Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Clinical Efficacy of Mind-Regulating Acupuncture on Post-Stroke Depression Based on the “Microbiota-Gut-Brain Axis” Theory: A Randomized Controlled Study
Authors Xie J , Li J, Sun Q, Jiang J
Received 27 February 2025
Accepted for publication 1 July 2025
Published 7 July 2025 Volume 2025:21 Pages 1349—1358
DOI https://doi.org/10.2147/NDT.S525238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Taro Kishi
Jingjun Xie,1 Jinxia Li,2 Qi Sun,1 Jie Jiang1
1Department of Acupuncture, The First People’s Hospital of Huzhou, Huzhou, Zhejiang Province, People’s Republic of China; 2Department of Acupuncture, Huzhou Hospital of Traditional Chinese Medicine, Affiliated to Zhejiang Chinese Medical University, Huzhou, Zhejiang Province, People’s Republic of China
Correspondence: Jie Jiang, Department of Acupuncture, The First People’s Hospital of Huzhou, No. 158, Guang Chang Hou Road, Huzhou, Zhejiang Province, 313000, People’s Republic of China, Email [email protected]
Objective: This study explored the clinical efficacy and mechanisms of Mind-Regulating Acupuncture (MRA) for post-stroke depression (PSD) based on the “microbiota-gut-brain axis” theory.
Methods: 92 PSD patients were randomly divided into an observation group (received conventional therapy + MRA) and a control group (conventional therapy only). After 8 weeks of treatment, multiple indicators including depression scores, neurological function scores, and levels of neurotransmitters and gut microbiota were compared.
Results: MRA significantly improved depressive symptoms, neurological function, and daily living abilities in PSD patients. It increased serum 5-HT and BDNF levels, and regulated gut microbiota, promoting beneficial bacteria and reducing harmful ones.
Conclusion: MRA effectively treats PSD, and its mechanism may involve regulating the “microbiota-gut-brain axis”, increasing beneficial gut bacteria, and enhancing 5-HT and BDNF levels.
Keywords: post-stroke depression, mind-regulating acupuncture, microbiota-gut-brain axis, gut microbiota
Introduction
Stroke is a major health concern globally, leading to a wide range of physical and psychological sequelae. Among these, post-stroke depression (PSD) has emerged as one of the most prevalent and debilitating complications. Epidemiological studies have indicated that PSD affects a substantial proportion of stroke survivors, with estimates ranging from 20% to 70% depending on the population studied and the diagnostic criteria employed.1,2 This high prevalence not only underscores the significance of PSD but also highlights its substantial impact on patients’ overall well-being.
The implications of PSD for patients are far-reaching. It not only exacerbates the physical symptoms associated with stroke but also significantly impairs the neurological recovery process. Patients with PSD often exhibit slower rehabilitation progress, reduced motivation for self-care, and a decreased quality of life. In addition, PSD has been linked to increased mortality rates among stroke survivors, further emphasizing the need for effective treatment strategies.
Current mainstream treatments for PSD, namely pharmacological and psychological therapies, have their limitations. Pharmacological agents, such as selective serotonin reuptake inhibitors (SSRIs), while effective in some cases, are associated with a variety of adverse effects. These can include nausea, insomnia, sexual dysfunction, and in some cases, an increased risk of suicidal ideation, which can lead to poor patient compliance. Psychological therapies, such as cognitive-behavioral therapy (CBT), require significant time commitment from both patients and therapists and may not be accessible to all, especially in resource-limited settings.
Acupuncture, a cornerstone of traditional Chinese medicine, has gained increasing attention in the treatment of PSD.3,4 It offers a non-pharmacological alternative with a relatively low risk of adverse effects. The theoretical basis of acupuncture in treating PSD lies in its ability to regulate the body’s qi and blood circulation, and recent research has also suggested that it may modulate neurotransmitter systems, which are closely related to mood regulation.
In recent years, the concept of the “Microbiota-Gut-Brain Axis” has revolutionized our understanding of the relationship between the gut microbiota and the central nervous system. A growing body of evidence from preclinical and clinical studies has shown that gut microbiota imbalance is closely associated with the development of various neurological and psychiatric disorders, including PSD.5 The gut microbiota can influence the brain through multiple pathways, such as the production of neurotransmitters, modulation of the immune system, and regulation of the hypothalamic-pituitary-adrenal (HPA) axis.
Despite these advancements in our understanding, there remain significant gaps in the literature. Existing studies on the treatment of PSD often focus on single-modality therapies, and there is a lack of comprehensive approaches that integrate different treatment modalities. In particular, the potential of acupuncture in modulating the Microbiota-Gut-Brain Axis in the context of PSD treatment has not been fully explored. This study aims to bridge these gaps by exploring the clinical efficacy and mechanisms of MRA (assuming MRA is a specific acupuncture-related intervention) on PSD patients based on the “Microbiota-Gut-Brain Axis” theory. By doing so, it hopes to provide novel insights and practical methods for the treatment of PSD, which could potentially improve the outcomes for stroke survivors suffering from depression.
Materials and Methods
Participant Selection and Grouping
The study was conducted at the First People’s Hospital of Huzhou from July 2023 to December 2024. A total of 92 patients diagnosed with post-stroke depression (PSD) were enrolled. This trial was registered at the Chinese Clinical Trial Center (Registration No. ChiCTR2300072096) and approved by the ethics committee of The First People’s Hospital of Huzhou (Approval No. 2023KYLL036).
Sample Size Calculation
The sample size was determined using the formula for comparing two independent proportions: 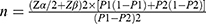
Based on preliminary data, the efficacy rates of Mind-Regulating Acupuncture (MRA) and pharmacological treatment were estimated at 80% (P1=0.8) and 60% (P2=0.6), respectively. With a significance level (α) of 0.05 (two-tailed), power (1−β) of 80% (Zα/2=1.96, Zβ=0.84), the calculation yielded a minimum requirement of 40 patients per group. To account for a potential 15% dropout rate, the final sample size was increased to 46 patients per group (total N=92).
Randomization and Blinding Procedures
Participants were randomly assigned to either the MRA or pharmacological treatment group using a computer-generated randomization sequence. To reduce performance bias and detection bias in this study, a double-blind design was employed. Physicians performing acupuncture were not involved in patient outcome assessments, and researchers responsible for outcome evaluations remained blinded to patient group assignments. During the assessment of HAMD scores, NIHSS scores, BI scores, serum 5-HT and BDNF levels, and gut microbiota test results, they were unaware of whether patients belonged to the MRA treatment group or the control group. Additionally, blinding procedures were applied to laboratory personnel to ensure the objectivity and accuracy of the test results.
Control Group
There were 46 patients in the control group. The baseline characteristics included: 27 males and 19 females, with ages ranging from 48 to 59 years old, having an average age of 54.2±4.9 years. The illness duration spanned from 13 to 19 days, averaging 17.8±1.4 days.
Observation Group
The observation group also consisted of 46 patients. The details were as follows: 28 males and 18 females, aged between 49 and 60 years old, with an average age of 53.6±5.1 years. The illness duration ranged from 15 to 20 days, averaging 18.1±1.6 days. There were no statistically significant differences in these baseline characteristics between the two groups (P>0.05), indicating comparability, as shown in Table 1.
 |
Table 1 Comparison of Baseline Characteristics Between Two Groups |
Participant Flow and Adherence
The CONSORT flow diagram (Figure 1) details participant enrollment, allocation, follow-up, and analysis: Randomization: 92 randomized (46 per group). Completion: 100% adherence in both groups (no dropouts). Analysis: All 92 participants included in intention-to-treat analysis.
 |
Figure 1 CONSORT flow diagram. |
Duration of Intervention Explanation
The setting of an 8-week intervention duration is primarily based on preliminary experimental results and clinical practice experience. In the pre-experiment, patients with post-stroke depression were subjected to Shen-calming acupuncture interventions of different durations, revealing significant improvement trends in depressive symptoms and neurological function by the 8th week. Additionally, referencing similar acupuncture treatments for mental disorders, an 8-week intervention duration demonstrates certain clinical feasibility and effectiveness. Although current studies suggest that an 8-week intervention may be insufficient to fully assess long-term dynamic changes in gut microbiota, this study aims to initially explore the mechanism by which Shen-calming acupuncture improves post-stroke depression through the “microbiota-gut-brain axis”. Long-term follow-up studies will be conducted to further clarify its impact on gut microbiota.
Diagnostic Criteria
Stroke Diagnosis
Based on the 《Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke 2018》and 《Chinese Guidelines for the Diagnosis and Treatment of Cerebral Hemorrhage 2019》, confirmed by cranial CT or MRI.6,7
Depression Diagnosis
Based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), with a Hamilton Depression Scale (HAMD) 17-item score ≥178.
Inclusion Criteria
①Met the above diagnostic criteria for stroke and depression.②First-time stroke within the last 6 months.③Aged between 45–75 years.④Patients and their families provided informed consent.
Exclusion Criteria
①History of psychiatric disorders, cognitive impairment, or dementia.②Severe dysfunction of the heart, liver, kidneys, or other organs.③Allergy to acupuncture or inability to cooperate with acupuncture treatment.④Currently taking medications that affect gut microbiota or psychiatric drugs.
Treatment Methods
Control Group
Conventional Pharmacological Treatment: Patients received standard medications, including antiplatelet therapy (Aspirin Enteric-Coated Tablets 100 mg, once daily), statins for lipid-lowering and plaque stabilization (Atorvastatin Calcium Tablets 20 mg, once daily), and management of blood pressure and blood glucose levels. Additionally, Venlafaxine Hydrochloride Capsules (75 mg/capsule, Pfizer) were administered, starting at an initial dose of 75 mg/day. The dosage was gradually increased to 150 mg/day based on the patient’s condition and tolerance, for a total treatment duration of 8 weeks. Conventional Rehabilitation Therapy: Personalized rehabilitation training programs were developed according to each patient’s specific condition. These programs included: Limb Function Training: Utilizing techniques such as Bobath and Brunnstrom methods, the training focused on joint mobility, muscle strength, and balance exercises to promote the recovery of limb function. Speech Training: For patients with speech impairments, the training included pronunciation exercises, vocabulary building, and sentence construction to enhance speech expression and comprehension. Cognitive Training: This involved exercises to improve attention, memory, and thinking skills, aimed at enhancing cognitive function. Rehabilitation sessions were conducted 5 times per week, with each session lasting 30–60 minutes, over a period of 8 weeks.
Observation Group
In addition to the control group’s treatment, patients received MRA.
Acupoint Selection
The main acupoints selected were Bai hui (GV20), Yin tang (EX-HN3), Shen men (HT7), Nei guan (PC6), and Tai chong (LR3). Bai hui, located at the top of the head, is the meeting point of all Yang meridians. It functions to awaken the mind, open the orifices, lift Yang, and calm the spirit. Yin tang, located between the eyebrows, is a point on the Governor Vessel (Du Meridian). It has the effects of awakening the mind, calming the spirit, and clearing the nasal passages. Shen men, the source point of the Heart Meridian of Hand Shao yin, nourishes the heart and calms the spirit, regulating the Qi and blood of the Heart Meridian. Nei guan, the Luo-connecting point of the Pericardium Meridian of Hand Jue yin, calms the mind, regulates Qi, and alleviates pain. Tai chong, the source point of the Liver Meridian of Foot Jue yin, soothes the liver, regulates Qi, and pacifies liver wind. Additional acupoints were selected based on the patient’s syndrome differentiation: For liver Qi stagnation, Dan zhong (CV17) and Qi men (LR14) were added. For heart-spleen deficiency, Xin shu (BL15), Pi shu (BL20), and Zu san li (ST36) were added. For phlegm-Qi stagnation, Feng long (ST40) and Zhong wan (CV12) were added.
Acupuncture Technique
Patients were placed in a supine position. After routine disinfection of the acupoint sites, sterile disposable acupuncture needles (0.25 mm × 40 mm) were used. Bai hui was needled horizontally backward to a depth of 15–20 mm using the reinforcing twisting method (thumb forward, index finger backward) at a frequency of 120–150 twists per minute, inducing a local sensation of soreness, numbness, or distension that radiated outward. Yin tang was needled horizontally downward to a depth of 5–10 mm using the reinforcing lifting-thrusting method (shallow to deep, heavy thrusting, and light lifting) with small amplitude and slow frequency, creating a tight, sinking sensation beneath the needle. Shen men was needled perpendicularly to a depth of 10–15 mm using the even reinforcing-reducing twisting method (uniform twisting with thumb and index finger) at a frequency of 100–120 twists per minute, inducing a local sensation of soreness and distension. Nei guan was needled perpendicularly to a depth of 15–20 mm using the even reinforcing-reducing lifting-thrusting method (equal amplitude, frequency, and force), with the needle sensation radiating toward the elbow or fingers. Tai chong was needled perpendicularly to a depth of 10–15 mm using the reducing twisting method (thumb backward, index finger forward) with greater force and faster frequency (150–180 twists per minute), inducing a pronounced local sensation of soreness and distension.
Needle Retention and Treatment Course
After achieving the desired Qi sensation (De qi), the needles were retained for 30 minutes, with needle manipulation repeated every 10 minutes to maintain the sensation. The manipulation techniques were consistent with the initial needling methods. Treatments were administered 5 times per week for a total of 8 weeks.
Observation Indicators
Hamilton Depression Scale (HAMD) Score
The HAMD-17 scale was used to assess depressive symptoms in both groups before treatment and after 8 weeks of treatment. Higher scores indicate more severe depressive symptoms.
National Institutes of Health Stroke Scale (NIHSS) Score
The NIHSS was used to evaluate the degree of neurological impairment in both groups before and after treatment. Higher scores indicate more severe neurological deficits.
Barthel Index (BI) Score
The BI was used to assess the ability to perform activities of daily living (ADL) in both groups before and after treatment. Higher scores indicate better ADL performance.
Serum 5-Hydroxytryptamine (5-HT) and Brain-Derived Neurotrophic Factor (BDNF) Levels
Fasting venous blood samples (5 mL) were collected from patients in both groups in the morning before treatment and after 8 weeks of treatment. Serum was separated by centrifugation, and enzyme-linked immunosorbent assay (ELISA) was used to measure serum 5-HT (The kit is manufactured by Shanghai Yan sheng Biotechnology Co., Ltd. (TSZ brand), with specifications of 48T/96T) and BDNF levels.
Gut Microbiota Analysis
Fresh stool samples were collected from patients in both groups after 8 weeks of treatment. The composition and quantity of gut microbiota were analyzed using 16S rRNA gene sequencing technology.
Statistical Methods
Data were analyzed using SPSS 22.0. Measurement data were expressed as mean± standard deviation ( ), with intergroup comparisons using independent samples t-tests and intragroup comparisons using paired samples t-tests. Count data were expressed as rates (%), with intergroup comparisons using χ²-tests. P<0.05 was considered statistically significant.
), with intergroup comparisons using independent samples t-tests and intragroup comparisons using paired samples t-tests. Count data were expressed as rates (%), with intergroup comparisons using χ²-tests. P<0.05 was considered statistically significant.
Results
Comparison of HAMD, NIHSS, and BI Scores Before and After Treatment
Before treatment, there were no significant differences in HAMD, NIHSS, and BI scores between the two groups (P>0.05). After 8 weeks, both groups showed reduced HAMD and NIHSS scores and increased BI scores, with the observation group showing more significant improvements (P<0.05) (Table 2).
 |
Table 2 Comparison of HAMD, NIHSS, and BI Scores Before and After Treatment Between the Two Groups |
Comparison of Serum 5-HT and BDNF Levels Before and After Treatment
Before treatment, there were no significant differences in serum 5-HT and BDNF levels between the two groups (P>0.05). After 8 weeks, both groups showed increased serum 5-HT and BDNF levels, with the observation group showing more significant increases (P<0.05) (Table 3).
 |
Table 3 Comparison of Serum 5-HT and BDNF Levels Before and After Treatment |
Comparison of the Abundance of Gut Microbiota Before and After Treatment
After 8 weeks of intervention, the relative abundance of Bifidobacterium and Lactobacillus in the observation group was significantly higher compared to the control group, while the abundance of Escherichia coli was significantly lower (P<0.05). (Table 4).
 |
Table 4 Comparison of the Abundance of Gut Microbiota Before and After Treatment |
Discussion
The pathogenesis of post-stroke depression (PSD) is complex and not yet fully understood. Modern medicine suggests that the occurrence of PSD is associated with various factors, including post-stroke neurobiological changes, neurotransmitter imbalances, and inflammatory responses.9–11 Among these, the “microbiota-gut-brain axis” plays a significant role in the pathogenesis of PSD. As a critical component of the “microbiota-gut-brain axis”, the gut microbiota maintains a close bidirectional communication with the brain. The gut microbiota can influence brain function and behavior through multiple pathways, such as producing neurotransmitters, regulating immune responses, and affecting the permeability of the blood-brain barrier.12 Studies have shown that PSD patients exhibit gut microbiota dysbiosis, characterized by a reduction in beneficial bacteria and an increase in harmful bacteria13.
From the perspective of Traditional Chinese Medicine (TCM), PSD falls under the categories of “depression syndrome” and “stroke”. Its pathogenesis is related to emotional disturbances, disruption of Qi and blood flow, and obstruction by phlegm and blood stasis. Mind-Regulating Acupuncture (MRA) is based on TCM meridian and organ theories, aiming to regulate mental states, unblock meridians, and harmonize Qi and blood by stimulating specific acupoints.14,15 In this study, acupoints such as Bai hui (GV20), Shen ting (GV24), and Shui gou (GV26) were selected, all located on the Governor Vessel (Du Meridian), which is known as the “sea of Yang meridians” and is closely related to the brain. Stimulating these acupoints can awaken the mind, open the orifices, and enhance mental functions. Nei guan (PC6) and Shen men (HT7), acupoints of the Heart and Pericardium meridians, have the effects of calming the mind, regulating Qi, and alleviating depression. Tai chong (LR3), the source point of the Liver Meridian, soothes the liver, regulates Qi, and pacifies liver wind. The combination of these acupoints synergistically regulates the mind, alleviates depression, and unblocks meridians.
The results of this study showed that after 8 weeks of treatment, the observation group had lower HAMD and NIHSS scores and higher BI scores compared to the control group, indicating that conventional treatment combined with MRA more effectively improved depressive symptoms, neurological function, and activities of daily living in PSD patients. Serum 5-hydroxytryptamine (5-HT) and brain-derived neurotrophic factor (BDNF) are neurotransmitters and neurotrophic factors closely associated with depression. 5-HT is involved in regulating physiological functions such as mood, sleep, and appetite, and its reduced levels are closely linked to the onset of depression.16,17 BDNF promotes neuronal survival, growth, and differentiation and regulates neuroplasticity, playing a crucial role in the pathogenesis and treatment of depression.18,19 The results of this study demonstrated that the observation group had higher serum 5-HT and BDNF levels than the control group after treatment. These findings suggest an association between MRA and increased levels of 5-HT and BDNF, implying that MRA may contribute to improving depressive symptoms in PSD patients by modulating these neurochemical markers, though the exact nature of this relationship requires further exploration.
Furthermore, this study found that after treatment, the observation group exhibited increased levels of Bifidobacterium and Lactobacillus and decreased levels of Escherichia coli in the gut microbiota compared to the control group, with statistically significant differences. Bifidobacterium and Lactobacillus are beneficial gut bacteria that can exert positive effects on the body by producing short-chain fatty acids and regulating immune function.20–22 Escherichia coli is a conditional pathogen in the gut, and its overgrowth may lead to intestinal inflammation and impaired barrier function. These results indicate an association between MRA and alterations in gut microbiota composition. However, it is important to note that the current study design only reveals correlations, and the observed changes in the gut microbiota may not be a direct result of MRA. Instead, they could potentially be secondary to the improvement in depressive symptoms or other confounding factors related to PSD recovery.
When comparing MRA with other acupuncture protocols or non-MRA methods for PSD, previous research has shown that traditional acupuncture can also improve depressive symptoms in stroke patients.23–25 For example, a meta-analysis indicated that conventional acupuncture led to a reduction in Hamilton Depression Rating Scale (HAMD) scores.26 In contrast, our study demonstrated a more substantial reduction in HAMD scores in the MRA group, suggesting that MRA may have a more pronounced effect on alleviating depressive symptoms. However, direct comparisons are challenging due to differences in study designs, sample characteristics, and outcome measures across studies. Additionally, compared to non-acupuncture interventions like antidepressant medications and psychological therapies,27,28 MRA offers a complementary approach with potentially fewer side effects, though more research is needed to fully assess its comparative efficacy and safety.
In summary, the current study suggests a potential association between MRA, changes in the gut microbiota, and modulation of neurochemical markers, which may contribute to its therapeutic effects on PSD. However, it is essential to acknowledge that the study design does not prove causation. The observed changes in the “microbiota-gut-brain axis” might be influenced by multiple factors and may not be directly caused by MRA. To validate causal relationships and support the mechanistic interpretation, additional experimental research, such as fecal microbiota transplantation studies, is necessary. Fecal samples from MRA-treated PSD patients with improved symptoms could be transplanted into animal models of PSD to observe whether similar improvements in depressive-like behaviors, neurological function, and neurochemical markers occur.
Although this study has limitations, such as a small sample size, insufficiently comprehensive observation indicators, and a short observation period, these limitations further underscore the need for larger, longer-term, multicenter trials. Such trials could include more comprehensive observation indicators, conduct extended follow-ups, and explore the dynamic interplay between MRA, gut microbiota, and neurochemical alterations over time. This would help confirm the effectiveness of MRA and elucidate its underlying mechanisms in PSD more accurately, providing a more solid foundation for its clinical application and guiding the development of more effective therapeutic strategies for PSD patients.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics Statement
This study has been approved by the Ethics Committee of The First People’s Hospital of Huzhou within which this study was undertaken. And that it conforms to the provisions of the Declaration of Helsinki. The treatment scheme adopted in this clinical study is simple and safe. The data obtained remain anonymous. The collection of research data complies with national laws, regulations, and social ethics.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Projects funded by Huzhou Science and technology planning project(2022GY16). Zhejiang Provincial Traditional Chinese Medicine Science and Technology Program (2024ZL1027).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Xu XM, Zou DZ, Shen LY, et al. Efficacy and feasibility of antidepressant treatment in patients with post-stroke depression. Medicine. 2016;95(45):e5349. doi:10.1097/MD.0000000000005349
2. Shen H, Tu X, Luan X, Zeng Y, He J, Tang W. Serum lipid profiles and post-stroke depression in acute ischemic stroke patients. Neuropsychiatr Dis Treat. 2019;15:1573–1583. doi:10.2147/NDT.S204791
3. Li M, Ran D, Yang X, et al. Efficacy of acupuncture in the treatment of post-stroke depression: a study protocol of a randomized controlled trial. PLoS One. 2024;19(8):e0303162. doi:10.1371/journal.pone.0303162
4. Wei C, Chen J, Yang Q, et al. Effects of manual acupuncture versus sham acupuncture in patients with post-stroke depression: a randomized clinical trial. Neurol Ther. 2024;13(6):1717–1735. doi:10.1007/s40120-024-00672-z
5. Durgan DJ, Lee J, McCullough LD, RM B. Examining the role of the microbiota-gut-brain axis in stroke. Stroke. 2019;50(8):2270–2277. doi:10.1161/STROKEAHA.119.025140
6. Chinese Medical Association Neurology Branch, Chinese Medical Association Neurology Branch Cerebrovascular Disease Group. Chinese guidelines for the diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. 2018;51(9):666–682.
7. Chinese Medical Association Neurology Branch, Chinese Medical Association Neurology Branch Cerebrovascular Disease Group. Chinese guidelines for the diagnosis and treatment of cerebral hemorrhage 2019. Chin J Neurol. 2019;52(12):994–1015.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5.
9. Dong Q, Wang N. Neurology.
10. Zhang MT, Tan K, Hu S, et al. Meta-analysis of acupuncture improving depressive state and daily living ability in post-stroke depression patients. Shanghai J Traditional Chin Med. 2021;55(1):13–19.
11. Chen K, Wang ZH, Luo Y. Research progress on the gut-brain-microbiota axis in post-stroke depression. Chin Med Herald. 2023;20(3):45–49.
12. He Y, Wang K, Su N, et al. Microbiota-gut-brain axis in health and neurological disease: interactions between gut microbiota and the nervous system.J. Cell Mol Med. 2024;28(18):e70099. doi:10.1111/jcmm.70099
13. Li Q, Zhang Y, Wang X, Dai L, Zhao W. Gut microbiota of patients with post-stroke depression in Chinese population: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2025;15:1444793. doi:10.3389/fcimb.2025.1444793
14. Huang WX, Yu XM, Zhi QM. Observation on the efficacy of Tong du mental acupuncture in the treatment of post-stroke depression. J Acupuncture Clin Med. 2017;33(04):13–16.
15. Su W, Qin LH. Research progress of “Tong du Tune Spirit” acupuncture in the treatment of sequelae of stroke. J Guangxi Univ Chin Med. 2024;27(04):66–69.
16. Sejbuk M, Siebieszuk A, Witkowska AM. The role of gut microbiome in sleep quality and health: dietary strategies for microbiota support. Nutrients. 2024;16(14):2259. doi:10.3390/nu16142259
17. Ejeda-Martínez AR, Ramos-Molina AR, Brand-Rubalcava PA, Flores-Soto ME. Involvement of serotonergic receptors in depressive processes and their modulation by β-arrestins: a review. Medicine. 2024;103(28):e38943. doi:10.1097/MD.0000000000038943
18. Qiu Y, Zhu L, Cai W, Zhu L. Research progress on BDNF and depression. ACS Chem Neurosci. 2025;16(11):2013–2023. doi:10.1021/acschemneuro.5c00193
19. Zarza-Rebollo JA, López-Isac E, Rivera M, Gómez-Hernández L, Pérez-Gutiérrez AM, Molina E. The relationship between BDNF and physical activity on depression. Prog Neuropsychopharmacol Biol Psychiatry. 2024;134:111033. doi:10.1016/j.pnpbp.2024.111033
20. Yang M, Cui X, Kong D, et al. The efficacy of Lactobacillus and Bifidobacterium in patients with schizophrenia: a meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2024. doi:10.1007/s00406-024-01935-4
21. Yun SW, Shin YJ, Ma X, Kim DH. Lactobacillus plantarum and bifidobacterium longum alleviate high-fat diet-induced obesity and depression/cognitive impairment-like behavior in mice by upregulating AMPK activation and downregulating adipogenesis and gut dysbiosis. Nutrients. 2024;16(22):3810. doi:10.3390/nu16223810
22. Wang W, Dong H, Chang X, et al. Bifidobacterium lactis and Lactobacillus plantarum enhance immune function and antioxidant capacity in cats through modulation of the gut microbiota. Antioxidants. 2024;13(7):764. doi:10.3390/antiox13070764
23. Li P, Zhao J, Wei X, et al. Acupuncture may play a key role in anti-depression through various mechanisms in depression. Chin Med. 2024;19(1):135. doi:10.1186/s13020-024-00990-2
24. Yang B, Miao R, Wang T, et al. The impact of acupuncture on the brain function of patients with mild to moderate major depressive disorder: a randomized controlled trial protocol. BMC Complement Med Ther. 2024;24(1):388. doi:10.1186/s12906-024-04690-0
25. Ma J, Yin X, Cui K, Wang J, Li W, Xu S. Mechanisms of acupuncture in treating depression: a review. Chin Med. 2025;20(1):29. doi:10.1186/s13020-025-01080-7
26. Zhang J, Zhao Y, Li H, Yang Y, Tang Q. Effectiveness of acupuncture plus music therapy for post-stroke depression: systematic review and meta-analysis. Medicine. 2024;103(37):e39681. doi:10.1097/MD.0000000000039681
27. Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101–112. doi:10.4068/cmj.2018.54.2.101
28. Lader MH. Tolerability and safety: essentials in antidepressant pharmacotherapy. J Clin Psychiatry. 1996;57 Suppl 2:39–44.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

