Back to Journals » Journal of Pain Research » Volume 18
Combined Functional and Structural Imaging of White Matter Reveals Brain Connectivity Alterations in Fibromyalgia Patients
Authors Gao Z, Xie X, Liu F, Xu T , Zhang N, Zhang X, Li Y, Kong Y, Lv D, Wu T
Received 16 December 2024
Accepted for publication 30 May 2025
Published 3 July 2025 Volume 2025:18 Pages 3361—3370
DOI https://doi.org/10.2147/JPR.S512581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Michael A Ueberall
Ziyi Gao,1 Xinyan Xie,1 Fuyuan Liu,2 Tong Xu,1 Nan Zhang,1 Xinran Zhang,1 Yun Li,1 Youyong Kong,2 Dongling Lv,3 Ting Wu1
1Department of Radiology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 2Jiangsu Provincial Joint Inter-School of Computer Science and Engineering, Southeast University, Nanjing, People’s Republic of China; 3Department of Cardiology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China
Correspondence: Ting Wu, Email [email protected] Dongling Lv, Email [email protected]
Background: Fibromyalgia syndrome (FMS) is a prevalent central chronic pain condition of unknown pathophysiology. White matter (WM) plays a crucial role in brain signal transmission, and WM structural abnormalities have been reported in FMS. However, functional WM changes in FMS patients have not been investigated. This study aimed to investigate whether functional neural changes in WM accompany structural changes observed in FMS.
Methods: The study included 43 FMS patients and 43 healthy controls. Structural and functional analyses of WM were assessed using fractional anisotropy (FA) and amplitude of low-frequency fluctuation (ALFF), respectively, to explore WM alterations in FMS patients.
Results: Structural analysis revealed altered FA values in the left corticospinal tract (CST), right cingulate/hippocampus, right fornix/stria terminalis (FX/ST), superior occipitofrontal fasciculus (SOFF), right superior corona radiata (SCR), right posterior corona radiata (PCR), sagittal stratum, and left medial lemniscus (ML) in FMS patients. Some regions showing structural changes also showed changes in resting-state functional activation. Functional analysis showed that FMS patients have reduced ALFF values in the left CST, left ML, right PCR, right cingulate/hippocampus, and right FX/ST. Furthermore, the degree of ALFF reduction in the right cingulate/hippocampus and left ML was positively correlated with anxiety, depression, and pain severity scores.
Conclusion: This study provides a preliminary exploration of the mechanisms underlying FMS in terms of WM functional signals. The observed reduction in WM functional activation offers novel insights into the pathophysiology of FMS and highlights potential targets for WM-focused therapeutic strategies.
Keywords: brain function, brain structure, fibromyalgia, white matter, ALFF
Introduction
Fibromyalgia syndrome (FMS) is a central chronic pain disorder characterized by widespread musculoskeletal pain, fatigue, and sleep disturbances.1,2 Compared to other chronic pain conditions like migraines and osteoarthritis, FMS has poorer treatment outcomes and is considered one of the most difficult chronic pain disorders to manage.3 Only 33–40% of FMS patients achieve relief with medication and approximately 75% of FMS patients worldwide endure lifelong, difficult-to-relieve pain.4 Recent studies have shown that fibromyalgia syndrome (FMS) is not only associated with chronic pain but also with significant cognitive impairments, including deficits in working memory and processing speed.5 One factor related to these poor outcomes is poor understanding of the pathophysiological mechanisms underlying FMS. Lee et al6 proposed FMS is caused by the abnormal amplification of pain signals at the central level (ie, central sensitization), which is a view currently accepted by most scholars. Prior studies7–10 have reported structural and functional alterations in gray matter of FMS patients. However, treatments targeting gray matter show limited efficacy,11,12 reflecting the complex pathophysiological mechanism of the condition. While previous studies have extensively explored gray matter (GM) alterations in FMS, the role of white matter (WM) in the pathogenesis of FMS remains understudied. Therefore, exploring WM abnormalities in FMS may provide new insights.
The neural fibers that comprise WM connect different regions of the brain, forming complex networks that facilitate rapid information transmission and processing. Studying WM is thus crucial for understanding brain connectivity13 and could reveal communication patterns between different brain regions and how these patterns support and regulate behavior. Notably, a growing body of research14–18 has demonstrated reliable blood-oxygen-level-dependent (BOLD) signals in WM, indicating neurovascular dynamic responses in WM during resting states.16,19
Alterations in brain connectivity can reflect the transition from acute to chronic pain.14 Mazerolle et al20 highlighted the potential value of integrating WM functional activation with structural changes to study brain connectivity in the pathogenesis of chronic pain. Based on prior findings, we hypothesize that brain connectivity alterations due to WM abnormalities may contribute to the onset and maintenance of FMS or reflect the primary clinical symptoms of FMS.
To test this hypothesis, we used fractional anisotropy (FA), which reflects brain microstructure, to investigate WM structural changes, and amplitude of low-frequency fluctuation (ALFF), which reflects brain activity intensity, to explore changes in WM functional activation in FMS patients. As a marker of resting-state functional activity, ALFF quantifies the intensity of spontaneous neural oscillations in local brain regions and has been widely applied in pain and neuropsychiatric research.21–24 Although traditionally dominated by GM analyses, recent studies have demonstrated that ALFF is equally valid for WM, effectively capturing its functional signatures.25,26 Such WM abnormalities may enhance our understanding of the pathophysiological mechanisms of FMS and provide insights for developing targeted therapeutic strategies.
Methods
Participants
A total of 43 FMS patients were recruited from the Affiliated Hospital of Nanjing University of Chinese Medicine and 43 healthy controls (HCs) were recruited from the community through advertisements. All FMS patients met the 2016 classification criteria established by the American College of Rheumatology (ACR).3 For FMS patients, the exclusion criteria were: (1) presence of other inflammatory rheumatic or autoimmune diseases; (2) history of psychiatric disorder, such as major depression or severe personality disorders; (3) history of seizures; and (4) presence of ferromagnetic elements or metal implants in the cranium or pacemaker implants at the time of enrollment. The criteria for HCs were (1) not meeting the criteria for any chronic pain condition and (2) no history of a class I psychiatric disorders (ie, major depression, schizophrenia) or substance abuse. The two groups were matched for age, gender, years of education, and handedness. All participants were provided with information about the study’s procedures and objectives and provided written informed consent. This study complies with the Declaration of Helsinki and was approved by the Research Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine (2021NL-193-02).
Clinical Assessment
FMS patients and HCs completed separate sets of assessments. To analyze pain levels and cognitive ability in FMS patients, the evaluations included the Fibromyalgia Impact Questionnaire (FIQ), Visual Analogue Scale (VAS) for pain, Hospital Anxiety and Depression Scale (HADS), Pittsburgh Sleep Quality Index (PSQI), and Montreal Cognitive Assessment (MoCA). The FIQ was administered by a rheumatologist and the other assessments were administered by a neurologist. For the HAMA and HAMD, total scores ≤ 7 were considered normal.27 For the MoCA, total scores ≥ 26 were considered normal.28
Imaging
We used a Verio 3.0 T (Siemens, Munich, Germany) superconducting magnetic resonance imaging (MRI) scanner with an eight-channel phased-array head coil to acquire T1, resting-state functional MRI (rs-fMRI), and diffusion tensor imaging (DTI) data. To reduce artifacts caused by head movement, foam padding was placed around the head coil. During the scanning process, participants were instructed to close their eyes, relax without falling asleep, and keep their head still. Resting-state fMRI data were acquired using an echo-planar imaging sequence with the following parameters: repetition time (TR) = 2310 ms, echo time (TE) = 221 ms, acquisition matrix = 64×64 mm, flip angle = 90°, field of view (FOV) = 124×100 mm, slice thickness = 3.5 mm, resolution = 3.43×3.43 × 5 mm, and scan duration = 8 min 10s. Diffusion weighted imaging was performed with pulse gradient spin-echo planar echo (EPI) with the following parameters: pulse TR =10,500 ms, TE =95 ms, voxel size =2×2×2mm, scanning field of view =256×100 mm, slice thickness =2.0 mm, and scanning time =5 min 59s. Sagittal three-dimensional T1-weighted imaging was performed using the following parameters: TR = 2,300 ms, TE = 2.19 ms, flip angle = 9°, matrix = 245×256 mm, slice thickness = 1 mm, sagittal slices = 176, slice gap = 0.5 mm, and scanning time = 7 min 16s.
Data Preprocessing
rsfMRI data were preprocessed using the Data Processing Assistant for Resting-State Function (DPARSF) MRI toolkit (https://rfmri.org/DPARSF). The preprocessing steps included the following: (1) correction for head motion (participants with head motion > 2.0 mm maximum displacement in 3 directions or 2.0 degrees of angular motion were excluded); (2) co-registration of T1 to functional images and reorientation; (3) for spatial normalization, T1-weighted anatomic images were segmented into WM, gray matter, and cerebrospinal fluid (CSF) and then normalized to Montreal Neurological Institute (MNI) space using transformation parameters estimated with a unified segmentation algorithm. These transformation parameters were applied to the functional images and then resampled with isotropic voxels of 3 mm; (4) detrending; (5) regressing out nuisance signals (CSF signals, head-motion parameters calculated by rigid body 6 correction) and spike regressors; and (6) temporal band-pass filtering (0.01–0.1Hz) to minimize low-frequency drift and filter high-frequency noise. ROIs were defined based on the JHU-ICBM WM Tractography Atlas.29
The FA values used for statistical analysis were acquired using PANDA software (http://www.nitrc. org/projects/panda/),30 a MATLAB toolbox implemented using FSL (FEAT; Oxford, UK; v6. 0 http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL). Data were skull-stripped and corrected for eddy-current and head motion artifacts. The FA images were calculated on a voxel-by-voxel basis. Individual FA images were non-linearly registered to the MNI space template using the FNIRT command in FSL. Then, the TBSS was conducted to create a mean FA skeleton (thresholded at 0.2) from the mean FA image generated by all aligned FA images. Subsequently, each individual FA image was projected onto this skeleton for voxel-wise statistical analysis, aiming to investigate white matter microstructural differences. An atlas-based segmentation approach was used to investigate diffusion changes in major WM tracts. Specifically, FA values were extracted for the whole brain values using the JHU-ICBM WM Tractography Atlas29 based on the mean FA skeleton.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Mac, version 22.0 (IBM Corp., Armonk, NY, United States). The Kolmogorov–Smirnov test was used to assess the normality of all continuous variables. Independent two-sample t-tests were used to compare demographic variables (age, education level), clinical symptom severity (pain duration, HAMD, HAMA, PSQI, MoCA, VAS), and whole-brain measurements between groups. Mean FA and ALFF values were extracted for each brain region and their relationships with clinical variables were then assessed using Pearson correlation analysis. Results with a p-value (two-tailed) < 0.05 were considered statistically significant. All results were corrected for multiple comparisons using the false discovery rate (FDR) with a threshold of p-value < 0.05.
Results
Participant Characteristics
The general characteristics of the FMS patients and HCs included in this study are presented in Table 1. All participants were women and the groups did not differ significantly in terms of age or education level (both p=0.19). FMS patients who experienced severe pain in the week before scanning had a mean VAS score(mm) of 67.78±24.85. FMS patients had significantly higher HAMD, HAMA, MoCA, PSQI, and VAS scores than HCs (all p < 0.001). The average duration of widespread pain was 45.68 months (range 3–240 months).
 |
Table 1 Clinical Characteristics of All Subjects |
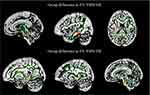
Integrity of WM Structure in FMS Patients
Figure 1 compares the spatial distribution of WM microstructural changes between FMS patients and HCs. TBSS analysis identified altered FA in numerous cerebral areas in FMS patients compared to HCs (p < 0.05, corrected). Specifically, FMS patients showed reduced FA values in the left CST, right cingulate/hippocampus, right FX/ST, and superior occipitofrontal fasciculus, but higher FA values in the right SCR, right PCR, sagittal stratum (including the inferior longitudinal fasciculus and inferior occipitofrontal fasciculus), and left ML.
WM Activation in FMS Patients
Some regions showing significant structural alterations in FMS patients also exhibited changes in resting-state functional activation, as shown by altered ALFF values compared to HCs (p < 0.05 corrected, Figure 2). WM regions showing abnormal activation are shown in Figure 3. Specifically, FMS patients exhibited reduced ALFF values in the left CST, left ML, right PCR, right cingulate/hippocampus, and right FX/ST. There were no areas where FMS patients showed significantly increased ALFF values compared to HCs.
Correlation Analyses
Figure 4 presents the relationships between changes in WM functional activation and clinical characteristics in FMS patients. For this analysis, we focused on brain regions that showed changes in functional activation. The degree of ALFF reduction in the right cingulate/hippocampus and left ML was positively correlated with anxiety and depression scores. Anxiety and depression scores were strongly correlated with each other. The degree of ALFF reduction in the right cingulate/hippocampus and left ML was positively correlated with pain severity. However, no statistically significant correlations were observed between ALFF values in any WM regions and neurocognitive performance (MoCA scores, all p > 0.05, corrected).
Discussion
This study investigated WM alterations in FMS patients. Distinct from previous research, we examined structural changes as well as abnormalities in WM functional activation. Our findings highlight the crucial role of WM alterations in the pathogenesis of FMS and provide deeper insights into the disorder in terms of whole-brain connectivity. Our results contribute to the literature on WM abnormalities in FMS, confirming and extending previous findings.11,31,32
While previous studies have reported WM structural abnormalities in FMS patients, our study is among the first to explore functional alterations. Recent studies report rsfMRI (BOLD signals) can also reflect WM activity, challenging the traditional view that associates these signals solely with gray matter. Ding et al16 first identified that variations in WM BOLD signals are associated with neural activity, while Huang Y et al33 confirmed a strong correlation between WM BOLD functional connectivity and intracranial electrophysiological connectivity. These findings provide evidence for the electrophysiological and structural basis of WM BOLD signals, supporting that WM BOLD signals could serve as biomarkers of neuropsychiatric disorders.
Research on the functional role of WM in pain mechanisms is currently limited. One study of patients with orthodontic pain31 found that, during pain stimulation, WM functional networks can mediate emotional and cognitive networks. Another study revealed that functional alterations in WM may disrupt signal transmission between neural centers in migraine patients, ultimately leading to impaired functional information integration between migraine-related brain centers.34 Taken together, these findings serve as a foundation for exploring changes in WM functional activation during pain.
WM Integrity Disruption and Connectivity Alterations
FA values, as a microstructural marker of WM, reflect the degree of organization of nerve fibers within WM.35 Decreased FA values typically indicate damage or degeneration of nerve fibers within WM tracts, which may lead to reduced efficiency in neural signal transmission and subsequently affect the communication and network connectivity between different brain regions.36 Such structural changes in FMS patients could be associated with chronic pain and long-term motor dysfunction.
Our findings of decreased FA values in WM regions including the CST, cingulum/hippocampus, and FX/ST in FMS patients are consistent with previous research on chronic pain and neurodegenerative diseases. For example, Ye et al37 also found that FMS patients exhibited reduced WM FA values, particularly in motor-related areas such as the CST. Barbas et al38 noted that WM integrity is fundamental to maintaining brain network connectivity and functional coordination, and WM damage is often accompanied by a decline in functional connectivity. Thus, impairment of WM pathways in FMS patients may lead to reduced connectivity between different brain regions, affecting functional integration.
Decreased FA values in specific regions such as the CST and cingulum/hippocampus may result in reduced effective connectivity during the execution of functions such as motor control, emotional regulation, and pain perception.39,40 This mechanism offers a neurobiological basis for the multidimensional symptoms of FMS, including motor dysfunction, cognitive impairment, and emotional disturbances.
Moreover, changes in WM FA values are often associated with remodeling or damage of brain functional networks. Increased FA values in certain brain regions in FMS patients, such as the SCR and PCR, may suggest compensatory neural remodeling.41,42 While this compensatory enhancement may temporarily sustain brain network function, in the long term, structural compensation may not restore normal functioning of brain networks. Especially in highly integrated and coordinated neural networks, compensatory changes may exacerbate functional dysregulation.
WM Functional Alterations and Brain Functional Connectivity
ALFF values reflect low-frequency fluctuations within a region and are commonly used to assess the level of functional activity in different brain regions during resting-state conditions.43 Although ALFF has been traditionally used in studies of gray matter, recent research suggests that ALFF in WM areas also reflects functional activity states.17 Although ALFF values primarily reflect the intensity of local brain region activity, they can, to some extent, indirectly indicate connectivity between brain networks. For example, restriction or enhancement of functional activity in a local region often affects information exchange and functional coordination of that region with other brain areas.44 Therefore, although ALFF values directly measure the functional activity of a specific region, changes in ALFF values can provide important information about the role of that region within the global brain network and its connectivity status.
We observed a significant reduction in ALFF values in several WM regions in FMS patients during resting-state conditions, including the CST and cingulum/hippocampus. We hypothesize that this decline in functional activity is related to WM structural damage, where the functional activity of these WM regions may be affected by disruptions in signal transmission, subsequently influencing their functional interactions with other brain areas.45 In the context of FMS, this could manifest as impairments in pain perception, motor control, and emotional regulation. For example, decreased ALFF values in the CST could relate to motor dysfunction, while reduced ALFF values in the cingulum/hippocampus could link to difficulties in emotional regulation and pain perception. Such reduction in functional activity may contribute to widespread pain and heightened sensory sensitivity in FMS because normal sensory signals are misinterpreted as pain signals—a concept that supports the theory of central sensitization.6,46 We did not find significant associations between WM functional alterations and neurocognitive performance. This may reflect limitations of the cognitive assessment tools (eg, the MoCA provides a global screening of cognitive function but may lack sensitivity to detect domain-specific deficits such as complex working memory5). Alternatively, WM functional changes in FMS may primarily mediate pain processing rather than direct cognitive control, as suggested by prior studies linking cingulate dysfunction to emotional regulation.40,46
The relationship between changes in ALFF and FA values reflects the profound impact of WM damage on overall brain network connectivity.47 Despite increased FA values in certain WM regions, such as the PCR and ML, the changes in ALFF values could still indicate suppression of functional activity. These findings suggest that even if the brain attempts to maintain function through neural network remodeling, efficiency of signal transmission and functional connectivity may still be compromised due to long-term damage.
Most studies on brain function in FMS continue to focus on gray matter, with WM function receiving comparatively limited attention. However, as the primary pathway for information transmission in the brain, changes in WM can have a profound impact on the overall brain network.48 Damage to WM and alterations in its functional activity not only affect the functional state of localized brain regions, but may also disrupt long-range connections and global coordination. Our findings underscore the importance of considering WM functional changes in FMS, supporting that their role in the pathophysiology of the disorder should not be overlooked.
Limitations
This study has several limitations. First, FMS is a gender-related condition with a significantly higher prevalence in women than men.49 Women FMS patients often exhibit distinct pathological features from men FMS patients, including more severe sleep disturbances, frequent fatigue, and widespread pain.50 However, since all participants in this study were women, our findings cannot be generalized to men FMS patients. Second, functional changes in WM typically occur in conjunction with gray matter interactions. Thus, examining WM in isolation may not fully capture its role in neural processes. Future research should investigate changes in pathways between gray and WM to provide a more comprehensive understanding. Additionally, the spatial resolution of fMRI may limit the precise separation of WM and GM signals, which could influence the interpretation of our results. Future studies using ultra-high-field MRI could better delineate WM-specific functional changes. Future studies could also explore the use of deep transcranial magnetic stimulation (dTMS) targeting WM as a potential treatment for FMS. By evaluating whether regular stimulation of targeted brain regions can improve functional activation deficits and reduce pain and mood symptoms, this approach could offer valuable insights for therapeutic strategies.
Conclusion
This study demonstrates that FMS is associated with both structural and functional alterations in key WM tracts, including the corticospinal tract and cingulate/hippocampus. These dual abnormalities likely disrupt signal transmission efficiency within pain-modulation networks, providing a mechanistic explanation for the persistence of chronic pain in FMS. By integrating WM-focused BOLD signal analysis (ALFF) with microstructural metrics (FA), our findings propose a novel framework for understanding FMS pathophysiology. Future research should examine the specific roles of these WM pathways and their associations with clinical symptoms, offering new insights into the comprehensive management of FMS.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by grants from the National Natural Science Foundation Project of China (No. 82172022), Jiangsu Province Social Development Project (No. BE2023795), Jiangsu Province Traditional Chinese Medicine Technology Development Plan Project (No. ZT202203), Jiangsu Graduate Research Innovation Program (No.2174).
Disclosure
The authors report no conflicts of interest in this work.
References
1. 1990 classification criteria of fibromyalgia from the American college of rheumatology. Report of the multicenter criteria committee. L’union Medicale du Canada. 1990;119(5):272.
2. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi:10.1002/acr.20140
3. Wolfe F, Clauw DJ, Fitzcharles MA, et al. Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi:10.1016/j.semarthrit.2016.08.012
4. Häuser W, Walitt B, Fitzcharles MA, Sommer C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther. 2014;16(1):201. doi:10.1186/ar4441
5. Fernández-Palacios FG, Pacho-Hernández JC, Fernández-de-Las-Peñas C, Gómez-Calero C, Cigarán-Méndez M. Evaluation of cognitive performance in patients with fibromyalgia syndrome: a case-control study. Life. 2024;14(5). doi:10.3390/life14050649
6. Lee U, Kim M, Lee K, et al. Functional brain network mechanism of hypersensitivity in chronic pain. Sci Rep. 2018;8(1):243. doi:10.1038/s41598-017-18657-4
7. Long Y, Xie X, Wang Y, et al. Atrophy patterns in hippocampal subregions and their relationship with cognitive function in fibromyalgia patients with mild cognitive impairment. Front Neurosci. 2024;18:1380121. doi:10.3389/fnins.2024.1380121
8. Mueller C, Fang YD, Jones C, et al. Evidence of neuroinflammation in fibromyalgia syndrome: a [18 F]DPA-714 positron emission tomography study. Pain. 2023;164(10):2285–2295. doi:10.1097/j.pain.0000000000002927
9. Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain J Neurol. 2008;131(Pt 12):3222–3231. doi:10.1093/brain/awn229
10. Farrell SF, Campos AI, Kho PF, et al. Genetic basis to structural grey matter associations with chronic pain. Brain J Neurol. 2021;144(12):3611–3622. doi:10.1093/brain/awab334
11. Lutz J, Jäger L, de Quervain D, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58(12):3960–3969. doi:10.1002/art.24070
12. Argaman Y, Granovsky Y, Sprecher E, Sinai A, Yarnitsky D, Weissman-Fogel I. Clinical effects of repetitive transcranial magnetic stimulation of the motor cortex are associated with changes in resting-state functional connectivity in patients with fibromyalgia syndrome. J Pain. 2022;23(4):595–615. doi:10.1016/j.jpain.2021.11.001
13. Hagmann P, Sporns O, Madan N, et al. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci USA. 2010;107(44):19067–19072. doi:10.1073/pnas.1009073107
14. Gawryluk JR, Mazerolle EL, D’Arcy RC. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front Neurosci. 2014;8:239. doi:10.3389/fnins.2014.00239
15. Ding Z, Huang Y, Bailey SK, et al. Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proc Natl Acad Sci USA. 2018;115(3):595–600. doi:10.1073/pnas.1711567115
16. Li M, Newton AT, Anderson AW, Ding Z, Gore JC. Characterization of the hemodynamic response function in white matter tracts for event-related fMRI. Nat Commun. 2019;10(1):1140. doi:10.1038/s41467-019-09076-2
17. Ji GJ, Liao W, Chen FF, Zhang L, Wang K. Low-frequency blood oxygen level-dependent fluctuations in the brain white matter: more than just noise. Sci Bull. 2017;62(9):656–657. doi:10.1016/j.scib.2017.03.021
18. Gore JC, Li M, Gao Y, et al. Functional MRI and resting state connectivity in white matter - a mini-review. Magnetic Resonance Imaging. 2019;63:1–11. doi:10.1016/j.mri.2019.07.017
19. Wang P, Meng C, Yuan R, et al. The organization of the human corpus callosum estimated by intrinsic functional connectivity with white-matter functional networks. Cerebral Cortex. 2020;30(5):3313–3324.
20. Mazerolle EL, Gawryluk JR, Dillen KN, et al. Sensitivity to white matter FMRI activation increases with field strength. PLoS One. 2013;8(3):e58130. doi:10.1371/journal.pone.0058130
21. Fortier A, Dumais A, Boisvert M, Zouaoui I, Chung CF, Potvin S. Aberrant activity at rest of the associative striatum in schizophrenia: meta-analyses of the amplitude of low frequency fluctuations. J Psychiatr Res. 2024;179:117–132. doi:10.1016/j.jpsychires.2024.09.012
22. Wang L, Xiong X, Liu J, et al. Gray matter structural and functional brain abnormalities in Parkinson’s disease: a meta-analysis of VBM and ALFF data. J Neurol. 2025;272(4):276. doi:10.1007/s00415-025-12934-3
23. Zhe X, Tang M, Ai K, Lei X, Zhang X, Jin C. Decreased ALFF and functional connectivity of the thalamus in vestibular migraine patients. Brain Sci. 2023;13(2). doi:10.3390/brainsci13020183
24. Chen XF, He P, Xu KH, et al. Disrupted spontaneous neural activity and its interaction with pain and emotion in temporomandibular disorders. Front Neurosci. 2022;16:941244. doi:10.3389/fnins.2022.941244
25. Guo Q, Duan J, Cai S, Zhang J, Chen T, Yang H. Desynchronized white matter function and structure in drug-naive first-episode major depressive disorder patients. Front Psych. 2022;13:1082052. doi:10.3389/fpsyt.2022.1082052
26. Yang X, Shang T, Ding Z, et al. Abnormal structure and function of white matter in obsessive-compulsive disorder. Prog Neuro Psychopharmacol Biol Psych. 2024;134:111061. doi:10.1016/j.pnpbp.2024.111061
27. Julian LJ. Measures of anxiety: state-trait anxiety inventory (STAI), beck anxiety inventory (BAI), and hospital anxiety and depression scale-anxiety (Hads-A). Arthritis Care Res. 2011;63(Suppl 11):S467–472. doi:10.1002/acr.20561
28. Applegate WB, Ouslander JG. Journal of the American geriatrics society: evolving strategies and processes. J Am Geriatr Soc. 2017;65(6):1132–1133. doi:10.1111/jgs.14951
29. Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39(1):336–347. doi:10.1016/j.neuroimage.2007.07.053
30. Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Human Neurosci. 2013;7:42. doi:10.3389/fnhum.2013.00042
31. Zhang F, Li F, Yang H, et al. Effect of experimental orthodontic pain on gray and white matter functional connectivity. CNS Neurosci Ther. 2021;27(4):439–448. doi:10.1111/cns.13557
32. Salans M, Tibbs MD, Karunamuni R, et al. Longitudinal change in fine motor skills after brain radiotherapy and in vivo imaging biomarkers associated with decline. Neuro-Oncol. 2021;23(8):1393–1403. doi:10.1093/neuonc/noab017
33. Huang Y, Wei PH, Xu L, et al. Intracranial electrophysiological and structural basis of BOLD functional connectivity in human brain white matter. Nat Commun. 2023;14(1):3414. doi:10.1038/s41467-023-39067-3
34. Qin Z, Liang HB, Li M, et al. Disrupted white matter functional connectivity with the cerebral cortex in migraine patients. Front Neurosci. 2021;15:799854. doi:10.3389/fnins.2021.799854
35. Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi:10.1016/j.neuroimage.2012.06.081
36. Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi:10.1002/nbm.782
37. Tu Y, Wang J, Xiong F, Gao F. Disrupted white matter microstructure in patients with fibromyalgia owing predominantly to psychological factors: a diffusion tensor imaging study. Pain Physician. 2022;25(8):E1305–e1313.
38. Bonilha L, Gleichgerrcht E, Fridriksson J, et al. Reproducibility of the structural brain connectome derived from diffusion tensor imaging. PLoS One. 2015;10(8):e0135247. doi:10.1371/journal.pone.0135247
39. Kim DJ, Lim M, Kim JS, Son KM, Kim HA, Chung CK. Altered white matter integrity in the corpus callosum in fibromyalgia patients identified by tract-based spatial statistical analysis. Arthritis Rheumatol. 2014;66(11):3190–3199. doi:10.1002/art.38771
40. Bubb EJ, Kinnavane L, Aggleton JP. Hippocampal - diencephalic - cingulate networks for memory and emotion: an anatomical guide. Brain Neurosci Adv. 2017;1(1). doi:10.1177/2398212817723443
41. Jakabek D, Power BD, Macfarlane MD, et al. Regional structural hypo- and hyperconnectivity of frontal-striatal and frontal-thalamic pathways in behavioral variant frontotemporal dementia. Human Brain Mapp. 2018;39(10):4083–4093. doi:10.1002/hbm.24233
42. Yang S, Chang MC. Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci. 2019;20(13):3130.
43. Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137–141. doi:10.1016/j.jneumeth.2008.04.012
44. Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi:10.1016/j.braindev.2006.07.002
45. Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front Human Neurosci. 2013;7:118. doi:10.3389/fnhum.2013.00118
46. Burgmer M, Pogatzki-Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B. Altered brain activity during pain processing in fibromyalgia. NeuroImage. 2009;44(2):502–508. doi:10.1016/j.neuroimage.2008.09.008
47. Dai H, Jiang C, Wu G, et al. A combined DTI and resting state functional MRI study in patients with postherpetic neuralgia. Japanese J Radiol. 2020;38(5):440–450. doi:10.1007/s11604-020-00926-4
48. Wang P, Wang J, Michael A, et al. White matter functional connectivity in resting-state fMRI: robustness, reliability, and relationships to gray matter. Cerebral Cortex. 2022;32(8):1547–1559.
49. Marschall U, Arnold B, Häuser W. Treatment and healthcare costs of fibromyalgia syndrome in Germany: analysis of the data of the Barmer health insurance (BEK) from 2008-2009. Schmerz. 2011;25(4):402–404,406–410. doi:10.1007/s00482-011-1079-3
50. Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3(2):128–134. doi:10.1007/s11926-001-0008-3
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.