Back to Journals » Cancer Management and Research » Volume 17
Clinical Use of Dostarlimab in Advanced Stage and Recurrent Endometrial Cancer: Patient Selection and Perspectives
Authors Bujnak AC, File B, Tewari KS
Received 19 April 2024
Accepted for publication 9 January 2025
Published 25 January 2025 Volume 2025:17 Pages 161—170
DOI https://doi.org/10.2147/CMAR.S341469
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Alyssa C Bujnak,1 Brittany File,2 Krishnansu S Tewari1
1Department of Gynecologic Oncology, University of California, Irvine-Medical Center, Orange, California, USA; 2Department of Obstetrics and Gynecology, University of California, Irvine-Medical Center, Orange, California, USA
Correspondence: Alyssa C Bujnak, University of California, Irvine-Medical Center, 3800 West Chapman Avenue, Suite 3400, Orange, CA, USA, Tel +1 714 456-8888, 92868, Fax +1 714 509-2257, Email [email protected]
Abstract: Endometrial cancer (EC) is the most common gynecologic cancer in developed nations with reported 420,368 new cases worldwide in 2022 and resulting in 97,723 deaths that year; it is also one of the few cancers with expected increases in incidence and mortality, which are expected to increase by 50% and 70%, respectively, by 2045. The mortality from EC can largely be attributed to the advanced stage and recurrent cases. Over the past decade, the standard of care for treatment of primary advanced stage and recurrent EC has been chemotherapy, resulting in a median overall survival (OS) of less than 3 years. Advances in molecular tumor profiling have highlighted that a portion of EC tumors release high levels of neoantigens, indicating an opportunity to harness the immune system in therapeutic strategies. On August 1st, 2024, the United States Food and Drug Administration expanded the indication of the immunologic anti-PD-1 checkpoint inhibitor, dostarlimab, and chemotherapy in endometrial cancer. This review summarizes the rationale, evidence, and indications for the use of dostarlimab in advanced and recurrent endometrial cancer.
Keywords: dostarlimab, pembrolizumab, durvalumab, endometrial cancer, mismatch repair deficiency, PD-1 inhibition, immune checkpoint inhibitor
Background
Endtrial cancer (EC) is the most common gynecologic cancer in developed nations with reported 420,368 new cases worldwide in 2022 and resulting in 97,723 deaths that year;1 it is also one of the few cancers with expected increases in incidence and mortality, which are expected to increase by 50% and 70%, respectively, by 2045.2,3
Molecular Classification in Endometrial Cancer
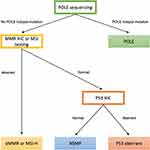
Classically, endometrial cancer has been categorized into two tumor groups based on histopathologic factors and prognosis, type I (estrogen-dependent and well-differentiated) and type II (estrogen-independent, less differentiated, poor prognosis).4 The use of molecular features as estimation of risk and recurrence was expanded upon when the Cancer Genome Atlas (TCGA) evaluated EC through whole-genome tumor sequencing and identified four molecular subgroups of endometrial cancer: DNA polymerase ε (POLE, ultramutated), microsatellite instability (MSI, hypermutated), copy-number low, and copy-number high (Table 1).5 The tumors were clustered by copy number variation and tumor exome mutational rate. The POLE/ultramutated tumors exhibited a very high mutational burden resulting from mutations in the POLE exonuclease domain with excellent prognosis, potentially indicating opportunities for de-escalation of management in the future. The MSI group exhibited high mutational burden, typically consequential from a deficiency in mismatch repair (MMR) proteins, with intermediate prognosis. The somatic copy-number alteration high group had a serous-like phenotype, with low mutation rate, frequent TP53 aberrations, and demonstrated poor prognosis.6 The molecular prognostic information from the TCGA highlights the opportunity for exploring therapeutic avenues for tumors with high tumor mutation burden, including POLE/ultramutated and the tumors deficient in MMR proteins and microsatellite instability high (MSI-H) (Figure 1).7
 |
Table 1 Endometrial Cancer Molecular Subgroups From the Cancer Genome Atlas |
Deficiency in Mismatch Repair and Microsatellite Instability
The MMR pathway repairs mismatched bases via nucleotide excision repair during DNA replication. This ensures genomic stability and prevents abnormal insertions and deletions of DNA, including within the non-coding microsatellite regions. This repair pathway depends on the combined function of four mismatch repair enzymes: mutL homologue 1 (MLH1), postmeiotic segregation increased 2 (PMS2), mutS homologue 2 (MSH2), and mutS 6 (MSH6). Dysfunction of these enzymes leads to the inability to excise and replace incorrect nucleotides and is referred to as MMR deficiency (dMMR), while normal functioning MMR proteins are termed proficient MMR (pMMR).8 The downstream effect of dMMR is the creation of multiple DNA mutations within the coding portion of the genome (including oncogenes) and microsatellite regions. Mutation accumulation at a rate of 100 to 1,000 fold increase from baseline leads to microsatellite instability, and the generation of non-self-appearing/abnormal proteins.9,10 Accordingly, dMMR can be viewed as the “cause” of MSI phenotype “effect”. Tumors with instability at one microsatellite locus are MSI-low, while those that demonstrate instability at 2 or more loci are MSI-high.
Immunotherapy in Mismatch Repair and Microsatellite Instability
The generation of non-self-appearing proteins by tumors deficient in MMR or MSI-H creates numerous neoantigens. Neoantigens can be detected by the immune system, leading to stimulation of an anti-tumor immune response. Given the tumor immunogenic properties of MMR/MSI-H tumors, the use of immunotherapy in these cancers is opportunistic. One of the most studied therapeutic targets has been in a class of immunotherapy drugs known as immune checkpoint inhibitors (ICIs). ICIs prevent cancer cells from undergoing immunologic escape, specifically effector T cell function, resulting in improved T cell anti-tumor activity. T cells, a component of the adaptive immune system, can attack and induce death of cancer cells. Antigen presenting cells collect and present cancer antigens to T cells, activating them to target and kill cancer cells displaying the antigens. Some cancer cells display the ligand, programmed death ligand 1 (PD-L1), that can bind to a receptor on the activated T cell, programmed death 1 (PD-1) receptor. This PD-L1 to PD-1 binding signals suppression of the T cell, resulting in avoidance of death by the cancer cell. Immunotherapies that inhibit PD-1, PD-L1 or other suppressive protein pathways on T cells are considered immune checkpoint inhibitors (Figure 2).11 ICIs with FDA approval in EC are dostarlimab, pembrolizumab and durvalumab.
Dostarlimab is a humanized anti-PD-1 immunoglobulin G4 monoclonal antibody that binds with high affinity to the PD-1 receptor on T cells, effectively blocking its interaction with PD-L1 and PD-L2 expressed on the surface of cancer cells. This alleviates T cell inhibition, and ultimately results in enhanced activity of T cells previously primed through tumor neoantigen exposure.12 In dMMR/MSI-H tumors, the expression of high levels of neoantigens leads to frequent opportunities for antigen presentation and T cell activation. With the addition of PD-1 inhibition by anti-PD-1 antibodies, these previously activated T cells can avoid suppression by PD-L when they interact with cancer cells, and ultimately emit cytotoxic effects. Hence, the PD-1 inhibition pathway is an ideal target for dMMR/MSI-H tumors. Pembrolizumab is also a humanized monoclonal IgG4 anti-PD1 antibody with demonstrated equipotency PD-1 inhibition to dostarlimab, as assessed by ex vivo IL-2 stimulation ratios.13 Durvalumab is another ICI, however, comparatively, it is a human IgG1 monoclonal antibody that binds to PD-L1 and CD80, hence enhancing T-cell response to cancer cells.14
Activity of Dostarlimab in dMMR Rectal Carcinoma
In June 2022, Cercek et al reported on a 100% complete response rate in dMMR rectal cancer treated with dostarlimab. NCT04165772 was a Phase II study that included patients with locally advanced mismatch repair–deficient (dMMR) rectal cancer, treated with 6 months of neoadjuvant anti–PD-1 immunotherapy agent, dostarlimab-gxly. Treatment with dostarlimab resulted in clinical, objective, and pathologic complete responses in 100% of the study’s first 14 patients. Prior to this, standard of care for locally advanced rectal cancer included neoadjuvant chemotherapy and radiation followed by surgical resection of the rectum.15 This astonishing response rate in dMMR rectal cancer provided immense promise for the use of dostarlimab in EC, with 34% of EC tumors being dMMR or MSI-H.5
Activity of PD-1 Inhibition in Endometrial Cancer
At the time of Cercek et al, first-line treatment in primary advanced stage and recurrent endometrial cancer was a cytotoxic treatment with carboplatin and paclitaxel, with a median overall survival (OS) of less than 3 years, as established by GOG0209, a Phase III non-inferiority study in which molecular tumor characteristics such as MMR/MSI/TMB were not assessed.16 There were no indications for the use of immunotherapy for EC in the primary advanced and/or first-line setting.
In the second-line setting, EC patients with dMMR/MSI-H or TMB unresectable or metastatic disease had an indication for pembrolizumab as monotherapy as supported by data from KEYNOTE 158 (NCT02628067). KEYNOTE 158 was a phase II study treating dMMR/MSI-H advanced non-colorectal cancers with pembrolizumab. Enrollment included 233 patients, 49 of which had a primary diagnosis of endometrial cancer. In the EC cohort, 16.3% achieved complete response and 40.8% had partial responses with a reported objective response rate (ORR) of 57%.17
Dostarlimab was also studied as second-line monotherapy in EC. The GARNET trial (NCT02715284) was a multicenter, open-label, multicohort, Phase I trial to assess the safety and antitumor efficacy of dostarlimab as monotherapy in EC, non–small cell lung cancer, and dMMR/MSI-H non- endometrial solid tumors. The endometrial cohort enrolled patients with advanced stage or recurrent previously treated EC and analyzed dMMR/MSI-H (N=143) and pMMR/MSS (N=156) tumors. Patients received dostarlimab monotherapy (500mg intravenously every three weeks for four doses), followed by dostarlimab maintenance (1000mg every six weeks) until progression or discontinuation. The initial results relayed an ORR 42% in the dMMR/MSI-H population and led to FDA accelerated approval of dostarlimab as monotherapy for advanced stage or recurrent dMMR EC as second-line following progression on prior platinum-containing regimen; more recently, the interim report demonstrated an ORR of dMMR/MSI-H (46%) and pMMR/MSS (15%).10,18 Interestingly, with the interim data, a post-hoc report included an analysis of biomarker-related response to dostarlimab. Each tumor was evaluated for PD-L1, TMB, and molecular classification (POLEmut, MMRd, NSMP, p53 aberrant). This hypothesis generating data suggested an ORR improvement in TMB-H regardless of MMR status and vice versa. This finding raises further questions regarding the role of TMB as a biomarker to utilize in determining appropriate therapy.18
Following the approval of dostarlimab and pembrolizumab for second-line advanced stage and recurrent dMMR/MSI-H EC, options for the pMMR/MSS population needed to be explored. It had been shown that monotherapy with PD-1 inhibition in this population was less effective and hence a trial of combination therapy developed. Study 309-KEYNOTE-775 was multicenter, open-label, Phase 3 clinical trial patients in which patients with advanced, staged or recurrent endometrial cancer (regardless of MMR/MSI status) were assigned to receive either lenvatinib plus pembrolizumab or physician’s choice of standard chemotherapy.19 Lenvatinib is a multiple receptor tyrosine kinase inhibitor with potent antiangiogenic properties secondary to its inhibition of VEGF receptor, which has been shown to prevent tumor angiogenesis and cancer cell proliferation.20 Of the 827 patients enrolled, 697 patients were found to be pMMR and 103 dMMR. The primary endpoints of PFS and OS were analyzed in the pMMR/MSS and overall population, and the study was not powered to evaluate the dMMR/MSI-H. In the pMMR/MSS population, both primary endpoints were significant with HR 0.60 for PFS and HR 0.68 for OS. In the overall population, PFS (HR 0.56) and OS (HR 0.62) were also significantly improved. No conclusions could be drawn about the dMMR/MSI-H group, however these findings resulted in FDA approval of lenvatinib plus pembrolizumab for second-line systemic therapy in pMMR/MSS advanced and recurrent EC.21
Checkpoint Inhibition in Frontline Advanced Stage and Recurrent EC
With the second-line option established for dMMR endometrial cancer using checkpoint inhibition monotherapy, in conjunction with demonstrable upfront activity of dostarlimab in dMMR rectal cancer, the logical next step was to study checkpoint inhibitors with chemotherapy in the frontline setting among patients with advanced or recurrent chemotherapy-naive endometrial cancer.
Dostarlimab
RUBY part 1 (NCT03981796) is a phase 3, global, randomized placebo-controlled trial that studied the efficacy and tolerability of dostarlimab in combination with chemotherapy and continued in the maintenance setting as first-line therapy for advanced and recurrent endometrial cancer. The concept of RUBY was to add dostarlimab to the standard of care chemotherapy (carboplatin and paclitaxel) regimen based on the premise that cytotoxic chemotherapy can produce immune activating effects to further enhance the anti-tumor effects of PD-1 inhibition.16,22 RUBY part 1 included patients with primary advanced stage IIIA, IIIB, or IIIC1 with measurable disease, stage IIIC1 with carcinosarcoma, clear-cell, serous, or mixed histology regardless of the presence of measurable disease, stage IIIC2 or stage IV, or first recurrent EC. The study enrolled 494 patients (118, 24% dMMR/MSI-H) with primary advanced stage III or IV, or first recurrent EC that were randomized to receive dostarlimab plus carboplatin and paclitaxel followed by dostarlimab versus carboplatin and paclitaxel plus placebo followed by placebo. The primary endpoints were PFS and OS in the overall and dMMR/MSI-H groups. Compared to the placebo group, the dMMR/MSI-H population was associated with a 72% lower risk of progression or death with an HR for the primary endpoint of PFS of 0.28 at 24 months. In the overall population that included both pMMR/MSS and dMMR/MSI-H, the HR for PFS was 0.64 at 24 months.22 In an interim analysis of patients with pMMR/MSS tumors, the HR for PFS was 0.76 at 24 months. RUBY part 1 also found significant improvement in OS at 36 months in the dMMR-MSI-H (HR 0.32), pMMR/MSS (HR 0.79), and the overall population (HR 0.69).23 RUBY part 1 PFS data first led to FDA approval for dostarlimab in combination with carboplatin and paclitaxel as first-line systemic therapy in dMMR/MSI-H advanced stage or recurrent EC, and as of August 2024, the approval was extended to all advanced stage or recurrent EC regardless of MMR or microsatellite status (Table 2).24,25
 |
Table 2 Clinical Trials Evaluating Dostarlimab in Endometrial Cancer |
RUBY part 2 uses the concept of forcible neoantigen creation combined with immunotherapy in the same patient population as RUBY part 1 and studied the addition of a PARP inhibitor (niraparib) to dostarlimab in the maintenance setting. The rationale was to create a regimen with greater benefit for the pMMR/MSS patient cohort in the frontline setting. Patients were randomized to dostarlimab plus chemotherapy followed by dostarlimab plus niraparib maintenance, versus placebo plus chemotherapy. The progression-free survival data at 42% maturity was presented at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer with improvements in PFS for both the dMMR/MSI-H (HR 0.48) and pMMR/MSS cohorts (HR 0.63).26 This was an important observation, offering an opportunity for benefit particularly in the pMMR/MSS group, however its clinical utility cannot fully be concluded due to the lack of dostarlimab in the comparison group, which was set as the new standard of care. Currently, there is no FDA indication for niraparib in addition to dostarlimab for maintenance therapy in EC.
Pembrolizumab
Pembrolizumab, an anti-PD-1 checkpoint inhibitor, was also studied as first-line treatment for advanced stage and recurrent endometrial cancer in GY018 NCT03914612. This was a phase 3 double-blinded, randomized trial including 816 patients with measurable disease stage III or IVA, stage IVB, or recurrent EC randomizing patients to receive pembrolizumab in combination with carboplatin and paclitaxel, followed by pembrolizumab maintenance, versus carboplatin and paclitaxel plus placebo, followed by placebo maintenance therapy. The study was powered to stratify and assess PFS as a primary endpoint in both dMMR/MSI-H and the pMMR/MSS cohorts. The study reported a significant improvement in PFS in both cohorts, HR 0.30 for dMMR/MSI and HR 0.54 for pMMR/MSS.27 This led to FDA approval of pembrolizumab with carboplatin and paclitaxel, followed by maintenance pembrolizumab, as first-line therapy for primary advanced or recurrent endometrial carcinoma, regardless of MMR/microsatellite status.28
Durvalumab
The addition of durvalumab, an anti-PD-L1 ICI, to standard of care chemotherapy followed by maintenance durvalumab plus olaparib, a PARP inhibitor, was also studied.
The DUO-E trial (NCT04269200) was a phase 3, global, double-blind, placebo-controlled trial that included patients with primary advanced or recurrent endometrial cancer randomized into one of the three groups: carboplatin/paclitaxel plus placebo followed by placebo maintenance, carboplatin/paclitaxel plus durvalumab followed by durvalumab maintenance, and carboplatin/paclitaxel plus durvalumab followed by durvalumab plus olaparib maintenance. Each investigative arm was compared to the chemotherapy alone group as a control, and the study was not powered to investigate the endpoints of the dMMR and pMMR groups separately. Interim overall survival results (maturity approximately 28%) were supportive of the primary outcomes with a significant benefit in PFS observed in the durvalumab (HR 0.71) and the durvalumab followed by durvalumab plus olaparib maintenance group (HR 0.55). In a subgroup analysis of the dMMR, pMMR, and PD-L1 groups, a PFS suggested improvement in the dMMR group for both durvalumab and durvalumab plus olaparib group when compared to control (HR 0.42 and 0.41, respectively). The pMMR group PFS was also suggested to be improved (HR 0.77 and 0.57, respectively). Interestingly, the subgroup analysis by PD-L1 positive status suggested improvement in PFS in both investigative arms (HR 0.63 and 0.42), while there was not a suggested improvement in PFS in the PD-L1 negative cohort.29 The applicability of this study is diminished by the lack of comparison between the two investigative arms, seeing as the new standard of care has become chemotherapy plus a checkpoint inhibitor for advanced stage and recurrent endometrial cancer. Based on the DUO-E trial, durvalumab was recently approved for first-line therapy for dMMR primary advanced or recurrent EC.30 There is currently no FDA indication for the use of olaparib plus durvalumab in EC, however hypothesis generating findings propose potential improvement in outcomes in the pMMR and PD-L1 positive groups.
Anti-PD-1 or Anti-PD-L1 in EC
The aforementioned trials have established the addition of ICI to chemotherapy in dMMR/MSI-H and pMMR/MSS advanced stage and recurrent EC. Bartoletti et al completed a meta-analysis to investigate the benefit of immunotherapy in combination with chemotherapy as well as an interesting concept of deciphering the effect of anti-PD-1 versus anti-PD-L1 therapy in EC cohorts. They investigated efficacy by MMR status and drug type (anti-PD1 vs anti-PD-L1). The analysis contained five studies including the following immunotherapies: anti-PD1 (pembrolizumab, dostarlimab), and anti-PD-L1 (durvalumab, atezolizumab, avelumab) in conjunction with traditional carboplatin-paclitaxel chemotherapy. Concordantly, they reported greater benefit in PFS when ICI was added to chemotherapy in the dMMR population (HR 0.34), and no difference was found when comparing the use of anti-PD-L1 vs anti-PD1 (HR 0.39 and 0.34). The analysis revealed a significant PFS benefit in pMMR patients (HR 0.77) as well, though, noted that this benefit was only significant when anti-PD-1 therapy was used and not in pMMR EC treated with anti-PD-L1 drugs.31 Currently, there are no anti-PD-L1 drugs approved for pMMR EC.
Ongoing Trials
DOMENICA study (NCT05201547) is an ongoing, international, randomized, open-label phase III trial in which patients with MMRd/MSI-H EC with Stage III or Stage IV or first recurrent disease without curative options are randomized to the current treatment standard with six cycles of carboplatin/paclitaxel chemotherapy versus dostarlimab monotherapy (500mg intravenously every three weeks for four doses), followed by dostarlimab maintenance (1000mg every six weeks) for up to two years (treatment). The primary endpoint to be studied is PFS. This study will add to the literature to assess how dMMR/MSI-H EC responds to dostarlimab monotherapy in the upfront setting; however, a major limitation in the design is the lack of immunotherapy in the comparison arm.32
Safety Profile of Dostarlimab
Interference with the PD-1/PD-L1 pathway disrupts a regulatory response of the immune system. This disinhibition of the immune response occurs not only on cancer cells but on non-cancer cells that express PD-L1 for the purpose of anti-inflammatory protection, including cells of the hematopoietic, skin, heart, pancreas, vascular endothelium, lung, muscle, liver, and eye tissues.33 Blockade of PD-1/PD-L1 can therefore lead to immune-mediated adverse effects and, occasionally, autoimmune diseases. Dostarlimab use, for example, has been associated with the development of immune-mediated pneumonitis, colitis, hepatitis, nephritis, and dermatitis. Reports have also shown immune-mediated endocrinopathies including adrenal insufficiency, hypophysitis, thyroid disorder, and type 1 Diabetes Mellitus (all uncommonly seen, <2%, except hypothyroidism at incidence 7%).34
In the GARNET study, the incidence of grade 3 or higher treatment-related adverse events (TRAEs) using dostarlimab as monotherapy was 12%, with anemia (3%) as the most common grade 3 AE and colitis (2%) as the most common serious AE. The most reported TRAEs overall were asthenia, diarrhea, fatigue and nausea. Immune-related adverse events occurred in 23% of patients with diarrhea (6%), hypothyroidism (6%) as most frequent, and pneumonitis reported in one patient. In RUBY part 1, with the addition of dostarlimab to chemotherapy, serious adverse events were more common in the dostarlimab group (38% vs 28%). The most common AEs leading to discontinuation of dostarlimab were maculopapular rash and infusion-related reactions. Immune-related AEs occurred in 41% of the dostarlimab plus chemotherapy arm, with hypothyroidism (11% vs 3%) and rash (7% vs 2%) being most frequent.22 In RUBY part 2, with the addition of niraparib to dostarlimab in the maintenance period, grade 3 TRAEs were more common (85% vs 50%) compared to placebo maintenance; most seen were anemia, neutropenia and thrombocytopenia. Despite these findings, overall, the safety profile of the addition of dostarlimab in these studies has been acceptable and expected.
Conclusion
Historically, systemic treatment options in the advanced and recurrent endometrial cancer setting were limited. With recent findings, dostarlimab and pembrolizumab are indicated and available in EC in combination with carboplatin/paclitaxel and subsequently as maintenance therapy for as first-line systemic therapy for dMMR/MSI-H and pMMR/MSS primary advanced and recurrent disease as supported by FDA approval. In first-line, use of dostarlimab and pembrolizumab in advanced and recurrent EC, the most prominent benefit was seen in the PFS for the dMMR/MSI-H population. For the dMMR/MSI-H population, dostarlimab, pembrolizumab or durvalumab can be chosen; however, RUBY part 1 analyzing the primary endpoint of OS for dMMR/MSI-H population makes this an attractive choice. Durvalumab would be less desirable as the study was not powered to evaluate the EC by MMR cohort status. For the overall population of primary advanced stage or metastatic EC, dostarlimab should be chosen in patients with carcinosarcoma histology as they were included in RUBY part 1. For first-line treatment in the pMMR/MSS population of primary advanced stage or metastatic EC, pembrolizumab should be considered, as GYO18 was the only study with a primary endpoint powered to evaluate the pMMR/MSS cohort and reported the most substantial PFS benefit in this population (Figure 2). Furthermore, pembrolizumab has been shown to be more cost-effective in both the pMMR/MSS and dMMR/MSI-H populations when evaluating the estimated incremental cost-effectiveness ratios based on progression-free-life-year saved, delineating a stronger point for its use in the pMMR/MSS population.35 In future settings, there may be a role for immunotherapy in combination with carboplatin/paclitaxel followed by maintenance immunotherapy combined with a PARP inhibitor in both the dMMR/MSI-H and pMMR/MSS patients, potentially with a promising effect in pMMR/MSS EC, though a comparison to the new standard of care is necessary.
In the second-line setting for advanced stage or metastatic dMMR/MSI-H EC dostarlimab or pembrolizumab as monotherapy should be utilized as monotherapy. An advantage of pembrolizumab as monotherapy in the second-line setting is its additional approval for TMB-H tumors. In the second-line setting for advanced stage or metastatic pMMR/MSS EC, pembrolizumab plus lenvatinib is the preferred regimen. There is maintained interest in studying a non-chemotherapy option (eg dostarlimab monotherapy or in combination with a novel agent) as primary therapy for newly diagnosed advanced and/or recurrent dMMR/MSI-H EC disease. Continued exploration of predictors of immunotherapy efficacy in EC beyond MMR and microsatellite status will remain important.
Disclosure
Dr Krishnansu S Tewari reports personal fees from GSK, during the conduct of the study; personal fees from Merck, Astra Zeneca, Eisai, GSK, Seagen/Genmab, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. World Cancer Resarch Fund International. Endometrial cancer statistics.
2. World Health Organization (WHO). Global Cancer Observatory. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj. https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf.
3. World Health Organization (WHO). Cancer Tomorrow. International Agency for Research on Cancer. https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=24&single_unit=5000&age_end=17&years=2045.
4. Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31(20):2607–2618. doi:10.1200/JCO.2012.48.2596
5. Getz G, Gabriel SB, Cibulskis K, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi:10.1038/nature12113
6. Berek JS, Matias-Guiu X, Creutzberg C, et al. FIGO staging of endometrial cancer: 2023. Int J Gynecol Obstet. 2023;162(2):383–394. doi:10.1002/ijgo.14923
7. Murali R, Delair DF, Bean SM, Abu-Rustum NR, Soslow RA. Evolving roles of histologic evaluation and molecular/genomic profiling in the management of endometrial cancer. J Natl Compr Canc Netw. 2018;16(2):201–209. doi:10.6004/jnccn.2017.7066
8. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):1–14. doi:10.1186/s13045-019-0738-1
9. Le DT, Durham JN, Smith KN, et al. HHS Public Access. 6349. 357:409–413.
10. Oaknin A, V. TA, Gilbert L, et al. Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients with Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: a Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020;6(11):1766–1772. doi:10.1001/jamaoncol.2020.4515
11. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi:10.1016/j.intimp.2018.06.001
12. Laken H, Kehry M, McNeeley P. Identification and characterization of TSR-042, a novel anti-human PD-1 therapeutic antibody. Eur J Cancer. 69. doi:10.1016/S0959-8049(16)32902-1
13. Austin D, Melhem M, Gandhi Y, Lu S, Visser S. Comparative analysis of PD-1 target engagement of dostarlimab and pembrolizumab in advanced solid tumors using ex vivo IL-2 stimulation data. CPT Pharmac yst Pharmacol. 2023;12(1):87–94. doi:10.1002/psp4.12878
14. Syed YY. Durvalumab: first Global Approval. Drugs. 2017;77(12):1369–1376. doi:10.1007/s40265-017-0782-5
15. Cercek A, Lumish M, Sinopoli J, et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386(25):2363–2376. doi:10.1056/nejmoa2201445
16. Miller DS, Filiaci VL, Mannel RS, et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol. 2020;38(33):3841–3850. doi:10.1200/JCO.20.01076
17. Marabelle A, Le DT, Ascierto PA, Maria A, Giacomo D, De J-AA. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability / Mismatch Repair – de fi cient Cancer: results From the Phase II KEYNOTE-158 Study abstract. J Cl. 2020;38(1):1–10. doi:10.1200/JCO.19.02105
18. Oaknin A, Pothuri B, Gilbert L, et al. Safety, Efficacy, and Biomarker Analyses of Dostarlimab in Patients with Endometrial Cancer: interim Results of the Phase I GARNET Study. Clin Cancer Res. 2023;29(22):4564–4574. doi:10.1158/1078-0432.CCR-22-3915
19. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med. 2022;386(5):437–448. doi:10.1056/nejmoa2108330
20. Suyama K, Iwase H. Lenvatinib: a Promising Molecular Targeted Agent for Multiple Cancers. Cancer Control. 2018;25(1):1–5. doi:10.1177/1073274818789361
21. FDA. FDA grants regular approval to pembrolizumab and lenvatinib for advanced endometrial carcinoma. Drug approvals and databases. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pembrolizumab-and-lenvatinib-advanced-endometrial-carcinoma.
22. Mirza MR, Chase DM, Slomovitz BM, et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med. 2023;388(23):2145–2158. doi:10.1056/nejmoa2216334
23. Powell MA, Auranen A, Willmott L, et al. Overall Survival in Patients with Primary Advanced or Recurrent Endometrial Cancer Treated with Dostarlimab plus Chemotherapy in Part 1 of the ENGOT-EN6-NSGO/GOG-3031/RUBY Trial (LBA1); 2024.
24. FDA. Jemperli (dostarlimab-gxly) injection, for intravenous use, prescribing information, GlaxoSmithKline; 2023. Drug approvals and databases chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761174s006lbl.pdf.
25. FDA. FDA expands endometrial cancer indication for dostarlimab-gxly with chemotherapy. Drug approvals and databases. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-endometrial-cancer-indication-dostarlimab-gxly-chemotherapy.
26. Mirza MR, Ghamande S, Hanker LC, et al. Dostarlimab plus Chemotherapy Followed by Dostarlimab plus Niraparib Maintenance Therapy in Patients with Primary Advanced or Recurrent Endometrial Cancer in Part 2 of the ENGOT-EN6-NSGO/GOG-3031/RUBY Trial (LBA2); 2024.
27. Eskander RN, Sill MW, Beffa L, et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N Engl J Med. 2023;388(23):2159–2170. doi:10.1056/nejmoa2302312
28. FDA. FDA approves pembrolizumab with chemotherapy for primary advanced or recurrent endometrial carcinoma. Drug approvals and databases. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-chemotherapy-primary-advanced-or-recurrent-endometrial-carcinoma.
29. Westin SN, Moore K, Chon HS, et al. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab With or Without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: the Phase III DUO-E Trial. J Clin Oncol. 2024;42(3):283–299. doi:10.1200/JCO.23.02132
30. FDA. FDA approves durvalumab with chemotherapy for mismatch repair deficient primary advanced or recurrent endometrial cancer. Drug approvals and databases. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-chemotherapy-mismatch-repair-deficient-primary-advanced-or-recurrent.
31. Bartoletti M, Montico M, Lorusso D, et al. Incorporation of anti-PD1 or anti PD-L1 agents to platinum-based chemotherapy for the primary treatment of advanced or recurrent endometrial cancer. A meta-analysis. Cancer Treat Rev. 2024;125:102701. doi:10.1016/j.ctrv.2024.102701
32. Choi CH, Ray-Coquard I, Rubio MJ, et al. DOMENICA study (GINECO-EN105b/ENGOT-en13): randomized phase III trial in MMR deficient (MMRd) endometrial cancer (EC) patients comparing chemotherapy (CT) alone versus dostarlimab in first line advanced/metastatic setting (APGOT-EN1). J Gynec Onc. 2024:P1. doi:10.3802/jgo.2024.35.s2.p1
33. Qin W, Hu L, Zhang X, et al. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front Immunol. 2019;10(October):1–16. doi:10.3389/fimmu.2019.02298
34. FDA. FDA approves dostarlimab-gxly with chemotherapy for endometrial cancer. Drug approvals and databases. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-dostarlimab-gxly-chemotherapy-endometrial-cancer.
35. Francoeur A, Richardson M, Tewari K, Chan J. Cost-Effectiveness Analysis of Immunotherapy Regimes in Upfront Treatment With Chemotherapy for Endometrial Cancer. Journal of Clinical Pathways. https://www.hmpgloballearningnetwork.com/site/jcp/news/cost-effectiveness-analysis-immunotherapy-regimes-upfront-treatment-chemotherapy.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.