Back to Journals » Infection and Drug Resistance » Volume 18
Coagulation Parameters in Elderly Patients with Severe Pneumonia: Correlation with Disease Severity and Prognosis
Authors Zhang Q, Liu Y, Tong C, Zhang L, Li R, Guo W, Li J
Received 29 October 2024
Accepted for publication 2 January 2025
Published 17 January 2025 Volume 2025:18 Pages 341—350
DOI https://doi.org/10.2147/IDR.S497755
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Qiuyue Zhang,1,* Yingchao Liu,1,* Chuntang Tong,2 Lina Zhang,3 Rongchen Li,1 Wenbin Guo,4 Jianliang Li5
1Department of Clinical Laboratory, The Second People’s Hospital of Liaocheng, Linqing, Shandong, 252600, People’s Republic of China; 2Department of Respiratory Medicine, The Second People’s Hospital of Liaocheng, Linqing, Shandong, 252600, People’s Republic of China; 3Department of Scientific Research Management Division, The Second People’s Hospital of Liaocheng, Linqing, Shandong, 252600, People’s Republic of China; 4Department of Intensive Care Unit, The Second People’s Hospital of Liaocheng, Linqing, Shandong, 252600, People’s Republic of China; 5Department of Thoracic Surgery, The Second People’s Hospital of Liaocheng, Linqing, Shandong, 252600, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianliang Li, Department of Thoracic Surgery The Second People’s Hospital of Liaocheng, 306 health Street, Linqing, Shandong, 252600, People’s Republic of China, Tel +86 15020622456, Email [email protected]
Objective: This study aimed to investigate the levels of coagulation parameters in elderly patients with severe pneumonia and analyse their correlation with disease severity and prognosis.
Methods: A retrospective study was conducted on 207 elderly patients (aged ≥ 60 years) with severe pneumonia admitted to our hospital between January 2022 and December 2023. Demographic data, clinical characteristics and coagulation parameters, including prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time and fibrinogen (FIB), were collected. Patients were divided into survivor and non-survivor groups based on 28-day mortality. The differences in coagulation parameters between groups and their correlation with disease severity and prognosis were analysed.
Results: The 28-day mortality rate was 52.2%. Non-survivors had significantly higher PT, APTT and D-dimer levels and lower FIB levels than survivors (p < 0.05). Multivariate logistic regression analysis showed that elevated PT (odds ratio [OR] = 1.218, 95% confidence interval [CI]: 1.076– 1.379, p = 0.002) and D-dimer (OR = 1.109, 95% CI: 1.032– 1.192, p = 0.005) were independent risk factors for 28-day mortality. The combined model using PT and D-dimer showed the highest predictive value for 28-day mortality (area under the curve = 0.801, 95% CI: 0.739– 0.863, p < 0.001), with a sensitivity of 0.759 and specificity of 0.758.
Conclusion: Coagulation dysfunction is common in elderly patients with severe pneumonia. Prothrombin time and D-dimer levels are closely associated with disease severity and can be valuable indicators for predicting prognosis in this population.
Keywords: elderly, severe pneumonia, coagulation parameters, disease severity, prognosis
Introduction
Pneumonia remains a leading cause of morbidity and mortality in the elderly population, with severe pneumonia posing a particularly significant threat to life.1 The susceptibility of elderly people to severe pneumonia is multifactorial, involving age-related changes in immune function, comorbidities and alterations in physiological reserves.2 As global demographics shift towards an ageing population, the incidence of severe pneumonia in elderly people is projected to rise, presenting substantial challenges to healthcare systems worldwide.3 As elderly patients are often associated with a variety of underlying diseases and reduced immune function, this leads to a poorer prognosis.4 Due to the limited physiological reserve of elderly patients, serious complications caused by pneumonia, such as respiratory failure and sepsis, may accelerate the deterioration of the condition and increase the risk of death.5 Therefore, timely assessment of the prognosis of severe pneumonia in elderly people is crucial. Searching for accurate prognostic factors can provide clinicians with a powerful aid to help optimise treatment options, guide disease monitoring and decision-making and improve patient outcomes and survival rates.
The intricate relationship between inflammation and coagulation plays a pivotal role in the pathogenesis of severe pneumonia.6 Coagulation dysfunction is a frequent complication observed in patients with severe pneumonia, contributing significantly to disease progression and poor outcomes.7 The inflammatory response triggered by severe pneumonia leads to the activation of the coagulation cascade, resulting in a hypercoagulable state. This state can potentially lead to microvascular thrombosis, further exacerbating lung injury and multi-organ dysfunction.8
The coagulation system undergoes several age-related changes, including increased levels of coagulation factors, decreased fibrinolytic activity and enhanced platelet reactivity.9 These alterations may predispose elderly individuals to a higher risk of thrombotic complications and more severe coagulation disturbances during acute illnesses such as pneumonia. Understanding the specific characteristics of coagulation dysfunction in elderly patients with severe pneumonia is crucial for improving risk stratification and guiding appropriate management strategies.
Several studies have demonstrated the prognostic value of coagulation parameters in various critical illnesses, including sepsis and acute respiratory distress syndrome (ARDS).10,11 These parameters, such as prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FIB) and D-dimer, have shown associations with disease severity and outcomes. However, limited research has focused specifically on the elderly population with severe pneumonia. Given the physiological changes and increased baseline coagulation activation in elderly people, it is crucial to investigate the clinical significance of coagulation parameters in this vulnerable group.12
In this study, we hypothesise that coagulation dysfunction is associated with poor prognosis and that certain coagulation parameters may serve as important prognostic indicators in this population. The study aims to assess the levels of coagulation parameters in elderly patients with severe pneumonia and to analyse their correlation with disease severity and prognosis; this can provide guidance for risk stratification and targeted management strategies for elderly patients with severe pneumonia.
Methods
Study Design and Participants
This retrospective study was conducted at our hospital between January 2022 and December 2023. The study protocol was approved by the hospital ethics committee (2023YLSNo.68), and the requirement for informed consent was waived due to the retrospective nature of the study.
The inclusion criteria were as follows: (1) age ≥60 years; (2) meeting the criteria for a diagnosis of severe pneumonia according to the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) guidelines.13 The diagnostic criteria for severe pneumonia included either one major criterion or three or more minor criteria. Major criteria comprised invasive mechanical ventilation or septic shock with the need for vasopressors. Minor criteria included respiratory rate ≥30 breaths/min, PaO2/FiO2 ratio ≤250, multilobar infiltrates, confusion/disorientation, uraemia (blood urea nitrogen [BUN] level ≥20 mg/dL), leukopenia (white blood cell [WBC] count <4000 cells/μL), thrombocytopenia (platelet count <100,000/μL), hypothermia (core temperature <36°C) or hypotension requiring aggressive fluid resuscitation.
The exclusion criteria were as follows: (1) age <60 years; (2) pregnancy; (3) haematological diseases that could affect coagulation parameters; (4) liver cirrhosis; (5) use of anticoagulant or antiplatelet drugs within 1 week before admission; or (6) incomplete clinical data.
Data Collection
Demographic data, clinical characteristics, laboratory findings and treatment information were extracted from electronic medical records by two independent researchers. Any discrepancies were resolved through discussion or consultation with a third researcher. The following data were collected:
Demographic Information – Age, Gender
Comorbidities – hypertension, diabetes mellitus, coronary heart disease, chronic obstructive pulmonary disease.
Clinical characteristics – mechanical ventilation status, intensive care unit (ICU) admission, oxygen therapy modalities, supportive treatments and length of hospital stay.
Laboratory parameters – (a) routine blood tests (WBC count, neutrophil percentage, lymphocyte percentage, platelet count); (b) inflammatory markers (C-reactive protein [CRP], procalcitonin [PCT]); (c) liver and kidney function tests (alanine aminotransferase, aspartate aminotransferase, total protein, albumin, globulin, BUN, creatinine); (d) arterial blood gas analysis (pH, PaO2, PaCO2, lactate); (e) coagulation parameters (PT, APTT, thrombin time [TT], FIB and D-dimer).
Coagulation parameters were measured on admission, day 3 and day 7 of hospitalisation. All laboratory tests were performed in the clinical laboratory of the hospital using standard methods. Coagulation tests were performed using an automated coagulation Werfen ACL TOP 750 analyser.
Definitions and Outcomes
Severe pneumonia was defined according to the IDSA/ATS guidelines as described in the participant selection criteria.13 The primary outcome was 28-day all-cause mortality. Patients were divided into survivor and non-survivor groups based on their 28-day outcome. Secondary outcomes included the need for mechanical ventilation, ICU admission and length of hospital stay.
Statistical Analysis
Statistical analysis was performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). The sample size was calculated based on the expected difference in PT levels between survivors and non-survivors, with a power of 80% and a significance level of 0.05. The calculated minimum sample size was 200 patients.
Continuous variables were tested for normality using the Kolmogorov–Smirnov test. Normally distributed variables were expressed as mean ± standard deviation and compared using Student’s t-test. Non-normally distributed variables were presented as median (interquartile range) and compared using the Mann–Whitney U-test. Categorical variables were presented as frequencies and percentages and compared using the chi-squared test or Fisher’s exact test as appropriate.
The dynamic changes of coagulation parameters over time were analysed using repeated measures analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons. The sphericity assumption was tested using Mauchly’s test, and the Greenhouse–Geisser correction was applied when the assumption was violated.
Univariate and multivariate logistic regression analyses were conducted to identify independent risk factors for 28-day mortality. Variables with a p-value of <0.1 in the univariate analysis were included in the multivariate model. The results were presented as OR with a 95% confidence interval (CI).
To identify potential factors affecting 28-day mortality, a one-way logistic regression analysis was first performed for each variable (including clinical characteristics and biochemical indicators). In the multifactorial analysis, variables with significance (p < 0.05) in the univariate analysis were selected as input variables, and variable screening and model construction were performed by stepwise regression. The OR for each variable and its 95% CI were calculated to quantify the independent effect of each factor on mortality. The performance of these models, including the combined model and one-factor model, was assessed using the area under the receiver operating characteristic curve (AUC), sensitivity, specificity, positive predictive value and negative predictive value. The optimal cut-off points were determined using the Youden index.
Spearman correlation analysis was performed to assess the relationship between coagulation parameters and other clinical indicators. The strength of correlations was interpreted as follows: 0–0.19 (very weak), 0.2–0.39 (weak), 0.4–0.59 (moderate), 0.6–0.79 (strong) and 0.8–1.0 (very strong).
A two-tailed p-value of <0.05 was considered statistically significant. To account for multiple comparisons, the Bonferroni correction was applied where appropriate, and the adjusted significance level was reported.
Results
Baseline Characteristics
A total of 207 elderly patients with severe pneumonia were included in the study. The mean age was 75.22 ± 7.69 years (range: 60–94 years), and 133 (64.3%) were male. The 28-day mortality rate was 52.2% (108/207). Table 1 presents the baseline characteristics of the study population stratified by 28-day survival status.
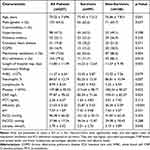 |
Table 1 Baseline Characteristics of the Study Population |
Comparison of Coagulation Parameters Between Survivors and Non-Survivors
Table 2 shows the comparison of coagulation parameters between survivors and non-survivors on admission, day 3 and day 7 of hospitalisation. The results showed that there were significant differences between survivors and non-survivors at admission, on day 3 and day 7 in PT, APTT, FIB and D-dimer, with the difference in D-dimer being particularly significant, suggesting that activation of the coagulation system may be closely related to the prognosis of the patients.
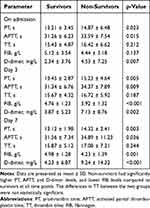 |
Table 2 Comparison of Coagulation Parameters Between Survivors and Non-Survivors |
Dynamic Changes of Coagulation Parameters
Repeated measures ANOVA was used to analyse the dynamic changes of coagulation parameters in survivors and non-survivors over the first week of hospitalisation. The results are summarised below (Table 3).
 |
Table 3 The Dynamic Changes of Coagulation Parameters in Survivors and Non-Survivors Over the First week of Hospitalization |
PT: A significant time effect (F = 12.34, p < 0.001) and group effect (F = 18.76, p < 0.001) were observed. The interaction between time and group was also significant (F = 4.52, p = 0.012), indicating that the pattern of change in PT differed between survivors and non-survivors. Non-survivors showed a more pronounced increase in PT levels over time.
APTT: There was a significant time effect (F = 8.65, p < 0.001) and group effect (F = 14.23, p < 0.001). The time-group interaction was not significant (F = 2.18, p = 0.114), suggesting that although non-survivors had consistently higher APTT levels, the pattern of change was similar in both groups.
FIB: A significant time effect (F = 15.87, p < 0.001) and group effect (F = 22.45, p < 0.001) were observed. The time-group interaction was significant (F = 6.34, p = 0.002), indicating that the pattern of change in FIB levels differed between survivors and non-survivors. Non-survivors showed a more significant decrease in FIB levels over time.
D-dimer: There was a significant time effect (F = 28.76, p < 0.001) and group effect (F = 31.54, p < 0.001). The time-group interaction was also significant (F = 9.87, p < 0.001), suggesting that non-survivors experienced a more pronounced increase in D-dimer levels over time compared with survivors.
TT: No significant time effect (F = 1.87, p = 0.156), group effect (F = 2.34, p = 0.128) or time–group interaction (F = 0.76, p = 0.469) was observed for TT levels.
These results demonstrate that non-survivors exhibited more severe and progressive coagulation dysfunction over the course of their hospital stay compared with survivors.
Risk Factors for Twenty-Eight-Day Mortality
Univariate and multivariate logistic regression analyses were performed to identify independent risk factors for 28-day mortality (Table 4).
 |
Table 4 Univariate and Multivariate Logistic Regression Analysis of Risk Factors for 28-Day Mortality |
Multivariate logistic regression analysis showed that age (OR = 1.048, 95% CI: 1.009–1.089, p = 0.016), albumin (OR = 0.934, 95% CI: 0.878–0.993, p = 0.029), PT (OR = 1.218, 95% CI: 1.076–1.379, p = 0.002) and D-dimer (OR = 1.109, 95% CI: 1.032–1.192, p = 0.005) were independent risk factors for 28-day mortality.
Predictive Value of Coagulation Parameters for Twenty-Eight-Day Mortality
Logistic regression models were used to evaluate the predictive value of coagulation parameters for 28-day mortality. The performance of these models is summarised in Table 5.
The combined model using PT and D-dimer showed the highest predictive value for 28-day mortality (AUC = 0.801, 95% CI: 0.739–0.863, p < 0.001), with a sensitivity of 0.759 and specificity of 0.758. This model demonstrated superior performance compared with individual coagulation parameters in predicting mortality.
 |
Table 5 Predictive Performance of Coagulation Parameters for 28-Day Mortality |
Correlation Between Coagulation Parameters and Other Clinical Indicators
Spearman correlation analysis was performed to assess the relationship between coagulation parameters and other clinical indicators (Table 6).
 |
Table 6 Correlation Between Coagulation Parameters and Other Clinical Indicators |
Prothrombin time, APTT and D-dimer showed positive correlations with age, neutrophil percentage, CRP, PCT and lactate and negative correlations with lymphocyte percentage and albumin. Fibrinogen was positively correlated with WBC, neutrophil percentage, platelet count, CRP and PCT and negatively correlated with lymphocyte percentage and albumin. These correlations highlight the complex interplay between coagulation dysfunction, inflammation and organ dysfunction in elderly patients with severe pneumonia.
Discussion
This comprehensive study investigated the clinical significance of coagulation parameters in elderly patients with severe pneumonia and their correlation with disease severity and prognosis. Our findings demonstrate that coagulation dysfunction is common in this population and that certain coagulation parameters, particularly PT and D-dimer, are closely associated with disease severity and can serve as valuable prognostic indicators.
The 28-day mortality rate in our study was 52.2%, which is higher than that reported in some previous studies on severe pneumonia.14,15 This discrepancy may be attributed to several factors. First, our study focused specifically on elderly patients (aged ≥60 years), who are known to have higher mortality rates from severe infections compared with younger populations.16 The advanced age of our cohort (mean age 75.22 years) likely contributed to the increased mortality rate. Second, our study included a high proportion of patients requiring mechanical ventilation (72.0%) and ICU admission (79.2%), reflecting the severity of their condition. These factors are known to be associated with increased mortality in patients with pneumonia.17
Our results showed that non-survivors had significantly higher PT, APTT and D-dimer levels and lower FIB levels compared with survivors. These findings are consistent with previous studies on sepsis and ARDS, which have reported similar alterations in coagulation parameters in non-survivors.18,19 The prolongation of PT and APTT reflects the consumption of coagulation factors and the overall activation of the coagulation system. This consumption coagulopathy can lead to a state of hypocoagulability, which may contribute to bleeding complications in patients with severe pneumonia.20
D-dimer has been widely recognised as a marker of disease severity and poor prognosis in various critical illnesses, including pneumonia.21 Our study confirms its prognostic value in elderly patients with severe pneumonia. The decrease in FIB levels observed in non-survivors may be due to its consumption in the coagulation process and its role as an acute-phase reactant.22
The dynamic changes of coagulation parameters over the first week of hospitalisation revealed significant differences between survivors and non-survivors. Non-survivors exhibited a more pronounced deterioration in coagulation function, characterised by a steeper increase in PT, APTT and D-dimer levels and a more significant decrease in FIB levels. These findings highlight the importance of serial measurements of coagulation parameters in monitoring disease progression and predicting outcomes in elderly patients with severe pneumonia.
The progressive worsening of coagulation parameters in non-survivors may reflect the development of disseminated intravascular coagulation (DIC), a severe complication associated with poor outcomes in critically ill patients.7 The International Society on Thrombosis and Haemostasis DIC score, which incorporates PT, platelet count, FIB and D-dimer, could be a valuable tool for assessing the development of DIC in this population.23 Future studies should investigate the incidence and impact of DIC in elderly patients with severe pneumonia.
Multivariate logistic regression analysis identified PT and D-dimer as independent risk factors for 28-day mortality, along with age and albumin levels. The association between advanced age and poor outcomes in pneumonia has been well-established in previous studies.24 Hypoalbuminaemia is a known predictor of mortality in critically ill patients and may reflect the severity of the underlying inflammatory response and nutritional status.25
The prognostic value of PT and D-dimer in our study is particularly noteworthy. Prolonged PT indicates a deficiency in the extrinsic coagulation pathway and has been associated with increased mortality in various critical illnesses.26 Our findings suggest that PT could be a valuable addition to existing severity assessment tools for pneumonia, such as the CURB-65 or Pneumonia Severity Index.27
D-dimer, a product of fibrin degradation, is not only a marker of coagulation activation but also reflects the severity of the inflammatory response.28 The strong predictive value of D-dimer observed in our study is consistent with previous research in community-acquired pneumonia and other critical illnesses.21 The combination of PT and D-dimer showed the highest predictive value for 28-day mortality in our study, suggesting that integrating these parameters could enhance prognostic accuracy in elderly patients with severe pneumonia.
The correlation analysis revealed significant associations between coagulation parameters and other clinical indicators of disease severity, such as inflammatory markers (CRP, PCT) and markers of organ dysfunction (albumin, lactate). These findings underscore the complex interplay between inflammation, coagulation and organ failure in severe pneumonia.29 The positive correlation between coagulation parameters and inflammatory markers supports the concept of immunothrombosis, where inflammation and coagulation act synergistically in the host’s response to infection.30
The relationship between coagulation dysfunction and poor outcomes in elderly patients with severe pneumonia may be explained by several mechanisms. First, the hypercoagulable state and microvascular thrombosis can lead to impaired tissue perfusion and organ dysfunction.6 Second, excessive consumption of coagulation factors may result in bleeding complications, further compromising patient outcomes.7 Third, the interplay between coagulation and inflammation can create a vicious cycle, exacerbating lung injury and systemic inflammation.8
Our study has several clinical implications. First, it highlights the importance of routine monitoring of coagulation parameters in elderly patients with severe pneumonia. Early identification of coagulation dysfunction may allow for timely interventions to prevent further deterioration. Second, the prognostic value of PT and D-dimer suggests that these parameters could be incorporated into existing risk stratification tools to improve their accuracy in predicting outcomes in this population.
Third, the strong association between coagulation dysfunction and poor outcomes raises the question of whether targeted anticoagulant therapies could improve outcomes in elderly patients with severe pneumonia. While the use of anticoagulants in sepsis and ARDS has shown mixed results,31,32 the specific characteristics of the elderly population with severe pneumonia may warrant further investigation.
Finally, our study underscores the importance of age-specific research in critical care. The coagulation system undergoes significant changes with ageing, and the response to severe infections may differ in elderly patients compared with younger populations.33 Developing age-specific diagnostic and therapeutic approaches could potentially improve outcomes in this vulnerable group.
Several limitations to our study should be acknowledged. First, its retrospective nature and single-centre design may limit the generalisability of our findings. Multicentre prospective studies are needed to validate our results in diverse healthcare settings. Second, we did not have data on some potentially relevant factors, such as the aetiology of pneumonia or the use of anticoagulant therapies during hospitalisation. Future studies should consider these factors to provide a more comprehensive understanding of coagulation dysfunction in elderly patients with severe pneumonia. Finally, although we identified PT and D-dimer as the most valuable prognostic indicators, the optimal cut-off values for these parameters in elderly patients with severe pneumonia require further validation. Larger studies with external validation cohorts are needed to establish robust, clinically applicable thresholds.
Conclusion
Our study demonstrates that coagulation dysfunction is common in elderly patients with severe pneumonia and is closely associated with disease severity and poor outcomes. Prothrombin time and D-dimer levels are independent risk factors for 28-day mortality and show promising predictive value for prognosis in this population. The dynamic changes in coagulation parameters over time provide valuable information about disease progression and may guide clinical decision-making.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of The Second People’s Hospital of Liaocheng (2023YLSNo.68). The requirement for informed consent was waived due to the retrospective nature of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Liaocheng City Key R&D Plan Policy Guidance Project (No.: 2023YD48).
Disclosure
The authors declare that they have no competing interests.
References
1. Tsoumani E, Carter JA, Salomonsson S, Stephens JM, Bencina G. Clinical, economic, and humanistic burden of community acquired pneumonia in Europe: a systematic literature review. Expert Rev Vaccines. 2023;22(1):876–884. doi:10.1080/14760584.2023.2261785
2. Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI. Community-acquired pneumonia. Lancet. 2021;398(10303):906–919. doi:10.1016/S0140-6736(21)00630-9
3. Zhao D, Sun Y, Li J, et al. Effectiveness of inactivated COVID-19 vaccines in preventing COVID-19-related hospitalization during the Omicron BF.7-predominant epidemic wave in Beijing, China: a cohort study. BMC Infect Dis. 2024;24(1):991. doi:10.1186/s12879-024-09889-7
4. Ocrospoma S, Restrepo MI. Severe aspiration pneumonia in the elderly. J Intensive Med. 2024;4(3):307–317. doi:10.1016/j.jointm.2023.12.009
5. Yoshimatsu Y, Thomas H, Thompson T, Smithard DG. Prognostic factors of poor outcomes in pneumonia in older adults: aspiration or frailty? Eur Geriatr Med. 2024;15(2):481–488. doi:10.1007/s41999-023-00929-0
6. Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi:10.1016/j.thromres.2016.11.007
7. Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192(5):803–818. doi:10.1111/bjh.17172
8. Rayes J, Bourne JH, Brill A, Watson SP. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res Pract Thromb Haemost. 2019;4(1):23–35. doi:10.1002/rth2.12266
9. Franchini M. Hemostasis and aging. Crit Rev Oncol Hematol. 2006;60(2):144–151. doi:10.1016/j.critrevonc.2006.06.004
10. Rodelo JR, De la Rosa G, Valencia ML, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30(9):1991–1999. doi:10.1016/j.ajem.2012.04.033
11. Vassiliou AG, Kotanidou A, Dimopoulou I, Orfanos SE. Endothelial damage in acute respiratory distress syndrome. Int J mol Sci. 2020;21(22):8793. doi:10.3390/ijms21228793
12. Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Exp Gerontol. 2008;43(2):66–73. doi:10.1016/j.exger.2007.06.014
13. Olson G, Davis AM. Diagnosis and treatment of adults with community-acquired pneumonia. JAMA. 2020;323(9):885–886. doi:10.1001/jama.2019.21118
14. Cilloniz C, Peroni HJ, Gabarrús A, et al. Lymphopenia is associated with poor outcomes of patients with community-acquired pneumonia and sepsis. Open Forum Infect Dis. 2021;8(6):ofab169. doi:10.1093/ofid/ofab169
15. Blanc E, Chaize G, Fievez S, et al. The impact of comorbidities and their stacking on short- and long-term prognosis of patients over 50 with community-acquired pneumonia. BMC Infect Dis. 2021;21(1):949. doi:10.1186/s12879-021-06669-5
16. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi:10.1097/01.ccm.0000194535.82812.ba
17. Ferrer M, Travierso C, Cilloniz C, et al. Severe community-acquired pneumonia: characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS One. 2018;13(1):e0191721. doi:10.1371/journal.pone.0191721
18. Giarratano A. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Aboutopen. 2022;9(1):58–60. doi:10.33393/ao.2022.2434
19. Zeineddin A, Dong JF, Wu F, Terse P, Kozar RA. Role of von Willebrand factor after injury: it may do more than we think. Shock. 2021;55(6):717–722. doi:10.1097/SHK.0000000000001690
20. Ikezoe T. Advances in the diagnosis and treatment of disseminated intravascular coagulation in haematological malignancies. Int J Hematol. 2021;113(1):34–44. doi:10.1007/s12185-020-02992-w
21. Cerda-Mancillas MC, Santiago-Germán D, Andrade-Bravo B, et al. D-dimer as a biomarker of severity and adverse outcomes in patients with community acquired pneumonia. Arch Med Res. 2020;51(5):429–435. doi:10.1016/j.arcmed.2020.04.014
22. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. doi:10.1007/s00281-011-0290-8
23. Iba T, Levi M, Thachil J, Levy JH. Disseminated intravascular coagulation: the past, present, and future considerations. Semin Thromb Hemost. 2022;48(8):978–987. doi:10.1055/s-0042-1756300
24. Lüthi-Corridori G, Boesing M, Roth A, et al. Predictors of length of stay, rehospitalization and mortality in community-acquired pneumonia patients: a retrospective cohort study. J Clin Med. 2023;12(17):5601. doi:10.3390/jcm12175601
25. Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237(3):319–334. doi:10.1097/01.SLA.0000055547.93484.87
26. Collins PW, Macchiavello LI, Lewis SJ, et al. Global tests of haemostasis in critically ill patients with severe sepsis syndrome compared to controls. Br J Haematol. 2006;135(2):220–227. doi:10.1111/j.1365-2141.2006.06281.x
27. Lim WS, Van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi:10.1136/thorax.58.5.377
28. Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):
29. Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989–1994. doi:10.1111/jth.14578
30. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34–45. doi:10.1038/nri3345
31. van der Poll T, Parker RI. Platelet activation and endothelial cell dysfunction. Crit Care Clin. 2020;36(2):233–253. doi:10.1016/j.ccc.2019.11.002
32. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. doi:10.1093/eurheartj/ehz405
33. Starr ME, SaitoH. Sepsis in old age: review of human and animal studies. Aging Dis. 2014;5(2):126–136. doi:10.14336/AD.2014.0500126
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

