Back to Journals » International Journal of Nanomedicine » Volume 19
Collagen-Based Nanoparticles as Drug Delivery System in Wound Healing Applications
Authors Kusnadi K, Herdiana Y , Rochima E, Putra ON, Mohd Gazzali A, Muchtaridi M
Received 7 July 2024
Accepted for publication 11 October 2024
Published 6 November 2024 Volume 2024:19 Pages 11321—11341
DOI https://doi.org/10.2147/IJN.S485588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lijie Grace Zhang
Kusnadi Kusnadi,1,2 Yedi Herdiana,1 Emma Rochima,3 Okta Nama Putra,1,4 Amirah Mohd Gazzali,5 Muchtaridi Muchtaridi1,6
1Department of Pharmacy Analysis and Medicinal Chemistry, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia; 2Department of Pharmacy, Politeknik Harapan Bersama, Tegal, Central Java, 52147, Indonesia; 3Department of Fishery, Faculty of Fisheries and Marine Sciences, Universitas Padjadjaran, Sumedang, West Java, 45363, Indonesia; 4Research Center for Agroindustry, National Research and Innovation Agency (BRIN), Cibinong, Jawa Barat, 16911, Indonesia; 5School of Pharmaceutical Sciences, Universiti Sains Malaysia, Gelugor, Penang, 11800, Malaysia; 6Research Collaboration Centre for Radiopharmaceuticals Theranostic, National Research and Innovation Agency (BRIN), Sumedang, West Java, 45363, Indonesia
Correspondence: Muchtaridi Muchtaridi, Email [email protected]
Background: Conventional wound dressings often adhere to wounds and can cause secondary injury due to their lack of anti-inflammatory and antibacterial properties. In contrast, collagen-based nanoparticles (NPs) as drug delivery systems exhibit both biocompatibility and biodegradability, presenting a promising avenue for accelerating wound healing processes.
Aims of Study: This review aims to provide a comprehensive overview of the mechanisms involved in wound healing, description of the attributes of ideal wound dressings, understanding of wound healing efficacy of collagen, exploring NPs-mediated drug delivery mechanisms in wound therapy, detailing the synthesis and fabrication techniques of collagen-based NPs, and delineating the applications of various collagen-based NPs infused wound dressings on wound healing.
Methodology: This review synthesizes relevant literature from reputable databases such as Scopus, Science Direct, Google Scholar, and PubMed.
Results: A diverse array of collagen-based NPs, including nanopolymers, metal NPs, nanoemulsions, nanoliposomes, and nanofibers, demonstrate pronounced efficacy in promoting wound closure and tissue regeneration. The incorporation of collagen-based NPs has not only become an agent for the delivery of therapeutics but also actively contributes to the wound healing cascade.
Conclusion: In conclusion, In brief, the use of collagen-based NPs presents a compelling strategy for expediting wound healing processes.
Keywords: wound healing, biodegradability, nanoparticles, fabrication techniques, collagen
Introduction
The skin serves as a crucial barrier to protect the internal tissues from mechanical trauma, microbial pathogens, ultraviolet radiation, and temperature extremes.1,2 Injuries are dermal disturbances arising from diverse origins such as physical trauma, chemical agents, incisive instruments, and thermal exposure.3,4 Wound healing is conventionally characterized by sequential phases. The phases cover hemostasis, inflammation, proliferation leading to angiogenesis and re-epithelialization, and remodeling. This intricate process is inherently varied and is temporally protracted.5–7 The process of wound healing encompasses a multitude of factors, such as angiogenesis, extracellular matrix (ECM) responsiveness, collagen synthesis, granulation tissue development, and re-epithelialization.8–11 However, the effectiveness of wound healing and wound control is significantly influenced by the dressing technique used.12,13
In general, wounds may be treated using various dressing techniques. They come with hydrogel, gauze film, foam, scaffold, and hydrocolloid, all of which are commonly prepared with antimicrobial properties.14–16 An ideal wound dressing material should demonstrate biocompatibility, adhere to the wound without leaving sticky residues, have optimal water absorption capacity, the adaptable to environmental changes such as pH or moisture levels, and have the ability to sustain a moist environment that is favorable for wound recovery.17–22 Nevertheless, an ideal wound dressing system requires the characteristics of a drug delivery vehicle that possesses antimicrobial activity to protect against wound infections.23 However, numerous traditional wound healing methods encounter challenges in meeting these standards as they encounter difficulties in reliably dispensing therapeutic agents to the wound site, consequently causing a prolonged wound-healing process.
Therefore, researchers aim to devise an innovative approach capable of addressing the challenges mentioned and expediting the wound-healing process. Nanotechnology is deemed a suitable approach, owing to its minute size, versatile characteristics, and ability to deliver drugs precisely to the wounded area.23,24 The molecular scale of materials usually ranges from 1 to 1.000 nm, with promising implications for biomedical applications.25,26 To date, a variety of nanomaterials have been used to expedite the wound-healing process. It is done with particular emphasis on nanoparticles (NPs). They have garnered considerable interest for their effectiveness in promoting wound recovery. In the advancement of wound dressings, NPs have been used either as drug delivery carriers or as bioactive components. Both of these aimed at improving wound healing outcomes.27,28 Numerous research findings indicate that polymer-based NPs drug delivery systems expedite wound healing by protecting therapeutic agents from degradation in specific wound conditions.29,30
Drug delivery systems using NPs derived from natural collagen polymers have garnered considerable attention, particularly in the biomedical field due to their safety and environmental friendliness.31,32 Collagen NPs have benefits in comparison to other natural polymeric NPs. They are attributed to their favourable biocompatibility, biodegradability, and low antigenicity.33,34 Collagen NPs exhibit a significant potential for high cationic charge density owing to their abundant amino groups, making them more advantageous compared to other synthetic and natural polymer NPs.35,36 Collagen NPs play a key role in their integration into tissue engineering systems and in their application in wound dressing systems to provide wound healing.37,38
Collagen is an environmentally friendly, safe, and efficient source of nanocarriers comparison to other types of polymer NPs. The high cationic properties and biodegradability of collagen-based NPs wound dressing systems facilitate the local administration of drug to the wound area by enhancing drug absorption, and promoting the natural healing process.39,40
Collagen has been the focus in many studies to determine its effectiveness in wound healing. A number of recent studies describe the application of collagen-based NPs for wound therapy, which shows the shift of interest towards collagen-based nanotherapeutics. Despite this positive trend, there is still no comprehensive scholarly work that analyze and synthesize the efficacy of collagen NPs as a drug delivery system in wound healing applications. This review aims to summarize the role of collagen-based nanoparticulate drug delivery systems in wound-healing, serving as a reference for scientists working in this field.
Methodology
This comprehensive review used a systematic examination of literature sourced from esteemed databases including Scopus, Google Scholar, and PubMed. This review processed targeted keywords such as “collagen nanoparticles wound”, “collagen nanoparticles wound drug delivery”, and “collagen nanoparticles”, Relevant articles were meticulously curated. The selected literature, comprising both review and research articles, underwent rigorous categorization, with emphasis placed on the relevance to specific thematic areas of interest. The final selection of articles, meticulously chosen based on their alignment with the outlined criteria, is outlined in Figure 1, illustrating the scope and depth of this review.
 |
Figure 1 Flowchart illustrating the methodology employed in this review. |
The Stages of Wound Healing Process
The wound healing process involves several sequential stages in repairing damaged tissue. The following is a brief description of each stage in the wound healing process, as shown in Figure 2.41 The hemostasis stage in the wound healing process begins immediately after the injury occurs. The main goal is to stop bleeding.42 Damaged blood vessels will contract to reduce blood flow, while blood clotting factors work to form blood clots or temporary clots. Platelet cells are the first to respond and form blood clots in the wound area.43 Damaged blood vessels will narrow and form temporary blood clots to stop bleeding.44 Endothelial cells in blood vessels also play a role in producing blood clotting factors.45
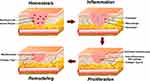 |
Figure 2 A diagram illustrating the primary stages of the wound healing process. Notes: Reprinted from Pharmacol Res, volume 174, Yazarlu O, Iranshahi M, Kashani HRK et al. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Copyright 2021, with permission from Elsevier.46 |
The subsequent stage of the inflammatory response begins once the bleeding has ceased, typically concluding within the initial 24–72 hours post-injury.47 During the inflammation stage, the wound area becomes red, swollen, and warm due to the body’s response to the injury.48 White blood cells, including neutrophils and macrophages, migrate to the wound area to clear debris, eliminate bacteria, and initiate the healing process.49 Growth factors such as fibroblast growth factor and proinflammatory cytokines are also produced to facilitate tissue regeneration.50
The proliferation phase begins with the fibrin matrix being replaced by a new matrix consisting of proteoglycans, collagen fibers, keratinocytes, and anti-inflammatory macrophages, thereby restoring tissue structure and function.51 Additional significant occurrences during this phase of healing involve the development of granulation tissue and epithelialization.52 Fibroblasts play a pivotal role in the proliferative phase of healing, as they generate and deposit collagen, proteoglycans, elastin, and other constituents forming the granulation tissue.53 Type III collagen is produced in large quantities in this stage, including other proteins in the ECM.54 This phase also covers blood clot formation, temporary ECM formation and various growth factors and proteins, including type I collagen, which are upregulated during this stage.55
The final stage of remodeling is done through the repair and strengthening of scar tissue formed during the proliferation stage. In this phase, the number of fibroblast cells decreases. Furthermore, the scar tissue becomes more organized to resemble the surrounding tissue.56 Collagen, produced during the proliferation stage, undergoes reorganization and repair to increase tissue strength.57 Type I collagen plays a key role in enhancing the tensile strength of scars. The important aspect of this stage is completed with the regeneration of elastic fibers within the ECM to maintain skin elasticity.58 Elastin, a protein present in the dermis layer, has various functions in mechanical and cellular interactions. This remodeling process can extend for months to years after the initial injury.59 A diagram illustrating the primary stages of the wound healing process can be seen in Figure 2.
Advantages and Disadvantages of Clinically Used Materials and the Ideal, Properties of Wound Dressing Excipients
Current Types and Characteristics of Wound Dressings
Various types of wound dressings are available in the clinic for various applications depending on the status and severity of the wound, with the ultimate aim of promoting wound healing (Figure 3). Over time, advancements in science and technology with a deeper comprehension of wound healing mechanisms, have led to continuous evolution and enhancement of wound dressing functionalities.60,61 Traditional wound dressings are primarily made of materials like cotton, polyester fiber, and gauze.62 This wound dressing material is easy to prepare, readily available, and effective in absorbing wound exudate to aid drainage of the wound.63 However, their drawbacks include limited protective and healing effects due to the lack of moisture and excessive water absorption, causing the wound to absorb and drain more quickly.64 Once the dressing dries, it adheres to the wound and has the potential to cause secondary injury during dressing changes. Moreover, their large pore structure fails to provide adequate isolation from environmental bacteria. In addition, as they lack anti-inflammatory and antibacterial properties, it is hence challenging to create an optimal sterile environment for wound healing.65,66 Consequently, modern wound dressings are increasingly being used in place of traditional ones due to their superior benefits.
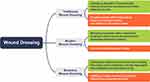 |
Figure 3 Development and characteristics of wound dressings. The advantages and disadvantages of various dressings are outlined in green and orange squares, respectively. |
The manufacture of modern wound dressings uses base layer materials that have superior characteristics. This wound dressing system reflects the structure of the natural extracellular matrix to some extent, thereby stimulating cell growth and facilitating wound healing.67 The materials used as the matrix for the dressing have also certain wound-healing properties.68 For example, collagen polymers have a role in controlling bleeding, retaining moisture, and facilitating gas exchange.59 Furthermore, by employing different types of materials as the matrix for the wound dressing preparation, different properties and functions can be imparted, thereby creating an improved wound microenvironment.22 The incorporation of bioactive substances has been shown to enhance the differentiation of biological functions, wound dressing properties, antibacterial and anti-inflammatory effects, and cellular proliferation.69,70 These dressings, which are known as bioactive dressings, can promote cell growth and wound healing by delivering bioactive substances to the wound surface.71
Properties of Ideal Excipients for Wound Dressings
Currently, available wound dressings can repair wounds to a certain extent and speed up healing. However, the availability of technology and materials is limited, so there is no perfect wound dressing product for use in clinical care.72 Traditional dressings, such as bandages, gauze, and cotton, have evolved into smart dressings, and modern tissue-engineered dressings, with improved the properties and functionalities.73 Traditional dressings mainly provide a physical barrier and drainage function for the wound but often adhere to the wound and can cause secondary injury due to their lack of anti-inflammatory and antibacterial properties.62
Modern dressings, which are made from natural materials like chitosan and collagen, as well as synthetic ones such as polyvinyl alcohol, have been devised to address these limitations.35 By using natural or synthetic ingredients to create hydrogel, hydrocolloid, and other forms, the dressings can enhance moisture retention and absorption properties. They can ensure wound conformity and a moisture-rich environment conducive to healing.74 Nevertheless, individual biological matrix materials have limited properties and cannot fully address wound inflammation, hemostasis, growth promotion, and bacterial infection.75 To enhance the efficacy of wound dressings, bioactive substances are incorporated into functional matrix materials such as chitosan and collagen.76 Incorporating silver ions into the matrix can enhance the dressing’s antibacterial properties, bolster the immune response, and accelerate wound healing.77 Similarly, the introduction of nerve growth factor (NGF) into the matrix triggers the release of endothelial cells, and keratinocytes, facilitating the migration and proliferation of fibroblasts, thereby promoting wound healing.78
Therefore, an ideal and good wound dressing system for skin wounds must have the following characteristics: (1) the ability to moisturize the skin well, (2) an optimal water absorption capacity, (3) sufficient air circulation, (4) an appropriate mechanical strength, (5) the ability to adhere to the wound without leaving a sticky residue, (6) antimicrobial properties, (7) good biocompatibility, (8) the ability to sustain a moist environment that is favorable for wound recovery, (9) affordable cost, and (10) the ability to support the growth and regeneration of skin cells in the skin tissue structure.79,80
Wound Healing Properties of Collagen
The structure of the collagen molecule consists of a triple helix arrangement formed from by three intertwined polypeptide chains. These chains are stabilized through hydrogen bonds in the CO and NH groups, along with electrostatic interactions (Figure 4). An important advantage of integrating collagen into wound dressings is its capacity to expedite the healing process, especially in challenging wound scenarios. Collagen promotes the migration of macrophages and fibroblasts to the wound area, facilitating the development of a new collagen framework.81 Additionally, collagen’s high hydrophilicity makes it suitable for wound dressings as it can absorb wound fluids containing diverse matrix metalloproteinase (MMPs) and growth factors, further aiding the healing process.82,83
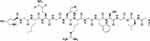 |
Figure 4 Molecular structure of collagen type I. Notes: Reprinted from Life Sci, volume 290, Sharma S, Rai VK, Narang RK, Markandeywar TS. Collagen-based formulations for wound healing: A literature review. 290. Copyright 2022, with permission from Elsevier.84 |
Collagen displays its wound healing efficacy through various well-researched mechanisms, including enhancing hemostasis, exhibiting antimicrobial properties, promoting proliferation, encouraging migration, and modulating the inflammatory response (Figure 5). The physicochemical characteristics of collagen, which characterized by the high cationic charge of its amino groups, enable it to enhance hemostasis at the wound site through several identified mechanisms.59,85 Collagen with its positive charge, can cluster negatively charged erythrocytes, platelets, and plasma proteins such as fibrinogen. These interactions contribute to hemostasis and blood clot formation.59 The wound-healing properties of collagen also include its ability to promote cell proliferation, stimulating the growth and development of new cells in the wound area.86 By facilitating cell proliferation, collagen helps accelerate tissue regeneration and the formation of new scar tissue in the wound area.87
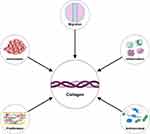 |
Figure 5 Summary of the healing attributes facilitated by collagen. |
Collagen promotes migration in the wound-healing process, facilitating new tissue growth and faster wound closure. Additionally, collagen aids in the formation of the extracellular matrix, which is essential for effective wound healing.88 Collagen contributes to preventing infections at wound sites by exerting antimicrobial effects through interactions between its positively charged amino groups and the negatively charged components of bacterial cell membranes.89 Additionally, its capability to bind trace amounts of ions on the surface membrane of bacterial cells also adds to its antimicrobial properties, although to a lesser degree.90 Prolonged inflammation beyond the normal duration can lead to chronic wounds and interfere with the natural wound-healing process.91 Collagen, possessing immunostimulatory properties, can regulate both pro-inflammatory and anti-inflammatory cytokines.92 This modulation plays a crucial role in controlling the inflammatory response at the wound site, which is essential for effective wound healing.93
Several strategies are hence crucial to enhance the efficacy of collagen in wound healing while also serving as a drug delivery vehicle.31 One effective technique in material development for wound healing involves converting collagen into NPs. These collagen-based NPs can effectively deliver drugs to wound areas, thereby enhancing drug absorption by the target tissue and expediting the wound-healing process.39,94
The Application of NPs as a Drug Delivery System in Wound Healing
NPs have become a significant asset in various realms of pharmaceutical and medical exploration due to their minute sizes, adaptable attributes, and capability to accomplish precise and controlled drug delivery.95 The vast potential of NPs stems from their ability to be tailored and precisely released.96 NPs are garnering growing interest for their use in wound dressing materials, serving both as carriers for drug delivery systems and as active agents, ultimately enhancing wound healing process,79,97 as shown in Figure 6.
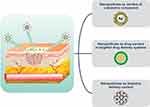 |
Figure 6 Overview of the application of NPs for wound healing. Notes: Reprinted from Mater Today Chem, volume 17, Gowda BHJ, Mohanto S, Singh A et al. Nanoparticle-based therapeutic approaches for wound healing: a review of the state-of-The-art. 17. Copyright 2023, with permission from Elsevier.98 |
NPs serve as excellent carriers for transporting active compounds to enhance wound healing outcomes as drug delivery vehicles.2 Likewise, NPs play an important role in protecting and extending the half-lives of short-lived therapeutic agents, such as growth factors and nitric oxide, which are susceptible to degradation by proteolytic enzymes and are transient.99 Furthermore, NPs have been instrumental in achieving precise and controlled delivery of antimicrobial agents and natural products for treating wounds, besides being used in gene therapy aimed at modifying the wound microenvironment by delivering specific genetic material to the site of injury.100 Interestingly, bioactive polymers like collagen offer an intriguing reservoir of important elements for NP systems, showcasing notable benefits in promoting wound healing.35,101,102 Overview of the application of NPs for wound healing can be seen in Figure 6.
Discussion - Techniques for Producing Collagen-Based NPs
Collagen NPs can be produced via three techniques: self-assembly, chemical, and physical. Chemical methods can be carried out by emulsification whilst, physical methods include electro spraying and electro spinning methods.103 In addition to these methods desolvation and milling will also be discussed to provide insight into the diverse production methods available for collagen-based NPs.31
Self Assembly
Proteins that undergo hydrophobic modification and are introduced into water-based solutions can autonomously assemble with the hydrophobic cores serving as pathways for active molecules.104 Self-assembly methods also involve dissolving individual protein chains in a solution that exceeds a critical micelle concentration, at a specific solution temperature suitable to the formation of nanoscale aggregates.105 Research has demonstrated that self-assembled wound dressings containing collagen stimulate fibroblast production and accelerate wound healing. By manipulating the self-assembly conditions of collagen constructs, the controlled release of biomolecules in these bioactive wound dressings can be achieved.106
Solvent Extraction/Emulsification
Polymers and drugs precipitate into droplets, and NPs will be formed by solvent extraction via evaporation. The double emulsion method for nanoparticle synthesis is both rapid and cost-effective.107 This technique involves using a surfactant to stabilize the emulsion during dispersion. Subsequently, the organic solvent is removed to preserve the NPs in an aqueous buffer. A stabilizing agent and surfactant are necessary to maintain the stability of the emulsion, which is typically thermodynamically unstable These components influence the interactions between the drug and the matrix, as well as the rate of drug release.108
Electrospraying
High voltage is employed to dispense a protein-containing, resulting in the ejection of a liquid jet stream from the nozzle and the formation of aerosolized droplets that comprise protein NPs of colloidal size.109 This process facilitates the incorporation of drugs into these NPs. Parameters such as applied voltage, distance of operation, gauge diameter of the needle, and flow rate may vary depending on the type of drug delivery system. A higher voltage is applied to ensure that the polymer solution exits the syringe as NPs. Electrospraying is an economical and easily implemented method with high encapsulation efficiency. It enables the production of stable NPs without concerns regarding biocompatibility and reduced encapsulation efficiency.110
Desolvation
Simple coacervation or desolvation is a self-assembly technique in the production of protein NPs, whereby a collagen solution containing a drug is mixed with a desolvation agent, such as alcohol or a natural salt.111 This agent induces changes in the collagen structure, reducing its solubility. Once a critical level of desolvation is reached, a crosslinking agent like glutaraldehyde is introduced to the collagen mixture, leading to the formation of NPs. Factors such as pH, protein concentration, desolvation rate, and temperature influence the size of the NPs produced through desolvation. Lower protein concentration and higher pH result in smaller NPs, achieved by decreasing protein solubility with the addition of the desolvation agent.112
Milling
Nanoscale collagen can be synthesized using a milling procedure. In this method, mechanical energy is applied to break down the polymer material into finer NPs.113 Grinding balls are utilized to generate high-energy mechanical impacts effectively disintegrating of the polymer. Cooling the grinding vessel is crucial to prevent material degradation or overheating. As collagen is temperature-sensitive, mechanical grinding with liquid nitrogen is employed to maintain a temperature below approximately −150°C preventing heat-induced denaturation.114
Electrospinning
One commonly employed method for fabricating collagen-based nanofibers is electrospinning. In electrospinning, a polymer solution, such as collagen, is ejected through a needle subjected to a high electrical charge. The electrically induced force attracts the polymer solution, resulting in the formation of exceedingly thin fibers that are drawn towards the surface of a collector.115 These fibers are then accumulated to create an extremely fine fiber matrix. This process produces nanometer-scale fibers with diameters typically ranging from hundreds of nanometers to several micrometers. In the production of collagen-based nanofibers, the collagen solution is initially prepared by dissolving it in an appropriate organic solvent. This solution is then fed into a pump and dispensed through the electrospinning needle. After fiber formation, chemical or physical treatments may be applied to enhance their mechanical properties and biocompatibility.116
Collagen-Based NPs as Drug Delivery Systems in Wound Healing
The literature documents various collagen formulations for wound healing, including sponges, hydrogels, and films, all of which utilize collagen as the primary ingredient. However, these larger structures cannot be compared to collagen-based NPs, which offer distinct advantages due to their significantly smaller particle size.117 The NPs nano-scale dimensions facilitate enhanced penetration through skin tissue, potentially reaching the wound area more effectively and leading to improved wound healing outcomes.118 In addition, collagen-based NPs possess a high cationic charge, stemming from the abundance of amino groups in their structure, thereby enabling various functions owing to their distinctive properties.32
This strong cationic charge result in good stability as electrostatic repulsion forces between similar charges prevent aggregation.40 It also allows effective interaction between the NPs and the cells involved in the wound healing process, including bacterial cells and mucosal tissue, which further promotes the wound-healing process.35,102 The development of collagen-based NPs is increasingly offering avenues to improve wound healing outcomes by enabling controlled release, precise delivery of therapeutic substances, and prolonging the activity of the encapsulated therapeutic agents.101 Compounds with therapeutic properties, when administered through collagen-based NPs, demonstrate the additional benefit of improved skin deposition and penetration.101 This indicates that the safety profile of therapeutic compounds may also be enhanced by utilizing formulations involving collagen-based NPs.119 There are six types of collagen-based NPs that are reported in the literature. These include collagen nanofibers, collagen nanopolymers, collagen nanoemulsions, nanocollagen films, collagen nanoliposomes, and collagen metal NPs (Figure 7).
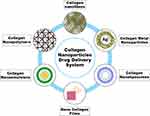 |
Figure 7 Different collagen-based NPs drug delivery systems in wound healing applications. |
Studies on Collagen-Based NPs as Drug Delivery Systems in Wound Healing Applications
Table 1 summarizes the previously reported studies of collagen-based NPs in wound healing applications.
 |
Table 1 Studies on Collagen-Based NPs as Drug Delivery Systems and Their Therapeutic Agent Effects |
Collagen-Based Polymer NPs
Polymeric NPs can be broadly classified as either synthetic or natural-based NPs. Natural-based NPs have attracted great attention for their potential in accelerating wound healing. Collagen is a type of natural polymer that can be prepared by assembling the particles themselves or mixing them with other particles using several reported methods.31 Natural polymers have a structure that is more similar to normal tissue than synthetic polymers, thus supporting the formation of extracellular matrix in wounds.131 Most natural polymers consist of polysaccharides or peptides, that have better adhesion and hydrophilic properties, and hence will better support the growth of cells attached to their surfaces.132 Furthermore, natural polymers also have a special role in facilitating the wound-healing process. As an example, collagen is a structural protein that has biodegradable properties, good biocompatibility, effective bioadhesion, and minimal immunogenicity. These properties further support its application in tissue regeneration for wound healing purposes.133
Based on the studies outlined in Table 1, the use of collagen-based polymeric NPs as drug delivery systems for wound healing has shown significant promise. In formulations designed for wound dressings, collagen is often combined with other materials, such as polyvinyl alcohol (PVA) and chitosan to enhance the formation of composite films and nanocomposites.35,119 For instance, Leng et al 35 developed a formulation to produce curcumin nanoparticles within a PVA/chitosan/collagen composite film, effectively accelerating skin wound healing. The incorporation of collagen-based NPs has been instrumental in promoting the healing process, as evidenced by macroscopic and histological examination, demonstrating their capability to enhance tissue regeneration and expedite wound closure.39
Additionally, Li et al102 introduced a novel strategy involving collagen-based NPs encapsulated within macrophagic membranes for targeted therapy against multidrug-resistant bacterial infections. Their research demonstrated robust antibacterial activity both in vitro and in vivo from collagen-based NPs containing chlorine against S. aureus. Another significant study by Huang et al90 explored collagen nanosheets containing antimicrobial peptides for treating bacterial keratitis, a condition characterized by corneal inflammation and infection. The authors highlighted the beneficial effects of various collagen-based NPs in wound healing applications, particularly in managing bacterial infections associated with corneal wounds. The synergistic action of collagen and antimicrobial peptides not only combats pathogenic bacteria but also creates an environment conducive to tissue regeneration and repair.
Collagen-Based Emulsion NPs
Emulsion NPs modified with natural polymers represent a formulation that combines nanoparticle technology with the benefits of natural polymers to enhance wound healing effectiveness.134 These emulsion NPs offer several advantageous characteristics for wound healing. Natural polymers share structural similarities with normal skin tissue, enabling modified emulsion NPs to create a conducive environment for cell growth and tissue regeneration.135
Collagen serves as a foundational material for modifying NPs in emulsions. As outlined in Table 1, collagen-based emulsion NPs have attracted attention due to their potential application in wound healing. As an example, Hou et al94 developed a nanoemulsion containing collagen peptides extracted from purified sturgeon fish skin to explore its potential for treating diabetes and promoting wound-healing in mice. This research demonstrated the therapeutic potential of collagen peptides with varying molecular weights and doses of nanoemulsion in wound-healing.
Collagen-Based Liposome NPs
Liposome NPs modified with natural polymers represent a formulation that integrates liposome technology with the benefits of natural polymers to enhance wound healing efficacy. A key advantage of these modified liposome NPs is their capability to deliver active compounds such as drugs or growth factors directly to the wound site.14 Acting as carriers, liposomes facilitate enhanced absorption of active compounds into the skin and their diffusion into damaged tissue. In addition, natural polymers incorporated into liposomes contribute to creating an environment conducive to wound healing.136 For example, collagen can serve as a fundamental material for modifying liposome NPs. Chang et al 120 introduce a novel ophthalmic formulation that combines a nanostructured lipid carrier containing moxifloxacin and dexamethasone with biodegradable collagen, alginate, and gelatin for long-term ophthalmic applications (Table 1). The results highlight the potential of collagen-based liposomal NPs as a promising anti-inflammatory formulation for treating eye diseases. Integrating liposomal NPs with collagen-based materials offers a viable strategy to address challenges in ocular drug delivery, such as rapid drug loss before reaching the cornea and the need for sustained release. Collagen-based liposomal NP drug delivery systems hold significant promise for enhancing therapeutic outcomes in managing corneal infections and promoting ocular wound healing.
Collagen-Based Metal NPs
Metal NPs modified with natural polymers hold significant potential for enhancing wound healing effectiveness. The primary advantage of natural polymers modified with metal NPs lies in their potent antibacterial and anti-inflammatory properties.137 Metal NPs, such as silver, exhibit robust antimicrobial characteristics, which reduce the risk of infection at the wound site. Moreover, natural polymers modified with metal NPs contribute to the formation of an extracellular matrix that facilitates cell proliferation and tissue regeneration, thereby accelerating the healing process and promoting optimal skin recovery. Furthermore, this technology also enables targeted delivery of drugs or growth factors to the wound site.138
Ragothaman et al101 focused on developing a bio-hybrid hydrogel combining collagen-coated silver and melatonin NPs to accelerate tissue regeneration in damaged skin. Similarly, research by Sankar et al40 aimed to enhance synergistic wound healing activity by formulating mupirocin-adsorbed collagen-stabilized silver NPs. This approach shows the potential for advancing wound care through an effective drug delivery system using collagen-stabilized silver NPs. Malathi et al122 investigated the application of collagen mixed with ZnO NPs embedded in niosome nanocomposites for wound healing applications. Their findings show the multifunctional potential of collagen-based metal NPs in wound management. Furthermore, silver nanoparticle-doped collagen-alginate biocomposites have been explored as potential wound dressings with antimicrobial properties. Integration of silver NPs into the collagen-alginate matrix enhances its antibacterial activity, making it an effective barrier against microbial colonization of wounds.121
Another research by Sucharita et al124 explored the use of Ag-collagen nanocomposites, offering a dual function as a biocompatible scaffold for tissue regeneration while exhibiting potent antibacterial and antibiofilm properties through silver NPs. These nanocomposites were evaluated in animal models under hyperglycemic conditions, with a particular focus on resistant pathogens like Acinetobacter baumannii. The study highlighted the effectiveness of these nanocomposites in addressing wound healing challenges in a diabetic environment while mitigating potential cytotoxicity. Recent advancements in collagen-based NPs for wound healing have demonstrated promising outcomes by combining collagen’s biocompatibility with the antimicrobial and regenerative properties of silver NPs.40,101,124 However, ongoing challenges include concerns regarding the scalability of production, consistent efficacy across different wound types, and the long-term safety of metal-based NPs.139 These issues underscore the need for comprehensive studies to ensure their safe and reliable use in clinical applications.138
Collagen-Based Nanofibers
Nanofibers can be considered a nanoparticle-based system due to their nanoscale dimensions and potential in drug delivery applications.140 These nanofibers are produced using electrospinning techniques to create ultra-fine fibers at the nanoscale. The primary advantage of collagen-based nanofibers lies in their structural similarity to normal skin tissue.128 As a natural component of the skin, collagen provides an environment conducive to cell growth and tissue regeneration.126 Furthermore, collagen-based nanofibers possess properties that support wound healing, including the ability to retain skin moisture, promote cell adherence to the wound area, and stimulate the proliferation of skin cells.115 These nanofibers are engineered to deliver therapeutic agents such as drugs or bioactive molecules directly to wound sites, thereby promoting tissue regeneration and accelerating the healing process.89
Serdar et al38 focused on the development of a three-layer doxycycline-collagen nanofiber wound dressing with promising characteristics for healing acute and chronic wounds. This wounds dressing, composed of core-shell nanofibers containing polycaprolactone, collagen, doxycycline, polyethylene oxide, chitosan, and sodium alginate, exhibited aligned nanofibers with excellent mechanical properties, bioadhesion, and biocompatibility. Similarly, research by Kandhasamy et al125 described the synthesis and fabrication of collagen-coated ostholamide electrospun nanofiber scaffolds for wound healing. The controlled release of ostholamide from these nanofibrous scaffolds, along with their cytocompatibility and demonstrated wound healing efficacy in vitro and in vivo, positions them as effective biomaterials for tissue engineering and wound repair. In addition, the development of biodegradable nanofiber scaffolds comprising silk fibroin, collagen, and silver-gold NPs showcased a dual therapeutic approach for treating bacterial infections and promoting wound healing. These composite nanofibers exhibited excellent antibacterial activity and enhanced cell proliferation, making them promising candidates for advanced wound healing applications.130
Overall, these studies underscore the promising role of collagen-based NPs as versatile platforms for drug delivery in wound healing applications.118 Through innovative fabrication techniques and the incorporation of bioactive compounds, such nanofiber scaffolds offer unique advantages such as controlled drug release, antimicrobial properties, and enhanced tissue regeneration.103 This positions them as crucial in the development of effective wound dressings and therapeutics in the field of regenerative medicine.129
Authors’ Perspective
The aim of this review is to explore potential of collagen-based NPs as a drug delivery system and revolutionary platform in wound healing applications. By harnessing the unique properties of collagen, the natural building block of the extracellular matrix, these NPs offer a multifaceted approach to accelerate and enhance the healing process.98 Existing literature indicates that collagen’s natural origin ensures excellent biocompatibility, minimizing the risk of adverse reactions.141,142 Additionally, its biodegradability eliminates the need for removal after wound healing. Collagen itself serves as a scaffold for cell growth and tissue repair, further accelerating wound closure.143 These collagen-based NPs can encapsulate therapeutic agents and deliver them directly to the wound site, improving their efficacy and reducing systemic side effects. The design of these NPs allows for sustained and controlled release of drugs over time, optimizing the healing process and minimizing the need for frequent dosing.
Combining the natural polymer collagen with NPs has the potential to revolutionize wound healing. Collagen-based NPs can improve treatment efficacy through targeted drug delivery and increasing bioavailability, while minimizing side effects by reducing systemic exposure to the therapeutic agent. Additionally, optimizes the healing process with controlled drug release and a biocompatible environment for tissue regeneration. The illustration of the application of collagen-based NPs for wound healing can be seen in Figure 8.
 |
Figure 8 The illustration of the application of collagen-based NPs for wound healing. |
Many studies have investigated various types of collagen-based NPs, including nanopolymers, metal NPs, nanoemulsions, nanoliposomes, and nanofibers, demonstrating efficacy in promoting wound closure and tissue regeneration.144 The incorporation of collagen-based NPs has not only serves an agent for the delivery of therapeutics but also actively contributes to the wound healing cascade. The application of collagen-based NPs paves the way for the development of new and highly effective therapeutic strategies in wound management. By harnessing the multifaceted properties of collagen-based NPs, a future can be envisioned where wound healing faster, more efficient, and minimal complications. This advancement will significantly improve patient outcomes and enhance the quality of life for individuals suffering from chronic or complex wounds.
Conclusion
Collagen-based polymeric NPs represent a promising approach in wound healing due to their ability to incorporate bioactive compounds that may help to enhance tissue regeneration and accelerate wound closure, thereby improving healing outcomes. These NPs leverage collagen’s favourable physicochemical attributes and biocompatibility, making them effective platforms for delivering therapeutic agents.
Emulsion NPs derived from collagen, particularly those sourced from fish skin collagen peptides, have shown promising properties in treating diabetes and promoting wound healing in animal models. These biomaterials highlight the therapeutic potential of collagen-based formulation, demonstrating their ability to address various health challenges effectively.
Collagen-based liposomal NPs offer another promising strategy for ocular drug delivery and wound management. They provide optimal therapeutic concentrations and sustained release profiles, which are crucial for promoting corneal wound healing and managing ocular conditions effectively.
Metal-based NPs incorporating collagen, especially those containing silver and melatonin, show promise in expediting tissue regeneration and addressing infected chronic wounds. These NPs demonstrate bactericidal efficacy against wound pathogens and modulate growth and inflammatory factors essential for wound healing processes. Furthermore, collagen-based nanofibers serve as a versatile platform for drug delivery in wound care applications. They offer a controlled release of therapeutic agents and promote wound healing by enhancing tissue regeneration and maintaining a conducive environment for cellular growth.
In summary, this review summarizes the important role of collagen-based NPs drug delivery systems in promoting tissue regeneration, accelerating wound closure, and addressing challenges in both wound management and ocular drug delivery. These innovative approaches hold promise for advancing therapeutic outcomes in various clinical settings, leveraging collagen’s natural properties and integrating them with advanced nanotechnology for enhanced medical treatments. While collagen-based NPs show significant promise for drug delivery in wound healing, they present several challenges. Key concerns include scalability in manufacturing, maintaining consistent nanoparticle size, and potential cytotoxicity, particularly with metal-based NPs. Additionally, long-term safety, storage stability, and controlled release mechanisms require further investigation. Overcoming these limitations will necessitate optimizing synthesis techniques and conducting comprehensive in vivo and clinical studies to ensure safe therapeutic application.
Acknowledgments
This work was supported by Department of Pharmaceutical Analysis and Medicinal Chemistry, Universitas Padjadjaran.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. He J, Wang C, Lin G, et al. Guard against internal and external: an antibacterial, anti-inflammation and healing-promoting spray gel based on lyotropic liquid crystals for the treatment of diabetic wound. Int J Pharm. 2023;646:123442. doi:10.1016/j.ijpharm.2023.123442
2. Deng Q-S, Gao Y, Rui B-Y, et al. Double-network hydrogel enhanced by SS31-loaded mesoporous polydopamine nanoparticles: symphonic collaboration of near-infrared photothermal antibacterial effect and mitochondrial maintenance for full-thickness wound healing in diabetes mellitus. Bioact Mater. 2023;27:409–428. doi:10.1016/j.bioactmat.2023.04.004
3. Ghosh D, Mondal S, Ramakrishna K. A topical ointment formulation containing leaves extract of aegialitis rotundifolia roxb. accelerates excision, incision and burn wound healing in rats. Wound Med. 2019;26(1):100168. doi:10.1016/j.wndm.2019.100168
4. Wathoni N, Sari DP, Suharyani I, et al. Enhancement of α-mangostin wound healing ability by complexation with 2-hydroxypropyl-β-cyclodextrin in hydrogel formulation. Pharmaceuticals. 2020;13(10):290. doi:10.3390/ph13100290
5. Čoma M, Fröhlichová L, Urban L, et al. Molecular changes underlying hypertrophic scarring following burns involve specific deregulations at all wound healing stages (inflammation, proliferation and maturation). Int J Mol Sci. 2021;22(2):897. doi:10.3390/ijms22020897
6. Wang Y, Luo M, Li T, Xie C, Li S, Lei B. Multi-layer-structured bioactive glass nanopowder for multistage-stimulated hemostasis and wound repair. Bioact Mater. 2023;25:319–332. doi:10.1016/j.bioactmat.2023.01.019
7. Chen H, Cheng R, Zhao X, et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Materials. 2019;11(1):3. doi:10.1038/s41427-018-0103-9
8. Zhang Q, Wang P, Fang X, Lin F, Fang J, Xiong C. Collagen gel contraction assays: from modelling wound healing to quantifying cellular interactions with three-dimensional extracellular matrices. Eur J Cell Biol. 2022;101(3):151253. doi:10.1016/j.ejcb.2022.151253
9. Tan Y, Xu C, Liu Y, Bai Y, Li X, Wang X. Sprayable and self-healing chitosan-based hydrogels for promoting healing of infected wound via anti-bacteria, anti-inflammation and angiogenesis. Carbohydr Polym. 2024;337:122147. doi:10.1016/j.carbpol.2024.122147
10. Neupane YR, Handral HK, Alkaff SA, et al. Cell-derived nanovesicles from mesenchymal stem cells as extracellular vesicle-mimetics in wound healing. Acta Pharmaceutica Sinica B. 2023;13(5):1887–1902. doi:10.1016/j.apsb.2022.10.022
11. Lou R, Chen J, Zhou F, et al. Exosomal miRNA-155-5p from M1-polarized macrophages suppresses angiogenesis by targeting GDF6 to interrupt diabetic wound healing. Mol Ther Nucleic Acids. 2023;34:102074. doi:10.1016/j.omtn.2023.102074
12. Shen S, Deng L, Du Y, et al. Analyzing and mapping the research status, hotspots, and frontiers of biological wound dressings: an in-depth data-driven assessment. Int J Pharm. 2022;629:122385. doi:10.1016/j.ijpharm.2022.122385
13. Duckworth PF, Rowlands RS, Barbour ME, Maddocks SE. A novel flow-system to establish experimental biofilms for modelling chronic wound infection and testing the efficacy of wound dressings. Microbiol Res. 2018;215:141–147. doi:10.1016/j.micres.2018.07.009
14. Ding Q, Ding C, Liu X, et al. Preparation of nanocomposite membranes loaded with taxifolin liposome and its mechanism of wound healing in diabetic mice. Int J Biol Macromol. 2023;241:124537. doi:10.1016/j.ijbiomac.2023.124537
15. Wang W, Ding D, Zhou K, et al. Prussian blue and collagen loaded chitosan nanofibers with NIR-controlled NO release and photothermal activities for wound healing. J Mater Sci Technol. 2021;93:17–27. doi:10.1016/j.jmst.2021.03.037
16. Asvar Z, Pirbonyeh N, Emami A, et al. Enhancing antibacterial activity against multi-drug resistant wound bacteria: incorporating multiple nanoparticles into chitosan-based nanofibrous dressings for effective wound regeneration. J Drug Delivery Sci Technol. 2024;95:105542. doi:10.1016/j.jddst.2024.105542
17. Cakmak HY, Ege H, Yilmaz S, et al. 3D printed styrax liquidus (liquidambar orientalis miller)-loaded poly (L-lactic acid)/chitosan based wound dressing material: fabrication, characterization, and biocompatibility results. Int J Biol Macromol. 2023;248:125835. doi:10.1016/j.ijbiomac.2023.125835
18. Zhu P, Yin H, Wei J, Wu J, Ping D, Zhang X. A bilayer biocompatible polycaprolactone/zinc oxide/Capparis spinosa L. ethyl acetate extract/polylactic acid nanofibrous composite scaffold for novel wound dressing applications. Int J Biol Macromol. 2023;242:125093. doi:10.1016/j.ijbiomac.2023.125093
19. Zhu J, Tang C, Zhang M, Zhang M, Fu L. Sulfated zwitterionic poly(sulfobetaine methacrylate)/κ-capacarragan double network hydrogel wound dressing for skin wound repair. Eur Polym J. 2024;207:112809. doi:10.1016/j.eurpolymj.2024.112809
20. Cai Y, Fu X, Zhou Y, et al. A hydrogel system for drug loading toward the synergistic application of reductive/heat-sensitive drugs. J Control Release. 2023;362:409–424. doi:10.1016/j.jconrel.2023.09.004
21. Swetha Menon NP, Kamaraj M, Anish Sharmila M, Govarthanan M. Recent progress in polysaccharide and polypeptide based modern moisture-retentive wound dressings. Int J Biol Macromol. 2024;256:128499. doi:10.1016/j.ijbiomac.2023.128499
22. Liu K, Kang Y, Dong X, et al. A simple yet effective hydrogel dressing for advanced microenvironmental management of diabetic wounds with intrinsic regulation. Chem Eng J. 2023;470:143987. doi:10.1016/j.cej.2023.143987
23. Kumar V, Sharma N, Janghu P, et al. Synthesis and characterization of chitosan nanofibers for wound healing and drug delivery application. J Drug Delivery Sci Technol. 2023;87:104858. doi:10.1016/j.jddst.2023.104858
24. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–124. doi:10.1038/s41573-020-0090-8
25. Zhang X-M, Zhang M, Xu -N-N, Zheng S-J, Cheng N. Multifunctional polydopamine/hemin-cyclodextrin reinforced chitosan nanocomposite hydrogel: a synergistic platform for wound healing. Int J Biol Macromol. 2024;260:129553. doi:10.1016/j.ijbiomac.2024.129553
26. Moaness M, Mabrouk M, Ahmed MM, Das DB, Beherei HH. Novel zinc-silver nanocages for drug delivery and wound healing: preparation, characterization and antimicrobial activities. Int J Pharm. 2022;616:121559. doi:10.1016/j.ijpharm.2022.121559
27. Polez RT, Ajiboye MA, Österberg M, Horn MM. Chitosan hydrogels enriched with bioactive phloroglucinol for controlled drug diffusion and potential wound healing. Int J Biol Macromol. 2024;265:130808. doi:10.1016/j.ijbiomac.2024.130808
28. Maatouk B, Jaffa MA, Karam M, et al. Sulfated alginate/polycaprolactone double-emulsion nanoparticles for enhanced delivery of heparin-binding growth factors in wound healing applications. Colloids Surf B. 2021;208:112105. doi:10.1016/j.colsurfb.2021.112105
29. Zhang H, Lin X, Cao X, Wang Y, Wang J, Zhao Y. Developing natural polymers for skin wound healing. Bioact Mater. 2024;33:355–376. doi:10.1016/j.bioactmat.2023.11.012
30. Narisepalli S, Salunkhe SA, Chitkara D, Mittal A. Asiaticoside polymeric nanoparticles for effective diabetic wound healing through increased collagen biosynthesis: in-vitro and in-vivo evaluation. Int J Pharm. 2023;631:122508. doi:10.1016/j.ijpharm.2022.122508
31. Arun A, Malrautu P, Laha A, Luo H, Ramakrishna S. Collagen nanoparticles in drug delivery systems and tissue engineering. Appl Sci. 2021;11(23):11369. doi:10.3390/app112311369
32. El-Sawah AA, El-Naggar NE-A, Eldegla HE, Soliman HM. Green synthesis of collagen nanoparticles by streptomyces xinghaiensis NEAA-1, statistical optimization, characterization, and evaluation of their anticancer potential. Sci Rep. 2024;14(1):3283. doi:10.1038/s41598-024-53342-3
33. Qiu M, Zhong G, Zhang J, et al. Biocompatible and biodegradable bletilla striata polysaccharides hydrogels crosslinked by BDDE for wound healing through the regulating of macrophage polarization. Int J Biol Macromol. 2024;254:128015. doi:10.1016/j.ijbiomac.2023.128015
34. Sivakumar S, Sadaiyandi V, Swaminathan S, Ramalingam R. Biocompatibility, anti-hemolytic, and antibacterial assessments of electrospun PCL/collagen composite nanofibers loaded with acanthophora spicifera extracts mediated copper oxide nanoparticles. Biocatal Agric Biotechnol. 2024;55:102983. doi:10.1016/j.bcab.2023.102983
35. Leng Q, Li Y, Pang X, et al. Curcumin nanoparticles incorporated in PVA/collagen composite films promote wound healing. Drug Delivery. 2020;27(1):1676–1685. doi:10.1080/10717544.2020.1853280
36. Jabeen N, Sohail M, Mahmood A, Ahmed Shah S, Mohammad Qalawlus AH, Khaliq T. Nanocrystals loaded collagen/alginate-based injectable hydrogels: a promising biomaterial for bioavailability improvement of hydrophobic drugs. J Drug Delivery Sci Technol. 2024;91:105291. doi:10.1016/j.jddst.2023.105291
37. Muthukumar T, Sreekumar G, Sastry TP, Chamundeeswari M. Collagen as a potential biomaterial in biomedical applications. Rev Adv Mater Sci. 2018;53(1):29–39. doi:10.1515/rams-2018-0002
38. Tort S, Acartürk F, Beşikci A. Evaluation of three-layered doxycycline-collagen loaded nanofiber wound dressing. Int J Pharm. 2017;529(1–2):642–653. doi:10.1016/j.ijpharm.2017.07.027
39. Shalaby M, Hamouda D, Khedr SM, Mostafa HM, Saeed H, Ghareeb AZ. Nanoparticles fabricated from the bioactive tilapia scale collagen for wound healing: experimental approach. PLoS One. 2023;18(10):e0282557–e0282557. doi:10.1371/journal.pone.0282557
40. Sankar V, Baby Roselin R, Vijaya Bhaskar RT. Formulation development of mupirocin adsorbed collagen stabilized silver nanoparticles to enhance synergistic wound healing activity. J Pharm Sci Res. 2020;12:469–474.
41. Yaghobee S, Pourhajibagher M, Bahrami R, Isaabadi M. Nano-emodin mediated photodynamic therapy for wound healing of donor site after free gingival graft: a parallel clinical trial. Photodiagn Photodyn Ther. 2024;45:103958. doi:10.1016/j.pdpdt.2023.103958
42. Wu S-H, Rethi L, Pan W-Y, Nguyen HT, Chuang AEY. Emerging horizons and prospects of polysaccharide-constructed gels in the realm of wound healing. Colloids Surf B. 2024;235:113759. doi:10.1016/j.colsurfb.2024.113759
43. Huang X, Lin L, Yang X, et al. Chitooligosaccharide-europium (III) functional micron complex with visualized inflammation monitoring, immunomodulation and pro-vascularization activities for effective wound healing of pressure ulcers injury. Appl Mater Today. 2022;26:101310. doi:10.1016/j.apmt.2021.101310
44. Gao Z, Qi Q, Li R, Li C, Xie X, Hou G. A nanofiber/sponge double-layered composite membrane capable of inhibiting infection and promoting blood coagulation during wound healing. Colloids Surf B. 2023;224:113209. doi:10.1016/j.colsurfb.2023.113209
45. Zhu G, Zhang T, Chen M, et al. Bone physiological microenvironment and healing mechanism: basis for future bone-tissue engineering scaffolds. Bioact Mater. 2021;6:4110–4140. doi:10.1016/j.bioactmat.2021.03.043
46. Yazarlu O, Iranshahi M, Kashani HRK, et al. Perspective on the application of medicinal plants and natural products in wound healing: a mechanistic review. Pharmacol Res. 2021;174:105841. doi:10.1016/j.phrs.2021.105841
47. Wang Y, Ding C, Zhao Y, et al. Sodium alginate/poly(vinyl alcohol)/taxifolin nanofiber mat promoting diabetic wound healing by modulating the inflammatory response, angiogenesis, and skin flora. Int J Biol Macromol. 2023;252:126530doi:10.1016/j.ijbiomac.2023.126530
48. Tan L, Li M, Chen H, et al. Dynamic hydrogel with environment-adaptive autonomous wound-compressing ability enables rapid hemostasis and inflammation amelioration for hemorrhagic wound healing. Nano Today. 2023;52:101962. doi:10.1016/j.nantod.2023.101962
49. He Z, Zhu Y, Ma H, et al. Hydrogen sulfide regulates macrophage polarization and necroptosis to accelerate diabetic skin wound healing. Int Immunopharmacol. 2024;132:111990. doi:10.1016/j.intimp.2024.111990
50. Benchaprathanphorn K, Muangman P, Chinaroonchai K, et al. Translational application of human keratinocyte-fibroblast cell sheets for accelerated wound healing in a clinically relevant type 2 diabetic rat model. Cytotherapy. 2024;26(4):360–371. doi:10.1016/j.jcyt.2024.01.003
51. Tripathi G, Park M, Lim H, Lee B-T. Natural TEMPO oxidized cellulose nano fiber/alginate/dSECM hybrid aerogel with improved wound healing and hemostatic ability. Int J Biol Macromol. 2023;243:125226. doi:10.1016/j.ijbiomac.2023.125226
52. Wang C-H, Hsieh D-J, Periasamy S, et al. Regenerative porcine dermal collagen matrix developed by supercritical carbon dioxide extraction technology: role in accelerated wound healing. Materialia. 2020;9:100576. doi:10.1016/j.mtla.2019.100576
53. Wietecha MS, Lauenstein D, Cangkrama M, et al. Phase-specific signatures of wound fibroblasts and matrix patterns define cancer-associated fibroblast subtypes. Matrix Biol. 2023;119:19–56. doi:10.1016/j.matbio.2023.03.003
54. You C, Zhang Z, Guo Y, et al. Application of extracellular matrix cross-linked by microbial transglutaminase to promote wound healing. Int J Biol Macromol. 2024;266:131384. doi:10.1016/j.ijbiomac.2024.131384
55. Zeng T, Liu L, Mo D, et al. Proteins extracted from pearl oyster (Pinctada martensii) with efficient accelerated wound healing in vitro through promoting cell proliferation, migration, and collagen formation. Heliyon. 2024;10(1):e24239–e24239. doi:10.1016/j.heliyon.2024.e24239
56. Oh EJ, Gangadaran P, Rajendran RL, et al. Extracellular vesicles derived from fibroblasts promote wound healing by optimizing fibroblast and endothelial cellular functions. Stem Cells. 2021;39(3):266–279. doi:10.1002/stem.3310
57. Moore AL, DesJardins-Park HE, Duoto BA, et al. Doxycycline reduces scar thickness and improves collagen architecture. Ann Surg. 2020;272(1):183–193. doi:10.1097/SLA.0000000000003172
58. Feng Z, Wang S, Huang W, Bai W. A potential bilayer skin substitute based on electrospun silk-elastin-like protein nanofiber membrane covered with bacterial cellulose. Colloids Surf B. 2024;234:113677. doi:10.1016/j.colsurfb.2023.113677
59. Cai P-F, Zheng B-D, Xu Y-L, et al. Multifunctional fish-skin collagen-based hydrogel sealant with dual-dynamic-bond cross-linked for rapid hemostasis and accelerated wound healing. Int J Biol Macromol. 2024;266:131179. doi:10.1016/j.ijbiomac.2024.131179
60. Laghezza Masci V, Taddei AR, Courant T, et al. Characterization of collagen/lipid nanoparticle–curcumin cryostructurates for wound healing applications. Macromol biosci. 2019;19(5). doi:10.1002/mabi.201800446
61. Hu T, Wu G-P, Bu H, et al. An injectable, adhesive, and self-healable composite hydrogel wound dressing with excellent antibacterial activity. Chem Eng J. 2022;450:138201. doi:10.1016/j.cej.2022.138201
62. Pereira RF, Bártolo PJ. Traditional therapies for skin wound healing. Adv Wound Care. 2016;5(5):208–229. doi:10.1089/wound.2013.0506
63. Chingwaru C, Bagar T, Maroyi A, Kapewangolo PT, Chingwaru W. Wound healing potential of selected Southern African medicinal plants: a review. J Herbal Med. 2019;17-18:100263. doi:10.1016/j.hermed.2019.100263
64. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care. 2021;10(12):685–698. doi:10.1089/wound.2020.1232
65. Mirhaj M, Labbaf S, Tavakoli M, Seifalian A. An overview on the recent advances in the treatment of infected wounds: antibacterial wound dressings. Macromol biosci. 2022;22(7). doi:10.1002/mabi.202200014
66. Wang F, Zhang W, Li H, Chen X, Feng S, Mei Z. How effective are nano-based dressings in diabetic wound healing? A comprehensive review of literature. Int j Nanomed. 2022;17:2097–2119. doi:10.2147/IJN.S361282
67. Singh H, Hassan S, Nabi SU, et al. Multicomponent decellularized extracellular matrix of caprine small intestine submucosa based bioactive hydrogel promoting full-thickness burn wound healing in rabbits. Int J Biol Macromol. 2024;255:127810. doi:10.1016/j.ijbiomac.2023.127810
68. Jia Y, Han Y, Zhang Y, Li L, Zhang B, Yan X. Multifunctional type lll recombinant human collagen incorporated sodium alginate hydrogel with sustained release of extra cellular vehicles for wound healing multimodal therapy in diabetic mice. Regener Ther. 2024;27:329–341. doi:10.1016/j.reth.2024.03.010
69. Nguyen QT, Nguyen VT, Nguyen DT, et al. Injectable hydrogel combining alginate and rhodomyrtus tomentosa medicine with antibacterial, anti-inflammatory and cellular proliferation properties as potential wound dressing material. Mater Today Commun. 2023;35:106243. doi:10.1016/j.mtcomm.2023.106243
70. Yuan Z, Zhang L, Jiang S, et al. Anti-inflammatory, antibacterial, and antioxidative bioactive glass-based nanofibrous dressing enables scarless wound healing. Smart Mater Med. 2023;4:407–426. doi:10.1016/j.smaim.2023.01.001
71. Ghosh D, Yaron JR, Abedin MR, et al. Bioactive nanomaterials kickstart early repair processes and potentiate temporally modulated healing of healthy and diabetic wounds. Biomaterials. 2024;306:122496. doi:10.1016/j.biomaterials.2024.122496
72. Laurano R, Boffito M, Ciardelli G, Chiono V. Wound dressing products: a translational investigation from the bench to the market. Eng Regen. 2022;3(2):182–200. doi:10.1016/j.engreg.2022.04.002
73. Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12(8):735. doi:10.3390/pharmaceutics12080735
74. e Souza IE, Barrioni BR, Costa MCet al. Development OF pH-sensitive wound dressings using PVA/PAA hydrogels and bioactive glass nanoparticles doped with cerium and cobalt. Mater Today Commun. 2024;38:107981. doi:10.1016/j.mtcomm.2023.107981
75. Yu Y, Xiao H, Tang G, et al. Biomimetic hydrogel derived from decellularized dermal matrix facilitates skin wounds healing. Mater Today Bio. 2023;21:100725. doi:10.1016/j.mtbio.2023.100725
76. Hu P, Lei Q, Duan S, et al. In-situ formable dextran/chitosan-based hydrogels functionalized with collagen and EGF for diabetic wounds healing. BiomateR Adv. 2022;136:212773. doi:10.1016/j.bioadv.2022.212773
77. Zhou C, Jiang T, Liu S, et al. AgNPs loaded adenine-modified chitosan composite POSS-PEG hybrid hydrogel with enhanced antibacterial and cell proliferation properties for promotion of infected wound healing. Int J Biol Macromol. 2024;267:131575. doi:10.1016/j.ijbiomac.2024.131575
78. Gostynska N, Pannella M, Rocco ML, Giardino L, Aloe L, Calzà L. The pleiotropic molecule NGF regulates the in vitro properties of fibroblasts, keratinocytes, and endothelial cells: implications for wound healing. Am J Physiol Cell Physiol. 2020;318(2):C360–C371. doi:10.1152/ajpcell.00180.2019
79. Fu C, Fan Y, Liu G, Li W, Ma J, Xiao J. One-step fabrication of an injectable antibacterial collagen hydrogel with in situ synthesized silver nanoparticles for accelerated diabetic wound healing. Chem Eng J. 2024;480:148288. doi:10.1016/j.cej.2023.148288
80. Hajialyani M, Tewari D, Sobarzo-Sánchez E, Nabavi SM, Farzaei MH, Abdollahi M. Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. Int j Nanomed. 2018;13:5023–5043. doi:10.2147/IJN.S174072
81. Ying H, Zhou J, Wang M, et al. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater Sci Eng. 2019;101:487–498. doi:10.1016/j.msec.2019.03.093
82. Yan L-P, Castaño IM, Sridharan R, et al. Collagen/GAG scaffolds activated by RALA-siMMP-9 complexes with potential for improved diabetic foot ulcer healing. Mater Sci Eng. 2020;114:111022. doi:10.1016/j.msec.2020.111022
83. Kulkarni P, Maniyar M. Utilization of fish collagen in pharmaceutical and biomedical industries: waste to wealth creation. Res J Life Sci Bioinform, Pharm Chem Sci. 2020;6:11–20. doi:10.26479/2020.0603.02
84. Sharma S, Rai VK, Narang RK, Markandeywar TS. Collagen-based formulations for wound healing: a literature review. Life Sci. 2022;290:120096. doi:10.1016/j.lfs.2021.120096
85. Zhou Y, Liu W, Gan B, et al. Non-cross-linked collagen type I microfibers for improved hemostasis and wound healing. J Mater Sci. 2022;57(28):13570–13585. doi:10.1007/s10853-022-07447-7
86. Zhou T, Wang N, Xue Y, et al. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf B. 2016;143:415–422. doi:10.1016/j.colsurfb.2016.03.052
87. Wu L, Zhang Q, Li Y, et al. Collagen sponge prolongs taurine release for improved wound healing through inflammation inhibition and proliferation stimulation. Ann Translat Med. 2021;9(12):1010. doi:10.21037/atm-21-2739
88. Bao L, Cai X, Zhang M, et al. Bovine collagen oligopeptides accelerate wound healing by promoting fibroblast migration via PI3K/Akt/mTOR signaling pathway. Journal of Functional Foods. 2022;90:104981. doi:10.1016/j.jff.2022.104981
89. Ashraf SS, Parivar K, Hayati Roodbari N, Mashayekhan S, Amini N. Fabrication and characterization of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16 produced exopolysaccharides for wound healing applications. Int J Biol Macromol. 2022;196:194–203. doi:10.1016/j.ijbiomac.2021.11.132
90. Huang H, Xie Y, Zhong J, et al. Antimicrobial peptides loaded collagen nanosheets with enhanced antibacterial activity, corneal wound healing and M1 macrophage polarization in bacterial keratitis. Composites Part B. 2024;275:111283. doi:10.1016/j.compositesb.2024.111283
91. Wang Y, Zhang Y, Yang Y-P, et al. Versatile dopamine-functionalized hyaluronic acid-recombinant human collagen hydrogel promoting diabetic wound healing via inflammation control and vascularization tissue regeneration. Bioact Mater. 2024;35:330–345. doi:10.1016/j.bioactmat.2024.02.010
92. Portela LCPN, Cahú TB, Bezerra TS, et al. Biocompatibility and immunostimulatory properties of fish collagen and shrimp chitosan towards peripheral blood mononuclear cells (PBMCs). Int J Biol Macromol. 2022;210:282–291. doi:10.1016/j.ijbiomac.2022.05.018
93. Hauck S, Zager P, Halfter N, et al. Collagen/hyaluronan based hydrogels releasing sulfated hyaluronan improve dermal wound healing in diabetic mice via reducing inflammatory macrophage activity. Bioact Mater. 2021;6(12):4342–4359. doi:10.1016/j.bioactmat.2021.04.026
94. Hou N-T, Chen B-H. Preparation of nanoemulsions with low-molecular-weight collagen peptides from sturgeon fish skin and evaluation of anti-diabetic and wound-healing effects in mice. Pharmaceutics. 2023;15:2304. doi:10.3390/pharmaceutics15092304
95. Bassam SM, Ali DE, Awwad ZM, Mahmoud SA, Abou-Taleb BA. Development and characterization of plant derived wastes nano-formulation loaded in thermo-reversible gel for burn healing: an effort towards sustainable development. J Drug Delivery Sci Technol. 2024;95:105543. doi:10.1016/j.jddst.2024.105543
96. Damian-Buda A-I, Nawaz Q, Unalan I, Beltrán AM, Boccaccini AR. Quaternary and pentanary mesoporous bioactive glass nanoparticles as novel nanocarriers for gallic acid: characterisation, drug release and antibacterial activity. Ceram Int. 2023;49(18):29923–29932. doi:10.1016/j.ceramint.2023.06.250
97. Sellappan LK, Manoharan S. Fabrication of bioinspired keratin/sodium alginate based biopolymeric mat loaded with herbal drug and green synthesized zinc oxide nanoparticles as a dual drug antimicrobial wound dressing. Int J Biol Macromol. 2024;259:129162. doi:10.1016/j.ijbiomac.2023.129162
98. Gowda BHJ, Mohanto S, Singh A, et al. Nanoparticle-based therapeutic approaches for wound healing: a review of the state-of-The-art. Mater Today Chem. 2023;27:101319. doi:10.1016/j.mtchem.2022.101319
99. Kolahreez D, Ghasemi-Mobarakeh L, Quartinello F, Liebner FW, Guebitz GM, Ribitsch D. Multifunctional casein-based wound dressing capable of monitoring and moderating the proteolytic activity of chronic wounds. Biomacromolecules. 2024;25(2):700–714. doi:10.1021/acs.biomac.3c00910
100. Xiao T, Liu J, Li Y, et al. Microenvironment-responsive Cu-phenolic networks coated nanofibrous dressing with timely macrophage phenotype transition for chronic MRSA infected wound healing. Mater Today Bio. 2023;22:100788. doi:10.1016/j.mtbio.2023.100788
101. Ragothaman M, Kannan Villalan A, Dhanasekaran A, Palanisamy T. Bio-hybrid hydrogel comprising collagen-capped silver nanoparticles and melatonin for accelerated tissue regeneration in skin defects. Mater Sci Eng. 2021;128:112328. doi:10.1016/j.msec.2021.112328
102. Li Y, Xiong J, Hu Y, Miao W, Huang H. Wrapping collagen-based nanoparticle with macrophage membrane for treating multidrug-resistant bacterial infection. J Leather Sci Eng. 2022;4(1):31. doi:10.1186/s42825-022-00106-2
103. Doodmani SM, Bagheri A, Natouri O, Nobakht A, Saghebasl S. Electrospinning-netting of spider-inspired polycaprolactone/collagen nanofiber-nets incorporated with Propolis extract for enhanced wound healing applications. Int J Biol Macromol. 2024;267:131452. doi:10.1016/j.ijbiomac.2024.131452
104. Zhu S, Yuan Q, Yin T, et al. Self-assembly of collagen-based biomaterials: preparation, characterizations and biomedical applications. J Mat Chem B. 2018;6(18):2650–2676. doi:10.1039/C7TB02999C
105. Liu S, Wen F, Muthukumaran P, et al. Self-assembled nanofibrous marine collagen matrix accelerates healing of full-thickness wounds. ACS Appl Bio Mater. 2021;4(9):7044–7058. doi:10.1021/acsabm.1c00685
106. Kandamachira A, Selvam S, Marimuthu N, Janardhanan Kalarical S, Fathima Nishter N. Collagen-nanoparticle interactions: type i collagen stabilization using functionalized nanoparticles. Soft Mater. 2015;13(1):59–65. doi:10.1080/1539445X.2015.1009550
107. Mendoza-Muñoz N, Alcalá-Alcalá S, Quintanar-Guerrero D. Preparation of Polymer Nanoparticles by the Emulsification-Solvent Evaporation Method: From Vanderhoff’s Pioneer Approach to Recent Adaptations. Cham: Springer International Publishing; 2016:87–121.
108. Sharkawy A, Barreiro MF, Rodrigues AE. New pickering emulsions stabilized with chitosan/collagen peptides nanoparticles: synthesis, characterization and tracking of the nanoparticles after skin application. Colloids Surf A. 2021;616:126327. doi:10.1016/j.colsurfa.2021.126327
109. Bi C, Li X, Xin Q, et al. Effect of extraction methods on the preparation of electrospun/electrosprayed microstructures of tilapia skin collagen. J Biosci Bioeng. 2019;128(2):234–240. doi:10.1016/j.jbiosc.2019.02.004
110. Mutlu ME, Ulag S, Sengor M, Daglılar S, Narayan R, Gunduz O. Electrosprayed collagen/gentamicin nanoparticles coated microneedle patches for skin treatment. Mater Lett. 2021;305:130844. doi:10.1016/j.matlet.2021.130844
111. Kim S, Yoo HY, Huang J, et al. Salt triggers the simple coacervation of an underwater adhesive when cations meet aromatic π electrons in seawater. ACS Nano. 2017;11(7):6764–6772. doi:10.1021/acsnano.7b01370
112. Si R, Gao C, Guo R, Lin C, Li J, Guo W. Human mesenchymal stem cells encapsulated-coacervated photoluminescent nanodots layered bioactive chitosan/collagen hydrogel matrices to indorse cardiac healing after acute myocardial infarction. J Photochem Photobiol B. 2020;206:111789. doi:10.1016/j.jphotobiol.2020.111789
113. Reese SP, Farhang N, Poulson R, Parkman G, Weiss JA. Nanoscale imaging of collagen gels with focused ion beam milling and scanning electron microscopy. Biophys J. 2016;111(8):1797–1804. doi:10.1016/j.bpj.2016.08.039
114. Zubieta-Otero LF, Rodriguez-Garcia ME. Obtention and characterization of nano bio-hydroxyapatite particles by combined hydrothermal alkaline and ultrasonic wet milling methods. Next Mat. 2023;1:100019. doi:10.1016/j.nxmate.2023.100019
115. Zhu L, Lin C, Chen Q. Facile construction of electrospun zein nanofiber loaded with rana chensinensis skin collagen for wound care after caesarean section surgery. Mater Res Express. 2023;10(1):015404. doi:10.1088/2053-1591/ac99bc
116. Elsherbini AM, Sabra SA. Nanoparticles-in-nanofibers composites: emphasis on some recent biomedical applications. J Control Release. 2022;348:57–83. doi:10.1016/j.jconrel.2022.05.037
117. Li R, Xu Z, Jiang Q, Zheng Y, Chen Z, Chen X. Characterization and biological evaluation of a novel silver nanoparticle-loaded collagen-chitosan dressing. Regen Biomater. 2020;7(4):371–380. doi:10.1093/rb/rbaa008
118. Zhou Y, Jia W, Bi J, et al. Sulfated hyaluronic acid/collagen-based biomimetic hybrid nanofiber skin for diabetic wound healing: development and preliminary evaluation. Carbohydr Polym. 2024;334:122025. doi:10.1016/j.carbpol.2024.122025
119. Tayel AA, Ghanem RA, Al-Saggaf MS, Elebeedy D, Abd El Maksoud AI. Application of fish collagen-nanochitosan-henna extract composites for the control of skin pathogens and accelerating wound healing. Int J Polym Sci. 2021;2021:1–9. doi:10.1155/2021/1907914
120. Chang M-C, Kuo Y-J, Hung K-H, Peng C-L, Chen K-Y, Yeh L-K. Liposomal dexamethasone–moxifloxacin nanoparticle combinations with collagen/gelatin/alginate hydrogel for corneal infection treatment and wound healing. Biomed Mater. 2020;15(5):055022. doi:10.1088/1748-605X/ab9510
121. Zhang H, Peng M, Cheng T, et al. Silver nanoparticles-doped collagen–alginate antimicrobial biocomposite as potential wound dressing. J Mater Sci. 2018;53(21):14944–14952. doi:10.1007/s10853-018-2710-9
122. Malathi S, Balashanmugam P, Devasena T, Kalkura SN. Enhanced antibacterial activity and wound healing by a novel collagen blended ZnO nanoparticles embedded niosome nanocomposites. J Drug Delivery Sci Technol. 2021;63:102498. doi:10.1016/j.jddst.2021.102498
123. Chandraprabha MN, Krishna RH, Samrat K, Pradeepa K, Patil NC, Sasikumar M. Biogenic collagen-nano zno composite membrane as potential wound dressing material: structural characterization, antibacterial studies and in vivo wound healing studies. J Inorg Organomet Polym Mater. 2022;32(9):3429–3444. doi:10.1007/s10904-022-02351-8
124. Sucharita Singh S, Jena B, Roy S, et al. Sprayable biogenic Ag-collagen nanocomposites with potent antibacterial and antibiofilm activity for acinetobacter baumannii infected wound healing under hyperglycemic condition. Chem Eng J. 2024;490:151788. doi:10.1016/j.cej.2024.151788
125. Kandhasamy S, Perumal S, Madhan B, et al. Synthesis and fabrication of collagen-coated ostholamide electrospun nanofiber scaffold for wound healing. ACS Appl Mater Interfaces. 2017;9(10):8556–8568. doi:10.1021/acsami.6b16488
126. Arumugam M, Murugesan B, Chinnalagu DK, Mahalingam S. Dual therapeutic approach: biodegradable nanofiber scaffolds of silk fibroin and collagen combined with silver and gold nanoparticles for enhanced bacterial infections treatment and accelerated wound healing. J Drug Delivery Sci Technol. 2024;95:105620. doi:10.1016/j.jddst.2024.105620
127. Tanha S, Rafiee‐Tehrani M, Abdollahi M, et al. G-CSF loaded nanofiber/nanoparticle composite coated with collagen promotes wound healing in vivo. J Biomed Mater Res Part A. 2017;105(10):2830–2842. doi:10.1002/jbm.a.36135
128. Dzikri MF, Armedya TP, Khairunisa SQ, et al. Design of cellulose acetate-collagen nanofiber and its in vitro assessment as wound dressing candidate. Digest J Nanomater Biostructures. 2019;14:203–212.
129. Emami M, Zomorodian K, Yazdanpanah S, Ghasemi Y, Mirzaei E, Derakhshan MA. Multifunctional Bi-layer collagen nanofiber-collagen/PLLA/Zataria multiflora essential oil nanofiber for wound healing: antibacterial, antifungal and antioxidant properties. J Drug Delivery Sci Technol. 2024;92:105285. doi:10.1016/j.jddst.2023.105285
130. Nikfarjam S, Aldubaisi Y, Swami V, et al. Polycaprolactone electrospun nanofiber membrane with skin graft containing collagen and bandage containing mgo nanoparticles for wound healing applications. Polymers. 2023;15(9):2014. doi:10.3390/polym15092014
131. Huang X, Zheng Y, Ming J, Ning X, Bai S. Natural polymer-based bioadhesives as hemostatic platforms for wound healing. Int J Biol Macromol. 2024;256:128275. doi:10.1016/j.ijbiomac.2023.128275
132. Jain S, Jain A, Jain R, Chauhan NS. Potential of natural polymeric materials in pharmaceutics. Pharml Res. 2024;2:100014. doi:10.1016/j.prenap.2024.100014
133. Li Z, Qian C, Zheng X, et al. Collagen/chitosan/genipin hydrogel loaded with phycocyanin nanoparticles and ND-336 for diabetic wound healing. Int J Biol Macromol. 2024;266:131220. doi:10.1016/j.ijbiomac.2024.131220
134. Thomas L, Zakir F, Mirza MA, Anwer MK, Ahmad FJ, Iqbal Z. Development of curcumin loaded chitosan polymer based nanoemulsion gel: in vitro, ex vivo evaluation and in vivo wound healing studies. Int J Biol Macromol. 2017;101:569–579. doi:10.1016/j.ijbiomac.2017.03.066
135. Chakraborty T, Gupta S, Nair A, Chauhan S, Saini V. Wound healing potential of insulin-loaded nanoemulsion with Aloe vera gel in diabetic rats. J Drug Delivery Sci Technol. 2021;64:102601. doi:10.1016/j.jddst.2021.102601
136. Victoria Schulte-Werning L, Singh B, Johannessen M, Einar Engstad R, Mari Holsæter A. Antimicrobial liposomes-in-nanofiber wound dressings prepared by a green and sustainable wire-electrospinning set-up. Int J Pharm. 2024;657:124136. doi:10.1016/j.ijpharm.2024.124136
137. Guo C, Cheng F, Liang G, et al. Copper-based polymer-metal–organic framework embedded with Ag nanoparticles: long-acting and intelligent antibacterial activity and accelerated wound healing. Chem Eng J. 2022;435:134915. doi:10.1016/j.cej.2022.134915
138. Paneysar JS, Barton S, Ambre P, Coutinho E. Novel temperature responsive films impregnated with silver nano particles (Ag-NPs) as potential dressings for wounds. J Pharmaceut Sci. 2022;111(3):810–817. doi:10.1016/j.xphs.2021.11.009
139. Huang Q, Chen Y, Ye M, et al. Metal-organic framework-based dressings: application and opportunities in wound healing. Mater Today Chem. 2024;40:102235. doi:10.1016/j.mtchem.2024.102235
140. Wathoni N, Rusdin A, Motoyama K, Joni IM, Lesmana R, Muchtaridi M. Nanoparticle drug delivery systems for alpha-mangostin. Nanotechnol Sci Appl. 2020;13:23–36. doi:10.2147/NSA.S243017
141. Xu N, Peng XL, Li HR, et al. Marine-derived collagen as biomaterials for human health. Front Nutr. 2021;8:702108. doi:10.3389/fnut.2021.702108
142. Zhang Y, Wang Y, Li Y, et al. Application of collagen-based hydrogel in skin wound healing. Gels. 2023;9(3):185. doi:10.3390/gels9030185
143. La Monica F, Campora S, Ghersi G. Collagen-based scaffolds for chronic skin wound treatment. Gels. 2024;10(2):137. doi:10.3390/gels10020137
144. Afshar M, Rezaei A, Eghbali S, et al. Nanomaterial strategies in wound healing: a comprehensive review of nanoparticles, nanofibres and nanosheets. Int Wound J. 2024;21(7):e14953. doi:10.1111/iwj.14953
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

