Back to Journals » Cancer Management and Research » Volume 17
Clinical Significance of the Peripheral Blood Neutrophil-to-Lymphocyte Ratio in Predicting Chemotherapy Outcomes for Small Cell Lung Cancer
Received 22 October 2024
Accepted for publication 9 January 2025
Published 22 January 2025 Volume 2025:17 Pages 113—119
DOI https://doi.org/10.2147/CMAR.S502242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Chien-Feng Li
Li Gao, Bin Liu
Department of Respiratory and Critical Care Medicine, Fuyang People’s Hospital, Fuyang, 236000, People’s Republic of China
Correspondence: Li Gao, Department of Respiratory and Critical Care Medicine, Fuyang People’s Hospital, No. 501, Sanqing Road, Qinghe Street, Yingzhou District, Fuyang, 236000, People’s Republic of China, Tel +86 0558-3010308, Email [email protected]
Objective: This study aims to assess the clinical significance of the peripheral blood neutrophil-to-lymphocyte ratio (NLR) in predicting chemotherapy outcomes for patients with small cell lung cancer (SCLC).
Methods: A cohort of 44 patients diagnosed with SCLC between January 2021 to June 2022 at Fuyang People’s Hospital was selected for analysis. All patients in this group received a first-line platinum-based doublet chemotherapy regimen. In parallel, a control group consisting of 44 healthy individuals undergoing routine physical examinations at the same hospital was also selected. Fasting venous blood samples were collected in the morning within one week before the initiation of chemotherapy, and a complete blood cell count was performed to calculate the NLR.
Results: The NLR in the plasma of patients with SCLC was significantly elevated compared to that of healthy individuals (P < 0.01). After two cycles of chemotherapy, there were no statistically significant differences in plasma NLR in SCLC patients compared to pre-chemotherapy levels (P > 0.05). However, in the subgroup of patients with a partial response (PR) to treatment, the NLR decreased to 2.625 (95% CI: 1.900, 3.625), down from a pre-chemotherapy level of 3.430 (2.688, 4.800) (Z = − 3.127, P = 0.002). Conversely, in patients whose disease progressed (PD) following chemotherapy, the NLR increased to 3.880 (95% CI: 2.953, 5.223) from a pre-chemotherapy level of 2.060 (1.915, 2.968) (Z = − 2.521, P = 0.012).
Conclusion: The dynamic variations in the peripheral blood NLR before and after chemotherapy in patients with SCLC are strongly associated with the efficacy of first-line chemotherapy regimens. These changes in NLR levels may serve as a crucial indicator for predicting the effectiveness of first-line chemotherapy in patients with SCLC.
Keywords: chemotherapy effectiveness, efficacy prediction, inflammatory response, neutrophil-to-lymphocyte ratio, small cell lung cancer
Introduction
Lung cancer remains a significant global public health challenge, marked by high incidence and mortality rates. In 2018, an estimated 2.1 million new cases of lung cancer were reported worldwide, accounting for 11.6% of all cancer diagnoses.1 In China, lung cancer has surpassed liver cancer as the leading cause of cancer-related deaths since 2008, with small cell lung cancer (SCLC) representing approximately 15% of all lung cancer cases. SCLC is characterized by rapid proliferation and early distant metastasis, with most patients presenting with hematogenous spread at the time of diagnosis, and only about one-third being diagnosed in the limited stage. Despite SCLC’s high sensitivity to radiotherapy and chemotherapy, the disease is prone to recurrence and metastasis, resulting in a 5-year survival rate of only 10% for patients with extensive-stage disease.2 Consequently, identifying biomarkers that can predict the efficacy of chemotherapy in SCLC is crucial for enabling precise and individualized treatment strategies for patients with lung cancer.
Inflammation plays a critical role in cancer progression, and the neutrophil-to-lymphocyte ratio (NLR) has emerged as a valuable indicator of systemic inflammatory response, providing important insights into the inflammatory and immune status of patients. NLR has been recognized as a prognostic biomarker across various cancer types, including urothelial tumors, prostate cancer, and non-small cell lung cancer (NSCLC).3–5 Previous studies related to SCLC have discussed the impact of NLR (Neutrophil-to-Lymphocyte Ratio). A retrospective study involving 139 SCLC patients showed that a high NLR is associated with poorer overall survival (OS).6 Another study on extensive-stage SCLC indicated that patients receiving first-line chemotherapy with an NLR < 3 could benefit in terms of progression-free survival (PFS).7 However, there is a paucity of research on the utility of NLR in predicting the effectiveness of first-line chemotherapy for SCLC. This study seeks to address this gap by conducting a retrospective analysis of 44 patients with SCLC, aiming to evaluate the predictive value of NLR in determining the efficacy of first-line chemotherapy in this patient population.
Materials and Methods
Clinical Data
A retrospective analysis was conducted on 44 patients diagnosed with SCLC who were treated at Fuyang People’s Hospital between January 2021 and June 2022. The cohort included 32 males and 12 females, with a mean age of 66.27 ± 8.48 years. All patients received first-line platinum-based doublet chemotherapy, and treatment efficacy was assessed following the completion of two chemotherapy cycles.
The inclusion criteria were: (1) treatment-naive patients diagnosed with SCLC confirmed by histopathological examination; (2) a performance status (PS) score of ≤ 2, with no significant abnormalities in complete blood count or liver and kidney function, and the ability to tolerate first-line chemotherapy for SCLC; (3) availability of complete blood count results for NLR evaluation, with blood collection conducted within one week before treatment; (4) an expected survival time exceeding three months; and (5) voluntary signed informed consent for chemotherapy and agreement to follow-up.
The exclusion criteria were: (1) patients with severe infections before admission; (2) individuals with impaired cardiac function or severe hepatic or renal dysfunction; (3) histopathological examination results indicating NSCLC; (4) patients with two or more concurrent malignancies; (5) those with comorbid hematological disorders; (6) individuals with immune system diseases; (7) patients with hepatitis B or C virus infection; and (8) those with a history of long-term steroid therapy.
During the same period, 44 healthy individuals were recruited from the physical examination center to serve as the healthy control group. This group included 33 males and 11 females, with a mean age of 65.23 ± 8.76 years. There were no significant differences in gender distribution or age between the patient and control groups (χ² = 0.059, P = 0.808; t = 0.569, P = 0.571). All participants, or their authorized representatives, provided signed informed consent, and the study adhered to the ethical guidelines outlined in the Helsinki Declaration of the World Medical Association.
Detection Methods
Within one week before the initiation of chemotherapy and again before the third cycle, 3 mL of fasting peripheral venous blood was collected from patients in the lung cancer group each morning. Similarly, healthy control subjects provided 3 mL of fasting venous blood on the day of their physical examination. All blood samples were placed in vacuum tubes with anticoagulant, mixed thoroughly, and analyzed within 4 hours. Blood cell parameters were measured using the BC-6900 hematology analyzer and associated reagents from Mindray. The NLRs were subsequently calculated for each group.
Efficacy Evaluation
The efficacy of chemotherapy was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST), classifying outcomes into four categories: complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD).
Observation Indicators
The NLR values for all subjects, as well as the efficacy of first-line chemotherapy in patients with SCLC, were as follows: Baseline NLR (NLR0) was recorded before the initiation of chemotherapy, while the NLR after two cycles of first-line chemotherapy (NLR2) was documented subsequently. The NLR values for the healthy control group were also measured. The effectiveness of the first-line chemotherapy was assessed based on the efficacy evaluation results obtained after two cycles of treatment.
Statistical Methods
Data were analyzed using SPSS 20.0 statistical software. Normally distributed quantitative data were expressed as the mean ± standard deviation 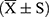 , while non-normally distributed data were reported as the median (interquartile range) [M (P25, P75)]. Comparisons between two groups were performed using the t-test for normally distributed data with homogeneity of variance, and the paired t-test was used to compare differences before and after chemotherapy within the same group. For non-normally distributed data or data with heterogeneity of variance, the Wilcoxon signed-rank test was applied. A P < 0.05 was considered statistically significant.
, while non-normally distributed data were reported as the median (interquartile range) [M (P25, P75)]. Comparisons between two groups were performed using the t-test for normally distributed data with homogeneity of variance, and the paired t-test was used to compare differences before and after chemotherapy within the same group. For non-normally distributed data or data with heterogeneity of variance, the Wilcoxon signed-rank test was applied. A P < 0.05 was considered statistically significant.
Results
Correlation Between Plasma NLR Levels and Clinical Characteristics in Patients With SCLC
In the SCLC group, no significant correlation was observed between plasma NLR levels and variables such as age, sex, smoking status, or TNM stage (P > 0.05), as shown in Table 1.
 |
Table 1 Correlation Between Plasma NLR Levels and Clinical Characteristics in Patients With SCLC |
Differences in Plasma NLR Levels Between Patients With SCLC and Healthy Individuals
The average NLR value in the 44 patients with SCLC was significantly higher compared to that in healthy individuals, measuring 3.055 (2.155, 4.588) versus 1.915 (1.490, 2.243), with a P < 0.01, as shown in Table 2.
 |
Table 2 Comparison of Plasma NLR Levels Between Patients With SCLC and Healthy Controls |
Differences in Plasma NLR Levels Before and After Chemotherapy in Patients With SCLC
The NLR levels in patients with SCLC were compared before and after two cycles of first-line chemotherapy. The results indicated that there was no significant difference in the average NLR values before chemotherapy [3.055 (2.155, 4.588)] and after chemotherapy [2.970 (2.178, 3.760)], among the 44 patients with SCLC (P > 0.05). Further details are shown in Table 3.
 |
Table 3 Comparison of NLR Levels Before and After Chemotherapy in Patients With SCLC |
Correlation Analysis Between Dynamic Changes in Plasma NLR and Therapeutic Efficacy
Among the 44 patients with SCLC who were followed up after two cycles of chemotherapy, the response distribution was as follows: 0 cases of CR, 36 cases of PR, 0 cases of SD, and 8 cases of PD.
A comparison of NLR changes before and after chemotherapy was conducted within the PR and PD subgroups. In the PR subgroup, the post-chemotherapy NLR was 2.625 (1.900, 3.625), which was significantly lower than the pre-chemotherapy NLR of 3.430 (2.688, 4.800) (P = 0.002). Conversely, in the PD subgroup, the post-chemotherapy NLR was 3.880 (2.953, 5.223), significantly higher than the pre-chemotherapy NLR of 2.060 (1.915, 2.968) (P = 0.012). Detailed results are shown in Table 4.
 |
Table 4 Comparison Between Fluctuations in NLR Levels and Treatment Efficacy |
Discussion
It is well established that inflammation plays a crucial role in the initiation and progression of various cancers, with systemic inflammation often correlating with poorer patient prognosis. Chronic inflammation can disrupt the regulation of multiple cytokines and chemokines, resulting in unregulated cell repair and impaired apoptosis, which can drive tumorigenesis. Research indicates that tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs) contribute to the inflammatory milieu of tumors, facilitating tissue remodeling, angiogenesis, and metastatic spread in several cancer types.8,9
The NLR, calculated as the ratio of neutrophil to lymphocyte counts, functions as a peripheral hematological marker indicative of systemic inflammation. An elevated NLR indicates a more pronounced inflammatory response, either due to elevated neutrophil counts or decreased lymphocyte counts. Elevated NLR has been associated with poorer prognoses in various solid tumors.10,11 The underlying mechanisms involve several factors: neutrophils secrete factors such as interleukin-8 (IL-8), vascular endothelial growth factor (VEGF), and elastase, which promote tumor growth and metastasis. Conversely, lymphocytes, which are pivotal in anti-tumor immunity, are typically reduced in patients with suppressed or deficient immune systems. Additionally, neutrophils have been shown to inhibit the quantity and function of lymphocytes and natural killer cells in vitro, contributing to immune suppression.12
Research into the peripheral blood NLR as a systemic inflammation marker in cancer patients is expanding, with NLR increasingly recognized as a significant indicator of systemic immune responses associated with inflammation and malignancy. Due to its rapid, widespread, and cost-effective detection, NLR has been extensively studied in lung cancer, particularly NSCLC.13–16 The meta-analysis by Yu et al indicated that an elevated NLR (Neutrophil-to-Lymphocyte Ratio) predicts poorer overall survival (OS) and progression-free survival (PFS) in lung cancer patients, suggesting that a higher NLR (for example, ≥4) could be considered a biomarker for poor prognosis in lung cancer patients.15 The meta-analysis by Yin et al, which included 14 studies and 2734 lung cancer cases, demonstrated that an elevated NLR (Neutrophil-to-Lymphocyte Ratio) predicts poorer overall survival (OS), supporting the notion that NLR is a good prognostic biomarker.16 However, there is a comparative paucity of studies focusing on SCLC.17,18 Pan et al have identified NLR as an independent predictor of progression-free survival (PFS) and overall survival (OS) in SCLC.19 Regarding whether NLR can be used to evaluate the therapeutic effects of SCLC, and to assess the recurrence or progression of SCLC, there is even less research. To further explore the value of NLR in predicting the efficacy of first-line chemotherapy for SCLC, this study conducted a retrospective analysis involving 44 treatment-naïve patients with SCLC. The results demonstrated that NLR levels in patients with SCLC were significantly higher compared to healthy individuals, aligning with Zhu et al’s findings that suggest NLR may be a useful biomarker for lung cancer diagnosis.20
In our study, we examined the relationship between NLR levels and clinical parameters in patients with SCLC. The analysis revealed no significant association between NLR and fundamental tumor characteristics such as gender, age, smoking history, or TNM stage. When evaluating the efficacy of first-line platinum-based doublet chemotherapy, no significant difference was observed between the pre-chemotherapy NLR (NLR0) and the NLR after two cycles of treatment (NLR2). However, subgroup analysis revealed that, in the PR subgroup, post-chemotherapy NLR was significantly lower than pre-chemotherapy NLR (P = 0.002), whereas, in the PD subgroup, post-chemotherapy NLR was significantly higher than pre-chemotherapy NLR (P = 0.012). These findings suggest that dynamic changes in NLR may reflect the effectiveness of chemotherapy. In the PD subgroup, the increase in NLR levels may be associated with tumor-induced inflammation, immune suppression, increased myelopoiesis, and tumor progression. In contrast, in the PR subgroup, effective chemotherapy may reduce tumor burden and inflammation, potentially restoring lymphocyte function, leading to a decrease in NLR levels. These changes reflect the complex interplay between the tumor microenvironment and the host immune response.16 The results of this study are consistent with those of Kang et al, who found that NLR levels significantly increased during disease progression in SCLC compared to after one cycle of chemotherapy, but did not analyze the differences in outcomes for patients who achieved remission. This study also found that after two cycles of chemotherapy, the NLR of patients who achieved remission was lower than before chemotherapy.21 Pan et al found that NLR could also help in selecting the optimal first-line treatment regimen, with patients having high NLR before treatment potentially benefiting more from EP therapy rather than EC therapy. This study did not statistically analyze the first-line chemotherapy regimens of EP or EC, which could be further analyzed in the future.19 Suh et al also observed that an NLR < 5 indicated better efficacy following 6 weeks of PD-1 antibody therapy in patients with NSCLC.22 These results suggest that NLR is a readily available and effective measurement method that may reflect tumor burden and the prognosis of patients with small cell lung cancer. Additionally, it can be helpful in assessing treatment responses and monitoring the recurrence or progression of small cell lung cancer. Thus, dynamic changes in NLR might be associated with the efficacy of first-line chemotherapy in SCLC, offering potential insights into chemotherapy effectiveness and patient prognosis.
However, this study has limitations, including a small sample size and a relatively short follow-up period with infrequent assessments. Future prospective studies with larger sample sizes and extended follow-up are needed to further validate these findings and establish the clinical utility of NLR as a predictive biomarker for chemotherapy response and prognosis in SCLC.
Conclusion
This study confirms that NLR is significantly elevated in SCLC patients compared to healthy individuals and correlates with the efficacy of first-line chemotherapy. As a simple and easily measured indicator of systemic inflammation and immune status, NLR shows potential for broader clinical use in SCLC management.
Abbreviation
NLR, neutrophil to lymphocyte ratio; SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; RECIST, response evaluation criteria in solid tumors; PD, progressive disease; SD, stable disease; PR, partial remission; CR, complete remission; TAM, tumor-associated macrophages; TANs, tumor-associated neutrophils; IL-8, Interleukin-8 VEGF, vascular endothelial growth factor; PFS, Progression-Free Survival; OS, overall survival; PD-1, programmed cell death protein-1.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Fuyang People’s Hospital (Approval Number: YLLSC [2024] 103). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Funding
No external funding was received to conduct this study.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current diagnosis and management of small-cell lung cancer. Mayo Clinic Proceedings. 2019;94(8):1599–1622. doi:10.1016/j.mayocp.2019.01.034
3. Suh J, Jung JH, Jeong CW, Kwak C, Kim HH, Ku JH. Clinical significance of pre-treated neutrophil-lymphocyte ratio in the management of urothelial carcinoma: a systemic review and meta-analysis. Front Oncol. 2019;9:1365. doi:10.3389/fonc.2019.01365
4. Guan Y, Xiong H, Feng Y, Liao G, Tong T, Pang J. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: a meta-analysis. Prostate Cancer Prostatic Dis. 2020;23(2):220–231. doi:10.1038/s41391-020-0209-3
5. Bryant AK, Sankar K, Strohbehn GW, et al. Prognostic and predictive value of neutrophil-to-lymphocyte ratio with adjuvant immunotherapy in stage III non-small-cell lung cancer. Lung Cancer. 2022;163:35–41. doi:10.1016/j.lungcan.2021.11.021
6. Liu D, Huang Y, Li L, Song J, Zhang L, Li W. High neutrophil-to-lymphocyte ratios confer poor prognoses in patients with small cell lung cancer. BMC Cancer. 2017;17(1):882. doi:10.1186/s12885-017-3893-1
7. Sakin A, Sahin S, Yasar N, Demir C, Arici S, Geredeli C. Cihan S.The relation between hemogram parameters and survival in extensive-stage small cell lung cancer. Oncol Res Treat. 2019;42(10):506–515. doi:10.1159/000501595
8. Nagahashi M, Abe M, Sakimura K, Takabe K, Wakai T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018;109(12):3671–3678. doi:10.1111/cas.13802
9. Yang LY, Luo Q, Lu L, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13(1):3. doi:10.1186/s13045-019-0836-0
10. Spiegel A, Brooks MW, Houshyar S, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6(6):630–649. doi:10.1158/2159-8290.CD-15-1157
11. Kumarasamy C, Tiwary V, Sunil K, et al. Prognostic utility of platelet-lymphocyte ratio, neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio in head and neck cancers: a detailed PRISMA compliant systematic review and meta-analysis. Cancers. 2021;13(16):4166. doi:10.3390/cancers13164166
12. Mishra V, Giri R, Hota S, Senapati U, Sahu SK. Neutrophil-to-lymphocyte ratio as a prognostic factor in oral squamous cell carcinoma - A single-institutional experience from a developing country. J Oral Maxillofac Pathol. 2021;25(2):322–326. doi:10.4103/0973-029X.325235
13. Nakaya A, Kurata T, Yoshioka H, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23(4):634–640. doi:10.1007/s10147-018-1250-2
14. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–894. doi:10.21037/tlcr.2019.11.16
15. Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: a meta-analysis of 7,219 patients. Mol Clin Oncol. 2017;7(3):498–506. doi:10.3892/mco.2017.1342
16. Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta-analysis. Clinics. 2015;70(7):524–530. doi:10.6061/clinics/2015(07)10
17. Chen C, Yang H, Cai D, Xiang L, Fang W, Wang R. Preoperative peripheral blood neutrophil-to-lymphocyte ratios (NLR) and platelet-to-lymphocyte ratio (PLR) related nomograms predict the survival of patients with limited-stage small-cell lung cancer. Transl Lung Cancer Res. 2021;10(2):866–877. doi:10.21037/tlcr-20-997
18. Liu F, Zhou S, Tan L, Jiang H, Huang Y. A retrospective cohort study on pretreated neutrophil-to-lymphocyte ratio and prognosis of small cell lung cancer: evidence of effect modification by chemotherapy regimen. Cancer Manag Res. 2020;12:10341–10352. doi:10.2147/CMAR.S263863
19. Pan Z, Zhang L, Liu C, et al. Cisplatin or carboplatin? Neutrophil to lymphocyte ratio may serve as a useful factor in small cell lung cancer therapy selection. Oncol Lett. 2019;18(2):1513–1520. doi:10.3892/ol.2019.10459
20. Zhu X, Song H, Chen Y, Han F, Wang Q, Cui Y. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in blood to distinguish lung cancer patients from healthy subjects. Dis Markers. 2020;2020:8844698. doi:10.1155/2020/8844698
21. Kang MH, Go SI, Song HN, et al. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer. 2014;111(3):452–460. doi:10.1038/bjc.2014.317
22. Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459–470. doi:10.1007/s00262-017-2092-x
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

