Back to Journals » Infection and Drug Resistance » Volume 18
Clinical Characteristics and Risk Factors for Multidrug-Resistant Enterobacter cloacae Complex Bacteremia in a Chinese Tertiary Hospital: A Decade Review (2013–2022)
Authors Han M, Hua M, Xie H, Li J, Wang Y, Shen H, Cao X
Received 8 November 2024
Accepted for publication 1 January 2025
Published 22 January 2025 Volume 2025:18 Pages 427—440
DOI https://doi.org/10.2147/IDR.S502509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Mei Han,1,2,* Miaomiao Hua,3,* Hui Xie,1 Jia Li,1 Yijun Wang,2,4 Han Shen,1 Xiaoli Cao1
1Department of Laboratory Medicine, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, Jiangsu, People’s Republic of China; 2Nanjing Field Epidemiology Training Program, Nanjing Municipal Center for Disease Control and Prevention, Nanjing, Jiangsu, People’s Republic of China; 3Department of Laboratory Medicine, The Affiliated Fuyang Hospital of Anhui Medical University, Fuyang, Anhui, People’s Republic of China; 4Nanjing Jiangning District Center for Disease Control and Prevention, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoli Cao; Han Shen, Email [email protected]; [email protected]
Objective: This study aimed to analyze the antimicrobial resistance profiles, clinical characteristics and risk factors of bacteremia caused by Enterobacter cloacae complex (ECC) strains.
Methods: We retrospectively collected clinical data from patients diagnosed with ECC bacteremia between 2013 and 2022 in a tertiary hospital in Jiangsu. Subgroup analyses were performed based on multidrug resistance (MDR), nosocomial acquisition, polymicrobial bacteremia, and mortality.
Results: Among 188 ECC strains, the highest resistance was to ceftriaxone (39.9%), followed by ceftazidime (36.7%) and aztreonam (31.2%), with low resistance to carbapenems (< 8.6%) and amikacin (1.6%). MDR ECC accounted for 30.9% (58/188). Previous antibiotic therapy was an independent risk factor for MDR ECC (OR = 3.193, P < 0.020), while appropriate antibiotic therapy significantly reduced the risk (OR = 0.279, P < 0.001). ICU admission was an independent risk factor for polymicrobial bacteremia, both endoscopy and blood transfusion were associated with mortality.
Conclusion: Carbapenems and amikacin are the most effective treatments for ECC bacteremia. Previous antibiotic therapy increases the risk of MDR ECC, while appropriate antibiotic therapy reduces it. ICU admission is an independent risk factor for polymicrobial bacteremia, both endoscopy and blood transfusion are linked to higher mortality. Effective control of MDR ECC bacteremia requires comprehensive strategies, including resistance detection, risk factor identification, and infection prevention.
Keywords: Enterobacter cloacae complex, bacteremia, multidrug-resistance, clinical characteristics, risk factors
Introduction
The Enterobacter cloacae complex (ECC), a member of the Enterobacter genus, is widely found in nature. To date, seven ECC species have been identified, including E. cloacae, E. hormaechei, E. asburiae, E. kobei, E. ludwigii, E. nimipressuralis, and E. mori.1 ECC is recognized as a significant opportunistic pathogen associated with a broad spectrum of hospital-acquired infections, affecting various organs and systems. These infections often arise from bacteremia, respiratory, urinary tract and wound infections, frequently linked to the use of invasive devices or procedures.2 Additionally, Enterobacter spp. is part of the ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp), notorious for their ability to “escape” the effects of antimicrobial agents and cause the majority of hospital-acquired infections.3
A major concern with ECC is its propensity to develop multidrug resistance (MDR), facilitated by the high expression of AmpC-type cephalosporinase and the acquisition of resistance genes through mobile genetic elements under antimicrobial pressure.1,4 The rise of MDR, including resistance to the carbapenems (meropenem, imipenem, and ertapenem), considered the last line of defense, has heightened worries about these pathogens.1,5 MDR strains, including those from the Enterobacterales family, impose significant clinical and economic burdens,6–10 making MDR ECC isolates particularly concerning for empiric therapy.11–13
In China, the epidemiology of ECC has garnered increasing attention due to the emergence and hospital transmission of MDR ECC strains, which have become a major clinical and public health concern.14 Recent studies highlight the prevalence of MDR ECC in various hospital settings, driven by factors such as the overuse of antibiotics and the dissemination of mobile genetic elements encoding resistance genes.14,15 Of great concern, the rise of carbapenem-resistant ECC strains, often considered the last line of defense, has further heightened the need for effective infection control strategies.
Bacteremia is a prevalent hospital-acquired infection that can lead to increased healthcare costs, prolonged hospital stays, and higher mortality rates, especially when associated with MDR or carbapenem resistance.16–19 Infections due to MDR Enterobacterales, including ECC, have been linked to high mortality rates, sometimes as high as 50%.20 Over recent decades, ECC has become the third most common and lethal Enterobacterales species causing bacteremia.21–24
Although many studies have assessed risk factors for bacteremia linked to MDR Enterobacterales, these recognized risk factors include admission to intensive care units (ICU), extended hospital stays, prior use of broad-spectrum antibiotics, history of resistant strain colonization, indwelling urethral catheterization, and central venous catheterization.25–27 However, these studies primarily focused on organisms like Klebsiella pneumoniae and Escherichia coli, less attention has been given to ECC.
This study retrospectively explores the antimicrobial resistance trends of ECC strains causing bacteremia. The risk and prognostic factors for these infections are also identified by reviewing medical records. The findings aim to facilitate the development and implementation of targeted strategies for nosocomial infection prevention and control.
Materials and Methods
Bacterial Identification and Antimicrobial Susceptibility Testing
Blood cultures were conducted using the BacT/ALERT 3D (BioMérieux, Missouri, USA). Identification of bacterial isolates was performed by Vitek 2.0 system (BioMérieux, Marcy- l’Étoile, France) and matrix-associated laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (BioMérieux, Craponne, France). Antimicrobial susceptibility was verified using the Vitek 2.0 system (BioMérieux, Marcy- l’Étoile, France) and Kirby-Bauer disk diffusion method.28 Results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) M100, 34th edition.29
Definitions and Clinical Data Collection
Patients diagnosed with bacteremia caused by ECC strains were enrolled in this study to collect related information such as demographic details, clinical diagnosis, treatment, and outcome by reviewing patients’ medical records and doctor’s advice by searching the hospital information system (HIS) and laboratory information system (LIS).
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of Nanjing Drum Tower Hospital (2023–390). The requirement for signed informed consent was waived due to the absence of any interventions.
An ECC strain was considered MDR-ECC if it is non-susceptible to three or more classes of antibiotics based on in-vitro sensitivity testing.30 Bacteremia was defined as the isolation of ECC from one or more blood cultures.31 Only the first episode of the bacteremia in each patient was considered. The date of onset of bacteremia was defined as the date when the first blood culture was collected from which ECC was isolated. If the positive blood culture was obtained 48h after admission, the bacteremia was considered nosocomial; otherwise, it was considered community acquired.32
Invasive procedures during the hospital stay included any therapeutic procedure (eg vascular catheter, urinary catheter, mechanical ventilation) or diagnostic procedure (eg bronchoscopy, colonoscopy) or invasive surgery or blood transfusion that could have caused transient bacteremia if conducted within 10 days before the infection.31
Treatments within one month included were chemotherapy or corticosteroid therapy (daily dose of 10 mg or higher for more than 10 days), or antibiotic therapy (receipt of any antibiotics for more than 48 h) performed within one month prior to the onset of bacteremia.31 Antibiotic therapy was considered as appropriate if the isolate was susceptible to any of the previous administered antibiotics according to the blood culture results.31
Statistical Analysis
Univariate analysis was performed using the χ2-test or the two-tailed Fisher’s exact test for categorical variables to identify risk factor. Significant parameters with P < 0.10 in univariate analysis were considered candidate predictors and were included in the logistic regression model for multivariate analysis.33 Differences were considered statistically significant if P < 0.05. All the statistical analyses were performed using SPSS software (v27.0).
Results
In total, 188 consecutive and non-duplicate ECC strains isolated from blood were collected from January 2013 to December 2022 within Nanjing Drum Tower Hospital (the affiliated hospital of Nanjing University Medical School), which was a tertiary hospital in Jiangsu with more than 4000 beds. Overall, the ECC strains showed the highest resistant rates to ceftriaxone (39.9%), followed by ceftazidime (36.7%) and aztreonam (31.2%) and low resistant rates to carbapenems (imipenem 8.6%, meropenem 5.0%, ertapenem 8.4%, doripenem 5.3%) and amikacin (1.6%) (Table 1).
 |
Table 1 Antimicrobial Resistance Rates of Strains Isolated from 188 Patients with Enterobacter cloacae Complex Bacteriemia (No. of Isolates Tested) |
Among the isolates, 30.9% (58/188) were identified as MDR ECC strains. The total number of ECC strains and the number of non-MDR strains exhibited a similar trend, while the number of MDR ECC remained relatively stable from 2013 to 2019 (Figure 1A). Notably, the number of MDR ECC detections showed an upward trend from 2019 to 2022. During this period, the number of isolates resistant to ceftazidime (CAZ), aztreonam (ATM), levofloxacin (LVX), and piperacillin-tazobactam (TZP) increased significantly, which may explain the rise in MDR ECC numbers (Figure 1B).
Male patients (58.0%,109/188) were more susceptible to ECC bacteremia. The median age was 60.5 years (range 15–94 years), and the mean age was 60.3 years. Among the patients, 33.0% (62/188) patients had been admitted to the ICU, and 18.6% (35/188) had infections caused by multiple bacteria.
As shown in Figure 2A, 53.2% of the patients with ECC bacteremia were from surgery wards, followed by internal medicine wards (35.1%), ICU (10.6%) and emergency ward (1.1%). Among the internal medicine wards, 34.9% of the patients with ECC bacteremia were from gastroenterology ward, followed by the hematology ward (19.7%), oncology ward (16.6%), geriatric ward (15.2%) (Figure 2B). Among the surgical wards, 34.0% of the patients with ECC bacteremia were from hepatological surgery ward, followed by cardio-thoracic surgery ward (25.0%), and gastrointestinal surgery ward (11.0%) (Figure 2C).
All 188 patients had one or more underlying diseases. The most common were malignant disease (100/188, 53.2%), followed by hypertension (77/188, 41.0%). The most common infection was biliary tract infection, affecting 53 patients (53/188, 28.2%). Invasive procedures had been performed prior to 168 episodes (168/188, 89.4%). Various types of catheters were used in 141 episodes (141/188, 75.0%), with the most common being vascular catheter (117/188, 62.2%), followed by urinary catheter (92/188, 48.9%). There was no significant difference in the use of different types of catheters between patients with MDR and non-MDR ECC bacteremia (Figure 3). A total of 87 patients underwent surgical operations, and 76 patients received recent blood transfusion.
 |
Figure 3 Distribution of vascular catheterization (Vas), urinary catheterization (Uri), mechanical ventilation (Ven), and combined use in patients with Enterobacter cloacae complex bacteremia. |
Within one-month prior to the onset of bacteremia, 146 (77.7%) patients received antibiotics, 19 (10.1%) underwent chemotherapy, and 18 (9.6%) were administered corticosteroids. The most commonly used antibiotics for previous infections in all patients with ECC bacteremia were carbapenems (31/188), followed by combinations (23/188), first-generation cephalosporins (16/188) and tigecycline (16/188). In patients with MDR bacteremia, carbapenems were the most commonly used antibiotics (11/58), followed by combinations (9/58) and first-generation cephalosporins (7/58). In non-MDR patients, carbapenems (20/130) were also the most commonly used antibiotics followed by combinations (14/130) and tetracyclines (9/130) (Figure 4).
 |
Figure 4 Antibiotic use in the month prior to Enterobacter cloacae complex bacteremia. |
Univariate analysis for bacteremia caused by MDR strains showed statistical differences in age over 60 years, hypertension, pulmonary infection, initial empirical antibiotic therapy, and appropriate therapy (Table 2). Further multivariate analysis revealed that previous antibiotic therapy was a robust and independent risk factor (OR = 3.193, 95% CI 1.203–8.479, P < 0.020) (Table 2) of leading to multidrug resistance in the bacteremia pathogens, while appropriate antibiotic therapy demonstrated a statistically significant impact (OR = 0.279, 95% CI 0.130–0.598, P < 0.001) on mitigating this risk.
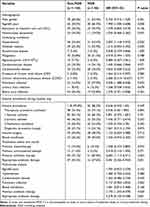 |
Table 2 Demographic and Clinical Characteristics of Patients with MDR-Negative and MDR-Positive Enterobacter cloacae Complex |
Univariate analysis for nosocomial acquired bacteremia showed statistical correlation with ICU admission (P = 0.038), biliary tract infection (P < 0.001), invasive procedures (P = 0.015), therapeutic procedure (catheter) (P < 0.001), vascular catheter (P = 0.002), urinary catheter (P = 0.003), diagnostic procedure (endoscopy) (P = 0.024), invasive surgery (P = 0.007), and blood transfusion (P = 0.036) (Table 3). Multivariate analysis showed a statistical difference in biliary tract infection (OR = 0.294, 95% CI 0.086–0.998, P = 0.050) (Table 3).
 |
Table 3 Demographic and Clinical Characteristics of Patients with Community and Nosocomial Acquired Enterobacter cloacae Complex |
Statistical differences were revealed in ICU admission (P < 0.001), malignancy (P = 0.013), chronic obstructive pulmonary disease (COPD) (P = 0.023), mechanical ventilation (P = 0.05), and previous chemotherapy (P = 0.027) (Table 4) in the univariate analysis of the risk factors for acquiring polymicrobial bacteremia. Multivariate analysis identified that previous ICU admission was an independent risk factor (P = 0.009) (Table 4).
 |
Table 4 Demographic and Clinical Characteristics of Patients with Non-Polymicrobial and Polymicrobial Bacteremia |
Univariate analysis in the mortality of ECC bacteremia showed a statistically significant association with invasive procedures (P = 0.048), urinary catheter (P = 0.030), mechanical ventilation (P = 0.009), diagnostic procedure (endoscopy) (P = 0.031), and blood transfusion (P < 0.001) (Table 5). Multivariate analysis indicated that diagnostic procedure (endoscopy) (P = 0.009) and blood transfusion (P = 0.003) were independent risk factors for mortality (Table 5).
 |
Table 5 Risk Factor Analysis for Survival and Mortality in Patients with Enterobacter cloacae Complex Bacteremia |
Discussion
The study highlighted notable patterns in antimicrobial resistance among ECC strains and identified significant risk factors for polymicrobial bacteremia, multidrug resistance, nosocomial acquisition and mortality associated with ECC bacteremia.
The resistance rates to ceftazidime and ceftriaxone in our study were obviously higher than those reported in Taiwan34 and Australian Group on Antimicrobial Resistance (AGAR) Bloodstream Infection Annual Report 2022.35 In Taiwan, resistance rates to ceftazidime and ceftriaxone were 27.2% and 29.3%, respectively, and in Australia, the resistant rates were 24.6% and 28.4% in order. The differences may come from the use of antimicrobial agents.36 Whereas, the consistency on the significant resistance to ceftriaxone, ceftazidime, and aztreonam across multiple analyses indicated that ceftriaxone, ceftazidime, and aztreonam consistently pose significant treatment challenges for ECC infections due to the high prevalence of resistant strains. Notably, the upward trend in MDR ECC strains from 2019 to 2022 is alarming, as it indicates a potential escalation in the complexity of clinical management of these cases. The stable prevalence of MDR strains prior to 2019 suggests that existing infection control measures and antimicrobial stewardship programs were initially effective, but the recent increase may signal a need for renewed strategies to prevent the spread of resistant strains. Notably, 30.9% of MDR ECC strains underscores the growing issue of MDR in clinical settings. This provided evidence to the reports that patients infected with third-generation or broad-spectrum cephalosporin-resistant isolates experience worse clinical response, longer hospital stay, poorer outcomes, and higher mortality rates.34 Furthermore, the finding that carbapenems and amikacin were the most effective antimicrobial agents against ECC in this study is in accordance with results from the China Antimicrobial Resistance Surveillance Trial (CARST) Program, 2011–2020,16 which suggests that carbapenems and amikacin may be considered primary options for empiric therapy in severe ECC infections with known resistance issues. However, clinicians must balance the need for effective immediate treatment with the long-term goal of preserving antibiotic efficacy.
Further, our study shows that male elderly patients are more susceptible to ECC bacteremia, suggesting a possible gender-related difference in susceptibility to ECC infections, which could be influenced by various biological and behavioral factors. Moreover, the high proportion of patients admitted to the ICU indicates the severity of the infections. In addition, the presence of underlying diseases in all patients highlights the vulnerability of these individuals to ECC bacteremia, and patients with compromised immune systems or chronic illnesses are at a higher risk. Lastly, the use of various types of catheters in 75.0% of the cases may indicate that medical interventions, particularly the use of various types of catheters are significant risk factors for infections. And the presence of these devices is a general risk factor for ECC bacteremia regardless of resistance status, since no significant difference in catheter use between MDR and non-MDR ECC bacteremia was observed.
MDR ECC is closely associated with prolonging hospitalization duration and worsening clinical outcome.11,12 Therefore, it is crucial to continuously monitor susceptibility profiles, clinical features and risk factors for MDR-ECC bacteremia to guide the formulation and implementation of effective infection control measures. Up to date, risk factors for MDR ECC bacteremia have not been extensively studied. Most studies focused on MDR Enterobacterales or Enterobacter bacteremia.7,31,37,38 For example, factors such as ICU admission, length of hospital stay, prior use of broad-spectrum antibiotics (eg, quinolones and cephalosporins), history of resistant strain colonization, indwelling urethral catheterization, and central venous catheterization are considered independent risk factors for bacteremia with MDR Enterobacterales.27 Previous third-generation cephalosporins therapy and prolonged perioperative prophylaxis are strong, independent risk factors for MDR Enterobacter bacteremia.31 ICU admission, drainage tube use, central venous catheterization, and carbapenem exposure are independent risk factors for carbapenem-resistant Enterobacter cloacae infection.33 Consistent with this study, the selective pressure of antibiotics leads to MDR ECC bacteremia, as patients receiving antibiotic therapy within one month were more likely to suppress sensitive bacteria while promoting the proliferation and spread of resistant strains.39 Notably, carbapenems were the most widely used antibiotic in this study. However, initial therapy with a carbapenem appears to be associated with improved clinical outcome in BSI due to ESBL-producing E. cloacae.20 Despite the improvement in clinical outcomes, the use of carbapenem antibiotics will inevitably select for carbapenem-resistant strains, resulting in the extensive proliferation of various resistant bacteria, and complicating subsequent antibiotic selection.
Multivariate analysis showed a statistical association between biliary tract infections and nosocomial ECC bacteremia. Interestingly, the data revealed that patients with community-acquired biliary tract infections accounted for 62.5% (15/24) of the cases, significantly higher than the 23.2% (38/164) observed for nosocomial-acquired infections. This suggests that patients with prior biliary tract infections, particularly those acquired in the community, may be predisposed to ECC bacteremia. This predisposition could be attributed to several factors. Patients with community-acquired biliary tract infections may already be colonized with ECC, which can translocate to the bloodstream during or after the infection due to disruption of the biliary tract barrier, potentially exacerbated by delayed diagnosis or incomplete treatment.40 Additionally, community-acquired infections may occur in patients with underlying conditions such as gallstones or biliary strictures, which increase their susceptibility to recurrent infections or prolonged colonization.40 In contrast, nosocomial ECC bacteremia may arise from diverse sources, such as indwelling devices or invasive procedures,41 thereby reducing the relative contribution of biliary tract infections in these cases.
ICU admission was identified as an independent risk factor for polymicrobial bacteremia. This could be due to several reasons. Firstly, patients in the ICU are usually in an immunocompromised state and exposed to multiple antimicrobial agents and undergo frequent invasive procedures, increasing the probability of polymicrobial bacteremia.33,42 Secondly, repeated exposure of ECC to antimicrobial agents more easily suppresses susceptible strains, facilitating the proliferation of resistant strains and promoting the dissemination of MDR ECC strains.32 Therefore, preventive and control measures for nosocomial infections should be implemented, and guidelines for invasive procedures should be strictly followed to mitigate the spread of MDR ECC.
Nosocomial infection has been independently associated with mortality of Enterobacter bacteremia.31 Solid tumors, septic shock and mechanical ventilation are significant predictors for 28-day mortality in carbapenem-resistant Enterobacter cloacae causing nosocomial infections.33 Lung infections, abdominal infections, central venous catheterization, and hormone use within 30 days increased the mortality rate of Enterobacterales BSIs.27 Both endoscopy and blood transfusion are associated with significant risks due to their invasive nature and the underlying conditions of the patients requiring these interventions. As we know that the resulted disruption of mucosal barriers, potential procedural contamination, immune modulation, and the severity of the underlying illness all contribute to the increased risk of mortality in patients with Enterobacter bacteremia undergoing these procedures. Understanding these risks underscores the importance of stringent infection control measures and careful patient monitoring during and after these interventions to mitigate the risk of adverse outcomes.
This study has several limitations. The study was conducted in a single center, and the prevalence of resistance found here might not be applicable to the entire region. As in any observational study, our analysis of clinical information in subject to confounding biases. However, it provides the foundation for future national research related to cooperative surveillance on resistance and risk factors to control further infection spread.
In conclusion, carbapenems and amikacin are the most effective treatments for ECC bacteremia. Previous antibiotic therapy was an independent risk factor, and appropriate therapy was a protective factor for patients with MDR ECC bacteremia. ICU admission was an independent risk factor for polymicrobial bacteremia. Both endoscopy and blood transfusion are associated with mortality of ECC Bacteremia. Control of MDR ECC bacteremia requires a cooperative and comprehensive approach, including strategies for improving the rate of pathogenic bacteria testing for antibiotic therapy in hospitalized patients, using antibiotics rationally based on susceptibility test results, risk factor detection and implementation strategies of infection-control and prevention.
Data Sharing Statement
The authors confirm that the data and material supporting the findings of this study are available within the article.
Acknowledgments
This paper has been uploaded to Research Square as a preprint:https://www.researchsquare.com/article/rs-4728109/v1, and the current version has been updated with additional statistical analyses and revisions based on reviewer feedback.
Funding
This work was supported by funding for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2021-LCYJ-PY-06).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Annavajhala MK, Gomez-Simmonds A, Uhlemann A-C. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front Microbiol. 2019;10. doi:10.3389/fmicb.2019.00044
2. Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol. 2012;7(7):887–902. doi:10.2217/fmb.12.61
3. Álvarez-Marín R, Lepe JA, Gasch-Blasi O, et al. Clinical characteristics and outcome of bacteraemia caused by Enterobacter cloacae and Klebsiella aerogenes: more similarities than differences. J Glob Antimicrob Resist. 2021;25:351–358. doi:10.1016/j.jgar.2021.04.008
4. Liu S, Fang R, Zhang Y, et al. Characterization of resistance mechanisms of Enterobacter cloacae complex co-resistant to carbapenem and colistin. BMC Microbiol. 2021;21(1). doi:10.1186/s12866-021-02250-x
5. Zhang Z, Sun Z, Tian L. Antimicrobial resistance among pathogens causing bloodstream infections: a multicenter surveillance report over 20 years (1998–2017). Infect Drug Resist. 2022;15:249–260. doi:10.2147/IDR.S344875
6. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of Carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2). doi:10.1128/AAC.01882-17
7. Seo H, Lee SC, Chung H, et al. Clinical and microbiological analysis of risk factors for mortality in patients with Carbapenem-resistant Enterobacteriaceae bacteremia. Int J Antimicrob Agents. 2020;56(4):106126. doi:10.1016/j.ijantimicag.2020.106126
8. Song KH, Kim CJ, Choi NK, et al. Clinical and economic burden of bacteremia due to multidrug-resistant organisms in Korea: a prospective case control study. J Glob Antimicrob Resist. 2022;31:379–385. doi:10.1016/j.jgar.2022.11.005
9. Lee CM, Lee S, Kim ES, et al. Disease burden of bacteremia with extended-spectrum beta-lactamase-producing and carbapenem-resistant Enterobacteriaceae in Korea. J Hosp Infect. 2023;114:85–93.
10. Stewardson AJ, Marimuthu K, Sengupta S, et al. Effect of carbapenem resistance on outcomes of bloodstream infection caused by Enterobacteriaceae in low-income and middle-income countries (PANORAMA): a multinational prospective cohort study. Lancet Infect Dis. 2019;19(6):601–610. doi:10.1016/S1473-3099(18)30792-8
11. Bush K. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr Opin Microbiol. 2010;13(5):558–564. doi:10.1016/j.mib.2010.09.006
12. Potter RF, D’Souza AW, Dantas G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updates. 2016;29:30–46. doi:10.1016/j.drup.2016.09.002
13. Giammanco A, Calà C, Fasciana T, Dowzicky MJ, Bradford PA. Global assessment of the activity of Tigecycline against multidrug-resistant gram-negative pathogens between 2004 and 2014 as part of the Tigecycline evaluation and surveillance trial. mSphere. 2017;2(1). doi:10.1128/mSphere.00310-16
14. Yan Z, Ju X, Zhang Y, et al. Analysis of the transmission chain of carbapenem-resistant Enterobacter cloacae complex infections in clinical, intestinal and healthcare settings in Zhejiang province, China (2022–2023). Sci Total Environ. 2024;920:170635. doi:10.1016/j.scitotenv.2024.170635
15. Zhou H, Wang S, Wu Y, et al. Carriage of the mcr-9 and mcr-10 genes in clinical strains of the Enterobacter cloacae complex in China: a prevalence and molecular epidemiology study. Int J Antimicrob Agents. 2022;60(4):106645. doi:10.1016/j.ijantimicag.2022.106645
16. Yan M, Zheng B, Li Y, Lv Y. Antimicrobial susceptibility trends among gram-negative Bacilli causing bloodstream infections: results from the China Antimicrobial Resistance Surveillance Trial (CARST) program, 2011–2020. Infect Drug Resist. 2022;15:2325–2337. doi:10.2147/IDR.S358788
17. Cai Y, Chen C, Zhao M, et al. High prevalence of Metallo-β-Lactamase-producing Enterobacter cloacae from Three Tertiary Hospitals in China. Front Microbiol. 2019;10. doi:10.3389/fmicb.2019.01610
18. Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by Enterobacter species: predictors of 30-day mortality rate and impact of broad-spectrum cephalosporin resistance on outcome. Clin Infect Dis. 2004;39(6):812–818. doi:10.1086/423382
19. Oka K, Matsumoto A, Tetsuka N, et al. Clinical characteristics and treatment outcomes of carbapenem-resistant Enterobacterales infections in Japan. J Glob Antimicrob Resist. 2022;29:247–252. doi:10.1016/j.jgar.2022.04.004
20. Qureshi ZA, Paterson DL, Pakstis DL, et al. Risk factors and outcome of extended-spectrum β-lactamase-producing Enterobacter cloacae bloodstream infections. Int J Antimicrob Agents. 2011;37(1):26–32. doi:10.1016/j.ijantimicag.2010.09.009
21. Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7). doi:10.1128/AAC.00355-19
22. Xu A, Zheng B, Xu YC, Huang ZG, Zhong NS, Zhuo C. National epidemiology of carbapenem-resistant and extensively drug-resistant Gram-negative bacteria isolated from blood samples in China in 2013. Clin Microbiol Infect. 2016;22(Suppl 1):S1–8. doi:10.1016/j.cmi.2015.09.015
23. Xi J, Jia P, Zhu Y, et al. Antimicrobial susceptibility to polymyxin B and other comparators against Gram-negative bacteria isolated from bloodstream infections in China: results from CARVIS-NET program. Front Microbiol. 2022;13:1017488. doi:10.3389/fmicb.2022.1017488
24. Davin-Regli A, Pagès JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:392. doi:10.3389/fmicb.2015.00392
25. Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20(7):1170–1175. doi:10.3201/eid2007.121004
26. Gallagher JC, Kuriakose S, Haynes K, Axelrod P. Case-case-control study of patients with carbapenem-resistant and third-generation-cephalosporin-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2014;58(10):5732–5735. doi:10.1128/AAC.03564-14
27. Li X, Ye H. Clinical and mortality risk factors in bloodstream infections with Carbapenem-resistant Enterobacteriaceae. Can J Infect Dis Med Microbiol. 2017;2017:6212910. doi:10.1155/2017/6212910
28. Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Tech Bull Regist Med Technol. 1966;36(3):49–52.
29. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100.
30. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
31. Ye Y, Li JB, Ye DQ, Jiang ZJ. Enterobacter bacteremia: clinical features, risk factors for multiresistance and mortality in a Chinese University Hospital. Infection. 2006;34(5):252–257. doi:10.1007/s15010-006-5038-3
32. Liu Y, Li F, Fang Y, et al. Clinical characteristics, prognosis and treatment of bloodstream infections with Enterobacter cloacae complex in a Chinese Tertiary Hospital: a retrospective study. Infect Drug Resist. 2024;17:1811–1825. doi:10.2147/IDR.S460744
33. Tian X, Huang C, Ye X, et al. Carbapenem-resistant Enterobacter cloacae causing nosocomial infections in Southwestern China: molecular epidemiology, risk factors, and predictors of mortality. Infect Drug Resist. 2020;13:129–137. doi:10.2147/IDR.S234678
34. Chang CY, Huang PH, Lu PL. The resistance mechanisms and clinical impact of resistance to the third generation cephalosporins in species of Enterobacter cloacae complex in Taiwan. Antibiotics. 2022;11(9):1153. doi:10.3390/antibiotics11091153
35. Bell JM, Fajardo lubian A, Partridge SR, et al. Australian Group on Antimicrobial Resistance (AGAR) Australian Gram-negative Surveillance Outcome Program (GnSOP) - bloodstream infection annual report 2022. Commun Dis Intell. 2023;47.
36. Huang Z, Liu S, Wang Y, et al. Comparison of prevalence, resistance, biofilm-forming ability and virulence between carbapenem-non-susceptible and carbepenem-susceptible Enterobacter cloacae complex in clusters. J Hosp Infect. 2023;139:168–174. doi:10.1016/j.jhin.2023.06.017
37. Lin T-C, Hung Y-P, Lin W-T, Dai W, Huang Y-L, Ko W-C. Risk factors and clinical impact of bacteremia due to carbapenem-nonsusceptible Enterobacteriaceae: a multicenter study in southern Taiwan. J Microbiol Immunol Infect. 2021;54(6):1122–1129. doi:10.1016/j.jmii.2021.05.005
38. Álvarez-Marín R, Navarro-Amuedo D, Gasch-Blasi O, et al. A prospective, multicenter case control study of risk factors for acquisition and mortality in Enterobacter species bacteremia. J Infect. 2020;80(2):174–181. doi:10.1016/j.jinf.2019.09.017
39. de Nies L, Kobras CM, Stracy M. Antibiotic-induced collateral damage to the microbiota and associated infections. Nat Rev Microbiol. 2023;21(12):789–804. doi:10.1038/s41579-023-00936-9
40. Liu J, Yan Q, Luo F, et al. Acute cholecystitis associated with infection of Enterobacteriaceae from gut microbiota. Clin Microbiol Infect. 2015;21(9):851.e1–9. doi:10.1016/j.cmi.2015.05.017
41. Tetsuka N, Hirabayashi A, Matsumoto A, et al. Molecular epidemiological analysis and risk factors for acquisition of carbapenemase-producing Enterobacter cloacae complex in a Japanese university hospital. Antimicrob Resist Infect Control. 2019;8:126. doi:10.1186/s13756-019-0578-3
42. Mrázová M, Spanik S, Trupl J, et al. Bacteremia due to Enterobacter spp. in cancer patients--analysis of 51 episodes. Int J Antimicrob Agents. 1997;8(4):277–285. doi:10.1016/S0924-8579(97)00024-1
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Clinical Characteristics and Risk Factors for in-Hospital Mortality in 240 Cases of Infective Endocarditis in a Tertiary Hospital in China: A Retrospective Study
Zhang X, Jin F, Lu Y, Ni F, Xu Y, Xia W
Infection and Drug Resistance 2022, 15:3179-3189
Published Date: 18 June 2022
Clinical Features of Human Brucellosis and Risk Factors for Focal Complications: A Retrospective Analysis in a Tertiary-Care Hospital in Beijing, China
Zhang Z, Zhang X, Chen X, Cui X, Cai M, Yang L, Zhang Y
International Journal of General Medicine 2022, 15:7373-7382
Published Date: 19 September 2022
Maternal and Fetal Risk Factors for Neonatal Hypoxic-Ischemic Encephalopathy: A Retrospective Study
Chen X, Chen H, Jiang D
International Journal of General Medicine 2023, 16:537-545
Published Date: 13 February 2023
Adolescent Non-Puerperal Mastitis: Risk Factors, Clinical Characteristics, and Prognosis Analysis
Tang H, Wu X, Feng J, Gao Q, Shao S, Qu W, Xie L, Sun J
Journal of Inflammation Research 2024, 17:487-495
Published Date: 24 January 2024
Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae: Risk Factors and Economic Burden Among Patients with Bloodstream Infections
Chen J, Allel K, Zhuo C, Luo W, He N, Yang X, Guo Y, Wang J, Yao L, Li J, Lin Y, Tu R, Yakob L, Zhuo C
Risk Management and Healthcare Policy 2024, 17:375-385
Published Date: 28 February 2024



