomedicine. 2022;40:102490. doi:10.1016/j.nano.2021.102490
89. Aib S, Iqbal K, Khan N, et al. pH-sensitive liposomes for colonic co-delivery of mesalazine and curcumin for the treatment of ulcerative colitis. J Drug Delivery Sci Technol. 2022;72:103335. doi:10.1016/j.jddst.2022.103335
90. Xian S, Zhu J, Wang Y, Song H, Wang H. Oral liposomal delivery of an activatable budesonide prodrug reduces colitis in experimental mice. Drug Deliv. 2023;30(1):2183821. doi:10.1080/10717544.2023.2183821
91. Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y. An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143(4):1027–36e3. doi:10.1053/j.gastro.2012.06.043
92. Ribeiro LN, Alcantara AC, Darder M, Aranda P, Araujo-Moreira FM, Ruiz-Hitzky E. Pectin-coated chitosan-LDH bionanocomposite beads as potential systems for colon-targeted drug delivery. Int J Pharm. 2014;463(1):1–9. doi:10.1016/j.ijpharm.2013.12.035
93. Prezotti FG, Cury BS, Evangelista RC. Mucoadhesive beads of gellan gum/pectin intended to controlled delivery of drugs. Carbohydr Polym. 2014;113:286–295. doi:10.1016/j.carbpol.2014.07.021
94. Chambin O, Dupuis G, Champion D, Voilley A, Pourcelot Y. Colon-specific drug delivery: influence of solution reticulation properties upon pectin beads performance. Int J Pharm. 2006;321(1–2):86–93. doi:10.1016/j.ijpharm.2006.05.015
95. Nie Y, Lin Q, Luo F. Effects of non-starch polysaccharides on inflammatory bowel disease. Int J mol Sci. 2017;18(7). doi:10.3390/ijms18071372
96. Kanwal S, Joseph TP, Aliya S, et al. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J Funct Foods. 2020;64. doi:10.1016/j.jff.2019.103641
97. Prabaharan M. Prospective of guar gum and its derivatives as controlled drug delivery systems. Int J Biol Macromol. 2011;49(2):117–124. doi:10.1016/j.ijbiomac.2011.04.022
98. Oshi MA, Naeem M, Bae J, et al. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease. Carbohydr Polym. 2018;198:434–442. doi:10.1016/j.carbpol.2018.06.107
99. Mohanbhai SJ, Sardoiwala MN, Gupta S, et al. Colon targeted chitosan-melatonin nanotherapy for preclinical inflammatory bowel disease. Biomater Adv. 2022;136:212796. doi:10.1016/j.bioadv.2022.212796
100. Kulkarni N, Jain P, Shindikar A, Suryawanshi P, Thorat N. Advances in the colon-targeted chitosan based multiunit drug delivery systems for the treatment of inflammatory bowel disease. Carbohydr Polym. 2022;288:119351. doi:10.1016/j.carbpol.2022.119351
101. Zhang S, Kang L, Hu S, et al. Carboxymethyl chitosan microspheres loaded hyaluronic acid/gelatin hydrogels for controlled drug delivery and the treatment of inflammatory bowel disease. Int J Biol Macromol. 2021;167:1598–1612. doi:10.1016/j.ijbiomac.2020.11.117
102. Li C, Zhao Y, Cheng J, et al. A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota. Adv Sci. 2019;6(18):1900610. doi:10.1002/advs.201900610
103. Wang QS, Wang GF, Zhou J, Gao LN, Cui YL. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int J Pharm. 2016;515(1–2):176–185. doi:10.1016/j.ijpharm.2016.10.002
104. Li Y, Fan L, Tang T, et al. Modified apple polysaccharide prevents colitis through modulating IL-22 and IL-22BP expression. Int J Biol Macromol. 2017;103:1217–1223. doi:10.1016/j.ijbiomac.2017.05.172
105. Han R, Wang L, Zhao Z, et al. Polysaccharide from Gracilaria Lemaneiformis prevents colitis in Balb/c mice via enhancing intestinal barrier function and attenuating intestinal inflammation. Food Hydrocoll. 2020;109. doi:10.1016/j.foodhyd.2020.106048
106.. Song W, Li Y, Zhang X, Wang Z. Potent anti-inflammatory activity of polysaccharides extracted from Blidingia minima and their effect in a mouse model of inflammatory bowel disease. J Funct Foods. 2019;61. doi:10.1016/j.jff.2019.103494.
107. Zhang X, Zhang N, Kan J, et al. Anti-inflammatory activity of alkali-soluble polysaccharides from Arctium lappa L. and its effect on gut microbiota of mice with inflammation. Int J Biol Macromol. 2020;154:773–787. doi:10.1016/j.ijbiomac.2020.03.111
108. Tang S, Liu W, Zhao Q, et al. Combination of polysaccharides from Astragalus membranaceus and Codonopsis pilosula ameliorated mice colitis and underlying mechanisms. J Ethnopharmacol. 2021;264:113280. doi:10.1016/j.jep.2020.113280
109. Sharma A, Kaur N, Sharma S, et al. Embelin-loaded guar gum microparticles for the management of ulcerative colitis. J Microencapsul. 2018;35(2):181–191. doi:10.1080/02652048.2018.1452991
110. Mutalik S, Suthar NA, Managuli RS, et al. Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int J Biol Macromol. 2016;86:709–720. doi:10.1016/j.ijbiomac.2015.11.092
111. Tang XH, Xie P, Ding Y, et al. Synthesis, characterization, and in vitro and in vivo evaluation of a novel pectin-Adriamycin conjugate. Bioorg Med Chem. 2010;18(4):1599–1609. doi:10.1016/j.bmc.2009.12.076
112. Xiao B, Xu Z, Viennois E, et al. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol ther. 2017;25(7):1628–1640. doi:10.1016/j.ymthe.2016.11.020
113. Liu Y, Sun Y, He S, et al. Synthesis and characterization of gibberellin-chitosan conjugate for controlled-release applications. Int J Biol Macromol. 2013;57:213–217. doi:10.1016/j.ijbiomac.2013.03.024
114. Wang X, Chen Y, Dahmani FZ, Yin L, Zhou J, Yao J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials. 2014;35(26):7654–7665. doi:10.1016/j.biomaterials.2014.05.053
115. Zhang M, Xu C, Liu D, Han MK, Wang L, Merlin D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J Crohn’s Colitis. 2018;12(2):217–229. doi:10.1093/ecco-jcc/jjx115
116. Vafaei SY, Esmaeili M, Amini M, Atyabi F, Ostad SN, Dinarvand R. Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa. Carbohydr Polym. 2016;144:371–381. doi:10.1016/j.carbpol.2016.01.026
117. Song Q, Wang Y, Huang L, et al. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res Int. 2021;140:109858. doi:10.1016/j.foodres.2020.109858
118. Kotla NG, Rana S, Sivaraman G, et al. Bioresponsive drug delivery systems in intestinal inflammation: state-of-the-art and future perspectives. Adv Drug Deliv Rev. 2019;146:248–266. doi:10.1016/j.addr.2018.06.021
119. Lozano-Perez AA, Rivero HC, Perez Hernandez MDC, et al. Silk fibroin nanoparticles: efficient vehicles for the natural antioxidant quercetin. Int J Pharm. 2017;518(1–2):11–19. doi:10.1016/j.ijpharm.2016.12.046
120. Zhao L, Wang F, Cai Z, et al. Improving drug utilization platform with injectable mucoadhesive hydrogel for treating ulcerative colitis. Chem Eng J. 2021;424:130464. doi:10.1016/j.cej.2021.130464
121. Bhavsar MD, Amiji MM. Oral IL-10 gene delivery in a microsphere-based formulation for local transfection and therapeutic efficacy in inflammatory bowel disease. Genet Ther. 2008;15(17):1200–1209. doi:10.1038/gt.2008.67
122. Mishra RK, Ahmad A, Kanika, et al. Caffeic acid-conjugated budesonide-loaded nanomicelle attenuates inflammation in experimental colitis. Mol Pharm. 2023;20(1):172–182. doi:10.1021/acs.molpharmaceut.2c00558
123. Wang CJ, Ko GR, Lee YY, et al. Polymeric DNase-I nanozymes targeting neutrophil extracellular traps for the treatment of bowel inflammation. Nano Converg. 2024;11(1):6. doi:10.1186/s40580-024-00414-9
124. Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. Exosomes horizontal line nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. 2022;16(11):17802–17846. doi:10.1021/acsnano.2c08774
125. Zhou X, Yu M, Ma L, et al. In vivo self-assembled siRNA as a modality for combination therapy of ulcerative colitis. Nat Commun. 2022;13(1):5700. doi:10.1038/s41467-022-33436-0
126. Deng Z, Rong Y, Teng Y, et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol ther. 2017;25(7):1641–1654. doi:10.1016/j.ymthe.2017.01.025
127. Anselmo AC, McHugh KJ, Webster J, Langer R, Jaklenec A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv Mater. 2016;28(43):9486–9490. doi:10.1002/adma.201603270
128. Praveschotinunt P, Duraj-Thatte AM, Gelfat I, Bahl F, Chou DB, Joshi NS. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat Commun. 2019;10(1):5580. doi:10.1038/s41467-019-13336-6
129. Zhou J, Li M, Chen Q, et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. 2022;13(1):3432. doi:10.1038/s41467-022-31171-0
130. Cao Z, Wang X, Pang Y, Cheng S, Liu J. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat Commun. 2019;10(1):5783. doi:10.1038/s41467-019-13727-9
131. Rutter JW, Dekker L, Owen KA, Barnes CP. Microbiome engineering: engineered live biotherapeutic products for treating human disease. Front Bioeng Biotechnol. 2022;10:1000873. doi:10.3389/fbioe.2022.1000873
132. Heavey MK, Hazelton A, Wang Y, et al. Targeted delivery of the probiotic Saccharomyces boulardii to the extracellular matrix enhances gut residence time and recovery in murine colitis. Nat Commun. 2024;15(1):3784. doi:10.1038/s41467-024-48128-0
133. Vandenbroucke K, de Haard H, Beirnaert E, et al. Orally administered L. lactis secreting an anti-TNF nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010;3(1):49–56. doi:10.1038/mi.2009.116
134. Watkins R, Wu L, Zhang C, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int J Nanomed. 2015;10:6055–6074. doi:10.2147/IJN.S92162
135. Khafaji M, Zamani M, Golizadeh M, Bavi O. Inorganic nanomaterials for chemo/photothermal therapy: a promising horizon on effective cancer treatment. Biophys Rev. 2019;11(3):335–352. doi:10.1007/s12551-019-00532-3
136. Obeid MA, Al Qaraghuli MM, Alsaadi M, Alzahrani AR, Niwasabutra K, Ferro VA. Delivering natural products and biotherapeutics to improve drug efficacy. Ther Deliv. 2017;8(11):947–956. doi:10.4155/tde-2017-0060
137. Sim S, Wong NK. Nanotechnology and its use in imaging and drug delivery (Review). Biomed Rep. 2021;14(5):42. doi:10.3892/br.2021.1418
138. Naha PC, Hsu JC, Kim J, et al. Dextran-coated cerium oxide nanoparticles: a computed tomography contrast agent for imaging the gastrointestinal tract and inflammatory bowel disease. ACS Nano. 2020;14(8):10187–10197. doi:10.1021/acsnano.0c03457
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Mesalazine–PAMAM Nanoparticles for Transporter-Independent Intracellular Drug Delivery: Cellular Uptake and Anti-Inflammatory Activity

Gorzkiewicz M, Marcinkowska M, Studzian M, Karwaciak I, Pulaski L, Klajnert-Maculewicz B
International Journal of Nanomedicine 2023, 18:2109-2126
Published Date: 24 April 2023

