Back to Journals » Infection and Drug Resistance » Volume 18
Clinical Characteristics of Vaginal Trichomoniasis Infection and Metronidazole Resistance in Vaginitis Patients
Received 7 November 2024
Accepted for publication 6 February 2025
Published 25 February 2025 Volume 2025:18 Pages 1161—1169
DOI https://doi.org/10.2147/IDR.S505326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Guixue Lv,* Xunrong Cao,* Chunfeng Zheng
Department of Gynecology, Jinan Second Maternal and Child Health Hospital, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xunrong Cao, Email [email protected]
Objective: This study aims to investigate the clinical characteristics of vaginal trichomoniasis infection among vaginitis patients and assess their resistance to metronidazole.
Methods: We conducted a retrospective study involving 687 vaginitis patients who visited our hospital from April 2022 to June 2024. Clinical data were collected through questionnaires that included information on age, occupation, season of infection, marital status, contraceptive methods, and frequency of vulvar hygiene. Vaginal secretions were examined for trichomoniasis, and the characteristics of the infection were analyzed. Multivariate logistic regression was employed to identify factors influencing vaginal trichomoniasis infection. Infected vaginal trichomonas samples were cultured in vitro, and metronidazole gradient concentration plates were prepared to determine the minimum lethal concentration (MLC) of metronidazole against vaginal trichomonas, allowing for an assessment of resistance.
Results: Out of 687 vaginitis patients, 65 were diagnosed with vaginal trichomoniasis, resulting in an infection rate of 9.46%. Significant differences in infection rates were observed based on age, occupation, season of infection, marital status, contraceptive methods, and frequency of vulvar hygiene (P < 0.05).
Conclusion: The infection rate of vaginal trichomoniasis is notably higher in younger vaginitis patients compared to middle-aged and elderly individuals, with a peak occurrence in spring. Higher rates were also noted among farmers, married individuals, those using oral contraceptives or no contraception, and those with infrequent vulvar cleaning. Additionally, resistance to metronidazole was identified in some cases. Clinical efforts should prioritize prevention and treatment strategies for high-risk populations.
Keywords: vaginitis, vaginal trichomoniasis infection, clinical characteristics, influencing factors, metronidazole, resistance
Introduction
Vaginal trichomoniasis is one of the most common non-viral sexually transmitted diseases worldwide, posing a serious threat to women’s reproductive health.1 After infection, patients may experience typical symptoms such as increased vaginal discharge, odor, itching, burning sensations, and vulvar redness. In severe cases, symptoms may extend to abdominal pain, frequent urination, and dysuria.2 Moreover, vaginal trichomoniasis not only endangers individual health but also has a significant public health impact. Studies3 have shown that some individuals with vaginal trichomoniasis infection have no obvious clinical symptoms, making asymptomatic carriers potential “sources of infection” and thereby increasing the public health burden. Consequently, early detection and effective control of vaginal trichomoniasis infection have become important public health topics worldwide.
In terms of treatment, metronidazole is currently the primary antiprotozoal drug, commonly used as a first-line treatment and demonstrating significant efficacy in early infections. It mainly works by being reduced to its active form within trichomonas cells, where it binds to DNA and leads to cell death.4,5 However, with the widespread clinical use of metronidazole, resistance has gradually increased, posing new challenges to treatment.6 This resistance not only reduces treatment effectiveness but also raises the risk of recurrence, significantly increasing the disease burden on patients. The mechanisms underlying resistance may be closely related to changes in cellular metabolic pathways, drug target alterations, and gene mutations.7,8 This trend has driven researchers to explore new treatment options and resistance suppression methods to combat the spread of resistant strains. Additionally, the prevalence of vaginal trichomoniasis infection is relatively high, with significant variations across different regions and populations.9,10 Based on these reasons, this study retrospectively analyzed the clinical data of 687 vaginitis patients at our hospital, aiming to explore the clinical characteristics of vaginal trichomoniasis infection and evaluate metronidazole resistance, providing insights into the prevalence characteristics of vaginal trichomoniasis infection among different populations in China and its resistance to metronidazole. This not only helps in identifying high-risk groups clinically but also provides a scientific basis for developing effective prevention and treatment strategies.
Materials and Methods
Clinical Data
A retrospective study was conducted on 687 patients diagnosed with vaginitis and treated at our hospital between April 2022 and June 2024. Inclusion criteria: ① meeting the clinical diagnostic criteria for vaginitis and Trichomonas vaginalis infection;11,12 ② age ≥ 18 years, with the ability to complete relevant examinations and data collection; ③ no treatment with metronidazole or other nitroimidazoles in the past three months; ④ complete medical records with authentic and analyzable data. Exclusion criteria: ① concurrent sexually transmitted infections (eg, Chlamydia, Gonorrhea, HIV) or other types of vaginal infections; ② chronic diseases affecting the immune system (eg, systemic lupus erythematosus, AIDS) or undergoing immunosuppressive therapy; ③ recent (within three months) use of antibiotics, antiprotozoal drugs, or other medications affecting vaginal microecology; ④ pregnancy or lactation; ⑤ cognitive, awareness, reading, or mental disabilities that could impair cooperation. This study was approved by the Jinan Second Maternal and Child Health Hospital’s Ethics Committee, approval number FK-24-GZ-011. Informed consent was obtained from all study participants. All the methods were carried out in accordance with the Declaration of Helsinki.
Methods
Data Collection
A case report form (CRF) was developed for this study, allowing for the collection of clinical data from the participants’ medical records. It is essential to clarify that the characteristics of the women were retrospectively collected from complete medical charts rather than through a dedicated questionnaire. This approach ensured comprehensive and accurate data retrieval.
Vaginal Secretion Examination Method
Following the method in reference,13 vaginal secretions were collected using a disposable sterile cotton swab by rotating it at the vaginal sidewall one-third area or posterior fornix. The collected sample was placed in a sterile saline tube (0.9% NaCl) and sent to the laboratory for testing immediately. The sample was smeared on a clean slide and examined under a microscope, starting with a low-power objective to identify abnormal cells or microorganisms. When a suspected motile Trichomonas body was observed under low magnification, a high-power objective was used for closer examination to identify typical Trichomonas morphology. Once confirmed under high magnification, Trichomonas vaginalis infection was diagnosed without further testing. For further analysis of Trichomonas vaginalis, the sample was cultured on a specialized medium in a 37°C incubator. To ensure culture quality, subculturing was performed after 48 hours and repeated three times to obtain a pure, viable Trichomonas sample.
Metronidazole Resistance Testing Method for Trichomonas Vaginalis
(1) Preparation of Metronidazole Gradient Concentration Plate: First, dissolve pure metronidazole standard to prepare a stock solution. The stock solution was serially diluted in the culture medium to create a gradient of metronidazole concentrations under sterile conditions. The gradient concentration solutions were stored in a refrigerator at 4°C. During the experiment, 100 μL of the prepared metronidazole solution was added to each well of a sterile 96-well plate, dried, and then frozen for storage. (2) Trichomonas vaginalis Preparation and Inoculation: Cultures of Trichomonas vaginalis in the logarithmic growth phase were centrifuged to remove the supernatant, and the precipitate was collected. The precipitate was resuspended in an appropriate amount of medium, and protozoa were counted to ensure cell density met detection requirements. The cell density was then adjusted to the standard level and aliquoted into the 96-well plate to ensure uniform Trichomonas concentration in each well. (3) Resistance Testing and Observation of Results: The adjusted Trichomonas suspension was inoculated into pre-prepared wells with different metronidazole concentrations, setting at least two parallel wells per concentration. The 96-well plate containing Trichomonas and metronidazole was incubated at 37°C to ensure adequate drug exposure. After incubation, a microscope was used to observe Trichomonas survival status in each concentration of metronidazole, and counting was performed. The minimal lethal concentration (MLC) was defined as the lowest metronidazole concentration at which Trichomonas morphology rounded, or movement ceased completely. Strains with MLCs of 100–300 μg/mL were marked as resistant strains.
Observation Indicators
(1) Collect clinical data of vaginitis patients and information on Trichomonas vaginalis infection. (2) Analyze infection rates of Trichomonas vaginalis among patients of different ages, occupations, seasons of infection, marital status, contraceptive methods, and vulvar cleaning frequency. (3) Summarize influencing factors of Trichomonas vaginalis infection in vaginitis patients using multivariate Logistic regression analysis. (4) Determine the MLC of metronidazole against Trichomonas vaginalis to assess the resistance status of Trichomonas vaginalis infection in vaginitis patients.
Statistical Analysis
GraphPad Prism 8 software was used for plotting, and SPSS 22.0 software was used for data processing. Count data were expressed as percentages (%) and analyzed by χ²-test or Fisher’s exact probability method. A P-value of <0.05 indicated statistically significant differences. Multivariate logistic regression analysis was utilized to identify associated factors for Trichomonas vaginalis infection among the participants. The dependent variable was the presence of Trichomonas vaginalis infection (1 = infected, 2 = not infected), while the independent variables included age, occupation, season of infection, marital status, contraceptive methods, and vulvar cleaning frequency.
To compute the logistic regression, variables were coded (eg, age <45 years = 1, age ≥45 years = 2), and the analysis was carried out to assess the odds ratios (OR) for each variable, with a significance level set at P < 0.05 indicating statistical significance. Count data were expressed as percentages, analyzed using the χ²-test or Fisher’s exact probability method.
Results
Clinical Data Information of Vaginitis Patients
The clinical data of 687 vaginitis patients, including age, occupation, season of infection, marital status, contraceptive method, and vulvar cleaning frequency, are shown in Table 1.
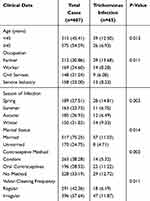 |
Table 1 Analysis of Clinical Characteristics of Trichomonas Vaginalis Infection in Vaginitis Patients [n (%)] |
Analysis of Clinical Characteristics of Trichomonas Vaginalis Infection in Vaginitis Patients
Among the 687 vaginitis patients, 65 cases were found to have Trichomonas vaginalis infection, with an infection rate of 9.46%. There were significant differences in the infection rates of Trichomonas vaginalis among patients of different ages, occupations, infection seasons, marital statuses, contraceptive methods, and vulvar cleaning frequencies (P<0.05), as shown in Figure 1 and Table 2.
 |
Figure 1 Trichomonas vaginalis. |
 |
Table 2 Clinical Characteristics Analysis of Vaginitis Patients Infected with Trichomonas vaginalis [n (%)] |
Associated Factors of Trichomonas Vaginalis Infection in Vaginitis Patients
Using whether vaginitis patients had Trichomonas vaginalis infection as the dependent variable (1=infection, 2=no infection) and taking the potential influencing factors identified in Table 2 as independent variables with assigned values (see Table 3), a multivariate logistic regression analysis model was established. The results indicated that being younger than 45, being a farmer, being infected in the spring, and being married were risk factors for Trichomonas vaginalis infection in vaginitis patients (OR>1, P<0.05). Using condoms and regular vulvar cleaning were protective factors (OR<1, P<0.05), as shown in Table 4.
 |
Table 3 Variable Assignment Table |
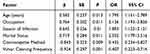 |
Table 4 Multivariate Logistic Regression Analysis of Influencing Factors of Trichomonas Vaginalis Infection in Vaginitis Patients |
MLC Value of Metronidazole for Trichomonas Vaginalis
The metronidazole MLC range for 30 samples was 1–300 μg/mL. Among them, 14 strains showed high sensitivity, with MLCs of 1–25 μg/mL; 11 strains had moderate sensitivity, with MLCs of 50–70 μg/mL; and 5 strains had low sensitivity, with MLCs of 100–300 μg/mL (Figure 2).
 |
Figure 2 MLC Values of Metronidazole for Trichomonas Vaginalis (μg/mL). |
Discussion
Our study shows that patients under the age of 45 have a significantly higher infection rate compared to those aged 45 and above, consistent with previous research.14–20 It is speculated that this may be due to the relatively high frequency of sexual activity and more exposure pathways in people under 45, thus increasing their infection risk. However, despite the lower frequency of sexual activity in patients over 45, a certain proportion of these patients are still infected with Trichomonas vaginalis. This could be attributed to the fact that in elderly women, reduced estrogen levels lead to changes in the vaginal microenvironment, weakening the mildly acidic environment, which compromises the immune defense barrier, creating favorable conditions for the survival and reproduction of Trichomonas vaginalis.21 Additionally, older individuals tend to have weaker immunity, making them more susceptible to infections, which contributes to the infection rate of Trichomonas vaginalis among elderly patients.22 The study also found significant seasonal differences in the infection rate of Trichomonas vaginalis, with a higher rate in the spring than in other seasons. This may be closely related to the warm and humid climate of spring, which favors the growth and reproduction of Trichomonas vaginalis, thereby increasing the infection risk for women during this season. Consequently, it is recommended that women exercise particular caution during this time and enhance their self-protection awareness. In terms of occupation, patients who were farmers had a higher infection rate of Trichomonas vaginalis. Due to limitations in education and economic conditions, farmers often have less access to health knowledge and lack strong hygiene habits. The lack of relevant health education results in low hygiene awareness and inadequate personal protection, leading to a higher infection rate in this population.23 Therefore, clinical efforts should focus on strengthening health knowledge dissemination among farmers, raising their awareness of preventing Trichomonas vaginalis infection, and helping them develop basic hygiene practices and health knowledge, especially regarding preventive measures for high-risk behaviors associated with infection. Marital status is also associated with the infection rate of Trichomonas vaginalis, with married women having a relatively higher frequency of sexual activity, which increases the likelihood of exposure to sources of infection.24 Therefore, married women are at a higher risk of infection with Trichomonas vaginalis. It is recommended that married women be more mindful of maintaining healthy and hygienic sexual practices to prevent infection. Regarding personal hygiene practices, women who clean their vulva regularly have a relatively lower infection rate, indicating that good hygiene habits have a positive effect on preventing Trichomonas vaginalis infection. Women should be encouraged to develop regular cleaning habits, particularly married women, who should pay extra attention to vulvar hygiene. Furthermore, in terms of contraceptive methods, it is recommended to prioritize the use of high-quality condoms. Condoms not only provide contraceptive protection but also help reduce the risk of Trichomonas vaginalis transmission to some extent, thereby protecting women’s health.25,26
Metronidazole is the primary drug used for treating Trichomonas vaginalis infections, and its efficacy has been widely applied and validated. However, with the widespread use of metronidazole in recent years, the issue of resistance in Trichomonas vaginalis to this drug has gradually emerged. Multiple studies27,28 have shown a gradual increase in metronidazole resistance rates of Trichomonas vaginalis globally, especially among patients with recurrent infections or those on prolonged medication. Studies on metronidazole-resistant Trichomonas vaginalis29 indicate that these resistant strains exhibit significantly elevated minimum inhibitory concentrations (MIC) for metronidazole, requiring higher doses of the drug to be effective. However, increasing the dose often brings more adverse reactions. This study determined the minimum lethal concentration (MLC) of metronidazole on Trichomonas samples and found that resistant strains accounted for 16.67%, further confirming a certain degree of resistance to metronidazole in Trichomonas vaginalis. The molecular mechanism of metronidazole resistance is not yet fully understood and mainly involves the metabolic activation of the drug and intracellular redox balance. However, several adaptive changes in resistant strains may weaken or inhibit this reduction process: ① Changes in redox pathways: Resistant strains of Trichomonas adjust their redox systems, such as by increasing intracellular oxygen concentration, disrupting metronidazole activation under anaerobic conditions, thereby preventing the drug from being reduced to its active form, which decreases its efficacy. ② Alterations in oxidases and antioxidative enzymes: The key mechanism of metronidazole action is inducing oxidative stress damage. Resistant strains may reduce oxidative stress damage to their DNA by upregulating antioxidant enzymes (eg, superoxide dismutase).30 Additionally, an increase in certain oxidases may also prevent metronidazole from completing metabolic activation in anaerobic environments, thereby enhancing resistance. ③ Plasmid or gene mutation: Some studies31 suggest that specific genes carried by plasmids may influence the function of metronidazole-metabolizing enzymes, thereby altering drug sensitivity. Furthermore, some resistant strains may have mutations in specific genes, reducing their capacity for metronidazole metabolism and activation, further weakening the drug’s effectiveness. The emergence of metronidazole resistance presents new challenges for treating Trichomonas vaginalis infections. This study suggests that an in-depth understanding of resistance mechanisms and influencing factors not only contributes to optimizing current treatment methods but also provides direction for developing new treatment regimens. Future studies could focus on the molecular characteristics of resistant strains, regulatory mechanisms of resistance, and the clinical efficacy of multidrug combinations to better control resistance and ensure treatment efficacy and reproductive health.
This study has the following limitations: ① Limited sample size: The number of patients included in this study was relatively small, particularly among specific populations (eg, elderly patients or groups with significant occupational differences), which may limit the representativeness of the study results. ② Lack of long-term follow-up data: This study is a cross-sectional analysis without long-term follow-up of patients, making it impossible to assess the long-term health impact of Trichomonas vaginalis infections on patients, especially the recurrence rate and its relationship with metronidazole resistance. ③ Single-center limitation: This study is based on data from a single research center, which may be influenced by regional factors, limiting the generalizability of the results. ④ Inadequate control of influencing factors: Although this study considered various potential influencing factors (eg, age, occupation, hygiene habits), some potential confounding factors (eg, number of sexual partners, history of other infections) were not included in the analysis, which may affect the accuracy and interpretability of the study results. ⑤ Limitations in resistance detection: The metronidazole resistance detection in this study was limited to certain patients, and the detection method used may have limitations, as it did not further analyze the resistance mechanisms. ⑥ Self-reporting bias: Some data (eg, frequency of cleaning, contraceptive methods) relied on patients’ self-reports, which may introduce subjective bias, affecting the accuracy of the study results.
Conclusion
In conclusion, our study sheds light on the multifaceted factors associated with Trichomonas infection. The higher prevalence among older adults, farmers, and unmarried individuals highlights the need for targeted public health interventions. Strategies to promote safe sex practices and improve access to healthcare, particularly in agricultural communities, are essential in addressing this public health concern. Additionally, as resistance to metronidazole is becoming increasingly prevalent, there is an urgent need for ongoing surveillance and research into alternative treatments. The findings contribute valuable insights into the epidemiology of Trichomonas vaginalis in China, paving the way for improved health outcomes through informed prevention and treatment strategies.
Future research should focus on longitudinal studies to better understand the dynamics of Trichomonas transmission and the efficacy of intervention programs. By considering both internal and external validity, future studies can further elucidate the complexities surrounding Trichomonas infection and inform effective strategies for prevention and control.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Funding
Project: Shandong Province Medicine and Health science and technology plan project. Project number: 202305011230.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Xu JB, Lu S-J, Ke L-J, et al. Trichomonas vaginalis infection impairs anion secretion in vaginal epithelium. PLoS Negl Trop Dis. 2021;15(4):e0009319. doi:10.1371/journal.pntd.0009319
2. Feleke DG, Yemanebrhane N. Trichomonas vaginalis infection in Ethiopia: a systematic review and meta-analysis. Int J STD AIDS. 2022;33(3):232–241. doi:10.1177/09564624211060176
3. Kim TG, Young MR, Goggins ER, et al. Trichomonas vaginalis in Pregnancy: patterns and Predictors of Testing, Infection, and Treatment. Obstet Gynecol. 2020;135(5):1136–1144. doi:10.1097/AOG.0000000000003776
4. Fang L, Lu X, Cui C, et al. Metronidazole-loaded nanoparticulate thermoreversible gel for gynecologic infection of Trichomonas vaginalis. Am J Transl Res. 2022;14(6):4015–4023.
5. Hernández Ceruelos A, Romero-Quezada LC, Ruvalcaba Ledezma JC, et al. Therapeutic uses of metronidazole and its side effects: an update. Eur Rev Med Pharmacol Sci. 2019;23(1):397–401. doi:10.26355/eurrev_201901_16788
6. Saito-Nakano Y, Umeki Y, Shimokawa C, et al. Prevalence and metronidazole resistance of Trichomonas vaginalis among Japanese women in 2021. IJID Reg. 2023;7:130–135. doi:10.1016/j.ijregi.2023.02.007
7. Marques-Silva M, Lisboa C, Gomes N, et al. Trichomonas vaginalis and growing concern over drug resistance: a systematic review. J Eur Acad Dermatol Venereol. 2021;35(10):2007–2021. doi:10.1111/jdv.17461
8. Mabaso N, Tinarwo P, Abbai N. Lack of association between mycoplasma hominis and Trichomonas vaginalis symbiosis in relation to metronidazole resistance. Parasitol Res. 2020;119(12):4197–4204. doi:10.1007/s00436-020-06930-x
9. YA Hawash, KA Ismail, NF Jaafer, et al. Prevalence and risk factors for trichomonas vaginalis infection among women: a population-based controlled study in Saudi Arabia. Clin Lab. 2022;68(6). doi:10.7754/Clin.Lab.2021.210913.
10. Nikpay S, Otaghi M, Azami M, et al. Trichomonas vaginalis infection among women attending laboratory centers in Ilam, Iran. Infect Disord Drug Targets. 2020;20(1):98–101. doi:10.2174/1871526519666190117120705
11. Shroff S. Infectious vaginitis, cervicitis, and pelvic inflammatory disease. Med Clin North Am. 2023;107(2):299–315. doi:10.1016/j.mcna.2022.10.009
12. Kissinger PJ, Gaydos CA, Seña AC, et al. Diagnosis and Management of Trichomonas vaginalis: summary of Evidence Reviewed for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines. Clin Infect Dis. 2022;74(Suppl_2):S152–s161. doi:10.1093/cid/ciac030
13. Marnach ML, Wygant JN, Casey PM. Evaluation and Management of Vaginitis. Mayo Clin Proc. 2022;97(2):347–358. doi:10.1016/j.mayocp.2021.09.022
14. Su RY, Ho L-J, Yang H-Y, et al. Association between Trichomonas vaginalis infection and cervical lesions: a population-based, nested case-control study in Taiwan. Parasitol Res. 2020;119(8):2649–2657. doi:10.1007/s00436-020-06759-4
15. S Xu, Z Wang, H Zhou, et al. High Co-Infection Rate of Trichomonas vaginalis and Candidatus Mycoplasma Girerdii in Gansu Province. China Healthcare. 2021;9(6):706.
16. Li T, Liu Z, Zhang Z, et al. Comparative analysis of the vaginal microbiome of Chinese women with Trichomonas vaginalis and mixed infection. Microb Pathog. 2021;154:104790. doi:10.1016/j.micpath.2021.104790
17. Lu H, He H, He X, et al. Prevalence and spatial heterogeneity of Trichomonas vaginalis infection among the female population and association with climate in Guangxi Zhuang autonomous region, Southern China. Acta Trop. 2022;225:106204. doi:10.1016/j.actatropica.2021.106204
18. Yu J, Zhou Y, Luo H, et al. Mycoplasma genitalium infection in the female reproductive system: diseases and treatment. Front Microbiol. 2023;14:1098276. doi:10.3389/fmicb.2023.1098276
19. da Silva Pinto GV, Bolpet ADN, Martin LF, et al. Factors associated with Trichomonas vaginalis infection in reproductive-aged women attending cervical screening in southeast of Brazil. Braz J Infect Dis. 2023;27(4):102794. doi:10.1016/j.bjid.2023.102794
20. Kim YH, Ahn H-J, Kim D, et al. Spatiotemporal Clusters and Trend of Trichomonas vaginalis Infection in Korea. Korean J Parasitol. 2022;60(2):97–107. doi:10.3347/kjp.2022.60.2.97
21. Tchankoni MK, Bitty-Anderson AM, Sadio AJ, et al. Prevalence and factors associated with trichomonas vaginalis infection among female sex workers in Togo, 2017. BMC Infect Dis. 2021;21(1):775. doi:10.1186/s12879-021-06432-w
22. Silva J, Cerqueira F, Teixeira AL, et al. Prevalence of Neisseria gonorrhoeae and Trichomonas vaginalis in Portuguese women of childbearing age. J Obstet Gynaecol. 2021;41(2):254–258. doi:10.1080/01443615.2020.1736014
23. Marous M, Huang W-Y, Rabkin CS, et al. Trichomonas vaginalis infection and risk of prostate cancer: associations by disease aggressiveness and race/ethnicity in the PLCO Trial. Cancer Causes Control. 2017;28(8):889–898. doi:10.1007/s10552-017-0919-6
24. Parvez R, Vins A, Radhakrishnan V, et al. Trichomonas vaginalis infection among married women of Andaman and Nicobar Islands. Infect Dis. 2023;55(12):874–879. doi:10.1080/23744235.2023.2236700
25. Barbosa MDS, Andrade de Souza IB, Schnaufer ECDS, et al. Prevalence and factors associated with Trichomonas vaginalis infection in indigenous Brazilian women. PLoS One. 2020;15(10):e0240323. doi:10.1371/journal.pone.0240323
26. Ghallab MMI, Alaa D, Morsy SM. Multiattribute Analysis of Trichomonas vaginalis Diagnostics and Its Correlation with Clinical Complaints and Contraceptive Methods in a Symptomatic Egyptian Cohort. Infect Dis Obstet Gynecol. 2021;2021:5525095. doi:10.1155/2021/5525095
27. Mabaso N, Abbai N. Distribution of genotypes in relation to metronidazole susceptibility patterns in Trichomonas vaginalis isolated from South African pregnant women. Parasitol Res. 2021;120(6):2233–2241. doi:10.1007/s00436-021-07177-w
28. Graves KJ, Ghosh AP, Schmidt N, et al. Trichomonas vaginalis Virus Among Women With Trichomoniasis and Associations With Demographics, Clinical Outcomes, and Metronidazole Resistance. Clin Infect Dis. 2019;69(12):2170–2176. doi:10.1093/cid/ciz146
29. Ramírez-Ledesma MG, Rodríguez MC, Alva-Murillo N, et al. The antimicrobial peptides LL-37, KR-20, FK-13 and KR-12 inhibit the growth of a sensitive and a metronidazole-resistant strain of Trichomonas vaginalis. Parasitol Res. 2022;121(12):3503–3512. doi:10.1007/s00436-022-07674-6
30. Vargas Rigo G, Petro-Silveira B, Devereux M, et al. Anti- Trichomonas vaginalis activity of 1,10-phenanthroline-5,6-dione-based metallodrugs and synergistic effect with metronidazole. Parasitology. 2019;146(9):1179–1183. doi:10.1017/S003118201800152X
31. Endres CT, Rigo GV, Loges LA, et al. Mass Spectrometry Metabolomics Approach Reveals Anti-Trichomonas vaginalis Scaffolds from Marine Fungi. Mar Biotechnol. 2022;24(5):1014–1022. doi:10.1007/s10126-022-10164-6
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

