Back to Journals » Journal of Inflammation Research » Volume 17
Clinical Significance of Abnormal Serum LGALS3BP Expression in Patients with Idiopathic Inflammatory Myopathies
Authors Huang L, Huang X, Zhou W, Jiang Y, Zhu H, Lao Y, Deng Z, Tang Y, Wang J, Li X
Received 2 September 2024
Accepted for publication 2 November 2024
Published 25 November 2024 Volume 2024:17 Pages 9697—9710
DOI https://doi.org/10.2147/JIR.S490210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Liuyi Huang,* Xiaoxia Huang,* Wei Zhou, Yanting Jiang, Haiqing Zhu, Yuehong Lao, Zhenjia Deng, Yuting Tang, Jian Wang, Xi Li
Department of Clinical Laboratory, the First Affiliated Hospital of Guangxi Medical University, Key Laboratory of Clinical Laboratory Medicine of Guangxi Department of Education, Nanning, Guangxi, 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi Li; Jian Wang, Department of Clinical Laboratory, the First Affiliated Hospital of Guangxi Medical University, Key Laboratory of Clinical Laboratory Medicine of Guangxi Department of Education, No. 6 Shuangyong Road, Nanning, Guangxi, 530021, People’s Republic of China, Email [email protected]; [email protected]
Objective: Idiopathic inflammatory myopathies (IIM) are classified into four subgroups: dermatomyositis (DM), anti-synthetase syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), and sporadic inclusion body myositis (sIBM); however, the role of LGALS3BP in IIM remains unclear. Our study aimed to explore the ability of LGALS3BP to discriminate between the IIM subtypes. The correlation between serum LGALS3BP levels, clinical features, and inflammatory markers in patients with IIM was also assessed.
Methods: Based on the Gene Expression Omnibus (GEO) database, we used bioinformatics analysis to screen for overlapping extracellular protein-differentially expressed genes between any two groups of DM, ASS, IMNM, and healthy controls (HCs). This study enrolled 84 patients with IIM and 36 HCs, and participant baseline data and laboratory parameters were recorded. Serum LGALS3BP levels were measured using an enzyme-linked immunosorbent assay (ELISA).
Results: Through bioinformatics analysis, LGALS3BP was selected as a potential biomarker for the identification of DM, ASS, IMNM, and HC. LGALS3BP expression decreased sequentially in the DM, ASS, IMNM, and HC groups, with significant differences among the two groups. The ELISA results were similar to those of the bioinformatics analysis; however, the difference in serum LGALS3BP expression between DM and ASS was not statistically significant. According to the receiver operating characteristic (ROC) curve analysis, using HC as the control group, the area under the curve (AUC) values of serum LGALS3BP levels for the diagnosis of IIM, DM, and ASS were > 0.8. In addition, patients with elevated serum LGALS3BP levels had a higher prevalence of interstitial lung disease (ILD). Serum LGALS3BP levels correlated with inflammatory markers.
Conclusion: LGALS3BP is differentially expressed in DM, ASS, IMNM, and HC and may assist in assessing the severity of IIM-ILD.
Keywords: LGALS3BP, idiopathic inflammatory myopathies, dermatomyositis, anti-synthetase syndrome, immune-mediated necrotizing myopathy, interstitial lung disease
Introduction
Idiopathic inflammatory myopathies (IIM), collectively known as myositis, are a heterogeneous group of diseases characterized by muscle involvement.1 In recent years, IIM has been mainly classified into four subgroups based on pathological features, myositis-specific autoantibodies (MSAs), and clinical manifestations: dermatomyositis (DM), anti-synthetase syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), and sporadic inclusion body myositis (sIBM).2,3 Muscle biopsy and MSA play leading roles in diagnosing and classifying IIM,4 although both have limitations. Muscle biopsy is an invasive procedure and complications may occur, including hematoma or infection at the biopsy site.5 MSA detection yet to be standardized, and new antibodies continue to be discovered.6 Notably, some patients with IIM may also be MSA-negative, and MSA is present in only 60% of patients with IIM,7 which makes it more difficult to clinically categorize this subset of patients. The early identification of IIM subtypes is important for clinicians to evaluate patients for individualized treatment options and prognostic decisions. Therefore, there is an urgent need for an easily detectable biomarker to aid the identification of IIM subtypes.
Because of its tendency to progress slowly, sIBM can be easily identified, whereas DM, ASS, and IMNM generally have an acute or subacute onset.8 Therefore, our first goal was to identify biomarkers that could recognize DM, ASS, and IMNM. We initially considered extracellular proteins because they are readily detectable and standardized. We identified differentially expressed genes (DEGs) in the IIM subtype using bioinformatics analysis and targeted some of these DEGs encoding extracellular proteins. LGALS3BP was initially recognized as an ideal extracellular protein that could assist in differentiating DM, ASS, IMNM, and healthy controls (HCs). LGALS3BP, also known as galectin 3 binding protein, Gal-3BP, or 90K, is a glycoprotein in both secreted and non-secreted forms that is widely expressed in many cell types.9 LGALS3BP has been identified as a ligand for LGALS3 that promotes intercellular adhesion and initiates pathological proinflammatory signaling cascades.10 LGALS3 has been shown to be associated with interstitial lung disease (ILD) in IIM as well as disease activity.11 However, the expression of LGALS3BP in the serum of patients with DM, ASS, and IMNM remains unknown. Therefore, the goal of our study was to detect serum LGALS3BP levels in patients with DM, ASS, IMNM, and HC, to determine the broader impact of the results from our bioinformatics analysis. Serum LGALS3BP levels were evaluated in relation to the clinical characteristics of patients with IIM, particularly ILD, and disease activity.
Methods
Dataset Collection, Preparation, and Identification of DEGs
Dataset GSE220915 was obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/). All data lacking transcript IDs and Multiple data with only one transcript ID were eliminated. Dataset GSE220915 includes 44 DM, 16 sIBM, 18 ASS, 54 IMNM, and 33 hC samples. Our study mainly targeted patients with DM, ASS, and IMNM, and HC. Two of these formed disease and disease control groups: DM-ASS, DM-IMNM, DM-HC, ASS-IMNM, ASS-HC, and IMNM-HC. DEGs from the six groups were evaluated using the R program “DESeq2.” Statistical significance was determined by |log2FoldChange| > 0.5 and an adjusted p-value< 0.05. The six groups of DEGs were evaluated using a Venn diagram, and the overlapping regions were called DAIH DEGs. Heatmaps of the DAIH DEGs were plotted using TBtools-II.12
Functional Enrichment Analysis
The DAIH DEGs were uploaded to the DAVID database (https://david.ncifcrf.gov/). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed. Finally, the enrichment results from the top six pathways were denoted using a Circos plot.
Protein-Protein Interaction (PPI) Network Construction
DAIH DEGs were prepared for PPI analysis using the STRING database (https://string-db.org/) and visualized using Cytoscape (version 3.10.0). A Molecular Complex Detection (MCODE) plug was used to identify essential subnetworks of the PPI network. We set the following parameters: node density cutoff = 0.1, node score cutoff = 0.2, k-core = 2, and depth = 100. The cluster with the highest MCODE score was used for the subsequent screening of extracellular proteins.
Screening for Extracellular Proteins
Extracellular protein gene lists were obtained from Wang13 and downloaded from the Human Protein Atlas (HPA) database (https://www.proteinatlas.org/) and UniProt (https://www.uniprot.org/) databases. A Venn diagram was constructed for the intersection between candidate genes in the PPI and the two extracellular protein gene lists, and extracellular protein DEGs (EP DEGs) were identified.
Participants and Ethical Approval
This study included 84 patients with IIM and 36 sex- and age-matched HCs. According to the classification criteria of the 239th Neuromuscular European Center (ENMC),5 patients with IIMs were categorized as having DM (n=47), ASS (n=16), and IMNM (n=21). IIM was diagnosed according to international standards established by Bohan and Peter.14,15 All patients were screened for malignant tumors. Patients under 18 years of age, with any malignancy or other autoimmune diseases were excluded from the analysis. This study was approved by the Ethical Review Committee of the First Affiliated Hospital of Guangxi Medical University (2023-E756-01) and informed consent was obtained from all participants. This study complied with the principles of the Declaration of Helsinki.
Definitions and Data Collection
Clinical manifestations and laboratory parameters were collected from patient electronic medical records. All data correspond to the dates on which patient sera were collected for LGALS3BP testing. Clinical manifestations include ILD, according to the American Thoracic Society and the European Respiratory Society, the diagnosis of ILD was determined by high-resolution computed tomography (HRCT); rapidly progressive interstitial lung disease (RP-ILD) was defined as worsening of imaging and pulmonary symptoms within three months.16 All patients included in the study underwent HRCT, and the presence of ILD/RP-ILD was diagnosed by a specialized imaging physician. Laboratory parameters included inflammatory indicators such as albumin and globulin.
Serum LGALS3BP and MSA Detection
Serum specimens were collected from the hospital laboratory and stored in a refrigerator at −80°C until testing. Following the manufacturer’s instructions, a human LGALS3BP enzyme-linked immunosorbent assay (ELISA) kit (2H-KMLJh314728; Nanjing, China) was used to measure LGALS3BP levels. Dilution of the serum with the sample diluent was not necessary. All samples were set up in compound wells and normalized, and the intra- and inter-assay coefficients of variation for ELISA were less than 12%.
MSA was assessed using a linear immunoblotting assay (Centuryyis, Hangzhou, China), according to the manufacturer’s protocol. The bands were scanned using EUROLineCamera (EUROIMMUN, Lubeck, Germany).
Statistical Analysis
Normality was assessed using the Kolmogorov–Smirnov test. Data was expressed as number (percentage), mean ± standard deviation, or median (1/4, 3/4). For comparisons between two groups, the independent samples t-test or Mann–Whitney U-test was performed. Matched samples were analyzed using the Wilcoxon signed-rank test. For comparisons among multiple groups, the ANOVA or the Kruskal–Wallis H-test was performed. Correlation analysis was performed using Pearson's correlation and Spearman correlation; an r-value of 0.3–0.5 was considered a moderate correlation, and >0.5 a strong correlation. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive value of continuous variables. IBM SPSS version 26.0 and GraphPad Prism 8 software were used to analyze all statistical data. DEGs were analyzed using the R software version v4.4.0. Statistical significance was set at a two-sided p-value <0.05 (two-sided) was considered statistically significant.
Results
Identification of DAIH DEGs and Functional Enrichment Analysis
Using |log2FoldChange| > 0.5 and an adjusted p-value< 0.05 as the screening criteria, 48 DAIH DEGs were identified in DM-ASS, DM-IMNM, DM-HC, ASS-IMNM, ASS-HC, and IMNM-HC (Figure 1A). A heat map of the DAIH DEGs is shown in Figure 1B. To understand the function of the DAIH DEGs, we performed GO and KEGG pathway enrichment analyses. The biological process (BP) of GO enrichment analysis illustrated that DAIH DEGs were mostly enriched in “defense response to virus”, “negative regulation of viral genome replication”,“innate immune response”, “response to virus”, “interleukin-27-mediated signaling pathway”, and “positive regulation of interferon-beta production” (Figure 1C). The cellular component (CC) and molecular function (MF) of GO enrichment analysis illustrated that DAIH DEGs were mostly located in the “cytoplasm” and related to “nucleotidyltransferase activity” (Figure 1D–E). KEGG pathway analysis showed that the DAIH DEGs were strongly associated with “Measles” (Figure 1G).
Screening of EP DEGs
To explore the interconnections among the DAIH DEGs, we uploaded 48 DAIH DEGs to the STRING database to construct a PPI network. The core module was screened using the MCODE plug, and 33 core genes of the core module were initially considered to aid in IIM subtype identification (Figure 2A). Our aim was easily detectable biomarkers, so we intersected the 33 candidate genes in PPI with 1903 extracellular proteins from the HPA database and 3684 extracellular proteins from the UniProt database via a Venn diagram to obtain three EP DEGs, namely LGALS3BP, IFI35, and LGALS9 (Figure 2B).
We did not find any research on LGALS3BP in the IIM; therefore, we focused on LGALS3BP. In the GSE220915 dataset, LGALS3BP expression in DM, ASS, IMNM, and HC is shown in Figure 2C. Significant differences were observed between the two groups (P<0.05). The expression of LGALS3 in the DM, ASS, IMNM, and HC groups is shown in Figure 2D. Compared to HC, LGALS3 was significantly elevated in DM, ASS, and IMNM (P<0.05), but there was no significant difference among subjects with DM, ASS, and IMNM (P>0.05). Therefore, LGALS3BP may be a more appropriate marker than LGALS3 for identifying the IIM subtypes. Receiver operating characteristic curves were plotted to assess the diagnostic value of LGALS3BP for each IIM subtype. When HC was used as the control group, the area under the curve (AUC) of LGALS3BP in the diagnosis of DM, ASS, and IMNM were all >0.8 (Figure 2E).
Baseline Characteristics of All Enrolled Participants
Table 1 shows the demographics, clinical manifestations, and laboratory parameters of the study participants. Eighty-four patients with IIM were included in the study, and the median disease duration was 6 (2.0, 17.8) months. There were no significant differences in sex or age between patients with IIM and HC. Based on clinical features, 44 patients (52.4%) had IIM-ILD, of whom six (7.1%) had RP-ILD The baseline characteristics of the patients with DM, ASS, and IMNM are shown in Supplementary Table S1. The patients with DM, ASS, and IMNM were predominantly female. Patients with DM and ASS present with ILD, rash, and muscle and joint involvement as the main clinical features. Patients with IMNM had significantly fewer ILD and rashes, with predominant muscle and joint injuries. Six patients with RP-ILD were identified as having DM, and none of the patients with RP-ILD were identified as having ASS or IMNM. All patients with IIM were treated with glucocorticoids and/or immunosuppressants, and 11 patients received immunoglobulin.
 |
Table 1 Baseline Characteristics of All Study Participants |
Characteristics of Serum LGALS3BP in Patients with IIM
The level of serum LGALS3BP was significantly higher in patients with IIM compared to HC [66.7 ± 15.4 vs 48.9 ± 15.8 ng/mL, P < 0.001] (Figure 3A). To assess the differential expression of LGALS3BP across IIM subtypes, we divided the IIM group into the DM, ASS, and IMNM groups. The serum LGALS3BP levels in patients with DM, ASS, and IMNM were 69.3 ± 13.9, 70.3 ± 10.3, and 58.1 ± 18.7 ng/mL, respectively. Patients in the DM and ASS groups had higher serum LGALS3BP levels than patients in the IMNM and HC groups [P < 0.05], and serum LGALS3BP levels were also higher in patients in the IMNM group than in those in the HC group [P = 0.028] (Figure 3B). Unfortunately, serum LGALS3BP levels were not significantly different between patients with DM and those with ASS [P=0.826], which did not correspond to our expected results. In addition, owing to the heterogeneity of MSA, we evaluated serum LGALS3BP levels stratified according to MSA. Serum LGALS3BP levels did not differ significantly between the MSA groups (Figure 3C).
ROC curves were constructed to determine the diagnostic value of serum LGALS3BP levels. According to the ROC curve, using HC as the control group, the AUCs of serum LGALS3BP for the diagnosis of IIM, DM, ASS, and DM + ASS were all > 0.8. Thus, LGALS3BP may be a good predictor for the diagnosis of IIM, DM, ASS, and DM + ASS. Subsequently, the optimal cutoff values were obtained by applying the Youden index, and the optimal cutoff values for serum LGALS3BP for the diagnosis of IIM, DM, ASS, and DM + ASS were 56.4, 53.4, 56.5,53.3, respectively. The AUCs for IMNM vs HC, DM vs IMNM, and ASS vs IMNM were 0.697, 0.684, and 0.726, respectively (P<0.05; Table 2).
 |
Table 2 The Diagnostic Performance of Serum LGALS3BP |
Demographic and Clinical Characteristics of Patients with Positive and Negative Serum LGALS3BP
Patients were categorized into LGALS3BP-positive (LGALS3BP+, n = 65) and LGALS3BP-negative (LGALS3BP-, n = 19) groups based on the cutoff values of the ROC curves for IIM versus HC. There were more male patients in the LGALS3BP-positive group than in the LGALS3BP-negative group. In addition, LGALS3BP-positive patients had higher levels of globulin, LDH, CRP, ESR, and ferritin (P < 0.05), which are indicators of inflammation that can represent myositis disease activity. Notably, we analyzed the association between serum LGALS3BP levels and all available clinical manifestations in patients with IIM, such as ILD, rash, and extramuscular complications, and found that only ILD was associated with LGALS3BP levels (Table 3).
 |
Table 3 Demographic and Clinical Characteristics of Patients with Positive and Negative Serum LGALS3BP Levels |
Association of Serum LGALS3BP Levels with IIM-ILD
ILD is a significant extramuscular complication of IIM and that associated with poor survival.17 RP-ILD is resistant to conventional therapy and associated with high mortality;18 therefore, we investigated the relationship between serum LGALS3BP levels and ILD in patients with IIM. As shown in Figure 4A, compared to IIM without ILD (Non-ILD), serum LGALS3BP levels were significantly higher in IIM-ILD [72.4 ± 11.3 vs 60.5 ± 16.9 ng/mL, P < 0.001]. Additionally, we categorized IIM-ILD into the RP-ILD and chronic interstitial lung disease (C-ILD) groups. Serum LGALS3BP levels were considerably higher in the RP-ILD group (80.1 ± 4.2 ng/mL) than in the C-ILD group (71.2 ± 11.6 ng/mL) and the non-ILD group (60.5 ± 16.9 ng/mL). Serum LGALS3BP levels were also significantly elevated in all patients with RP-ILD (Figure 4B). The ROC analysis showed that the optimal threshold for differentiating patients with IIM from those without ILD was 73.7 ng/mL, with a sensitivity of 54.5%, specificity of 82.5%, and AUC of 0.715 (P = 0.001) (Figure 4C).
Serum LGALS3BP Level Correlation with Disease Activity Indicators in Patients with IIM
Next, we assessed the relationship between serum LGALS3BP levels and disease activity in patients with IIM. There was a moderate correlation between serum LGALS3BP levels and albumin [r = −0.449, P < 0.001], globulin [r = 0.375, P = 0.001], LDH [r = 0.443, P < 0.001], CRP [r = 0.442, P < 0.001], ESR [r = 0.351, P = 0.001], and ferritin [r = 0.477, P < 0.001] and a strong correlation between serum LGALS3BP levels and IL-6 [r = 0.511, P < 0.001] (Table 4).
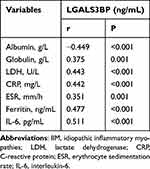 |
Table 4 Correlation Analysis of Serum LGALS3BP Levels and Laboratory Parameters in Patients with IIM |
Discussion
Proteomics, transcriptomics, and machine learning are evolving at an astonishing rate to provide a deeper understanding of the underlying pathophysiology of IIM. Integrated bioinformatics analysis and machine learning tools are increasingly used to explore potential diagnostic/prognostic biomarkers. We resorted to bioinformatics analysis methods to search for extracellular proteins that can assist in the identification of DM, ASS, IMNM, and HC, based on the R program “DESeq2” and the MCODE plugin of the PPI network and identified proteins encoded by three key genes (LGALS3BP, IFI35, and LGALS9) as candidate biomarkers. In a study by Bianchi,19 IFI35 was identified as a potential genetic risk locus for IIM, particularly in the DM subgroup, whereas ASS was not associated with IFI35. In a study by Liang,20 serum LGALS9 levels in patients with IIM were measured using ELISA, demonstrating that LGALS9 is an important marker for identifying patients with DM, IMNM, and HC. These findings are consistent with the results of the present study. We find no studies on LGALS3BP in myositis; therefore, we focused on LGALS3BP. Our study demonstrated that serum LGALS3BP levels were significantly elevated in patients with IIM. The differences in serum LGALS3BP levels were statistically significant in the DM, ASS, IMNM, and HC groups, except for those in DM and ASS groups, which were not significant. Unfortunately, the serum LGALS3BP levels were unable to identify DM or ASS, which is a limitation of this study. Taken together, serum LGALS3BP is an easily detectable extracellular protein, and the aberrant expression of serum LGALS3BP in IIM may provide additional evidence for its classification.
We found that LGALS3BP, IFI35, and LGALS9 were interferon (IFN)-regulated genes.21 In addition, pathway enrichment analysis of the 48 DAIH DEGs revealed a significant correlation with the interferon pathway. Differences in interferon (IFN) expression have been characterized in various IIM subtypes. Type 1 IFN are predominant in patients with DM. According to one report, IFN-I scores were significantly higher in patients with MDA5+ DM, and there was also a significant difference between IFN-I scores in patients with MDA5-DM and HC; however, there was no significant difference in IFN-I scores between the ASS, IMNM, and HC groups.22 Additionally, type I interferon-induced stimulated genes (ISGs) accounted for 21 of the 25 highly expressed genes in the skin of patients with DM.23 Patients with ASS were predominantly characterized by type 2 IFN pathway activation, whereas patients with IMNM showed low levels of interferon pathway activation regardless of the type.24,25 In summary, previous findings suggest that specific drugs tailored to each subtype can be explored based on differences in interferon expression in different myositis subtypes. Our data more precisely defined the targets in the pathway at the gene level, and inhibitors of the type 1 IFN pathway are currently being investigated for their use in patients with DM.4 Furthermore, drugs targeting LGALS3BP have been developed. Recently, LGALS3BP has been recognized as a viable target for antibody-drug conjugate (ADC) therapy, Anti-LGALS3BP ADC exhibits dose-dependent antitumor activity against neuroblastoma, oral squamous cell carcinoma, and adenoid cystic carcinoma, with no signs of toxicity.26–28 Drugs targeting LGALS3BP are mainly used in oncology research and provide new ideas for IIM drug research.
LGALS3BP has been extensively studied in tumors and is known to be involved in processes such as cell adhesion and metastasis.29,30 In addition to carcinogenesis, high LGALS3BP levels have been linked to several autoimmune disorders. Significantly elevated serum, urine, platelets, and circulating microvesicle LGALS3BP expression levels have been observed in patients with systemic lupus erythematosus (SLE). A positive association was also observed between disease activity and LGALS3BP levels.31–34 It has been reported that patients with inflammatory bowel disease (IBD) have higher serum levels of LGALS3BP, a novel predictive biomarker of responsiveness to infliximab treatment in IBD patients, which correlates positively with inflammatory markers, such as CRP.35 We observed similar results in patients with IIM with significantly elevated serum LGALS3BP levels. More importantly, we found that serum LGALS3BP levels in patients with IIM correlated with disease activity markers, including CRP. In addition, serum LGALS3BP has a good diagnostic value in patients with IIM, suggesting that LGALS3BP may be a promising marker for IIM disease activity. We must recognize that It is not possible to rely on a single indicator for subtype classification of IIM, assessment of myositis disease activity, or response to treatment. LGALS3BP can only assist in disease identification and assessment, and a definitive diagnosis of IIM subtypes requires a comprehensive analysis of the combination of clinical manifestations, laboratory and pathological data, and imaging findings.
In the present study, LGALS3BP was found to be involved in IIM-ILD pathogenesis, and this conclusion is based on the following two points. First, our study demonstrated that serum LGALS3BP levels were higher in patients with ILD than in those without ILD and in those with RP-ILD than in those with C-ILD. Secondly, several studies have reported that LGALS3 is highly expressed in various fibrotic tissues and is a marker of fibrosis. LGALS3 induces pulmonary fibrosis by promoting the activity of TGF-β, which is a crucial mediator of fibrosis.36 TD139, an inhibitor of LGALS3, has recently entered clinical trials. The antifibrotic potential of TD139 lies mainly growth inhibition of LGALS3-secreting macrophages and TGF-β impairment, which has been shown to slow the progression of fibrosis.37 Our study also revealed that LGALS3 was abnormally highly expressed in the IIM, which is consistent with the results reported by Watanabe.11 Interestingly, LGALS3BP is the most studied interaction partner of LGALS3, and is a possible candidate biomarker of fibrosis.
In our study, a strong correlation was observed between LGALS3BP and IL-6 levels. IL-6 is a significant contributor to DM-ILD and can indicate response to therapy in patients with DM.38 Increasing evidence suggests that IL-6 is produced by LGALS3BP stimulation. D2, a recombinant fragment of the LGALS3BP-binding region, stimulates IL-6 expression in colon and lung epithelial cell lines, and treatment with an anti-LGALS3BP monoclonal antibody significantly reduces the increase in IL-6 secretion following D2 stimulation.39 Furthermore, LGALS3BP stimulation of macrophages leads to a significant increase in IL-6 expression, particularly, in the presence of IFN-I.33 In conclusion, these data suggest that LGALS3BP stimulates IL-6 production, thereby participating in the inflammatory process in the IIM.
This study had some limitations that should be noted when interpreting our findings. First, there were limited datasets that contained DM, ASS, IMNM, and HC, and we found only one suitable dataset that should be validated using more datasets. Second, IIM is a rare disease, and the sample sizes of the included studies were relatively small. All patients were from a single center, and the results may be biased and require large-scale experiments for further validation. Third, LGALS3BP may be significantly elevated in IIM; however, we lacked a disease control group, and the results of this study could not definitively distinguish IIM from other diseases. Fourth, we excluded patients with concomitant tumors because LGALS3BP expression was elevated in most patients with tumors, so some antibodies were underrepresented, such as TIF1γ, and the probability of synchronous tumors was significantly higher in TIF1γ-positive patients.40,41 Finally, most of the data were obtained from cross-sectional studies of patients with DM. Future studies should focus on larger longitudinal cohorts of DM patients to assess the value of LGALS3BP as a predictive biomarker.
Conclusion
In summary, by combining bioinformatics analysis and ELISA results, we showed that LGALS3BP is differentially expressed in DM, ASS, IMNM, and HC. LGALS3BP is significantly elevated in IIM, correlates with inflammatory markers and ILD parameters, and maybe a promising marker for assessing IIM-ILD severity.
Compliance with Ethical Standards
The study was approved by the Ethics Committee of the first affiliated Hospital of Guangxi Medical University (approval number:2023-E756-01), and all patients signed an informed agreement.
Data Sharing Statement
Data will be made available on request.
Acknowledgments
We thank all the colleagues in our department for their kind cooperation in this article. We are very grateful to the patients and their families for their cooperation and for giving consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the Natural Science Foundation of Guangxi Zhuang Autonomous Region (2022GXNSFBA035544, 2022GXNSFAA035637), Health Department of Guangxi Zhuang Autonomous Region (S2017022), Scientific Research Projects of Health Commission of Guangxi Zhuang autonomous region (Z-A20220436), and the National Natural Science Foundation of China (81660275).
Disclosure
The authors report no conflicts of interest in this work. PREPRINT available at Research Square [https://doi.org/10.21203/rs.3.rs-4248146/v1].
References
1. Plotz PH, Rider Lg Fau - Targoff IN, Targoff In Fau Raben N, Raben N, Fau O’Hanlon TP, Tp Fau – Miller O. NIH conference. Myositis: immunologic contributions to understanding cause, pathogenesis, and therapy.
2. Tanboon J, Uruha A, Stenzel W, Nishino I. Where are we moving in the classification of idiopathic inflammatory myopathies? 1473–6551.
3. Mariampillai K, Granger B, Amelin D, et al. Development of a New Classification System for Idiopathic Inflammatory Myopathies Based on Clinical Manifestations and Myositis-Specific Autoantibodies. JAMA Neurol.
4. Connolly CM, Gupta L, Fujimoto M, Machado PM, Paik JJ. Idiopathic inflammatory myopathies: current insights and future frontiers. Lancet Rheumatol. 2665–9913.
5. Mammen AL, Allenbach Y, Stenzel W, Benveniste O
6. Zanframundo G, Selva-O’Callaghan A, González-Gay M, Montecucco C, Cavagna L. Issues in the classification of myositis patients: an ongoing process. Clini Experim Rheum. 2024. doi:10.55563/clinexprheumatol/8u8p8x
7. Lundberg IA-O, Fujimoto MA-O, Vencovsky JA-O, et al. Idiopathic inflammatory myopathies.
8. Schmidt J Current Classification and Management of Inflammatory Myopathies. 2214–3599.
9. White MJ, Roife D, Gomer RH Galectin-3 Binding Protein Secreted by Breast Cancer Cells Inhibits Monocyte-Derived Fibrocyte Differentiation.
10. Inohara H, Raz A. Identification of human melanoma cellular and secreted ligands for galectin-3. Biochem Bioph Rese Commu. 1994;201:1366–1375. doi:10.1006/bbrc.1994.1854
11. Watanabe E, Kato K, Gono T, Chiba E, Terai C, Kotake S. Serum levels of galectin-3 in idiopathic inflammatory myopathies: a potential biomarker of disease activity. J Immun. 2020;204. doi:10.4049/jimmunol.1900681
12. Chen C, Wu Y, Li J, et al. TBtools-II: a “one for all, all for one”. Bioinfo Platform Biol Big-Data Mining. 1752–9867.
13. Zhou X, Zhang Y, Wang N. Systematic identification of key extracellular proteins as the potential biomarkers in lupus nephritis. 1664–3224.
14. Bohan A F, Peter JB, Peter JB Polymyositis and dermatomyositis (first of two parts). 0028–4793.
15. Bohan A F, Peter JB, Peter JB Polymyositis and dermatomyositis (second of two parts). 0028–4793.
16. Travis WD, Costabel U, Hansell DM, et al. An Official American Thoracic Society/European Respiratory Society Statement: update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am J Resp Crit Care. 2013;188(6):733–748. doi:10.1164/rccm.201308-1483ST
17. Douglas WW, Tazelaar Hd F, Hartman TE, et al. Polymyositis-dermatomyositis-associated interstitial lung disease. (1073–449X.
18. Ida TA-OX, Furuta S, Takayama A, et al. Efficacy and safety of dose escalation of tofacitinib in refractory anti-MDA5 antibody-positive dermatomyositis. LID. doi:10.1136/rmdopen-2022-002795
19. Bianchi MA-O, Kozyrev SA-O, Notarnicola AA-O, et al. Contribution of Rare Genetic Variation to Disease Susceptibility in a Large Scandinavian Myositis Cohort. Arthritis Rheumatol. 2022
20. Liang L, Zhang YM, Shen YW, et al. Aberrantly Expressed Galectin-9 Is Involved in the Immunopathogenesis of Anti-MDA5-Positive Dermatomyositis-Associated Interstitial Lung Disease. Front Cell Develop Biol.
21. Samarajiwa SA, Forster S, Fau - Auchettl K, Auchettl K, Fau - Hertzog PJ. INTERFEROME: the database of interferon regulated genes. 1362–4962.
22. Qian J, Lu L, Fu Q, et al. Type I interferon score is associated with the severity and poor prognosis in anti-MDA5 antibody-positive dermatomyositis patients. Sensors (Basel, Switzerland). 2013;13(2):1664–3224. doi:10.3390/s130201664
23. Wong D, Kea B, Fau - Pesich R, et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. 1932–6203.
24. Pinal-Fernandez I, Casal-Dominguez M, Derfoul A, et al. Identification of distinctive interferon gene signatures in different types of myositis. 1526.
25. Bolko LA-O, Jiang W, Tawara N, et al. The role of interferons type I. II III myositis. 1750–3639.
26. Dufrusine BA-O, Capone E, Ponziani S, et al. Extracellular LGALS3BP: a potential disease marker and actionable target for antibody-drug conjugate therapy in glioblastoma. Mole Oncolo
27. Cela IA-O, Caponio VA-O, Capone E, et al. LGALS3BP is a potential target of antibody-drug conjugates in oral squamous cell carcinoma. 1601–1825.
28. Capone E, Perrotti V, Cela I, et al. Anti-LGALS3BP antibody-drug conjugate treatment induces durable and potent antitumor response in a preclinical model of adenoid cystic carcinoma. Oral Oncol. 2024.
29. Choi YS, Kim MJ, Choi EA, et al. Antibody-mediated blockade for galectin-3 binding protein in tumor secretome abrogates PDAC metastasis. Chemosphere. 2012;87(10):1091–6490. doi:10.1016/j.chemosphere.2012.02.003
30. Song Y, Wang M, Tong H, et al. Plasma exosomes from endometrial cancer patients contain LGALS3BP to promote endometrial cancer progression. Oncogene. 2021.
31. Peretz ASR, Rasmussen NS, Jacobsen S, Sjöwall C, Nielsen CT. Galectin-3-binding protein is a novel predictor of venous thromboembolism in systemic lupus erythematosus. Clin Exp Rheumatol. 2021;39(6):1360–1368. doi:10.55563/clinexprheumatol/ol0vqj
32. Ding H, Shen Y, Lin C, et al. Urinary galectin-3 binding protein (G3BP) as a biomarker for disease activity and renal pathology characteristics in lupus nephritis. 1478–6362.
33. El Bannoudi HA-O, Cornwell MA-O, Luttrell-Williams E, et al. Platelet LGALS3BP as a Mediator of Myeloid Inflammation in Systemic Lupus Erythematosus. 2326–5205.
34. Rasmussen NS, Draborg AH, Houen G, Nielsen CT. Human herpesvirus infections and circulating microvesicles expressing galectin-3 binding protein in patients with systemic lupus erythematosus. Clinical and Experimental Rheumatology. 2022;40(1):158–161. doi:10.55563/clinexprheumatol/s364rt
35. Pesole PA-O, Liso MA-O, Donghia RA-OX, et al. 90K/Mac-2 BP Is a New Predictive Biomarker of Response to Infliximab Therapy in IBD Patients. Int J Mol Sci. 3955; doi:10.3390/ijms24043955
36. Mackinnon AC, Gibbons Ma Fau - Farnworth SL, Farnworth Sl Fau - Leffler H, et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. 1535–4970.
37. Hirani N, MacKinnon AA-O, Nicol L, et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. LID. doi:10.1183/13993003.02559-2020
38. Kogami MA-OX, Abe Y, Ando T, Makiyama A, Yamaji K, Tamura NA-O. Changes in anti-MDA5 antibody titres and serum cytokine levels before and after diagnosis of anti-MDA5 antibody-positive dermatomyositis.
39. Mendes-Frias A, Gallo V, Iacobelli V, et al. Galectin-3 binding protein stimulated IL-6 expression is impeded by antibody intervention in SARS-CoV-2 susceptible cell lines. ScienRepor. 2045–2322.
40. NTM L, Ma N, Ueda-Hayakawa I, et al. Clinical and laboratory parameters predicting cancer in dermatomyositis patients with anti-TIF1γ antibodies. J Derma Scie. 2021. 1873–569X.
41. De Vooght J, Vulsteke JB, De Haes P, Bossuyt X, Lories R, De Langhe E. Anti-TIF1-γ autoantibodies: warning lights of a tumour autoantigen. Rheumatolo. 2020.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Risk Factors and Predictive Model for Dermatomyositis Associated with Rapidly Progressive Interstitial Lung Disease
Wang K, Tian Y, Liu S, Zhang Z, Shen L, Meng D, Li J
Pharmacogenomics and Personalized Medicine 2022, 15:775-783
Published Date: 1 September 2022
Sarcoplasmic Myxovirus Resistance Protein A: A Study of Expression in Idiopathic Inflammatory Myopathy
Waisayarat J, Wongsuwan P, Tuntiseranee K, Waisayarat P, Dejthevaporn C, Khongkhatithum C, Soponkanaporn S
Journal of Inflammation Research 2023, 16:5417-5426
Published Date: 20 November 2023
Immune-Related Genes Associated with Interstitial Lung Disease in Dermatomyositis
Liu C, Ge Y
International Journal of General Medicine 2024, 17:5261-5271
Published Date: 14 November 2024
Clinical Features and Prognosis of Double-Positive Anti-MDA5 and Anti-CCP Antibodies in Dermatomyositis: A Retrospective Study
Xu X, Zhu L, Li S, Wang G, Ge Y
Journal of Inflammation Research 2025, 18:1929-1939
Published Date: 10 February 2025





