Back to Journals » Infection and Drug Resistance » Volume 17
Clinical Characteristics, Species Distribution, and Drug Resistance of Non-Tuberculous Mycobacteria Lung Disease in Qingdao, China
Authors Chu Y, Wang X, Dou M, Wang J, Wang B, Wang H, Lv S, Lu S, Li T
Received 22 April 2024
Accepted for publication 18 October 2024
Published 1 November 2024 Volume 2024:17 Pages 4807—4814
DOI https://doi.org/10.2147/IDR.S475015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yan Chu,1,* Xiaomin Wang,2,* Min Dou,1 Jin Wang,2 Baoqian Wang,1 Hairong Wang,1 Shasha Lv,1 Shuihua Lu,2,* Tongxia Li1,*
1Qingdao Chest Hospital, Qingdao, Shandong, People’s Republic of China; 2National Clinical Research Center for Infectious Diseases, Shenzhen Third People’s Hospital, Shenzhen, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tongxia Li; Shuihua Lu, Email [email protected]; [email protected]
Objective: To analyze the clinical characteristics, species distribution and drug resistance of patients with non-tuberculous mycobacteria (NTM) lung disease in Qingdao, China.
Methods: Clinical data of patients with NTM lung disease and pulmonary tuberculosis (TB) treated at Qingdao Chest Hospital from July 2021 to July 2023 were retrospectively analyzed.
Results: The prevalence of NTM lung disease was 8.03%, with a high rate of drug resistance during the study period. Patients with NTM lung disease had higher rates of older age, bronchiectasis, malignancy, HIV infection and bronchial dilatation shadow and lower rates of hollow shadow compared to patients with pulmonary TB.
Conclusion: Comprehensive understanding of NTM lung disease, improved laboratory testing techniques and appropriate treatment regimens are essential for the management of NTM lung disease.
Keywords: non-tuberculous mycobacteria, pulmonary tuberculosis, clinical characteristics, drug resistance
Introduction
Non-tuberculous mycobacteria (NTM) are all mycobacteria other than Mycobacterium tuberculosis (MTB) and Mycobacterium leprae, which are widely existed in nature, and are also called environmental mycobacteria.1–3 More than 190 species of NTM have been identified, most of which are non-pathogenic, and only a few of them are conditionally pathogenic.4,5 When the immune system is compromised, it is susceptible to infected NTM and causes the tissue or organ lesions known as NTM disease, of which lung disease is the most common type.6–9
In recent years, with increased awareness of NTM lung disease and improved laboratory testing techniques, the incidence of this disease has increased significantly worldwide.10 Epidemiologic data from the Tuberculosis Department of the Chinese Medical Association showed that the isolation rate of NTM in China increased from 4.3% in 1979 to 11.1% in 2000 and then to 22.9% in 2010, showing a remarkable upward trend. Sun et al reported the incidence of NTM increased each year, from 4.24% in 2014 to 12.68% in 2021 in Northern China.11 Wang et al demonstrated that NTM detection rose significantly each year from January 2017 to December 2022 in Southwest China.12 These evidences indicated that the incidence of NTM diseases is still on the rise in China.
However, the lack of characteristic clinical manifestations of NTM lung disease and the similarity of its symptoms to those of tuberculosis (TB) make it difficult to distinguish, resulting in many patients with NTM lung disease being misdiagnosed with TB and given the inappropriate medication and treatment regimen, delaying the patient’s optimal treatment time.13,14 Therefore, to raise awareness of NTM lung disease, this study analyses the clinical characteristics of patients with NTM lung disease and TB, as well as the distribution of NTM species and drug resistance at Qingdao Chest Hospital, China.
Materials and Methods
Patients
This retrospective study enrolled all patients with bacteriologically diagnosed NTM lung disease as well as pulmonary TB admitted to Qingdao Chest Hospital between July 2021 and July 2023. Patients were diagnosed according to the Chinese Guidelines for Diagnosis and Treatment of Non-Tuberculous Mycobacteria Disease (2020 edition)15 and the Diagnostic Criteria for Tuberculosis in China (WS288-2017).16 According to the Chinese Guidelines for Diagnosis and Treatment of Non-Tuberculous Mycobacteria Disease, NTM lung disease is defined as identical NTM species from more than 2 sputum specimens collected at different time points, and imaging evidence of pulmonary cavitation or multiple bronchial dilatation with micronodularity. The clinical symptoms, laboratory tests and imaging results of all patients were collected and analyzed.
MTB and NTM Species Identification
All samples were tested by Mycobacterium nucleic acid detection kit (CapitaiBio Technology, China) based on PCR fluorescent probe assay to differentiate Mycobacterium tuberculosis complex from NTM. The strain growing on p-nitrobenzoic acid (PNB) selective medium was initially identified as NTM. Species identification of NTM was performed using matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) by Conlight Medical (Shanghai, China).
Drug Susceptibility Testing
Drug susceptibility of NTM strains was determined by the proportion method in Lowenstein-Jensen (LJ) medium with drug concentrations of isoniazid (INH) 0.2ug/mL, streptomycin (SM) 4ug/mL, rifampicin (RFP) 40ug/mL, ethambutol (EMB) 2ug/mL, para-aminosalicylic acid (PAS) 1ug/mL, amikacin (AMK) 30ug/mL, prothionamide (PTO) 40ug/mL, levofloxacin (LFX) 2ug/mL, Moxifloxacin (MFX) 2ug/mL, capreomycin (CAP) 40ug/mL and cycloserine (CS) 40ug/mL.
Statistics
The Shapiro–Wilk test was used to analyse whether the data for continuous variables followed a normal distribution. Comparisons of continuous variables were performed using t-tests for variables with a normal distribution, or the Mann–Whitney U-test for variables without a normal distribution. Categorical variables were compared using the Pearson chi-square test. All analyses were performed using SPSS software (version 23.0), and results with a p-value less than 0.05 were considered statistically significant.
Results
NTM Lung Disease and TB Patients
From July 2021 to July 2023, a total of 280 patients were isolated NTM strains at Qingdao Chest Hospital. Of these, seven patients with a concurrent diagnosis of tuberculosis were excluded. In addition, 56 patients had second sputum culture results that were inconsistent with the first sputum culture results, 35 patients had no imaging evidence of NTM lung disease (absence of pulmonary cavitation or multiple bronchiectasis with micronodules), 9 patients had contaminated cultures, and 8 patients did not have complete clinical data, and all of these patients were excluded. Finally, 165 patients were diagnosed with NTM lung disease. Meanwhile, 1890 patients diagnosed with pulmonary TB during the same period were included. NTM lung disease accounts for 8.03% (165/2055) of mycobacterial lung disease.
Comparison of NTM Lung Disease and Pulmonary TB Patients
Compared with pulmonary TB patients, NTM lung disease patients had a higher proportion of patients aged over 60 years (χ2=65.60, P<0.001), bronchiectasis (χ2=11.00, P=0.001), malignancy (χ2=27.43, P<0.001), HIV infection (χ2=9.16, P=0.002) and bronchial dilatation shadow (χ2=12.43, P<0.001), but a lower proportion of patients with hollow shadow (χ2=5.64, P=0.018) (Table 1). In terms of clinical symptoms, there was no statistical difference between NTM lung disease and pulmonary TB patients. Among laboratory indicators, lymphocyte percentage (P<0.001) and albumin concentration (P=0.027) of NTM lung disease patients were higher than those of pulmonary TB patients, while white blood cell count (P=0.039) and neutrophil percentage (P=0.002) of NTM lung disease patients were lower (Table 2).
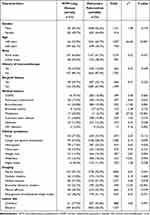 |
Table 1 Comparison of Clinical Characteristics of NTM Lung Disease and Pulmonary Tuberculosis Patients |
 |
Table 2 Comparison of Laboratory Indicators of NTM Lung Disease and Pulmonary Tuberculosis Patients |
Distribution of NTM Species
A total of seven NTM species were isolated from 165 patients with NTM lung disease, including five slow-growing (M. intracellulare, M. avium, M. kansasii, M. bolletii, and M. gordonae) and two fast-growing (M. chelonae/abscessus and M. fortuitum) mycobacteria. The proportions of slow-growing and fast-growing mycobacteria were 81.2% (134/165) and 18.8% (31/165), respectively. The three most common NTMs are M. intracellulare (38.2%), M. avium (22.4%) and M. chelonae/abscessus (17.6%) (Table 3).
 |
Table 3 Distribution of NTM Species |
Drug Resistance Profiles of NTM Isolates
Since NTM lung disease is easily misdiagnosed as pulmonary TB, especially in primary hospitals where NTM diagnostic techniques are not available, this study analyzed the anti-TB drug resistance profiles of NTM isolates (Table 4). As the most prevalent species of NTM, M. intracellulare has a high resistance rate to first-line anti-TB drugs, all above 60%, and a relatively low resistance rate to second-line anti-TB drugs such as amikacin, capreomycin, cycloserine and moxifloxacin, all below 40%. Fast-growing NTM, represented by M. chelonae/abscessus, were 100% resistant to isoniazid, rifampicin and streptomycin, and more than 50% resistant to other anti-TB drugs.
 |
Table 4 Drug Resistance Profiles of NTM Species |
Discussion
This retrospective study investigated the clinical characteristics, species distribution and drug resistance profiles of patients with NTM lung disease in Qingdao, China. Compared with patients with pulmonary TB, patients with NTM lung disease had differences in several characteristics including age, medical history, imaging and laboratory indicators. These results will help in the diagnosis and treatment of NTM lung diseases.
NTM lung disease accounts for 8.03% of total mycobacterial lung disease diagnosed at Qingdao Chest Hospital in this study. In recent years, the prevalence of NTM in Shandong province (including Qingdao) has increased,17 which may be related to the rising awareness of NTM disease among doctors and the improvement of laboratory techniques.18–20 In this study, patients were diagnosed according to the Chinese Guidelines for Diagnosis and Treatment of Non-Tuberculous Mycobacteria Disease (2020 edition).15 The Chinese Guidelines are based on clinical symptoms, radiological features and microbiological tests, which are consistent with the ATS Guidelines.4 All detailed diagnostic criteria were consistent, except that positive molecular biological tests were also accepted for microbiological tests in the Chinese guidelines. Molecular biological tests are less time-consuming than culture and can directly identify the species,21 which is advantageous in the diagnosis of NTM. Even molecular biological tests for TB, such as Xpert, can be very helpful in diagnosing NTM. Patients with positive smears but negative molecular tests are likely to be infected with NTM. Nevertheless, the proportion of NTM in this study was lower than the data from the 5th National Tuberculosis Epidemiological Survey in 2010.22 This may be due to regional differences in climate, geography and other factors, leading to differences in the infection rate of NTM in China.
NTM lung disease has similar symptoms to pulmonary TB, with clinical manifestations such as fever, cough and sputum, hemoptysis and other non-specific symptoms, which is an important reason for NTM to be easily misdiagnosed and mistreated.23,24 In this study, compared with pulmonary TB patients, we found that a greater proportion of NTM lung disease patients were older, and had bronchiectasis, malignancy or HIV infection, suggesting that these populations may be at high risk for NTM lung disease, which is consistent with the findings of other studies.25–27 The high incidence of NTM in patients with a history of comorbid bronchiectasis may be due to hormones often used in this disease to reduce the inflammatory response, leading to reduced immune function and increased risk of NTM infection. In addition, NTM disease may be associated with alpha 1-antitrypsin deficiency in patients with bronchiectasis.28,29 Alpha-1-antitrypsin inhibits the rapid growth of mycobacterial infection in macrophages by enhancing phagosome-lysosome fusion and autophagy, expressing anti-inflammatory and antimicrobial properties, thereby enhancing macrophage immunity against NTM.29,30 However, alpha-1 antitrypsin has not been widely tested in China. The relationship between alpha-1 antitrypsin and NTM disease need for further research.
In imaging, compared to pulmonary TB, patients with NTM lung disease had a lower incidence of cavitation, higher bronchodilated shadows, and a wider cumulative range that tended to invade both lobes of the lungs, which is consistent with previous studies.31,32 We also found that the white blood cell counts of patients with NTM lung disease were lower than those of patients with pulmonary TB, with a lower proportion of neutrophils but a higher proportion of lymphocytes, suggesting that NTM induces a milder inflammatory response than TB, but the mechanism is not clear and needs to be further investigated.
NTM is characterized by a very broad spectrum of drug resistance, which may be related to mutations in cell wall barriers, efflux pump systems and mutations in drug target genes,33,34 resulting in a very limited number of drugs available to treat NTM infections. The study also found that NTM strains were generally resistant to first-line anti-TB drugs. In contrast, these strains had relatively low resistance rates to amikacin, capreomycin and cycloserine, suggesting that these drugs could be candidates for NTM treatment. M. chelonae/abscessus is one of the NTM species with the highest rate of resistance, which is an important reason for the difficulty of clinical treatment.35 We found that all M. chelonae/abscessus strains were resistant to isoniazid, rifampicin and streptomycin, and more than 50% of them were resistant to the remaining drugs. Therefore, accurate identification of NTM species and drug susceptibility testing to select appropriate treatment regimens are very important to improve the outcome of NTM disease treatment.
This study had several limitations. First, despite being the major TB designated hospital in the region, this study only analysed mycobacterial isolates from one pilot study site in Qingdao. The results of this study do not fully reflect the situation in the whole city. Second, only NTM species rather than subspecies were identified in this study, which could lead to an overestimation of NTM patients. Patients with isolates belonging to the identical species but different subspecies cannot be considered to have NTM disease.4 Third, outcome is a key indicator of NTM treatment, but we were unable to analyse the outcome of NTM treatment because we did not follow up our patients.
In conclusion, this retrospective study analysed clinical data from patients with NTM and TB disease diagnosed at Qingdao Chest Hospital in China over a 2-year period. We found that patients with NTM lung disease in this study had higher rates of older age, bronchiectasis, malignancy, HIV infection and bronchial dilatation shadow and lower rates of hollow shadow compared to patients with pulmonary TB. It is hoped that these results will raise awareness of NTM disease.
Research Ethics
This observational retrospective study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Qingdao Chest Hospital (No. QDSPH/SW-05/3.0). As the diagnostic tests were routinely used in clinical practice and all individual patient information was removed before analysis, the Ethics Committee of Qingdao Chest Hospital waived the requirement for informed consent from individual patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Shandong Province Key R&D Program (Major Science and Technology Innovation Project) (2021SFGC0504). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Falkinham JO 3rd. Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23(3):529–551. doi:10.1016/s0272-5231(02)00014-x
2. Jamal F, Hammer MM. Nontuberculous mycobacterial infections. Radiol Clin North Am. 2022;60(3):399–408. doi:10.1016/j.rcl.2022.01.012
3. Prasla Z, Sutliff RL, Sadikot RT. Macrophage signaling pathways in pulmonary nontuberculous mycobacteria infections. Am J Respir Cell Mol Biol. 2020;63(2):144–151. doi:10.1165/rcmb.2019-0241TR
4. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71(4):905–913. doi:10.1093/cid/ciaa1125
5. Gopalaswamy R, Shanmugam S, Mondal R, et al. Of tuberculosis and non-tuberculous mycobacterial infections - a comparative analysis of epidemiology, diagnosis and treatment. J Biomed Sci. 2020;27(1):74. doi:10.1186/s12929-020-00667-6
6. Cruz-Aguilar M, Castillo-Rodal AI, Arredondo-Hernandez R, et al. Non-tuberculous mycobacteria immunopathogenesis: closer than they appear. a prime of innate immunity trade-off and NTM ways into virulence. Scand J Immunol. 2021;94(2):e13035. doi:10.1111/sji.13035
7. Mortaz E, Moloudizargari M, Varahram M, et al. What Immunological defects predispose to non-tuberculosis mycobacterial infections? Iran J Allergy Asthma Immunol. 2018;17(2):100–109.
8. Porvaznik I, Solovic I, Mokry J. Non-tuberculous mycobacteria: classification, diagnostics, and therapy. Adv Exp Med Biol. 2017;944:19–25. doi:10.1007/5584_2016_45
9. Tan S, Kasperbauer S. Nontuberculous Mycobacteria. Semin Respir Crit Care Med. 2021;42(4):567–586. doi:10.1055/s-0041-1730997
10. Sharma SK, Upadhyay V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J Med Res. 2020;152(3):185–226. doi:10.4103/ijmr.IJMR_902_20
11. Sun Q, Yan J, Liao X, et al. Trends and species diversity of non-tuberculous mycobacteria isolated from respiratory samples in northern China, 2014-2021. FRONT PUBLIC HEALTH. 2022;10:923968. doi:10.3389/fpubh.2022.923968
12. Wang DM, Liu H, Zheng YL, et al. Epidemiology of nontuberculous mycobacteria in tuberculosis suspects, southwest of China, 2017-2022. Front Cell Infect Microbiol. 2023;13:1282902. doi:10.3389/fcimb.2023.1282902
13. Echeverria G, Rueda V, Espinoza W, et al. First case reports of nontuberculous mycobacterial (NTM) lung disease in Ecuador: important lessons to learn. PATHOGENS. 2023;12(4):507. doi:10.3390/pathogens12040507
14. Park M, Lee Y, Kim S, et al. Distinguishing nontuberculous mycobacterial lung disease and Mycobacterium tuberculosis lung disease on X-ray images using deep transfer learning. BMC Infect Dis. 2023;23(1):32. doi:10.1186/s12879-023-07996-5
15. Sheng-sheng TS-j LIU. Interpretation of the Guidelines for diagnosis and treatment of non-tuberculous mycobacteria disease (2020 edition). J Tuberc Lung Dis. 2021;2(2):108–115. doi:10.3969/j.issn.2096-8493.2021.02.004
16. National Health and Family Planning Commission of the People’s Republic of China. Diagnosis for pulmonary tuberculosis (WS 288-2017). Chin J Infect Contr. 2018;17(7):642–652.
17. Xu D, Han C, Wang MS, et al. Increasing prevalence of non-tuberculous mycobacterial infection from 2004-2009 to 2012-2017: a laboratory-based surveillance in China. J Infect. 2018;76(4):422–424. doi:10.1016/j.jinf.2017.12.007
18. Chindam A, Vengaldas S, Srigiri VR, et al. Challenges of diagnosing and treating non-tuberculous mycobacterial pulmonary disease [NTM-PD]: a case series. J Clin Tuberc Other Mycobact Dis. 2021;25:100271. doi:10.1016/j.jctube.2021.100271
19. Larsson LO, Polverino E, Hoefsloot W, et al. Pulmonary disease by non-tuberculous mycobacteria - clinical management, unmet needs and future perspectives. Expert Rev Respir Med. 2017;11(12):977–989. doi:10.1080/17476348.2017.1386563
20. McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria: current state and new insights. Chest. 2015;148(6):1517–1527. doi:10.1378/chest.15-0458
21. Huh HJ, Kim SY, Jhun BW, et al. Recent advances in molecular diagnostics and understanding mechanisms of drug resistance in nontuberculous mycobacterial diseases. Infect Genet Evol. 2019;72:169–182. doi:10.1016/j.meegid.2018.10.003
22. Technical Guidance Group of the Fifth National TB Epidemiological Survey. The office of the fifth national TB epidemiological survey. the fifth national tuberculosis epidemiological survey in 2010. Chin J Antituberculosis. 2012;34(8):485–508.
23. Karamat A, Ambreen A, Ishtiaq A, et al. Isolation of non-tuberculous mycobacteria among tuberculosis patients, a study from a tertiary care hospital in Lahore, Pakistan. BMC Infect Dis. 2021;21(1):381. doi:10.1186/s12879-021-06086-8
24. Pennington KM, Vu A, Challener D, et al. Approach to the diagnosis and treatment of non-tuberculous mycobacterial disease. J Clin Tuberc Other Mycobact Dis. 2021;24:100244. doi:10.1016/j.jctube.2021.100244
25. Drysdale M, Choate R, Brunton AE, et al. Nontuberculous mycobacterial (NTM) infections in bronchiectasis patients: a retrospective US registry cohort study. Pulm Pharmacol Ther. 2023;83:102260. doi:10.1016/j.pupt.2023.102260
26. Yoo JW, Jo KW, Kang BH, et al. Mycobacterial diseases developed during anti-tumour necrosis factor-alpha therapy. Eur Respir J. 2014;44(5):1289–1295. doi:10.1183/09031936.00063514
27. Zhang L, Xing W, Zhou J, et al. Characteristics of tuberculosis patients in the integrated tuberculosis control model in Chongqing, China: a retrospective study. BMC Infect Dis. 2020;20(1):576. doi:10.1186/s12879-020-05304-z
28. Kim JS, Tanaka N, Newell JD, et al. Nontuberculous mycobacterial infection: CT scan findings, genotype, and treatment responsiveness. Chest. 2005;128(6):3863–3869. doi:10.1378/chest.128.6.3863
29. Bai X, Bai A, Honda JR, et al. Alpha-1-antitrypsin enhances primary human macrophage immunity against non-tuberculous mycobacteria. Front Immunol. 2019;10:1417. doi:10.3389/fimmu.2019.01417
30. Janciauskiene SM, Bals R, Koczulla R, et al. The discovery of alpha 1-antitrypsin and its role in health and disease. Respir Med. 2011;105(8):1129–1139. doi:10.1016/j.rmed.2011.02.002
31. Glodic G, Samarzija M, Sabol I, et al. Risk factors for nontuberculous mycobacterial pulmonary disease (NTM-PD) in Croatia. Wien Klin Wochenschr. 2021;133(21–22):1195–1200. doi:10.1007/s00508-021-01923-x
32. Varley CD, Winthrop KL. Nontuberculous mycobacteria: diagnosis and therapy. Clin Chest Med. 2022;43(1):89–98. doi:10.1016/j.ccm.2021.11.007
33. van Ingen J, Boeree MJ, van Soolingen D, et al. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat. 2012;15(3):149–161. doi:10.1016/j.drup.2012.04.001
34. Saxena S, Spaink HP, Forn-Cuni G. Drug resistance in nontuberculous mycobacteria: mechanisms and models. Biology. 2021;10(2):96. doi:10.3390/biology10020096
35. Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36(1):67–78. doi:10.1016/j.ccm.2014.10.004
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Factors Related to Complying with Anti-TB Medications Among Drug-Resistant Tuberculosis Patients in Indonesia
Yani DI, Juniarti N, Lukman M
Patient Preference and Adherence 2022, 16:3319-3327
Published Date: 17 December 2022
Screening and Drug Resistance Analysis of Non-Tuberculous Mycobacteria in Patients with Suspected Pulmonary Tuberculosis on the Hainan Island, China
Wang J, Chen Z, Xu Y, Qiu W, Chen S, Pei H, Zhong Y
Infection and Drug Resistance 2023, 16:463-476
Published Date: 25 January 2023
Clinical Characteristics Analysis of 30 Cases of Interferon-γ Autoantibody-Positive Patients with Concurrent Mycobacterial Infection: A 6-Year Retrospective Study
Zhao CY, Song C, He HW, Huang XZ, Meng XY, Huang AC, Xu CY, Luo LL, Xi SY, Lan YQ, Li WW, Lin YR, Zhu QD
Infection and Drug Resistance 2025, 18:1097-1110
Published Date: 25 February 2025

