Back to Journals » Infection and Drug Resistance » Volume 18
Clinical Features, Outcomes, and Antifungal Susceptibility Profiles of Invasive Candida Infections in a Tertiary Care Hospital in China
Authors Yao D , Chen J, Zhang G
Received 4 December 2024
Accepted for publication 18 April 2025
Published 5 May 2025 Volume 2025:18 Pages 2271—2282
DOI https://doi.org/10.2147/IDR.S510389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi Ruan
Dongting Yao,1,2,* Jia Chen,3,* Guanyi Zhang3
1Department of Laboratory Medicine, The International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200030, People’s Republic of China; 2Shanghai Key Laboratory of Embryo Original Diseases, Shanghai, 200030, People’s Republic of China; 3Department of Laboratory Medicine, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guanyi Zhang, Department of Laboratory Medicine, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200030, People’s Republic of China, Tel/Fax +86-021-64385700, Email [email protected] Dongting Yao, Department of Laboratory Medicine, The International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200030, People’s Republic of China, Tel/Fax +86-021-64070434, Email [email protected]
Purpose: Given the increasing incidence of invasive Candida infection worldwide, particularly among immunocompromised and critically ill patients, we aimed to assess the distribution of Candida species as well as their clinical features and responses to common antifungal agents through a retrospective analysis of patient data in a Chinese traditional medicine hospital.
Patients and Methods: In this retrospective single-center study, we analyzed data from 301 patients with invasive Candida infection at our hospital between 2020 and 2022, We report the clinical characteristics, species distribution, and in-vitro susceptibility profiles of Candida isolates to eight antifungal agents. Logistic regression analysis was employed for multivariate assessments to analysis the correlation between clinical symptoms and prognosis. Kaplan–Meier survival analysis was used for survival analysis.
Results: Candida albicans was the most prevalent species (38.9%, 117/301), followed by C. tropicalis (28.2%, 85/301) and C. glabrata (22.9%, 69/301). Age, department of admission, underlying disease, and presence of risk factors differed significantly among patients with different Candida infections. Kaplan–Meier survival analysis showed that C. krusei infection was associated with a higher seven-day mortality than other Candida spp. infections. Multivariate logistic regression analyses showed that age, presence of sepsis, insertion of the central venous catheter, and administration of total parenteral nutrition were independent predictors of mortality. C. tropicalis was most resistant to azoles, with 36.26% of the strains being fluconazole-resistant, 35.16% being non-wild type to itraconazole, and 34.52% being non-wild type to voriconazole. Non-susceptibility to echinocandins was found in 11 C. glabrata strains (10.39%, 3.90%, and 1.30% of isolates for caspofungin, micafungin, and anidulafungin, respectively).
Conclusion: Our findings underscore the need for close monitoring of azole resistance in C. tropicalis and echinocandin resistance in C. glabrata, and highlight age, sepsis, CVC insertion, and parenteral nutrition as key predictors of mortality in invasive Candida infections.
Keywords: fungal disease, resistant, azoles, echinocandins
Introduction
Invasive Candida infection (ICI) is a common fungal disease, and its prevalence increases annually owing to growing susceptible populations.1 The mortality rate of patients with ICI is approximately 20%, and patients in intensive care units (ICUs) have an even higher mortality rate.2 Candida has a strong adhesive capacity and easily colonizes polystyrene, epithelium, and other surfaces to form biofilms, thereby becoming pathogenic.3 Risk factors for pathogen colonization include central venous catheter (CVC) and administration of total parenteral nutrition. Candida not only causes mucosal diseases but also invades the internal organs or blood, causing systemic infections in patients with compromised immunity, such as those with diabetes and neutropenia, as well as in those who have received organ transplants.4
Although C. albicans is the most common pathogen associated with ICI, the pathogenicity rate for non-albicans Candida is increasing annually.5 Moreover, the rate of drug resistance in Candida spp. is increasing owing to the widespread and long-term use of antifungals.6 Notably, emerging drug resistance patterns, such as C. tropicalis resistance to azoles and C. glabrata resistance to echinocandins, have become critical issues in the management of ICI. The distribution of Candida spp. and susceptibility patterns vary according to country, region, center, and clinic, making comparisons among studies difficult.7–9 Therefore, local epidemiologic information on ICI is critical for therapeutic management of the disease.
In traditional Chinese medicine hospitals, where the use of antifungal agents may differ from conventional medical settings, there is a significant gap in regional data regarding the epidemiology of ICI and antifungal resistance. This study aims to address this gap by providing detailed information on the species distribution and in vitro susceptibility of Candida isolates to eight antifungal agents in our hospital. In this study, we retrospectively analyzed data from patients with ICI at our hospital between 2020 and 2022. We aim to inform the selection of optimal clinical treatment options, enhance empirical antifungal treatment protocols, and support infection control measures, thereby improving overall clinical management of ICI.
Materials and Methods
Collection of Clinical Data
Clinical data from 301 patients with ICI were gathered retrospectively, encompassing demographics, admission departments, underlying conditions, potential risks, and outcomes. These patients were admitted to Longhua Hospital in Shanghai, China from January 2020 to December 2022. Longhua Hospital is a university hospital, affiliated to Shanghai University of Traditional Chinese Medicine, with 1000 beds, featuring 48 departments such as Internal Medicine, Surgery, Oncology, and Emergency Medicine. To ensure data completeness and relevance, the following exclusion criteria were applied: Patients with incomplete medical records that lacked essential information on demographics, underlying conditions, or treatment outcomes; patients who received antifungal treatment prior to sample collection, as this could influence the accuracy of antifungal susceptibility testing. In addition to the 301 inpatient samples, we included 55 Candida spp. strains isolated from outpatients. These outpatient samples were collected from various clinics within Longhua Hospital, shown in Table S1.
Ethical Approval and Data Confidentiality
Ethical approval for this study was obtained from the Medical Research Ethics Committee of Longhua Hospital. To ensure data confidentiality and patient anonymity, all patient records were de-identified prior to analysis, and only anonymized data were used in the study. Access to the data was restricted to the research team, and all data were stored securely in compliance with local data protection regulations.
Strains and Culture Conditions
Collectively, 301 Candida spp. strains were isolated from patients with ICI, and 55 strains were collected from outpatients at Long Hua Hospital. Species were identified through the use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. The strains were cultured on a yeast-peptone-dextrose medium at 30°C, as detailed previously.10
Antifungal Sensitivity Testing
Antifungal sensitivity was assessed using the broth microdilution technique, following the Clinical and Laboratory Standards Institute (CLSI) guidelines M27-A3.11 Quality control was ensured by including reference strains (Candida parapsilosis ATCC 22019 and Candida krusei ATCC6258) in each batch of susceptibility testing. Susceptibility breakpoints were assessed following the CLSI M27-M44S guidelines,12 when applicable, while epidemiological cutoff values were determined based on CLSI M60,13 CLSI M59,14 and CLSI M27-S3.15
Statistical Analysis
A power analysis was conducted prior to the study to ensure adequate sample size for detecting significant associations. Based on previous studies and pilot data, we estimated that a sample size of 301 patients would provide 99% power to detect a significant association with a medium effect size (w = 0.3) at a significance level of P < 0.05. This calculation was performed using G*Power software (version 3.1).16 Statistical analyses were performed using SPSS (version 24.0). The chi-squared test was employed to analyze proportions, and Fisher’s exact test was used to analyze incomplete data. A statistical significance threshold was established at P < 0.05. Variables that were biologically plausible and P < 0.1 in the univariate analysis were incorporated into the multiple logistic regression model. Variables such as age, gender, and treatment regimens were included in the model to adjust for confounding effects. Kaplan–Meier survival analysis was used for survival analysis. Statistical significance was assessed using the Log rank test to compare survival differences between groups, with P < 0.05 indicating significant differences. During data analysis, we assessed the dataset for missing data points. No missing data existed in the covariates included in the analysis.
Results
Clinical Characteristics
Of the 301 strains isolated from patients with ICI, C. albicans (38.9%, 117/301) was the most common Candida species, followed by C. tropicalis (28.2%, 85/301) and C. glabrata (22.9%, 69/301).
Table 1 summarizes the characteristics and predisposing factors of the study participants. The mean age for the entire sample population was 77.86 years, and 160 (53.2%) of the patients were male. Patients with C. parapsilosis infection, of whom half (10/20) were aged 70 years or younger, were on average nine years younger than patients with other types of Candida infections. The incidence of ICI increased with age and was prevalent among patients aged 81–90 years (35.6%, 108/301). Moreover, 120 of the 301 patients (39.9%) were admitted to the ICU, where C. albicans was the most common species. Regarding the probable sources of ICI, the urinary tract (37.2%, 112/301) was the most frequently detected portal of entry, followed by the upper respiratory (including sputum and nasopharyngeal swabs) (29.4%, 89/301) and lower respiratory (including tracheal aspirates and bronchoalveolar lavage fluid) (16.5%, 50/301) tracts.
 |
Table 1 Clinical Characteristics of Patients With Infections Attributed to Different Candida Species |
All patients had at least one underlying disease, the most common being lung infection (73.8%, 222/301), followed by hypertension (64.5%, 194/301) and cardiovascular disease (54.2%, 163/301). The most common risk factor for Candida infection was previous antimicrobial use (84.7%, 2255/301), followed by insertion of a CVC (56.5%, 170/301), use of a urinary catheter (54.2%, 163/301), and previous surgery (54.2%, 163/301). Significant differences in the percentages of lung disease, sepsis, kidney disease, and urinary tract infection, among underlying diseases, and total parenteral nutrition and dialysis, among risk factors, were observed among patients with different Candida infections. Patients infected with C. albicans were likely to suffer from sepsis and were commonly administered total parenteral nutrition. Patients infected with C. glabrata were likely to have urinary tract infections, patients infected with C. parapsilosis were likely to have kidney disease and require dialysis, and patients infected with C. krusei were likely to have lung disease.
Information on antifungal treatments administered to patients with ICI was collected and analyzed. The most commonly used antifungal agent was fluconazole (93.0%, 280/301). Other antifungal agents itraconazole and ketoconazole were used less frequently (2.3%, 7/301 and 3.0%, 9/301). Additionally, five patients were treated with a combination of two antifungal agents, including three treated with fluconazole and caspofungin.
Correlation Between Clinical Symptoms and Prognosis
The overall in-hospital mortality rate among the patients was 27.6% (83/301). Mortality was evaluated at different time intervals to provide a comprehensive understanding of the prognosis: 13 (4.3%) patients died within seven days after the diagnosis with Candida infection, 51 (16.9%) died within 7–30 days, and 19 (6.3%) died after 30 days. Patients with C. krusei, C. parapsilosis, and C. glabrata infections had an overall mortality rate of 50% (5/10), 35.0% (7/20), and 29.0% (20/69), respectively, whereas those with C. albicans and C. tropicalis infections had mortality rates of 27.4% (32/117) and 22.4% (19/85), respectively. Kaplan–Meier survival analysis showed that C. krusei infection led to a higher seven day-mortality rate than infection caused by other Candida spp. (P = 0.0122, Figure 1).
Table 2 shows the results of the univariate and multivariate logistic regression analyses performed to identify risk factors for mortality. For the primary endpoint analysis of mortality, we focused on the overall mortality rate during the entire follow-up period, which provides a comprehensive assessment of the long-term impact of invasive Candida infections. The statistical analysis showed no significant differences between the sexes. Among the three age groups, patients older than 85 years exhibited the highest mortality rate (33.7%, 35/104). Patients admitted to the ICU had a higher mortality rate (38.3%, 46/120). Among patients treated with fluconazole, the mortality rate was 28.9% (81/280), while those treated with a combination of antifungal agents was 40.0% (2/5). The univariate logistic regression analyses revealed that lung and cardiovascular disease and sepsis, among the underlying diseases, and use of antimicrobial, presence of CVC, urine catheter, or stomach tube, and use of total parenteral nutrition, among the risk factors, were correlated with mortality. Among the patients with underlying diseases, those with sepsis had the highest mortality rate (44.9%, 48/107). Among the patients with risk factors, those administered total parenteral nutrition had the highest overall mortality rate (46.3%, 38/82). The multivariate logistic regression analyses showed that age, sepsis, CVC insertion, and total parenteral nutrition were independent predictors of mortality.
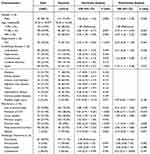 |
Table 2 Univariate and Multivariate Logistic Regression Analysis of Risk Factors for Mortality |
Antifungal Susceptibility
The results of the in-vitro susceptibility testing of the Candida isolates are summarized in Table 3. A total of 356 isolates were analyzed, including 55 collected from outpatients. All strains were generally sensitive to amphotericin and 5-fluorocytosine.
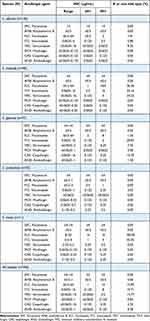 |
Table 3 In-Vitro Susceptibility of 356 Candida Isolates to Eight Antifungal Agents |
Among the azoles, the minimum inhibitory concentration value required to inhibit 90% of the isolates (MIC90) was lower for voriconazole (0.5 mg/L) than for fluconazole (16 mg/L) or itraconazole (8 mg/L). Of the isolates studied, 15.95% were fluconazole-resistant, 19.71% were itraconazole-non-wild type (NWT), and 14.77% were voriconazole-NWT. C. tropicalis was the most resistant to azoles, with 36.26% of the strains being fluconazole-resistant, 35.16% being itraconazole-NWT, and 34.54% being voriconazole-NWT.
All echinocandins showed good activity against most Candida spp. Overall, caspofungin had higher MIC90 values (0.25 mg/L) than micafungin (0.125 mg/L) and anidulafungin (0.125 mg/L). Non-susceptibility to echinocandins was found in 11 C. glabrata isolates (10.39%, 3.90%, and 1.30% for caspofungin, micafungin, and anidulafungin, respectively).
Discussion
Despite the availability of diagnostic and treatment methods, the risk of Candida infections has recently increased due to the use of broad-spectrum antimicrobial and immunosuppressive agents, total parenteral nutrition, and mechanical ventilation. The prevalence of ICI has increased from 3.8 to 29.3% since the 1990s,17 whereas the rate of infection by non-albicans Candida among all pathogens has been increasing annually.18 This study revealed that Candida isolates comprised five invasive species, of which C. albicans was the most abundant, followed by C. tropicalis and C. glabrata, and then C. parapsilosis and C. krusei. This strain distribution is consistent with that observed in several previous studies,1,19 but also differed from that of other studies.20–22 While our findings are consistent with global trends, they provide critical local data that can inform regional treatment protocols and infection control strategies.
This study describes the demographic and clinical characteristics of patients with ICI, the distribution of Candida species in patient specimens, and patient outcomes at our hospital between 2020 and 2022, along with the in-vitro susceptibility profiles of these isolates to eight antifungal agents. The Candida strains were mainly isolated from the urine and respiratory tracts of ICI patients. The use of urinary and tracheal catheters for patients with poor urine flow and inability to cough sputum increases the risk of Candida colonization, making the urinary and respiratory systems common sites of infection. Xu et al23 and Ai Er Ken et al24 obtained specimens from the blood. Strains isolated from blood are associated with high drug resistance and mortality rates, as can be deduced from the increased detection rate of non-albicans Candida in bloodstream infections in recent years,7,25 complicating the treatment of candidiasis.
Our results showed that the Candida spp. strains had varying distributions across wards. ICI occurred more frequently among patients hospitalized in general wards than in those from ICUs. Among the patients admitted to the ICU with severe underlying diseases, older patients tended to have a high likelihood of becoming infected and developing ICI.10,26,27 This is due to their relatively low level of immunity, which often necessitates the use of endotracheal tubes, CVCs, and other invasive procedures that facilitate Candida colonization. Previous studies have shown that Candida can be easily isolated from patients with impaired immunity,28–30 which is consistent with our results.
ICI etiology varied according to age, hospitalization status, or underlying conditions of the patients. Clinical data were compared between patients with ICI caused by different species of Candida. Patients with C. parapsilosis infection were young and were likely to experience kidney disease or to require dialysis. Infection with C. albicans occurred frequently in patients with sepsis and in those who required administration of total parenteral nutrition. The mortality rate was higher after C. krusei infection than after infections caused by other Candida spp., indicating that infection by this species is more difficult to treat than those by other species. Infection is not only related to the pathogenicity of Candida species but also to complications associated with the patient’s underlying disease. In our study, patients with one or more underlying diseases had increased mortality rates. The multivariate logistic regression analysis showed that age, presence of sepsis, CVC use, and administration of total parenteral nutrition were associated with increased mortality. Other studies have also shown a correlation between high mortality, underlying diseases, and the presence of risk factors.31,32 The use of catheters facilitates Candida colonization, which further complicates treatment. Moreover, underlying diseases and immune-related disorders suppress the immune system and increase the risk of infection.33 While the identified mortality predictors are consistent with existing literature, they reinforce the importance of these factors in local clinical practice and can guide the development of targeted interventions.
The interaction between age, sepsis, and antifungal resistance patterns is complex. Older patients often have comorbidities and weakened immune systems, which increase their susceptibility to infections and antifungal resistance. Sepsis, as a severe systemic inflammatory response, can further impair immune function and increase the likelihood of Candida colonization and subsequent infection. Additionally, prolonged hospital stays and the use of invasive devices in older patients with sepsis can lead to higher exposure to antifungal agents, potentially contributing to resistance. Future studies should explore these interactions in more detail to better understand the underlying mechanisms.
Our study examines the antifungal treatments employed in patients with ICI and their potential influence on patient mortality. Our analysis revealed no significant differences in mortality rates between patients treated with different antifungal agents. This finding may be attributed to the high prevalence of fluconazole use, which limits the statistical power to detect differences in mortality rates associated with other antifungal agents. Fluconazole remains a viable empirical treatment option for many patients, although its efficacy may be influenced by factors such as antifungal resistance and the severity of underlying conditions. Despite the lack of significant differences in mortality, our findings highlight the importance of considering alternative antifungal agents, particularly in patients with high-risk factors or those who do not respond to initial fluconazole therapy. The use of combination therapy, such as fluconazole and caspofungin, may be beneficial in certain cases, although further studies are needed to confirm its efficacy and safety.
The sensitivity of Candida species to antifungal agents was also analyzed; azoles and echinocandins are commonly used antifungal drugs for treating ICI. The results showed that although most strains were sensitive to azoles, resistance to itraconazole was the highest (19.71%), whereas the rates of strain resistance to fluconazole and voriconazole were 15.95% and 14.77%, respectively. C. tropicalis was the most resistant to azoles, and C. glabrata was non-susceptible to echinocandins. The drug resistance exhibited by these strains may be due to the long-term use of antifungal drugs.34,35 All Candida strains were sensitive to both 5-flucytosine and amphotericin. Although 5-flucytosine and amphotericin are not first-line antifungals in the treatment of ICI in most clinical contexts, they may substitute azoles or echinocandins or be used in salvage therapy.36,37 The resistance patterns observed in our study have significant implications for empirical therapy and antifungal stewardship. Empirical treatment protocols should consider these resistance patterns, especially in high-risk populations such as ICU patients and those with underlying sepsis. Antifungal stewardship programs should be implemented to monitor and optimize antifungal use, reducing the risk of resistance development.
To contextualize our findings within the broader scope of research on ICI, we compared our results on antifungal resistance rates with those from other studies conducted in China. In our study, C. tropicalis exhibited relatively high resistance rates to azole antifungal, which are similar to the national data reported by Xiao et al,1 but higher than that reported by Li et al38 in a Tertiary Care Hospitals in Western China, where the fluconazole resistance rate was 5.9%. For echinocandin antifungals, resistance was only observed in C. glabrata, consistent with the findings of Zhang et al39 and Song et al.40 However, the resistance rates varied across studies. The disparities in resistance rates could be connected to the regional use of antifungals, the types of hospitals, and the demographics of the patient populations. This highlights the importance of local epidemiology and drug resistance surveillance in guiding clinical management and treatment strategies.
The findings of this study carry significant implications for clinical practice. First, given the high azole resistance rate in C. tropicalis and the presence of echinocandin-non-susceptible C. glabrata strains, we propose revising local empirical antifungal treatment guidelines: for critically ill or high-risk patients (eg, those with long-term central venous catheters or receiving total parenteral nutrition), initial therapy should prioritize echinocandins over fluconazole to cover potential resistance risks; for non-critical patients, a step-down approach guided by susceptibility testing is recommended. Second, infection control strategies must be strengthened, including strict adherence to catheter insertion and maintenance protocols (eg, daily assessment of catheter necessity), implementation of active fungal colonization screening in high-risk wards (eg, ICUs), and establishment of real-time resistance surveillance networks to detect nosocomial transmission early. Furthermore, this study underscores the urgency of Antifungal Stewardship Programs (ASPs). For instance, azole resistance in C. tropicalis may correlate with prolonged exposure. ASP-driven strategies, such as restriction policies for broad-spectrum azoles, therapeutic drug monitoring, and duration optimization, could attenuate resistance development. Finally, integrating rapid molecular diagnostics (eg, MALDI-TOF MS combined with resistance gene detection) could significantly shorten species identification and susceptibility reporting times. For example, FKS gene mutation testing in C. glabrata could guide targeted echinocandin use, avoiding treatment delays and unnecessary drug exposure. The comprehensive implementation of these measures may improve outcomes in invasive candidiasis and curb the spread of resistant strains.
Our research had certain constraints. First, the retrospective design inherently carries risks of incomplete documentation of clinical details and microbiological data, as well as potential recall bias during data collection. Second, the single-center nature of the investigation may limit the generalizability of our findings, as regional variations in antifungal resistance patterns and population-specific risk factors might not be adequately represented. Third, the lack of standardized protocols for patient management protocols during the study period, including antifungal treatment regimens and ICU care protocols, introduces potential variability in treatment outcomes that could not be fully accounted for in the analysis. Nevertheless, we are confident that our findings offer clinicians valuable insights prior to devising empirical treatment plans for patients with ICI and contribute to understanding the epidemiology and risk factors associated with ICI.
Conclusion
Our study provides insights into the epidemiology, clinical outcomes, and antifungal resistance patterns of ICI in a traditional Chinese medicine hospital setting, highlighting the importance of local data for guiding clinical practice. Our findings emphasize the need for tailored empirical antifungal therapy, particularly prioritizing echinocandins (eg, anidulafungin) for high-risk patients, such as those with prolonged central venous catheterization or total parenteral nutrition, while adopting de-escalation strategies for non-critical cases guided by rapid susceptibility testing. Based on these results, the implementation of antifungal stewardship programs (ASPs) is essential to mitigate resistance trends. This is particularly achieved through restriction policies for broad-spectrum azoles, therapeutic drug monitoring, and optimized treatment durations. Additionally, enhanced infection control measures are also imperative to curb nosocomial transmission. These measures include standardized catheter maintenance protocols, proactive fungal colonization surveillance in high-risk units, and real-time resistance monitoring networks. The public health significance of the increasing antifungal resistance is extremely important. The rising prevalence of non-albicans Candida species and their resistance to first-line antifungals pose a substantial threat to global health, particularly in immunocompromised populations. Our findings call for heightened vigilance and coordinated efforts to monitor resistance patterns, promote rational antifungal use, and integrate advanced diagnostic tools (eg, MALDI-TOF MS and FKS mutation assays) into routine clinical workflows. These measures are vital for improving patient outcomes and preserving the efficacy of existing antifungal agents. This study has limitations, including its single-center, retrospective design, which may limit the generalizability of the findings. Future multicenter, prospective studies are needed to validate our observations and explore the impact of integrated traditional Chinese medicine-Western therapies on antifungal resistance.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article. The datasets are available from the corresponding author on reasonable request.
Ethics Approval
This retrospective study was approved by the Medical Research Ethics Committee of Longhua Hospital; individual data were collected anonymously and the requirement to obtain informed written consent was waived. This study was conducted in accordance with the Declaration of Helsinki.
Consent to Publish
Patient consent to publish was waived due to the retrospective nature of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the three-year action plan for Strengthening the Construction of Public Health System in Shanghai (2023-2025; GWVI-11.2-YQ28), Medical Science and Technology Talents Support Project of IPMCH (Honghu Plan) (HHJH2401), and Longhua Hospital Affiliated with Shanghai University of Chinese Medicine ‘Longyi Healer’ (grant number KY22029).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xiao M, Chen SC, Kong F, et al. Distribution and antifungal susceptibility of Candida species causing candidemia in China: an update from the CHIF-NET study. J Infect Dis. 2020;221:S139–S147. doi:10.1093/infdis/jiz573
2. Hernández-Carreón O, Hernández-Howell C, Hernández-Hernández G, et al. Highly specific and rapid molecular detection of Candida glabrata in clinical samples. Braz J Microbiol. 2021;52:1733–1744. doi:10.1007/s42770-021-00584-2
3. Pereira R, Dos Santos Fontenelle RO, de Brito EHS, de Morais SM. Biofilm of Candida albicans: formation, regulation and resistance. J Appl Microbiol. 2021;131:11–22. doi:10.1111/jam.14949
4. Chesdachai S, Yetmar ZA, Ranganath N, et al. Antifungal susceptibility pattern of Candida glabrata from a referral center and reference laboratory: 2012–2022. J Fungi. 2023;9(8):821. doi:10.3390/jof9080821
5. Sig AK, Sonmezer MC, Gülmez D, et al. The emergence of echinocandin-resistant Candida glabrata exhibiting high MICs and related FKS mutations in Turkey. J Fungi. 2021;7(9):691. doi:10.3390/jof7090691
6. Stefanini I, Stoakes E, Hht W, et al. Genomic assembly of clinical Candida glabrata (Nakaseomyces glabrata) isolates reveals within-species structural plasticity and association with in vitro antifungal susceptibility. Microbiol Spectr. 2022;10:e0182722. doi:10.1128/spectrum.01827-22
7. Dalyan Cilo B. Species distribution and antifungal susceptibilities of Candida species isolated from blood culture. Cureus. 2023;15:e38183. doi:10.7759/cureus.38183
8. Seyoum E, Bitew A, Mihret A. Distribution of Candida albicans and non-albicans Candida species isolated in different clinical samples and their in vitro antifungal susceptibility profile in Ethiopia. BMC Infect Dis. 2020;20:231. doi:10.1186/s12879-020-4883-5
9. Aziz HSA, Ismail DK, Mohammed NSA, et al. Distribution and antifungal susceptibility profiles of Candida species isolated from candidemia patients admitted to Egyptian tertiary hospitals: a cross-sectional study. BMC Infect Dis. 2024;24:1177. doi:10.1186/s12879-024-10007-w
10. Torres R, Barreto-Santamaría A, Arévalo-Pinzón G, et al. In vitro antifungal activity of three synthetic peptides against Candida auris and other Candida species of medical importance. Antibiotics. 2023;12:1234. doi:10.3390/antibiotics12081234
11. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard.
12. Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts.
13. Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts.
14. Clinical and Laboratory Standards Institute. Epidemiological Cutoff Values for Antifungal Susceptibility Testing.
15. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Third Informational Supplement Document M27-S3. Wayne, PA: CLSI; 2008.
16. Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. doi:10.3352/jeehp.2021.18.17
17. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17:e383–e392. doi:10.1016/S1473-3099(17)30316-X
18. Wang Q, Li Y, Cai X, et al. Two sequential clinical isolates of Candida glabrata with multidrug-resistance to posaconazole and echinocandins. Antibiotics. 2021;10:1217. doi:10.3390/antibiotics10101217
19. Gil HI, Yang B, Lee T, et al. Clinical characteristics and treatment outcome of Candida tracheobronchitis. Medicine. 2021;100:e24606. doi:10.1097/MD.0000000000024606
20. Qiu J, Roza MP, Colli KG, et al. Candida-associated denture stomatitis: clinical, epidemiological, and microbiological features. Braz J Microbiol. 2023;54:841–848. doi:10.1007/s42770-023-00952-0
21. Elbaz M, Chikly A, Meilik R, Ben-Ami R. Frequency and clinical features of candida bloodstream infection originating in the urinary tract. J Fungi. 2022;8:123. doi:10.3390/jof8020123
22. García-Salazar E, Acosta-Altamirano G, Betancourt-Cisneros P, et al. Detection and molecular identification of eight Candida species in clinical samples by simplex PCR. Microorganisms. 2022;10:374. doi:10.3390/microorganisms10020374
23. Xu H, Yu SY, Zhou ML, et al. Epidemiology and antifungal susceptibility patterns of invasive fungal infections from 2012 to 2014 in a teaching hospital in Central China. Infect Drug Resist. 2019;12:3641–3651. doi:10.2147/IDR.S227839
24. Er Ken ABB A, Ma ZH, Xiong DQ, Xu PR. Clinical features of invasive candidiasis and risk factors for Candida bloodstream infection in children: a multicenter study in Urumqi, China. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:414–418. doi:10.7499/j.issn.1008-8830.2017.04.011
25. Gorgun S, Bilgin M, Kilic SS. Distribution and antifungal susceptibility of candida species isolated from blood cultures. J Pak Med Assoc. 2021;71:1601–1604. doi:10.47391/JPMA.1464
26. Wang B, He X, Lu F, et al. Candida isolates from blood and other normally sterile foci from ICU patients: determination of epidemiology, antifungal susceptibility profile and evaluation of associated risk factors. Front Public Health. 2021;9:779590. doi:10.3389/fpubh.2021.779590
27. Alenazy H, Alghamdi A, Pinto R, Daneman N. Candida colonization as a predictor of invasive candidiasis in non-neutropenic ICU patients with sepsis: a systematic review and meta-analysis. Int J Infect Dis. 2021;102:357–362. doi:10.1016/j.ijid.2020.10.092
28. Lopes JP, Lionakis MS. Pathogenesis and virulence of Candida albicans. Virulence. 2022;13:89–121. doi:10.1080/21505594.2021.2019950
29. Le Bars P, Kouadio AA, Bandiaky ON, Guéhennec L L, de La Cochetière MF, de La Cochetière M-F. Host’s immunity and Candida species associated with denture stomatitis: a narrative review. Microorganisms. 2022;10:1437. doi:10.3390/microorganisms10071437
30. Solis NV, Wakade RS, Filler SG, Krysan DJ. Candida albicans oropharyngeal infection is an exception to iron-based nutritional immunity. mBio. 2023;14:e0009523. doi:10.1128/mbio.00095-23
31. Thomas-Rüddel DO, Schlattmann P, Pletz M, Kurzai O, Bloos F. Risk factors for invasive candida infection in critically ill patients: a systematic review and meta-analysis. Chest. 2022;161:345–355. doi:10.1016/j.chest.2021.08.081
32. Zhong L, Dong Z, Liu F, et al. Incidence, clinical characteristics, risk factors and outcomes of patients with mixed Candida/bacterial bloodstream infections: a retrospective study. Ann Clin Microbiol Antimicrob. 2022;21:45. doi:10.1186/s12941-022-00538-y
33. Gómez-Gaviria M, Ramírez-Sotelo U, Mora-Montes HM. Non-albicans Candida species: immune response, evasion mechanisms, and new plant-derived alternative therapies. J Fungi. 2022;9:11. doi:10.3390/jof9010011
34. Alikhani T, Daie Ghazvini R, Mirzaii M, et al. Drug resistance and biofilm formation in Candida species of vaginal origin. Iran J Public Health. 2022;51:913–918. doi:10.18502/ijph.v51i4.9253
35. Castanheira M, Deshpande LM, Davis AP, Carvalhaes CG, Pfaller MA. Azole resistance in Candida glabrata clinical isolates from global surveillance is associated with efflux overexpression. J Glob Antimicrob Resist. 2022;29:371–377. doi:10.1016/j.jgar.2022.05.004
36. Lim HJ, Choi MJ, Byun SA, et al. Whole-genome sequence analysis of Candida glabrata isolates from a patient with persistent fungemia and determination of the molecular mechanisms of multidrug resistance. J Fungi. 2023;9(5):515. doi:10.3390/jof9050515
37. Badrane H, Cheng S, Dupont CL, et al. Genotypic diversity and unrecognized antifungal resistance among populations of Candida glabrata from positive blood cultures. Nat Commun. 2023;14:5918. doi:10.1038/s41467-023-41509-x
38. Li K, Yang X, Li L, et al. Candidaemia: a 9-year retrospective analysis of epidemiology and antimicrobial susceptibility in Tertiary Care Hospitals in Western China. Infect Drug Resist. 2024;17:3891–3900. doi:10.2147/IDR.S477815
39. Zhang S, Zhang L, Yusufu A, et al. Clinical distribution and drug susceptibility characterization of invasive Candida isolates in a Tertiary Hospital of Xinjiang Province. Infect Drug Resist. 2024;17:1345–1356. doi:10.2147/IDR.S450933
40. Song Y, Chen X, Yan Y, et al. Prevalence and antifungal susceptibility of pathogenic yeasts in China: a 10-year retrospective study in a teaching hospital. Front Microbiol. 2020;11:1401. doi:10.3389/fmicb.2020.01401
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


