Back to Journals » Journal of Inflammation Research » Volume 18
Clinical Significance of the Serum 3′tRF-AlaAGC, Neutrophil to High-Density Lipoprotein Ratio, and Lymphocyte-to-Monocyte Ratio in Breast Cancer with Lymph Node Metastasis
Authors Mo D , Zhu Y, Mao X, Li C, Yang Y, Zheng J, Yan F
Received 23 January 2025
Accepted for publication 26 April 2025
Published 7 May 2025 Volume 2025:18 Pages 5979—5991
DOI https://doi.org/10.2147/JIR.S518232
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qing Lin
Dongping Mo,1,2 Yurong Zhu,1 Xuelian Mao,1 Cong Li,1 Yining Yang,1 Junyu Zheng,1 Feng Yan1,2
1Department of Clinical Laboratory, Jiangsu Cancer Hospital & Nanjing Medical University, Affiliated Cancer Hospital & Jiangsu Institute of Cancer Research, Nanjing, People’s Republic of China; 2Jiangsu Key Laboratory of Molecular and Translational Cancer Research, Nanjing, People’s Republic of China
Correspondence: Dongping Mo, Department of Clinical Laboratory, Jiangsu Cancer Hospital & Nanjing Medical University, Affiliated Cancer Hospital & Jiangsu Institute of Cancer Research, Baizi Ting No. 42, Nanjing, Jiangsu, 210009, People’s Republic of China, Email [email protected] Feng Yan, Department of Clinical Laboratory, Jiangsu Cancer Hospital & Nanjing Medical University, Affiliated Cancer Hospital & Jiangsu Institute of Cancer Research, Baizi Ting No. 42, Nanjing, Jiangsu, 210009, People’s Republic of China, Email [email protected]
Objective: Breast cancer (BC) is a common malignant tumor among women, the local recurrence, lymph node metastasis (LNM), and distant metastasis are the key factors affecting the prognosis of patients. tRNA-derived small RNAs (tDRs) are non-coding small RNA fragments [16– 40 nucleotides (nt) in length] that play an important role in carcinogenesis and can serve as novel biological markers for the diagnosis and prognosis of various tumors. Accumulating evidence suggests that blood-based inflammatory indicators are linked with the pathogenesis of BC. However, the clinical significance of the combination of tDRs and inflammatory indicators in BC patients with LNM is still unclear.
Methods: The serum samples were collected from 175 patients with BC admitted to our hospital during June 2021 and May 2024, and 94 age-matched healthy women, and the clinical data of the research subjects were recorded. Serum 3′tRF-AlaAGC levels were measured using quantitative real-time PCR (qRT-PCR) and the blood-based inflammatory indicators were calculated from peripheral blood samples. Lasso-cox regression and multiple logistic regression were employed for variable selection. Receiver operating characteristic (ROC) was used to calculate the cut-off value of variables. Spearman correlation test was used to examine the correlation between 3′tRF-AlaAGC levels and neutrophil to HDL-C ratio (NHR), lymphocyte-to-monocyte ratio (LMR). A nomogram model for risk assessment of LNM in BC was established by using the rms package of R software.
Results: Serum 3′tRF-AlaAGC levels in BC patients with LNM were significantly higher than that in without LNM [5.17 (1.79, 16.55) vs 11.68 (2.64, 58.74), P=0.009]. The variables screened by Lasso-cox regression including 3′tRF-AlaAGC, NHR and LMR, with optimal cut-off values of 18.78, 2.94 and 5.41, respectively. NHR levels were significantly negatively associated with LMR in low 3′tRF-AlaAGC expression groups (r=− 0.209, P=0.021). Multivariate logistic regression analysis confirmed that 3′tRF-AlaAGC (OR: 3.242, 95% CI: 1.583– 6.641, P=0.001), NHR (OR: 3.305, 95% CI: 1.543– 7.079, P=0.002), and LMR (OR: 0.329, 95% CI: 0.150– 0.723, P=0.006) were independent risk factors of BC with LNM. The C-statistic of the nomograms model was 0.704, with a sensitivity of 57.14% and a specificity of 77.14%.
Conclusion: 3′tRF-AlaAGC > 18.78, NHR > 2.94, and LMR ≤ 5.41 were the independent risk factors of BC with LNM. The nomogram model incorporating 3′tRF-AlaAGC, NHR and LMR can effectively predict the risk of LNM of BC patients.
Keywords: 3′tRF-Ala-AGC, neutrophil to high-density lipoprotein ratio, NHR, lymphocyte-to-monocyte ratio, LMR, lymph node metastasis, breast cancer
Introduction
Breast cancer (BC) is the most common malignant tumor and the second main cause of cancer-related deaths in women worldwide.1 Recent data have suggested that the incidence rate of BC increased during 2015–2019 by 0.6% - 1% annually, accounting for approximately 32% of new cases and 15% of deaths from female cancers.2 For about half a century, in the field of clinical oncology, in addition to traditional surgery, radiotherapy and chemotherapy, new treatments for cancer targeting the tumor immune microenvironment have also rapidly developed.3 Despite this, more than 19% of BC patients still have recurrence and metastasis 3 years after surgery, and the 5-year survival rate for metastatic is only 14%.4 Lymph nodes are parts of the locoregional metastasis and the main doorway for tumor cell escape from the primary site to other regions of the body.5 Patients diagnosed with lymph node metastasis (LNM) are prone to distant metastasis and have poorer overall survival rate compared to those without LNM. However, the early-stage BC is potentially curable.6 Therefore, there is an urgent need for early detection and discovery of new therapeutic targets to reduce the metastasis rate and mortality of BC.7
tRNA-derived small RNAs (tDRs) are small non-coding RNA molecules produced by specific nucleases, such as Dicer, ELAC2/RNase Z, and angiogenin. It is derived from specific cleavage of precursors or mature tRNA and can participate in various tumorigenesis and development.8,9 In addition, the increasing evidence reveals that tDRs are abnormally expressed in metabolic diseases, neurological diseases, and malignant tumor.10 Specifically, research has suggested that i-tRF-AsnGTT has a low and stable expression level in gastric cancer (GC), which can differentiate between patients with GC and gastritis and healthy donors with better diagnostic efficacy,11 while serum tRF31-79MP9P9NH57SD appears to be overexpressed in non-small cell lung cancer (NSCLC) patients and related to both clinical stage and lymph node metastasis.12 Moreover, tRF-33-RZYQHQ9M739P0J was highly expressed in hepatocellular carcinoma (HCC), and its expression was correlated with metastasis, tumor-node-metastasis (TNM) stage, Barcelona Clinic Liver Cancer (BCLC) stage, and vein invasion. High serum tRF-33-RZYQHQ9M739P0J levels are associated with low survival rates.13 Our previous study identified that a 3′tRF fragment, 3′tRF-AlaAGC, was significantly overexpressed in the serum of BC patients, and higher expression of 3′tRF-AlaAGC was associated with TNM stage and LNM.14 Therefore, it is reasonable to believe that 3′tRF-AlaAGC can serve as candidate molecular marker for monitoring BC.
Over the past decade, inflammation has been demonstrated to play essential role in the process of carcinogenesis.15 Chronic inflammation promotes the initiation and progression of cancer by altering the tumor microenvironment and activating endogenous or exogenous signaling involving multiple inflammatory mediators and proteins.16 Several biomarkers for measuring systemic inflammation such as, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), LMR, systemic immune-inflammation index (SII) have been proved to be critical progression indicators of many cancers, including bladder cancer, urothelial carcinoma, and colorectal cancer.17–19 Recent researches have confirmed that high-density lipoprotein cholesterol (HDL-C) plays a crucial role in anti-inflammatory, anti-oxidation and anti-apoptotic functions.20,21 Reduced HDL-C levels have been shown to be associated with poor prognosis in several disease. The neutrophil to HDL-C ratio (NHR), platelet to HDL-C ratio (PHR), and monocyte to HDL-C ratio (MHR) have emerged as prognostic biomarker in cardiovascular events, cancer, and metabolic syndrome.22–25 It is important that these biomarkers could be easily measured through the routine blood tests of cancer patients. Despite this, research on the clinical potential significance of these inflammatory markers in BC patients with LNM is limited. Furthermore, to date, there has been little research on the combined diagnostic accuracy of inflammatory markers and other serum tumor biomarkers in BC.
This is the first study to simultaneously detect the levels of serum 3′tRF-AlaAGC and calculate the inflammatory markers based on peripheral blood test in BC patients. Additionally, we employed the Lasso-cox regression and multiple logistic regression to screen parameters to construct a nomogram model. Our present study may provide a new less-invasive method with highly accurate for the diagnosis of BC patients with LNM, which is of great potential value in improving the prognosis of BC patients.
Materials and Methods
Serum Samples
The present study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethical Committee of Nanjing Medical University. We collected serum samples from patients who visited the thoracic surgery department of Jiangsu Cancer Hospital from June 2021 and May 2024. The diagnosis was based on the tissue sample obtained during breast pathological biopsy and confirmed by postoperative pathology. Patients were included if they met the following criteria:26 (1) Female patients; (2) Age at 18–75 years; (3) Primary breast cancer; (4) Not receiving any therapeutic procedures, including surgery, chemotherapy, neoadjuvant chemotherapy and other anti-tumor treatments; (5) Complete collection of clinical or pathological data; (6) No distant metastasis; (7) No history of malignant hematologic disease, severe hepatic or renal disease; (8) No history of serious immune disorders or hyperlipidemia. A total of 175 patients were involved in this study, and 105 patients without lymph node metastasis, seventy patients with lymph node metastasis, according to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) 8th edition staging system. The clinicopathological characteristics of these 175 cases were shown in Table S1. Furthermore, serum from 94 cases of healthy controls was also obtained. Serum samples were extracted from whole blood after centrifugation (2800 g, 10 min) and stored at −80 °C until further processing. A flow chart of the study is provided in Figure 1.
 |
Figure 1 The flowchart of the study design and analysis. Abbreviation: LNM, lymph node metastasis. |
RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR
Total RNAs of serum samples were extracted with TRIzol LS reagent, according to the manufacturer’s protocol. The OD 260/280 absorbance ratios of all the samples were between 1.8 and 2.0. Then, the RNA was quantified using a riboSCRIPT™ Reverse Transcription Kit (Ribobio, China) following the manufacturer’s protocol and reverse transcribed to cDNA with Bulge-loop™ miRNA qRT-PCR primers (Ribobio, China) specific for 3′tRF-AlaAGC. SYBR Green Mix was used to perform qPCR in a volume of 10 µL. After adding forward primer and reverse primer, the mixtures were incubated at 95°C for 10 min, followed by 40 cycles at 95°C for 10s, 60°C for 20s, and 70°C for 10s. RNU6B was used for 3′tRF-AlaAGC template normalization. RT-qPCR was performed as previously described.14
Laboratory Data Extraction
Laboratory indicators measured for the first time on admission of the patients: neutrophils (NE), lymphocytes (LY), monocytes (MO), platelets (Plt), fibrinogen (Fbg), albumin (Alb), lactic dehydrogenase (LDH), high-density lipoprotein cholesterol (HDL-C). Besides, the tumor markers (CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19–9, and CA125: carbohydrate antigen 125) were also measured.
The calculation formula of peripheral blood derived inflammatory marker including fibrinogen-to-lymphocyte ratio (FLR), NLR, PLR, LMR, SII, prognostic nutritional index (PNI), the systemic inflammation response index (SIRI), aggregate index of systemic inflammation (AISI), (neutrophil + monocyte)-to-lymphocyte ratio (NMLR), NHR, MHR, lymphocyte to HDL-C ratio (LHR) and PHR according to the following equations: FLR = Fbg/LY, NLR = NE/LY, PLR = Plt/LY, LMR = LY/MO, SII = (NE × Plt)/LY, PNI = Alb(g/L) + 5×LY, SIRI = (NE × MO)/LY, AISI = (NE × Plt×MO)/LY, NMLR = (NE+MO)/LY, NHR = NE/HDL-C, MHR= MO/HDL-C, LHR = LY/HDL-C, and PHR = Plt/HDL-C.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics Version 20.0, GraphPad Prism v9.4.1 and R version 4.2.1. To analyze the distribution of 3′tRF-AlaAGC and inflammatory markers of patients, results were compared using Mann–Whitney U-test, and the data are reported as median (range). Categorical variables were presented as frequencies and percentages and compared with Chi-square tests. Lasso-cox regression and multiple logistic regression were employed for variable selection and nomogram model construction. Spearman correlation test was used to examine the correlation between 3′tRF-AlaAGC levels and NHR, LMR. In addition, the optimal cut-off values for the 3′tRF-AlaAGC, NHR and LMR were selected using receiver operating characteristic analysis. Sensitivity, specificity and Youden’s index for prediction of LNM were calculated for 3′tRF-AlaAGC, NHR and LMR. Youden’s index (in percent) was calculated as (sensitivity + specificity − 1) × 100.27 Finally, the nomogram model was constructed from the multivariate logistic regression results, and its discriminatory ability was determined by receiver operating characteristic-area under curve (ROC-AUC). Two-sided p-value of less than 0.05 was considered statistically significant.
Results
Evaluation of Serum 3′tRF-AlaAGC Level in Patients with BC
We have expanded the serum sample size on the basis of the original.14 A total of 269 serum samples, including those from patients with BC (n = 175), and healthy controls (n = 94) were examined by quantitative real-time PCR. Serum 3′tRF-AlaAGC levels were significantly higher in BC patients compared to healthy controls [7.79 (1.86, 26.96) vs 1.09 (0.31, 5.81), P<0.001]. Meanwhile, higher expression of 3′tRF-AlaAGC was observed in patients with LNM [5.17 (1.79, 16.55) vs 11.68 (2.64, 58.74), P=0.009] (Figure 2).
Baseline Characteristics of BC Patients According to Status of LNM
In the present study, seventy BC patients (40%) were pathologically diagnosed with LNM. As shown in the Table 1, the medians of 3′tRF-AlaAGC, CA153, FLR, NLR, LMR, SII, SIRI, AISI, NMLR, NHR, MHR and PHR and the difference between the two groups were statistically significant (all P<0.05). In contrast, age (P=0.503), BMI (P=0.086), CEA (P=0.478), CA125 (P=0.163), LDH (P=0.609), PLR (P=0.122), PNI (P=0.610) and LHR (P=0.555) showed no statistically significant differences between the two groups. What’s more, there was no statistically significant difference in the positive rates of ER, PR, HER-2 and Ki-67 between patients with LNM and those without LNM (P>0.05).
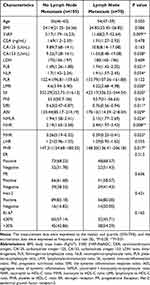 |
Table 1 Comparison of Clinicopathological Characteristics in Breast Cancer Patients with or without Lymph Node Metastasis |
Prediction Model Built Based on Lasso-Cox Regression
We analyzed 13 variables with differences between the two groups of LNM and without LNM to screen parameters using Lasso-cox regression, and the variation characteristics of the coefficient of these variables were shown in Figure 3A. The 10-fold cross validation method was applied to the iterative analysis, and a model with excellent performance but minimum number of variables was obtained when λ was 0.058 (Log λ= −1.237) (Figure 3B). Finally, we screened 3 variables (3′tRF-AlaAGC, NHR and LMR) through Lasso regression to construct the new prediction model.
ROC Curve for Screening Variables of LNM in BC Patients
In order to evaluate the predictive values of 3′tRF-AlaAGC, NHR and LMR for the presence and severity of LNM, we performed an area under of ROC-AUC analysis. As shown in Table 2, the ROC-AUC of 3′tRF-AlaAGC for differentiating LNM from without LNM was 0.617 (95% CI: 0.530–0.703). The Yonden’s index was 0.21, and was associated with a cut-off value of 18.78 for 3′tRF-AlaAGC, corresponding to a sensitivity of 42.86% and a specificity of 78.10%. And then, the optimal cut-off value of NHR and LMR were 2.94 and 5.41, respectively. The AUC of NHR was 0.619, the 95% CI was 0.532–0.706, the sensitivity was 37.14%, and the specificity was 84.76%. The AUC of LMR was 0.604, the 95% CI was 0.519–0.688, the sensitivity was 82.86%, and the specificity was 38.10%.
 |
Table 2 Diagnostic Efficiency of Hematological Indicators for BC Patients with Lymph Node Metastasis |
Correlation Analysis of Serum 3′tRF-AlaAGC, NHR and LMR
The Spearman correlation test was used to examine the correlation between levels of 3′tRF-AlaAGC, NHR and LMR. Unfortunately, there is no significant correlation between 3′tRF-AlaAGC and NHR, LMR in the present study. But the levels of NHR was significantly negative correlated with LMR (r=−0.188, P=0.012, Figure 4A). Subsequently, we divided the patients into high and low expression groups based on 3′tRF-AlaAGC’s cut-off value of 18.78, with 122 patients showing low expression and 53 showing high expression. As shown in Figure 4B, NHR levels was significantly negative associated with LMR in low 3′tRF-AlaAGC expression groups (r=−0.209, P=0.021). However, no similar results were observed in the high expression groups (r=−0.141, P=0.313).
Multivariate Analysis of Logistic Regression Model for Predicting LNM
We used the occurrence of LNM as the dependent variable (negative = 0, positive = 1), 3′tRF-AlaAGC (≤18.78 = 0, >18.78 = 1), NHR (≤2.94 = 0, >2.94 = 1) and LMR (≤5.41 = 0, >5.41 = 1), as independent variables for multivariable logistic regression analysis, employing a likelihood ratio test with maximum partial likelihood estimation (forward: LR). The results showed that 3′tRF-AlaAGC (OR: 3.242, 95% CI: 1.583–6.641, P=0.001), NHR (OR: 3.305, 95% CI: 1.543–7.079, P=0.002), and LMR (OR: 0.329, 95% CI: 0.150–0.723, P=0.006) were independent risk factors of BC with LNM (Table 3). Subsequently, we obtained a classification discriminant equation using the above results to ascertain whether patients BC with LNM, as follow: logit (P) = −0.786+1.176*3′tRF-AlaAGC+1.195*NHR-1.111*LMR (χ2= 28.951, P<0.01), for which the critical value is 0.50, thus, if the logit (P) of a case is larger than 0.50, it belongs to the BC with LNM, and the prediction accuracy is 69.14%. The calibration ability of the prediction model was evaluated through the Hosmer-Lemeshow goodness of fit test. The results indicated that there is no statistically significant difference between the predicted values of the model and the actual observed values (χ2= 2.781, P=0.595).
 |
Table 3 Multivariate Analysis of Logistic Regression Model for Predicting Lymph Node Metastasis |
Nomogram Model for Risk Assessment of BC with LNM
The logistic regression model of 3′tRF-AlaAGC, NHR, and LMR was established by the R language rms package, and the C statistic of its evaluation was 0.704, the 95% CI was 0.627–0.781, with a sensitivity of 57.14%, a specificity of 77.14% and an accuracy of 69.14%, indicating that the prediction model had certain accuracy (Figure 5A). And then, the plotting function was constructed and the nomogram was plotted (Figure 5B). A score of 3′tRF-AlaAGC > 18.78 was 98 points, while a score of 3′tRF-AlaAGC ≤ 18.78 was 0 points; a score of NHR >2.94 was 100 points, while a score of NHR ≤ 2.94 was 0 points; a score of LMR > 5.41 was 0 points, while a score of LMR ≤ 5.41 was 93 points. The total score was 291 points, suggesting that the probability of LNM in BC was greater than 80%. The risk of BC with LNM can be predicted based on the total points (Table 4).
 |
Table 4 Relationship Between Total Points and Risk of LNM for BC Patients |
Discussion
BC is the most common malignant tumor among women worldwide, with lymph node metastasis being the primary cause of death.28 Currently, pathological biopsy is the gold standard for identifying LNM in patients with BC.29 Moreover, sentinel lymph node biopsy (SLNB) is the standard clinical procedure for pathological biopsy in BC patients. However, this procedure is invasive and may lead to overtreatment in SLN-negative BC. In addition, there are still some complications, such as lymphedema, impaired shoulder range of motion, shoulder/arm pain, infection and seroma, etc.30,31 Besides, the most common tumor markers CEA, CA125 or CA153 are not the unique biomarkers for BC diagnosis because of their poor sensitivity and specificity. Therefore, it is crucial to identify feasible, less-invasive, effective and easily accessible indicators. Research has revealed that serum miRNA profiles (miR-629-5p, miR-629-3p, miR-4710, and miR-4492) may be useful for the diagnosis of axillary lymph node metastasis in BC before surgery in a less-invasive manner than sentinel lymph node biopsy.26 tDRs is an active process resulting from the cleavage of tRNAs by multiple enzymes, which plays important roles in regulating transcription and translation, similar to miRNAs.32,33 Importantly, tDRs are shorter in length and less susceptible to degradation by RNAs enzymes compared to miRNAs, making them more ideal low-invasive biomarker.11
Previous study revealed that the AUC of i-tRF-AspGTC and tRF-1-SerCGA for predicting the early diagnostic efficiency of NSCLC were 0.656 and 0.688, respectively. They could be used as predictive indicators for diagnosis of NSCLC.34 Mao et al analyzed 115 patients with GC and confirmed that the diagnostic value of tRF-17-18VBY9M was higher than that of CEA, CA199 and CA724, suggesting that tRF-17-18VBY9M has good diagnostic potency in GC serum.35 In BC, tRF-Gly-CCC-046, tRF-Tyr-GTA-010 and tRF-Pro-TGG-001 were downregulated in both tissues and serum from BC patients, and AUC of the combined tDRs for differentiating early diagnosis of BC was 0.799.36 Despite this, there is little research on tDRs for diagnosing LNM in BC. In our present study, we showed that 3′tRF-AlaAGC levels were significantly higher in the serum of BC patients, and the higher expression levels of 3′tRF-AlaAGC was associated with LNM. Additionally, 3′tRF-AlaAGC was one of the variables characteristics related to LNM of BC screened by Lasso-cox regression. It is reasonable to believe that 3′tRF-AlaAGC has diagnostic potency of monitoring LNM in BC patients.
Current evidence shows that chronic inflammation is inextricably linked to different stages of tumorigenesis, proliferation, invasion, metastasis, and apoptosis.37,38 Inflammatory responses play decisive roles at different stages of tumor development and also affect immune surveillance and responses to therapy.39 When immune cells, including lymphocytes and neutrophils, are activated, they release pro-inflammatory and anti-inflammatory mediators.40 Neutrophils are the first line of inflammatory response, producing cytokines that affect lymphocytes and monocytes.41 Due to the release of chemokines and cytokines such as vascular endothelial growth factor (VEGF), neutrophils can accelerate angiogenesis, enhance tumor cell adhesion, and promote distant metastasis and tumor metastasis.42 Lymphocytes play a crucial role in anti-tumor immune activity and tumor related immune response, and tumor infiltrating T lymphocytes inhibit tumor cell growth and invasion by enhancing apoptosis.43,44 Beyond that, activated monocytes interact with damaged endothelial cells, inducing the secretion of pro-inflammatory cytokines and adhesion molecules.45 In addition, many studies indicated that HDL-C plays important anti-inflammatory and antioxidant roles in preventing the progression of atherosclerosis,46 as it is able to inhibit the activation and transformation of monocytes, thereby inhibiting the inflammatory response.47 These also explains the elevated levels of NHR and reduced levels of LMR in patients with LNM in the current study. The NHR is an effective biomarker of systemic inflammation and oxidative stress, and its prognostic ability in various diseases (such as cardiovascular diseases, Parkinson’s disease, diabetes, etc.) has been studied, but its clinical significance in malignant tumors has rarely been investigated.22,23,48,49 In hepatocellular carcinoma, NHR was a significant independent factor for 3-year mortality, and patients with NHR ≥ 3.5 have a high mortality rate.23 Previous studies have shown that LMR can be used as diagnostic and prognostic markers for laryngeal carcinoma.50 Huang et al confirmed that the level of LMR in colorectal cancer (CRC) were significantly lower than those in non-CRC subjects, and the ROC-AUC for differentiating CRC was 0.77 (sensitivity = 0.72 and specificity = 0.73).51 In this study, NHR and LMR were both selected as variables associated with LNM in BC by Lasso-cox regression screening. What’s more, when all patients were divided into high and low expression groups based on the cut-off value of 3′tRF-AlAGC, NHR levels was significantly negative associated with LMR in low 3′tRF-AlaAGC expression groups. The above results suggest that 3′tRF-AlaAGC is correlated with blood inflammation indicators.
The nomogram model in this study demonstrated a favorable identification effect for both LNM and without LNM cases, basing an AUC value of 0.704. The calibration curve and decision curve analyses further affirmed the nomogram model’s excellent discriminatory ability and clinical utility. Although the previous study demonstrated that 5′-tiRNAVal can distinguish BC patients with LNM from healthy controls,52 the discriminative power of tDRs between LNM and without LNM in BC, especially in combination with inflammatory markers, has not yet been conducted. Nomograms integrate several predicting factors and allow the score for each factor to be calculated using a scale, so that the total score can be used to predict the risk of a specific event. Nomograms have significantly benefited in diagnosis and prognosis of various malignancies.53 In head-neck squamous cell carcinoma, the survival nomogram models were established based on Aminoacyl tRNA Synthetase Complex Interacting Multifunctional Protein 1 (AIMP1) and Cornichon Family AMPA Receptor Auxiliary Protein 4 (CNIH4), respectively, for clinical applications.54,55 In glioma, a nomogram was constructed for the prediction of 1-,3-,5- year survival based on multivariate Cox analysis.56 The approach was conductive to creation of a genuinely reliable prediction model before or after treatment, and help clinicians make accurate and wise clinical decisions.
Overall, the present study is a prospective study with a relatively large sample size and complete clinical pathological information, which reduces the selection bias and ensures the accuracy of diagnosis. For all that, our study has some limitations. First, this study was a single-institution one with clinical data collection, and it is necessary to conduct a Multi-institutional study with a wider patient population. Then, the cut-off value of 3′tRF-AlaAGC, inflammatory markers and other tumor markers was calculated only by mathematical methods, and the sensitivity and specificity of these laboratory data in predicting LNM need to be further verification in a multicenter large-scale prospective randomized controlled trial. Finally, this study divided the subjects into two groups based on whether or not they had LNM, without further subgrouping according to the degree of metastasis. This will be investigated more thoroughly in subsequent work.
Conclusions
This is the first study to apply tDRs and inflammatory markers to predict LNM of BC and to establish risk assessment models for LNM of BC. We identified 3′tRF-AlaAGC >18.78, NHR > 2.94, and LMR ≤ 5.41 as independent risk factors for LNM in BC patients. And the nomogram model constructed by 3′tRF-AlaAGC, NHR and LMR can correctly predict the risk of LNM of BC. In sum, we provide a new perspective for the combination of tDRs and inflammatory markers in the early diagnosis of LNM in BC patients.
Data Sharing Statement
The data that support the results of this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Our study complied with the Declaration of Helsinki (as revised in 2013). The clinical isolates from patients in this study were part of routine hospital procedure, and the use of these samples was approved by the Ethical Committee of Nanjing Medical University (No. 2019-030 and No. 2020-130).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (NO. 82472363) and the Research Fund of Jiangsu Cancer Hospital (No: ZJ202312) and the Research Project of Jiangsu Cancer Hospital (No. ZJ202119).
Disclosure
All authors have no conflicts of interest of declare for this work.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820;
3. Sonkin D, Thomas A, Teicher BA. Cancer treatments: past, present, and future. Cancer Genet. 2024;286-287:18–24. doi:10.1016/j.cancergen.2024.06.002
4. Giaquinto AN, Sung H, Newman LA, et al. Breast cancer statistics 2024. CA Cancer J Clin. 2024;74(6):477–495. doi:10.3322/caac.21863
5. de Boer M, van Dijck JA, Bult P, et al. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. J Natl Cancer Inst. 2010;102(6):410–425. doi:10.1093/jnci/djq008
6. Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi:10.1016/S0140-6736(16)31891-8
7. Obeagu EI, Obeagu GU. Breast cancer: a review of risk factors and diagnosis. Medicine (Baltimore). 2024;103(3):e36905. doi:10.1097/MD.0000000000036905
8. Pekarsky Y, Balatti V, Croce CM. tRNA-derived fragments (tRFs) in cancer. J Cell Commun Signal. 2023;17(1):47–54. doi:10.1007/s12079-022-00690-2
9. Mao M, Chen W, Huang X, et al. Role of tRNA-derived small RNAs(tsRNAs) in the diagnosis and treatment of malignant tumours. Cell Commun Signal. 2023;21(1):178. doi:10.1186/s12964-023-01199-w
10. Oberbauer V, Schaefer MR. tRNA-derived small RNAs: biogenesis, modification, function and potential impact on human disease development. Genes (Basel). 2018;9(12):607. doi:10.3390/genes9120607
11. Jiang X, Li X, Li Y, et al. Systematic assessment of serum i-tRF-AsnGTT in gastric cancer: a potential clinical biomarker. Carcinogenesis. 2024;46(1):bgae044.
12. Li J, Cao C, Fang L, et al. Serum transfer RNA-derived fragment tRF-31-79MP9P9NH57SD acts as a novel diagnostic biomarker for non-small cell lung cancer. J Clin Lab Anal. 2022;36(7). doi:10.1002/jcla.24492
13. Li X, Li Y, Yuan J, et al. Serum tRF-33-RZYQHQ9M739P0J as a novel biomarker for auxiliary diagnosis and disease course monitoring of hepatocellular carcinoma. Heliyon. 2024;10(9):e30084. doi:10.1016/j.heliyon.2024.e30084
14. Mo D, Tang X, Ma Y, et al. tRNA-derived fragment 3’tRF-AlaAGC modulates cell chemoresistance and M2 macrophage polarization via binding to TRADD in breast cancer. J Transl Med. 2024;22(1):706. doi:10.1186/s12967-024-05513-z
15. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi:10.1038/nrc3611
16. Zhou J, Wei S, Guo X, et al. Correlation between preoperative peripheral blood NLR, PLR, LMR and prognosis of patients with head and neck squamous cell carcinoma. BMC Cancer. 2023;23(1):1247. doi:10.1186/s12885-023-11752-y
17. Karan C, Yaren A, Demirel BC, et al. Pretreatment PLR is preferable to NLR and LMR as a predictor in locally advanced and metastatic bladder cancer. Cancer Diagn Progn. 2023;3(6):706–715. doi:10.21873/cdp.10275
18. Zheng J, Peng L, Zhang S, et al. Preoperative systemic immune-inflammation index as a prognostic indicator for patients with urothelial carcinoma. Front Immunol. 2023;14:1275033. doi:10.3389/fimmu.2023.1275033
19. Nakamoto S, Ohtani Y, Sakamoto I, et al. Systemic immune-inflammation index predicts tumor recurrence after radical resection for colorectal cancer. Tohoku J Exp Med. 2023;261(3):229–238. doi:10.1620/tjem.2023.J074
20. Tanaka S, Couret D, Tran-Dinh A, et al. High-density lipoproteins during sepsis: from bench to bedside. Crit Care. 2020;24(1):134. doi:10.1186/s13054-020-02860-3
21. Santos-Gallego CG, Badimon JJ, Rosenson RS, et al. Beginning to understand high-density lipoproteins. Endocrinol Metab Clin North Am. 2014;43(4):913–947. doi:10.1016/j.ecl.2014.08.001
22. Pan X, Zhang X, Ban J, et al. Association of neutrophil to high-density lipoprotein cholesterol ratio with cardiac ultrasound parameters and cardiovascular risk: a cross-sectional study based on healthy populations. J Inflamm Res. 2023;16:1853–1865. doi:10.2147/JIR.S406102
23. Shi K, Hou J, Zhang Q, et al. Neutrophil-to-high-density-lipoprotein-cholesterol ratio and mortality among patients with hepatocellular carcinoma. Front Nutr. 2023;10:1127913. doi:10.3389/fnut.2023.1127913
24. Jialal I, Jialal G, Adams-Huet B. The platelet to high density lipoprotein -cholesterol ratio is a valid biomarker of nascent metabolic syndrome. Diabetes Metab Res Rev. 2021;37(6):e3403. doi:10.1002/dmrr.3403
25. Zhou F, Wu L, Shen G, et al. Association between monocyte to high-density lipoprotein-cholesterol ratio and osteoporosis: an analysis of the national health and nutrition examination survey 2013-2014. J Investig Med. 2024;72(1):3–12. doi:10.1177/10815589231204057
26. Shiino S, Matsuzaki J, Shimomura A, et al. Serum miRNA-based prediction of axillary lymph node metastasis in breast cancer. Clin Cancer Res. 2019;25(6):1817–1827. doi:10.1158/1078-0432.CCR-18-1414
27. Michels S, Widmann P, Rapp D, et al. Predictive parameters of early respiratory decline in amyotrophic lateral sclerosis. Eur J Neurol. 2022;29(11):3170–3176. doi:10.1111/ene.15486
28. Chen W, Hoffmann AD, Liu H, et al. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precis Oncol. 2018;2(1):4. doi:10.1038/s41698-018-0047-0
29. Charalampoudis P, Markopoulos C, Kovacs T. Controversies and recommendations regarding sentinel lymph node biopsy in primary breast cancer: a comprehensive review of current data. Eur J Surg Oncol. 2018;44(1):5–14. doi:10.1016/j.ejso.2017.10.215
30. Tan H, Wu Y, Bao F, et al. Mammography-based radiomics nomogram: a potential biomarker to predict axillary lymph node metastasis in breast cancer. Br J Radiol. 2020;93(1111):20191019. doi:10.1259/bjr.20191019
31. Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245(3):452–461. doi:10.1097/01.sla.0000245472.47748.ec
32. Balatti V, Nigita G, Veneziano D, et al. tsRNA signatures in cancer. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(30):8071–8076. doi:10.1073/pnas.1706908114
33. Martinez G, Choudury SG, Slotkin RK. tRNA-derived small RNAs target transposable element transcripts. Nucleic Acids Research. 2017;45(9):5142–5152. doi:10.1093/nar/gkx103
34. Peng J, Zhang Y, Zhou G, et al. Circulating serum exosomes i-tRF-AspGTC and tRF-1-SerCGA as diagnostic indicators for non-small cell lung cancer. Clin Transl Oncol. 2024;26(8):1988–1997. doi:10.1007/s12094-024-03423-6
35. Mao C, Zhang Z, Fang R, et al. A novel tRNA-derived fragment tRF-17-18VBY9M works as a potential diagnostic biomarker for gastric cancer. J Cancer Res Clin Oncol. 2024;150(5):263. doi:10.1007/s00432-024-05792-5
36. Zhang Y, Bi Z, Dong X, et al. tRNA-derived fragments: tRF-Gly-CCC-046, tRF-Tyr-GTA-010 and tRF-Pro-TGG-001 as novel diagnostic biomarkers for breast cancer. Thorac Cancer. 2021;12(17):2314–2323. doi:10.1111/1759-7714.14072
37. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
38. Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi:10.4103/aam.aam_56_18
39. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
40. Li JY, Yao RQ, Liu SQ, et al. Efficiency of monocyte/high-density lipoprotein cholesterol ratio combined with neutrophil/lymphocyte ratio in predicting 28-day mortality in patients with sepsis. Front Med (Lausanne). 2021;8:741015. doi:10.3389/fmed.2021.741015
41. Rosales C. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol. 2020;108(1):377–396. doi:10.1002/JLB.4MIR0220-574RR
42. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14(1):173. doi:10.1186/s13045-021-01187-y
43. Feng F, Zheng G, Wang Q, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018;18(1):148. doi:10.1186/s12876-018-0877-9
44. Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi:10.1200/JCO.2010.30.5037
45. Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62(17):1541–1551. doi:10.1016/j.jacc.2013.07.043
46. Krentz AJ. Lipoprotein abnormalities and their consequences for patients with type 2 diabetes. Diabetes Obes Metab. 2003;5 Suppl 1:S19–S27. doi:10.1046/j.1462-8902.2003.0310.x
47. Ucar FM. A potential marker of bare metal stent restenosis: monocyte count - to- HDL cholesterol ratio. BMC Cardiovasc Disord. 2016;16(1):186. doi:10.1186/s12872-016-0367-3
48. Liu Z, Fan Q, Wu S, et al. Compared with the monocyte to high-density lipoprotein ratio (MHR) and the neutrophil to lymphocyte ratio (NLR), the neutrophil to high-density lipoprotein ratio (NHR) is more valuable for assessing the inflammatory process in Parkinson’s disease. Lipids Health Dis. 2021;20(1):35. doi:10.1186/s12944-021-01462-4
49. Song Y, Zhao Y, Shu Y, et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more valuable for predicting peripheral arterial disease in type 2 diabetic patients: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1100453. doi:10.3389/fendo.2023.1100453
50. Li P, Li H, Ding S, et al. NLR, PLR, LMR and MWR as diagnostic and prognostic markers for laryngeal carcinoma. Am J Transl Res. 2022;14(5):3017–3027.
51. Huang M, Deng S, Li M, et al. Clinical diagnostic value of methylated SEPT9 combined with NLR, PLR and LMR in colorectal cancer. BMC Gastroenterol. 2024;24(1):240. doi:10.1186/s12876-024-03332-6
52. Mo D, Jiang P, Yang Y, et al. A tRNA fragment, 5’-tiRNAVal, suppresses the Wnt/β-catenin signaling pathway by targeting FZD3 in breast cancer. Cancer Lett. 2019;457:60–73. doi:10.1016/j.canlet.2019.05.007
53. Liu J, Huang X, Chen S, et al. Nomogram based on clinical characteristics for preoperative prediction of perineural invasion in gastric cancer. J Int Med Res. 2020;48(1):300060519895131. doi:10.1177/0300060519895131
54. Li Y, Liu H. Clinical powers of aminoacyl tRNA synthetase complex interacting multifunctional protein 1 (AIMP1) for head-neck squamous cell carcinoma. Cancer Biomark. 2022;34(3):359–374. doi:10.3233/CBM-210340
55. Liu H, Li Y. Potential roles of cornichon family AMPA receptor auxiliary protein 4 (CNIH4) in head and neck squamous cell carcinoma. Cancer Biomark. 2022;35(4):439–450. doi:10.3233/CBM-220143
56. Liu H, Weng J. A comprehensive bioinformatic analysis of cyclin-dependent kinase 2 (CDK2) in glioma. Gene. 2022;822:146325. doi:10.1016/j.gene.2022.146325
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





