Back to Journals » International Journal of Nanomedicine » Volume 20
Combined Graphene Oxide with 2-Methoxyestradiol for Effective Anticancer Therapy in-vitro Model
Authors Uzdrowska K , Knap N, Konieczna L, Kamm A, Kuban-Jankowska A , Gierałtowska J, Belka M, Baran M, Chlanda A , Kowiorski KM, Żołnierski A, Gulczynski J , Lipińska L, Bączek T, Izycka-Swieszewska E, Górska-Ponikowska M
Received 2 October 2024
Accepted for publication 20 December 2024
Published 22 January 2025 Volume 2025:20 Pages 933—950
DOI https://doi.org/10.2147/IJN.S498947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Farooq A. Shiekh
Katarzyna Uzdrowska,1 Narcyz Knap,1 Lucyna Konieczna,2 Anna Kamm,1 Alicja Kuban-Jankowska,1 Joanna Gierałtowska,3 Mariusz Belka,2 Magdalena Baran,3 Adrian Chlanda,3 Krystian Michał Kowiorski,3 Aleksander Żołnierski,4 Jacek Gulczynski,5 Ludwika Lipińska,3 Tomasz Bączek,2 Ewa Izycka-Swieszewska,5 Magdalena Górska-Ponikowska1
1Department of Medical Chemistry, Faculty of Medicine, Medical University of Gdansk, Gdansk, Poland; 2Faculty of Pharmacy, Medical University of Gdansk, Gdansk, Poland; 3Łukasiewicz Research Network - Institute of Microelectronics and Photonics, Warsaw, Poland; 4Institute of Economics of the Polish Academy of Sciences, Warsaw, Poland; 5Faculty of Health Sciences with the Institute of Maritime and Tropical Medicine, Medical University of Gdansk, Gdansk, Poland
Correspondence: Magdalena Górska-Ponikowska, Email [email protected]
Introduction: This article describes the invention of graphene oxide (GO) or reduced graphene oxide (rGO) functionalised with 2-methoxy estradiol. The presence of polar hydroxyl groups enables the binding of 2-ME to GO/rGO through hydrogen bonds with epoxy and hydroxyl groups located on the surface and carbonyl and carboxyl groups located at the edges of graphene flake sheets.
Methods: The patented method of producing the subject of the invention and the research results regarding its anticancer effectiveness via cytotoxicity in an in vivo model (against A375 melanoma and 143B osteosarcoma cells) are described.
Results: It was shown that the inhibition of PTP1B phosphotyrosine phosphatase is one of the mechanisms of action of GO functionalised with 2-ME (GO-2-ME). This is a very important result, considering the fact that 2-ME itself has no inhibitory properties against this phosphatase.
Discussion: Graphene oxide flakes embroidered with 2-methoxyestradiol molecules may be a promising solution, bringing a new and important effect in the form of improving the bioavailability of the therapeutic substance, ie 2-ME. An appropriate dosage of GO-2-ME/rGO-2-ME, in which GO/rGO is a carrier of 2-methoxyestradiol (2-ME), can ensure effective penetration of the active substance through biological boundaries/membranes and controlled modification of cell signalling, ultimately leading to the selective elimination of malignant cells.
Keywords: new graphene-based anticancer drugs, graphene-based oncological therapy, new anticancer treatment, biomedical innovations, drugs delivery systems
Introduction
Chemotherapy is one of the primary forms of cancer treatment; however, its effectiveness is limited by systemic toxicity, suboptimal bioavailability or efficacy, and cancer cells’ chemoresistance. For this reason, new means and more selective therapeutic methods are still being sought, including those based on nanoparticles as drug carriers with unique biophysicochemical properties, especially in the area of binding xenobiotics and physiological metabolites, including absorption and penetration through biological membranes, volume of distribution, and, consequently, the bioavailability and potential selectivity of the chemotherapeutic.1–4
Melanoma of the skin and mucous membranes is an important oncological problem in terms of the unpredictable course of the disease and relatively low effectiveness of the therapy for tumours at higher advanced clinical stages. In turn, osteosarcoma is a malignant bone tumour in children and adolescents. Despite applicable medical advances, due to its resistance to chemotherapy, osteosarcoma still constitutes a serious therapeutic challenge.5–8 2-Methoxyestradiol (2-ME) is a natural estradiol metabolite with relatively strong antineoplastic properties with a broad cytotoxic spectrum of action against various types of cancer, with the pleiotropic mechanism of action still being the subject of many biomedical studies. The therapeutic effectiveness of 2-ME (trade name Panzem® NCD) has been documented in Phase II clinical trials for the treatment of advanced kidney, prostate, ovarian, and carcinoid cancers.9–11
In addition, in vitro studies have confirmed the anti-tumour potential of 2-ME in the treatment of breast, colon, and lung cancers. The potential therapeutic efficacy of 2-ME is related, inter alia, to the influence of this compound on microtubule polymerisation, its inhibition of superoxide dismutase activity and induction of stress nitroxide, eventually leading to the death of malignant cells. 2-ME has not entered Phase III clinical trials due to its low oral bioavailability, so research is ongoing to develop new derivatives and forms of the 2-ME drug.12–18
In recent years, graphene and its derivatives have become relatively accessible organic materials with well-defined structures with promising application opportunities in the context of targeted anti-tumour therapy.19–23 The cytotoxic properties of graphene oxides (GO) and reduced graphene oxides (rGO) have been demonstrated against a variety of malignancies, including melanoma.24–27 In these studies, GO and rGO are most often found to be the main component of a composite, on the surface of which there are nanoparticles or polymers, bonded by adhesive or covalently bonded forces, as well as immobilised substances with the properties of anti-cancer drugs, such as doxorubicin and cisplatin. At the moment, there are known solutions for the production of graphene complexes with doxorubicin and other anti-cancer compounds.27–31 There are also known studies on the possibility of using graphene biocomposites with hyaluronic acid, dsDNA (double-stranded DNA) or PEG (polyethylene glycol), or other cytostatics in oncology therapy. Hyaluronic acid or PEG can serve as carriers for drugs and as a plasticiser for graphene membranes. Dressings made of a biodegradable hydrogel are also being developed to allow the release/delivery of active substances related to various forms of graphene to aid healing wounds.32–35 2-ME is cytotoxic while being relatively non-toxic to normotypical cells. A significant problem in the use of 2-ME in cancer therapies is its limited bioavailability, therefore the aim of the research is to provide structures with improved availability and possibly selective action on cancer cells. In the course of the research and development, stable derivatives of 2-ME were obtained, which solve the problem mentioned above.36–38
The solution brings a new and surprising effect in the form of improving the bioavailability of the therapeutic substance, ie 2-ME, through its complexation with graphene nanoflakes, while minimising the potential cytotoxicity of graphene nanoflakes to normotypical cells. The lipophilic structure of the graphene carrier enables more efficient interaction of the 2-ME-graphene complex with lipid membranes and, consequently, more efficient penetration into the cell interior, compared to the isolated 2-ME molecule. Graphene nanoflakes constitute a carrier platform with unique biological and biophysical properties, which enable the regulated and selective distribution of the associated chemotherapeutic agent, thus increasing the effectiveness of treatment and minimising the side effects. The lipophilic nature of 2-ME (logP = 3.59) and the relatively small polar surface of the molecule (PSA = 49.69 Å2) ensure the formation of a stable complex of 2-ME with the hydrophobic surface of graphene oxide nanoflakes (especially reduced graphene oxide (rGO)), which was experimentally confirmed (analysis of the durability of graphene oxide and reduced graphene oxide complexes with 2-methoxyestradiol).39–41
Graphene oxide is formed by the oxidation and exfoliation of graphite. The structure of graphene is a densely packed crystal lattice with a thickness of one atom, where all atoms are sp2-hybridised carbons and form six-membered rings arranged in a kind of honeycomb lattice.42–44 Graphene and graphene-based materials are seeing increasing interest for their potential in biomedical applications because of their excellent biocompatibility, cargo capability, and surface functionalisation. Various oxygen functional groups (hydroxyl, carbonyl, carboxyl, epoxide) have been attached to the graphene surface. It is known that hydrophilic GO groups, which include hydroxyl, carbonyl, carboxyl, and carboxylate functional groups, enable enhanced interaction with proteins (eg growth factors, extracellular matrix proteins, receptors) through hydrogen, electrostatic, and covalent bonds. It is also known that such improved interactions between GO and proteins significantly improve cell adhesion to GO surfaces.21,45–48 As a result of the reduction reaction, functionalised groups of GO are partially removed, and the product of this reaction is reduced graphene oxide (rGO). rGO is a material with a moderate degree of oxygen enrichment, which is an intermediate form between unoxidised graphene and graphene oxide. Apart from trace impurities, both GO and rGO are made of carbon, oxygen, and hydrogen atoms. 2-ME is composed of the same atoms, but it also contains an aromatic ring and hydroxyl groups, which facilitates the intermolecular interaction of the drug with the carrier.47–49
A significant problem in the use of 2-ME in cancer therapies is its limited bioavailability, therefore the aim of the invention is to provide structures with improved availability and possibly a selective effect on cancer cells. In the course of the research and development works, stable derivatives of 2-ME were obtained, which solve the above problem. The subject of the research is an invention constituting GO-2-ME2 or rGO-2-ME, which are a permanent combination of 2-methoxyestradiol (2-ME) particles with GO graphene oxide or reduced graphene oxide rGO. The preferred mass ratio of the 2-ME to GO or rGO in the reaction mixture to obtain the complex is 2:1 (according to the invention under study). The formation of the GO-2 ME follows the reaction:
In the complex constituting the subject of the tested invention, the graphene oxide (GO) or reduced graphene oxide (rGO) is in the form of flakes with dimensions from 2 nm to 6–5 μm.50
Materials and Methods
Description of the Synthesis of Graphene Oxide Using the Modified Hummers Method
To synthesise GO, the modified Hummers method was used. Drastic reaction conditions must be considered to achieve the required partial oxidation of graphene. They involve the use of high temperatures, strong oxidants, and sulfuric acid. The following reagents were used in this procedure: graphite with flake sizes from 40 to 150 μm (Asbury Carbons USA, catalogue number 635), sulfuric acid 96% (Chempur) 750 mL, sodium nitrate (Chempur) 16.5 g, potassium permanganate (Chempur) 90 g, hydrogen peroxide 30% (Chempur) 30 mL, hydrochloric acid 3% (Chempur). In the first step of the synthesis, sodium nitrate was added to concentrated sulfuric acid, and the contents were mixed until a clear solution was obtained, to which flaky graphite was added and mixing was continued. Then, the temperature of the colloidal system was lowered in an ice bath to 5°C, and potassium permanganate was added in several portions. The reaction mixture was heated to 35°C and then stirred for several hours. Finally, the temperature of the mixture was raised to 95°C and held for 15 minutes. The post-reaction suspension was diluted with water, and then hydrogen peroxide was added. Purification of the graphene oxide was carried out by washing it three times in hydrochloric acid and then by washing it several times with deionised water. The pre-purified graphene oxide was exfoliated with an ultrasonic probe (500 W, 5 min). Graphene oxide was then obtained in the form of flakes, with sizes ranging from 2 nm to 6 μm.51
Description of the Synthesis of Reduced Graphene Oxide by Chemical Reduction
This process consists of chemical reduction, ie remove oxygen from the graphene oxide. As a result, reduced graphene oxide (rGO) is obtained, being a material with an intermediate degree of oxygen enrichment, which is an intermediate form between graphene oxide and unoxidized graphene. Sodium hypophosphite (NaH2PO2·H2O, Chempur) and hydrochloric acid (HCl, Chempur, 35%) were used to reduce the graphene oxide. The reduction was carried out using a magnetic stirrer at pH ≈ 0 for 4 hours at 95°C. Reduced graphene oxide flakes were obtained in a mould with sizes ranging from 2 nm to 6 μm, which were later cleaned in a vacuum filtration system. Reduced graphene oxide (rGO) suspensions were then prepared with a concentration of 0.5 mg/mL in ethanol and dimethyl sulfoxide (DMSO).52,53
Synthesis and Durability Analysis of Graphene Oxide and Reduced Graphene Oxide Complexes with 2-Methoxyestradiol
The aqueous suspension of graphene oxide was concentrated to remove the water, resulting in a graphene oxide paste with a dry matter content of 2.5%, from which a suspension of graphene oxide with a concentration of 0.5 mg/mL in ethanol (EtOH, 96%, Stanlab) and a suspension of graphene oxide with a concentration of 0.5 mg/mL, which was prepared in dimethyl sulfoxide (DMSO, ≥99.9%, Sigma Aldrich). At the same time, solutions of 2-methoxyestradiol (2-ME, Selleckchem) were prepared with a concentration of 1 mg/mL in ethanol and dimethyl sulfoxide, respectively. GO (in EtOH or DMSO, respectively) and 2-ME solutions were combined at a volume ratio of 1:1. Three mL of 2-ME solution with a concentration of 1 mg/mL was added dropwise to 6 mL of GO solution with a concentration of 0.5 mg/mL and mixed. The mixture was left for 24 hours at room temperature until a GO-2-ME complex was formed, and then the mixture was centrifuged (20 min, 1200 rpm) and the supernatant from the sediment was poured off. Samples were cleared of unadsorbed 2-ME by centrifugation once with an appropriate solvent (EtOH/DMSO). The sample was topped up with an appropriate solvent (EtOH/DMSO) to a volume of 6 mL. The following suspensions were obtained:
- GO-2-ME in ethanol at a concentration of 0.25 mg/mL (based on the mass of graphene oxide).
- GO-2-ME in DMSO at a concentration of 0.25 mg/mL (based on the weight of graphene oxide).50
Three mL of the previously prepared 2-ME solution was added dropwise to 6 mL of a 0.5 mg/mL suspension of reduced graphene oxide (rGO) in ethanol and dimethylsulfoxide and the whole was mixed. The material was left for 24 hours at room temperature. In the next step, the sample was centrifuged (20 min, 1200 rpm). Due to the low efficiency of centrifugation, the sample containing the rGO-2-ME was left at room temperature for another 2 days for the sedimentation of unbound rGO, after which the supernatant solution was decanted. The rGO samples with adsorbed 2-ME were purified similarly to the samples with graphene oxide. The following suspensions were obtained:
- rGO-2-ME in ethanol at a concentration of 0.25 mg/mL (based on the weight of rGO).
- rGO-2-ME in DMSO at a concentration of 0.25 mg/mL (based on the weight of rGO).50
Yield of Graphene Oxide and Reduced Graphene Oxide Complexes with 2-Methoxyestradiol
Liquid chromatography combined with mass spectrometry (Liquid Chromatography – Mass Spectrometry; LC-MS) analysis with 2-ME-specific ion monitoring was used to determine the concentration of unbound 2-ME. Based on the amount of 2-ME used in the reaction (3 mg), it was possible to calculate the fraction of 2-ME bound to GO and rGO, respectively.
Information About Cell Lines and Reagents
Human melanoma A375 cells (CRL-1619) were purchased from the American Type Culture Collection (Manassas, VA, USA). The human osteosarcoma 143B cell line (ATTC-8303) was purchased from Sigma Aldrich (Poland). Phosphatase PTP1B (No. SRP0215) was obtained from Sigma Aldrich, Schnelldorf, Germany. Human dermal fibroblast (HDF) was obtained courtesy of R Sadej, IFB, UG&MUG, Gdansk, Poland. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Aldrich (Poland)
Culture Conditions of Malignant Melanoma Cell Line
The malignant melanoma cells were grown in an incubator under the defined conditions: temperature 37°C, 5% CO2. A culture medium (Dulbecco’s Modified Eagle’s Medium, high glucose, Sigma Aldrich (Poland)), supplemented with 10% heat-inactivated foetal calf serum (FBS), enriched with antibiotics (penicillin [100 μg/mL]/streptomycin [100 μg/mL] was used for breeding (Sigma Aldrich, Poland)).
Culture Conditions of the Osteosarcoma Cell Line
The osteosarcoma cells were grown in an incubator under the defined conditions: temperature 37°C, 5% CO2. A culture medium (Dulbecco’s Modified Eagle’s Medium, low glucose, Sigma Aldrich (Poland)), supplemented with 10% heat-inactivated foetal calf serum (FBS), enriched with antibiotics (penicillin [100 μg/mL]/streptomycin [100 μg/mL] was used for breeding (Sigma Aldrich, Poland)).
Culture Conditions of the Human Dermal Fibroblast Cell Line
Human dermal fibroblast cells were grown in an incubator under the defined conditions: temperature 37°C, 5% CO2. A dedicated culture medium (Fibroblast Growth Medium, Cell Applications, INC), supplemented with 10% heat-inactivated foetal calf serum (FBS), enriched with antibiotics (penicillin [100 μg/mL]/streptomycin [100 μg/mL], was used for the culture, Sigma Aldrich (Poland)).
Cells of all tested lines were kept in the log phase by regular passage into new culture vessels after the culture was approximately 80% confluent. The cell monolayer, after removing the culture medium, was washed with 0.9% NaCl. A 0.25% EDTA trypsin solution was then added in an amount appropriate to the area of the culture vessel in question (300–500 μL). Cells were incubated with trypsin for 3–5 min in a CO2 incubator, and the trypsinisation process was monitored under a microscope. After trypsinisation was completed, the culture medium was added in a volume appropriate to the size of the new culture vessel, and the suspension, after mixing thoroughly, then transferred to new, sterile culture vessels.
Viability Analysis of Malignant Melanoma, Osteosarcoma, Dermal Fibroblast Cell Lines Using the MTT Test
Cultures of appropriately confluent cells after trypsinisation (0.25% trypsin and 0.02% EDTA) were passaged, then incubated for 24 hours. After this time, the medium was replaced with a new one containing the test compounds. The untreated cells were controls; however, to exclude the solvent effect (DMSO, ethanol), control cells were treated with the solvent at a concentration corresponding to the system with cells treated with the test compounds. After the specified incubation time, cells were harvested and analysed as described below. The cell incubation medium contained only 1% FBS inactivated with activated carbon (charcoal-stripped FBS) and an antibiotic cocktail (penicillin [100 µg/mL]/streptomycin [100 µg/mL], Sigma Aldrich (Poland)). Cells of all lines were passaged into 96-well plates at a density of 10,000 cells per well and grown for 24 hours. The medium was then removed, and the cells were treated with test compounds for 24 hours in the system:
- GO in DMSO
- GO + 2-ME complex in DMSO 20
- GO in ethanol
- GO + 2-ME complex in ethanol
- rGO in DMSO
- rGO + 2-ME complex in DMSO
- rGO in ethanol 25
- rGO + 2-ME complex in ethanol
- 2-ME
The following dilutions of the obtained mixtures were used: 1:10, 1:20, 1:40, 1:80. It was assumed that the reference system would be a 2-ME solution (0.5 mg/1 mL) appropriately diluted from the initial concentration of 2-ME obtained during the formation of the complex. After the appropriate incubation time, 0.5 mg/mL of 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was added. The plates were then incubated at 37°C for 4 hours and the supernatant was removed after prior centrifugation (700 × g for 10 min). Then, 100 µL DMSO (Sigma Aldrich, Poland) was added to dissolve the formazan crystals. Absorbance at 570 nm was read using a microplate reader (Bio-Tek Instruments, Inc., USA). The number of cells was calculated from the absorbance values, and the results are shown as percentages of the control. Each experiment was repeated 3 times.
Assessment of PTP1B Phosphatase Activity
Measurement of the phosphatase activity was performed using the synthetic substrate p-nitrophenylphosphate (pNPP) by the colorimetric method – test for the activity of recombinant PTP1B phosphatase with the use of a substrate, resulting in a product with a maximum absorbance shifted relative to the substrate. Human recombinant protein tyrosine phosphatase PTP1B was purchased from Sigma-Aldrich. Recombinant phosphatase PTP1B solution was prepared in 10 mm HEPES buffer pH 7.4. The final concentration of phosphatase in the reaction samples was 1.5 µg/mL (3.3 nM). The PTP1B enzyme was untreated (control) or treated with various graphene derivatives at a concentration of 125 µg/mL. The assay was performed in 96-well microplates, and the final volume of each sample was 100 μL. The enzymatic activity of the PTP1B was measured using 1 mm chromogenic para-nitrophenol phosphate (pNPP) substrate in 10 mm HEPES buffer pH 7.4 at 37°C. Phosphatase hydrolyses pNPP to p-nitrophenol and inorganic phosphate. In an alkaline environment (pH 7.4), the p-nitrophenol formed is intensely yellow, which enables the colorimetric assessment of the concentration of the product formed in the sample over a given period. The value of the absorbance coefficient measured spectrophotometrically at a wavelength of 405 nm is directly proportional to the concentration of the enzymatic activity of the PTP1B phosphatase. Each experiment was repeated 3 times.
Transdermal Diffusion Test Using a Franz Chamber
The transcutaneous GO diffusion test was implemented using a Strat-M® membrane (Merck Poland), which makes it possible to predict the diffusion of substances in human skin. The initial concentration of the GO in saline was 1 μg/mL. A diffusion test of this solution through an artificial skin model (Strat-M® membrane) was performed using a Franz diffusion chamber. Samples for chromatographic analysis were taken after 0.5 and 1 hour of the membrane diffusion test. Three samples were taken from each stage of the research. The GO content in physiological saline after diffusion through the artificial skin model was determined using liquid chromatography combined with mass spectrometry (Liquid Chromatography–Mass Spectrometry; LC-MS).
X-Ray Photoelectron Spectroscopy (XPS) of Graphene Oxide and Reduced Graphene Oxide Complexes with 2-Methoxyestradiol
Samples of GO-2ME and rGO-2ME were analysed under high vacuum conditions in a multi-chamber UHV system by Prevac. After mounting the samples on the molybdenum carrier, the samples were degassed at room temperature to a constant high vacuum in the UHV system loading lock. Then, after transferring the carrier with the sample to the UHV system analytical chamber, the proper XPS analysis was performed. The source of the excitation radiation was an aluminium (Al) anode generating monochromatic radiation with a characteristic Al Kα line and energy of 1486.7 eV. To standardise the spectroscopic measurements, the X-axes of the spectra (corresponding to the binding energy, EB) were calibrated using the aliphatic carbon C1s peak. It is most often assumed that the binding energy corresponding to this peak is EB = 285.0 eV. Depending on the needs or possibilities, the energy scale of the spectra can be standardised using other reference values of the binding energy (eg EB = 284.8 eV, or EB = 284.7 eV).
Results
Analysis of the Durability of Graphene Oxide and Reduced Graphene Oxide Complexes with 2-Methoxyestradiol
Since it is difficult to find a spectral technique that is able to distinguish carbon and oxygen atoms belonging to 2-ME and GO or rGO, a method was sought to quantify the adsorbed 2-ME on the GO or rGO surface. It was determined that the FTIR technique is impossible to use. The most reliable quantitative method was found to be liquid chromatography combined with mass spectrometry (LC-MS technique). It was therefore this technique that was used to assess the amount of adsorbed 2-ME on the surface of the GO and rGO complexes. The performed analysis made it possible to assess what fraction of the 2-ME was bound by graphene by determining the concentration of free 2-ME, and thus to estimate the synthesis efficiency and indirectly the stability of the obtained complexes.
The previously prepared GO-2-ME DMSO, GO-2-ME EtOH, rGO-2-ME DMSO, and rGO-2-ME EtOH solutions were filtered with a syringe filter, and then a 100-fold dilution was obtained using ethanol as a solvent (which is necessary for chromatography and mass spectrometry). The obtained samples were analysed by means of the LC-MS technique with the use of selected ion monitoring that is characteristic for 2-ME. Each experiment was repeated 3 times. The yield of 2-ME/graphene complex formation was calculated as (1-fold determined concentration of 2-ME)/1 * 100%. About 0.5 mg/mL was assumed to be the initial concentration of 2-ME used during the formation of the complex (at 0.25 mg/mL graphene concentration in the reaction mixture). The results for the concentration of 2-ME in the obtained solutions using ethanol as the solvent are shown in Table 1. The results for the concentration of 2-ME in the obtained solutions using DMSO as the solvent are shown in Table 2.
 |
Table 1 Concentration of 2-ME in the Obtained Solutions Using Ethanol as Solvent |
 |
Table 2 Concentration of 2-ME in the Obtained Solutions Using DMSO as Solvent |
Anticancer Efficacy
In studies on the viability of A375 melanoma cells, A375 melanoma cells incubated with 2-methoxyestradiol (2-ME), graphene oxide (GO), or reduced graphene oxide (rGO) in sizes ranging from 2 nm–6 μm and derivative complexes (GO-2-ME, rGO-2-ME) were used for 24 hours. The cytotoxicity was assessed using the MTT cell viability assay. Cells treated with the solvent at a concentration corresponding to the successive test systems were used as controls. It should be emphasised that the studies showed a significant impact of the choice of solvent (environment) on the anticancer potential of each of the tested compounds. The use of ethanol as a solvent decreased the anticancer effectiveness of the GO, rGO and their derivatives compared to the use of DMSO as a solvent (Figure 1). Interestingly, the largest difference in cytotoxicity towards A375 cells depending on the solvent used was seen for the rGO-2ME complex.
Graphs A, B, C, and D (Figure 2) show the cytotoxicity towards A375 cells of the graphene-based compounds, respectively: GO, GO-2ME, rGO, and rGO-2ME in DMSO solvent. The concentrations presented in the chart, expressed in g/mL units, correspond to subsequent dilutions of the starting compounds at the ratios 1:10, 1:20, 1:40, and 1:80. In the case of GO-2ME (Figure 2B) and rGO-2ME (Figure 2D), their exact concentrations were calculated from the initial concentration of 0.25 g/mL after their preparation, taking into account the production efficiency (Table 2). For example, as a result of the procedure for obtaining the GO-2ME, we will finally achieve a compound of 0.25 mg/mL (based on the weight of graphene oxide), as a suspension in DMSO. If we take into account the efficiency of obtaining the complex (Table 2), then its content in the solvent was 0.181 mg/mL. In the case of subsequent dilutions (1:10, 1:20, 1:40, 1:80), the concentrations would be 0.0181 mg/mL, 0.00905 mg/mL, 0.004525 mg/mL, and 0.002625 mg/mL, respectively, which is marked on the chart. Concentrations were determined in a similar way for the rGO-2ME (Figure 2D). The cytotoxicity results of the GO-2ME and rGO-2ME in the graphs (Figure 2B and D) were also correlated with the cytotoxicity values of the 2-ME in DMSO. The 2-ME concentrations included in the comparisons are the initial 2-ME concentration used in the graphene oxide flakes embroidered with the 2-methoxyestradiol molecules preparation process – ie 1 mg/mL, as well as the concentration corresponding to the 2-ME content in the GO-2ME (Table 3). Therefore, all results included in the graphs in Figure 2 take into account the use of DMSO as a solvent for the tested compounds.
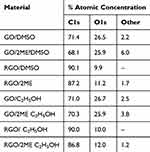 |
Table 3 XPS Analysis Data |
It is observed that GO-2 ME shows higher anticancer efficacy against A375 cells than GO (Figure 2A and B). Similarly, rGO-2ME shows higher anticancer efficacy against A375 cells than rGO (Figure 2C and D). Interestingly, the cytotoxicity of the GO-2ME in DMSO (Figure 2B) at a concentration of 0.0181 mg/mL (1:10 dilution) against A375 cells was comparable to that of 2-ME (at more than a sevenfold higher concentration). This leads to the conclusion that by using graphene carriers in complex compounds with anticancer drugs, the drugs’ effectiveness can be increased. It is also worth noting that the cytotoxic effect of the RGO-2ME at a dilution of 1:10 (0.02 mg/mL) is higher than the effect of 2-ME at the starting concentration for creating a 1 mg/mL complex (Figure 2D). Cytotoxic effects against A375 cells were also observed for GO nanoflakes alone in DMSO at dilutions of 1:20 (Figure 2A) and rGO in DMSO at all dilutions of 1:10, 1:20, 1:40 (Figure 2C). A cytotoxic effect of GO and rGO nanosheets alone was observed in ethanol only at a dilution of 1:10. At a 1:80 dilution, no significant changes were observed in the viability of the A375 cells treated with graphene GO, rGO, or its derivatives (Figure 2).
In the osteosarcoma cell viability studies, 143B osteosarcoma cells were incubated with 2-methoxyestradiol (2-ME), graphene oxide (GO), or reduced graphene oxide (rGO) in sizes ranging from 2 nm to 6 µm, and their complex derivatives (GO-2-ME, rGO-2-ME) for a period of 24 hours. Cytotoxicity assessment was performed using the MTT test. The control consisted of cells treated with a solvent at a concentration corresponding to the subsequent test systems. Similarly to the cytotoxicity test of the tested compounds against melanoma cells, a significant influence of the choice of solvent (environment) on the anticancer potential of each of the tested compounds was also observed against the osteosarcoma cells (Figure 3). In this case, the use of ethanol as a solvent for the tested compounds (GO, rGO, and their derivatives) also reduced their anticancer effectiveness compared to the use of DMSO as a solvent. Another similarity is that the largest difference in cytotoxicity towards 143B cells depending on the solvent used was determined for the rGO-2ME.
Graphs A, B, C, and D (Figure 4) show the cytotoxicity of the graphene-based compounds: GO, GO-2ME, RrGO, and RrGO-2ME in DMSO solvent against 143B cells. The results obtained on the lines of melanoma and osteosarcoma correlate with each other. It is observed that GO-2 ME shows higher anticancer efficacy against 143B cells than GO (Figure 4A and B). Similarly, rGO-2ME shows higher anticancer efficacy against 143B cells than rGO (Figure 4C and D). In addition, the concentration (dose) impact on the anti-tumor activity of GO, rGO, and their derivatives as well have been also documented. It is worth noting that the applied dilutions of 1:10, 1:20, and 1:40 of the GO-2ME resulted in higher cytotoxicity than the effect of the 2-ME contained in the GO-2ME (0.135 mg/mL) (Figure 4B). Similarly to the previously discussed tests, with the test results for the 143B line, the concentrations presented in the chart, expressed in g/mL units, correspond to subsequent dilutions of the starting compounds in the proportions 1:10, 1:20, 1:40, and 1:80. A similarity is also observed in the calculation of the exact concentrations of GO-2ME (Figure 4B) and rGO-2ME (Figure 4D). The cytotoxicity results of the GO-2ME and rGO-2ME in the graphs (Figure 4B and D) were also compared with the cytotoxicity values of the 2-ME in DMSO. The 2-ME concentrations included in these comparisons are the initial 2-ME concentration used in the complex preparation process – ie 1 mg/mL, as well as the concentration corresponding to the 2-ME content in the GO-2ME (Table 3). Also, in the case of the tests for the 143B line, all results included in the analysis take into account the use of DMSO as a solvent for the tested compounds.
Moreover, the use of the GO-2ME hybrid at a dilution of 1:10 (0.0181 g/mL) produces a similar cytotoxic effect to the use of 2ME at the initial concentration for hybrid formation (1 mg/mL). Therefore, the conducted studies showed that the GO-2-ME hybrid produces, on average, the greatest reduction in the viability of the 143B osteosarcoma cells at a dilution of 1:10. The use of a 1:10 or 1:20 dilution of the rGO-2ME hybrid resulted in higher cytotoxicity compared to the 2-ME at the initial concentration in the process of obtaining this hybrid – 1 mg/mL (Figure 4D). Moreover, some cytotoxic effects of the GO nanoflakes alone and the rGO in DMSO were also observed against 143B cells at concentrations of 0.0125 mg/mL and 0.00625 mg/mL.
It is extremely important to note that subsequent studies have shown that the use of an appropriate technique of complexing 2-ME with graphene (the type of solvent and the type of graphene nanoflakes) makes it possible to obtain a significant cytotoxic effect (50%) against neoplastic cells at relatively low concentrations, ie whose toxicity against normotypical cells of the carrier itself (graphene oxide) is negligible, as demonstrated in in vitro studies (Figure 5A–D).
It should be particularly emphasised that, irrespective of the dilution, no cytotoxic effect of GO, rGO, or their derivatives was demonstrated on human dermal fibroblasts (Figure 5), indicating the extremely interesting selectivity of the action of GO/ rGO complexes with 2-ME against tumour cells. This creates a rational clinical potential with a significant safety margin for the use of test compounds in the treatment of chronic wounds, especially in ulcerative skin tumours. It is worth noting that the lack of a toxic effect on human skin fibroblasts was demonstrated for the tested graphene derivatives using both DMSO and ethanol as a solvent.
These results indicate that the attachment of 2-ME to the GO/rGO graphene nanoflakes in most of the tested systems provides greater cytotoxicity of the biocomposite compared to pure graphene nanoflakes. Another very important observation is that GO-2ME and rGO-2ME at a dilution of 1:10 provide comparable or higher cytotoxicity against malignant cells compared to the 2-ME at an initial concentration of 1 mg/mL, which was used in the complex preparation process. This is important in the context of potential clinical applications for increasing the selectivity of the treatment effectiveness of malignant melanoma, osteosarcoma, and other malignancies, including ulcerative skin tumours. It is worth noting that the 1:10 dilution of the GO-2ME and rGO-2ME, which produces comparable results of anticancer effectiveness to the 2-ME concentration of 1 mg/mL, is 50 times and 55 times lower, respectively. As a result, we can talk about developing a drug delivery solution that optimises the dose of its use and the selectivity of its action.
The performed physicochemical analysis of graphene complexes with 2-ME correlates with the cytotoxicity parameters, indicating a significantly greater reduction in the viability of cancer cells for the GO-2ME in DMSO as compared to the rGO-2-ME in DMSO in certain concentration ranges. This result suggests a possible mechanism of sustained release of 2-ME from the rGO-2ME due to the greater lipophilicity of the graphene carrier and/or more efficient penetration of the hydrophobic complex through lipid membranes (greater bioavailability). Nevertheless, both forms of complex are valuable from a clinical point of view – the possibility of regulating the duration of the drug’s action and obtaining, if necessary, the pharmacological effect of prolonged release of the active substance (the so-called depot form).
Phosphotyrosine Phosphatase PTP1B Activity Assay
In addition, the inhibition of phosphotyrosine phosphatase PTP1B was shown to be one of the mechanisms of action of the GO-2-ME (Figure 6). As a result of the research, a decrease in the activity of PTP1B phosphatase to 50%, 61%, 72%, and 73% was shown as a result of incubation with GO suspended in DMSO, GO-2-ME suspended in DMSO, GO suspended in ethanol, and GO-2- ME suspended in ethanol, respectively. This is a very significant result considering that 2-ME has no inhibitory properties against this phosphatase (Figure 6). Due to the absorbance reading threshold being exceeded, the researchers were unable to objectively assess the activity of the rGO and rGO-2-ME. Therefore, it can be assumed that the inhibitory potential of PTP1B phosphatase depends on the type of graphene form used for the synthesis of the GO/rGO, and on the type of solvent.
Transdermal Diffusion Test Using a Franz Chamber
The transdermal GO diffusion test was performed on an artificial skin model using a Franz diffusion chamber. The results of the analysis were presented in the form of chromatograms obtained from an LC-MS chromatography (Figure 7A–G). It was not detected in the analysed samples at a retention time of 7.9 min, and this peak is visible in the calibration curve, the height of which varies in proportion to the concentration. Therefore, the presented test results prove that GO does not undergo transdermal diffusion. This limits the risk of GO accumulation in body tissues.
Results of X-Ray Photoelectron Spectroscopy (XPS) of Graphene Oxide and Reduced Graphene Oxide Complexes with 2-Methoxyestradiol
GO is expected to have a lower carbon content compared to its reduced counterpart, while showing a higher oxygen content relative to rGO. As such, it can be stated that this is the most important difference, which can be directly translated to the removal of oxygen groups upon the reduction of graphene oxide. The modification of the pristine flakes with 2-ME resulted in additional peaks in the XPS spectra, originating from the attached molecules (Table 3). It can be stated that in the case of materials based on GO (regardless of the implemented solvent), an increase in the values of other components (besides C1s, O1s) was observed for the modified samples. In the case of materials based on rGO, the XPS analysis indicated that regardless of the type of the solvent, the unmodified samples contained only carbon and oxygen, while after modification (2-ME attachment), other components also appeared.
Discussion
The conducted tests showed a relatively high durability of the manufactured product complex of graphene nanoflakes with 2-ME. The highest complex formation efficiency was obtained for the rGO in DMSO, which was above 80% (Table 2). The differences in complex formation efficiency are not very big; however, it can be seen that in the tested systems, the rGO showed a greater affinity to 2-ME than GO, which may be related to the relatively high lipophilicity (measured with the calculated lipophilicity coefficient logP = 3.59; MarvinSketch v.20.21.0 5 ChemAxon) and the relatively small polar surface of the molecule (PSA = 49.69 Å2; MarvinSketch v.20.21.0 ChemAxon). Moreover, the results indicate that DMSO to a lesser extent destabilises the complex of graphene nanoflakes with 2-ME, increasing its stability in the biological system and thus enhancing the potential therapeutic effect of the complex carrier-drug system. The relatively small polar surface area of 2-ME is due to the larger hydrophobic area relative to its polar part. RGO has a high surface area with a combination of hydrophobic and polar regions. The hydrophobic moieties of 2-ME can interact favourably with the hydrophobic domains of rGO, resulting in stable binding or adsorption.
In the anticancer in vitro efficacy studies, it was observed that the solutions of GO-2ME and rGO-2ME in DMSO increased their anticancer efficacy against A375 and 143B cells compared to their solutions in ethyl alcohol. This is because DMSO is effective against cancer cells. Dimethyl sulfoxide (DMSO) is an amphipathic molecule that displays a diversity of anti-tumour activities. Previous studies have demonstrated that DMSO can modulate transcription factor AP-1 and lead to cell cycle arrest at the G1 phase.54
The successful creation of GO and rGO embroidered with 2-ME was confirmed using XPS survey data. This fact is reflected in the numerical values presented in Table 3. In addition, the C/O ratio was also compared to the results obtained using combustion elemental analysis. The results gathered in Table 3 correlate with the XPS data: the GO was characterised by a higher oxygen content in relation to the rGO and a lower content of carbon. This is direct evidence of GO reduction. At the same time, it should be noted that the data presented in Table 4 were recorded for pristine GO and rGO powders, whereas the XPS data was acquired for flakes suspended in ethanol and DMSO, thus influencing the registered carbon-to-oxygen ratio.
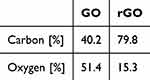 |
Table 4 Combustion Elemental Analysis Data |
According to the invention, GO-2-ME or rGO-2-ME can be used as a therapeutic system with the regulated bioavailability and controlled release of the active component, 2-ME. The results of the GO and rGO complexes studied with 2-ME indicate increased bioavailability of 2-ME and the relative selectivity of potential anticancer therapy by targeting the active compound directly at the tumour site. Thus, preferably, the GO-2-ME or rGO-2-ME can be used for the local treatment of tumours, in particular malignant melanoma or other tumours of the skin and mucous membranes. The same is true for the treatment of advanced tumours of the soft tissues and bones, for instance, osteosarcoma, or as an aid in healing chronic wounds, bedsores, and ulcerated malignancies. Therefore, the developed GO and rGO have clinically significant application potential.
Their advantage over the currently used anticancer drugs is undoubtedly the lack of a toxic effect of the tested compounds on normotypical cells, which was demonstrated on the human skin fibroblast line. The use of complexes according to the invention makes it possible to obtain a relatively selective cytotoxic effect on neoplastic cells, thus minimising the potential side effects related to the impact on normotypical cells. The challenge for further work on the invention is to design an anticancer drug with practical application in the treatment of oncological patients.
Computer analysis was performed using computational chemistry methods regarding the possible extra-receptor bioactivity of 2-ME compared to other natural metabolites of estradiol in terms of drug safety in the context of the potential induction of oxidative stress and/or DNA damage. Molecular modelling and optimisation of the geometry of 17-β estradiol derivative molecules were performed in the Gaussian 09 program (revision D.01) made available on high-performance computing clusters as part of a grant from the PLGrid academic supercomputer network using quantum-mechanical methods, including the Hartree-Fock (RHF) computational method and density functional theory (DFT).55 The optimisation of the geometric structure and energy parameters of the tested molecules was performed at the B3LYP density functional level with the 6–31G(d) functional basis based on the Koopmans theorem56 in terms of the density functional theory according to Kohn and Sham57 by the method described by Parr.58 The potential reactivity with weak cytoprotective nucleophiles and the tendency to form adducts with nucleophilic amino groups of DNA were assessed by calculating the electrophilic potential of estradiol derivative molecules, including 17-β estradiol, 2-hydroxyestradiol, 2-methoxyestradiol (2-ME), 2.3-ortho-benzoquinone estradiol and its methine isomer, as well as 4-hydroxyestradiol, 4-methoxyestradiol (4-ME), 3.4-ortho-benzoquinone estradiol, and analogous oestrone derivatives.54,55
It has been shown that the expected reactivity of 2-ME towards weak cytoprotective nucleophiles (glutathione, protein thiolates) and the amino groups of proteins and nucleic acids is extremely low (electrophilic potential ω = 0.53 eV) compared to the highly reactive electrophile, potentially mutagenic estradiol 3.4-ortho-benzoquinone (ω = 3.63 eV).54,59 Therefore, it can be assumed that the methoxy group of 2-ME effectively protects against the spontaneous oxidation of catechol to highly reactive quinone derivatives, and thus against uncontrolled enzymatic, extra-receptor damage to biomolecules that are important for maintaining homeostasis at the cellular level, which makes 2-ME appear to be a relatively safe drug with a predictable and experimentally confirmed mechanism of action. The lipophilic nature of 2-ME (logP = 3.59) and the relatively small polar surface of the molecule (PSA = 49.69 Å2) ensure the formation of a stable complex with the hydrophobic surface of GO nanoflakes (especially rGO), which has been experimentally confirmed.54,60 The presence of polar hydroxyl groups enables the binding of 2-ME to GO/rGO through hydrogen bonds with hydroxyl and epoxy groups located on the surface, and carbonyl and carboxyl groups located at the edges of the graphene flake sheets. The appropriate dosage of GO-2-ME/rGO-2-ME, where GO/rGO is a carrier of 2-methoxyestradiol (2-ME), ensures efficient penetration of the active substance through biological membranes and controlled modification of cell signalling, ultimately leading to the selective elimination of cancer cells. The complexes according to the invention, depending on their type, size, or type of solvent, can therefore be used as anticancer therapeutic systems with relative selectivity, adjustable bioavailability of the active substance and duration of action, which is particularly important in the treatment of certain types of cancers, in particular skin and bone cancers and soft tissues, including osteosarcoma, malignant melanoma, and ulcerative cancer lesions of the skin or mucous membranes. Thus, this creates the possibility of implementing personalised anticancer therapy, which is extremely important, especially in the case of tumours that are resistant to standard treatment.
Conclusion
The use of Go-2ME and rGO-2ME as drugs in anticancer therapy for melanoma is a solution to the problem of the toxicity of 2-ME itself towards healthy skin cells. According to the literature, 2-ME inhibits the level of proliferation of keloid fibroblasts through p38 in the MAPK/Erk signalling pathway.61,62 Another study also proved that 2ME inhibits the proliferation of and promotes apoptosis in keloid fibroblasts. However, in this study, Go-2ME and rGO-2ME were shown to have no toxicity to human skin fibroblasts at any of the concentrations tested (Figure 6). The selective anticancer effect of the designed complexes is a promising solution for innovative oncological therapies for skin cancer in epidermal or subcutaneous administration. However, this is certainly an issue that requires further research. A particularly important issue for further research on of Go-2ME and rGO-2ME is the development of optimal drug forms for the potential clinical use of these molecules and monitoring their activity under in vivo test conditions.
Acknowledgments
AKJ and MGP acknowledge the support from st46 funding (Medical University of Gdansk). Studies under graphene have been performed thanks to 71-01421 IDUB (Medical University of Gdańsk).
Disclosure
Mrs Magdalena Baran reports a patent P.438737 issued to Medical University of Gdańsk, Łukasiewicz – the Institute of Microelectronics and Photonics, The Institute of Economics of the Polish Academy of Sciences. Dr Krystian Kowiorski reports a patent P.438737 issued to Medical University of Gdańsk, Łukasiewicz – the Institute of Microelectronics and Photonics, The Institute of Economics of the Polish Academy of Sciences. Dr Jacek Gulczynski reports a patent P.438737 pending to Urząd Patentowy RP;. Prof. Dr. Magdalena Górska-Ponikowska reports a patent PCT/PL2022/050050 (2022) issued to N/A, a patent P.449413 (2024) issued to N/A, a patent P.449413 pending to N/A; The authors declare no other conflicts of interest.
References
1. Amjad MT, Chidharla A, Kasi A. Cancer chemotherapy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
2. DeVita VT, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–8653. doi:10.1158/0008-5472.CAN-07-6611
3. Morelli MB, Bongiovanni C, Da Pra S, et al. Cardiotoxicity of anticancer drugs: molecular mechanisms and strategies for cardioprotection. Front Cardiovasc Med. 2022;9:847012.
4. Mercurio V, Pirozzi F, Lazzarini E, et al. Models of heart failure based on the cardiotoxicity of anticancer drugs. J Card Fail. 2016;22(6):449–458.
5. Ahmed B, Qadir MI, Ghafoor S. Malignant melanoma: skin cancer-diagnosis, prevention, and treatment. Crit Rev Eukaryot Gene Expr. 2020;30(4):291–297. PMID: 32894659. doi:10.1615/CritRevEukaryotGeneExpr.2020028454
6. Mısır AF, Durmuşlar MC, Zerener T, Gün BD. Primary malignant melanoma. Saudi Med J. 2016;37(4):446–449. PMID: 27052289; PMCID: PMC4852024. doi:10.15537/smj.2016.4.15017
7. Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma: fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1–10. PMID: 33359211. doi:10.1016/j.canlet.2020.12.024
8. Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The osteosarcoma microenvironment: a complex but targetable ecosystem. Cells. 2020;9(4):976. PMID: 32326444; PMCID: PMC7226971. doi:10.3390/cells9040976
9. Gorska-Ponikowska M, Kuban-Jankowska A, Daca A, Nussberger S. 2-Methoxyestradiol reverses the pro-carcinogenic effect of L-lactate in osteosarcoma 143B cells. Cancer Genomics Proteomics. 2017;14(6):483–493. PMID: 29109098; PMCID: PMC6070326. doi:10.21873/cgp.20058
10. Kamm A, Przychodzeń P, Kuban-Jankowska A, et al. 2-Methoxyestradiol and its combination with a natural compound, ferulic acid, induces melanoma cell death via downregulation of Hsp60 and Hsp90. J Oncol. 2019;2019:9293416. PMID: 32082378; PMCID: PMC7012217. doi:10.1155/2019/9293416
11. Patravale V, Dandekar P, Jain R. Nanoparticulate Drug Delivery: Perspectives on the Transition from Laboratory to Market. Elsevier; 2012.
12. Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23(2):165–172. PMID: 12587805. doi:10.1592/phco.23.2.165.32088
13. Awan ZA, AlGhamdi SA, Alhakamy NA, et al. Optimized 2-methoxyestradiol invasomes fortified with apamin: a promising approach for suppression of A549 lung cancer cells. Drug Deliv. 2022;29(1):1536–1548. PMID: 35612292; PMCID: PMC9154778. doi:10.1080/10717544.2022.2072412
14. Zhang Y, Mi Y, He C. 2-methoxyestradiol restrains non-small cell lung cancer tumorigenesis through regulating circ_0010235/miR-34a-5p/NFAT5 axis. Thorac Cancer. 2023;14(22):2105–2115. PMID: 37439026; PMCID: PMC10396792. doi:10.1111/1759-7714.14993
15. Parks M, Tillhon M, Donà F, Prosperi E, Scovassi AI. 2-Methoxyestradiol: new perspectives in colon carcinoma treatment. Mol Cell Endocrinol. 2011;331(1):119–128. PMID: 20816916. doi:10.1016/j.mce.2010.08.017
16. Sweeney C, Liu G, Yiannoutsos C, et al. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res. 2005;11(18):6625–6633. PMID: 16166441. doi:10.1158/1078-0432.CCR-05-0440
17. LaVallee TM, Zhan XH, Herbstritt CJ, et al. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62(13):3691–3697.
18. Alhakamy NA, Ahmed OA, Fahmy UA, et al. Development, optimization and evaluation of 2-methoxy-estradiol loaded nanocarrier for prostate cancer. Front Pharmacol. 2021;12:682337. PMID: 34335251; PMCID: PMC8322574. doi:10.3389/fphar.2021.682337
19. Patel SC, Lee S, Lalwani G, Suhrland C, Chowdhury SM, Sitharaman B. Graphene-based platforms for cancer therapeutics. Ther Deliv. 2016;7(2):101–116. PMID: 26769305; PMCID: PMC4976992. doi:10.4155/tde.15.93
20. Shafiee A, Iravani S, Varma RS. Graphene and graphene oxide with anticancer applications: challenges and future perspectives. MedComm. 2022;3(1):e118. PMID: 35281783; PMCID: PMC8906468. doi:10.1002/mco2.118
21. Oz Y, Barras A, Sanyal R, et al. Functionalization of reduced graphene oxide via thiol–maleimide “click” chemistry: facile fabrication of targeted drug delivery vehicles. ACS Appl Mater Interfaces. 2017;9:34194–34203. doi:10.1021/acsami.7b08433
22. Pramanik N, Ranganathan S, Rao S, et al. Composite of hyaluronic acid-modified graphene oxide and iron oxide nanoparticles for targeted drug delivery and magnetothermal therapy. ACS Omega. 2019;28:9284–9293. doi:10.1021/acsomega.9b00870
23. Elham M, Soheila K, Nasim M. A targeted drug delivery system based on dopamine functionalized nano graphene oxide. Chem Phys Lett. 2017;668:56–63. doi:10.1016/j.cplett.2016.12.019
24. Shim G, Kim J-Y, Han J, et al. Reduced graphene oxide nanosheets coated with an anti-angiogenic anticancer low-molecular-weight heparin derivative for delivery of anticancer drugs. J Control Release. 2014;189:80–89. doi:10.1016/j.jconrel.2014.06.026
25. Yang K, Wan J, Zhang S, Tian B, Zhang Y, Liu Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials. 2012;33:2206–2214. doi:10.1016/j.biomaterials.2011.11.064
26. Yunus MA, Ramli MM, Osman NH, Mohamed R. Stimulation of innate and adaptive immune cells with graphene oxide and reduced graphene oxide affect cancer progression. Arch Immunol Ther Exp. 2021;69(1):1–16. doi:10.1007/s00005-021-00625-6
27. Kavinkumar T, Varunkumar K, Ravikumar V, Manivannan S. Anticancer activity of graphene oxide-reduced graphene oxide-silver nanoparticle composites. J Colloid Interface Sci. 2017;505:1125–1133. PMID: 28704918. doi:10.1016/j.jcis.2017.07.002
28. Lu Y-J, Lin P-Y, Huang P-H, et al. Magnetic graphene oxide for dual targeted delivery of doxorubicin and photothermal therapy. Nanomaterials. 2018;8(4):193. doi:10.3390/nano8040193
29. Taheri-Kafrani A, Shirzadfar H, Kajani AA, et al. Functionalized graphene oxide/Fe3O4 nanocomposite: a biocompatible and robust nanocarrier for targeted delivery and release of anticancer agents. J Biotechnol. 2021;331:26–36. doi:10.1016/j.jbiotec.2021.03.005
30. Giusto E, Žárská L, Beirne DF, et al. Graphene oxide nanoplatforms to enhance cisplatin-based drug delivery in anticancer therapy. Nanomaterials. 2022;12(14):2372. PMID: 35889596; PMCID: PMC9321599. doi:10.3390/nano12142372
31. Ashrafizadeh M, Saebfar H, Gholami MH, et al. Doxorubicin-loaded graphene oxide nanocomposites in cancer medicine: stimuli-responsive carriers, co-delivery and suppressing resistance. Expert Opin Drug Deliv. 2022;19(4):355–382. PMID: 35152815. doi:10.1080/17425247.2022.2041598
32. Nahain -A-A, Lee J-E, Jeong JH, Park SY. Photoresponsive fluorescent reduced graphene oxide by spiropyran conjugated hyaluronic acid for in vivo imaging and target delivery. Biomacromol. 2013;14(11):4082–4090. doi:10.1021/bm4012166
33. Poudel K, Banstola A, Tran TH, et al. Hyaluronic acid wreathed, trio-stimuli receptive and on-demand triggerable nanoconstruct for anchored combinatorial cancer therapy. Carbohydr Polym. 2020;249:116815. doi:10.1016/j.carbpol.2020.116815
34. Bellier N, Baipaywad P, Ryu N, Lee JY, Park H. Recent biomedical advancements in graphene oxide- and reduced graphene oxide-based nanocomposite nanocarriers. Biomater Res. 2022;26(1):65. PMID: 36435846; PMCID: PMC9701399. doi:10.1186/s40824-022-00313-2
35. Ghosh S, Chatterjee K. Poly(Ethylene Glycol) functionalized graphene oxide in tissue engineering: a review on recent advances. Int J Nanomed. 2020;15:5991–6006. PMID: 33192060; PMCID: PMC7656781. doi:10.2147/IJN.S249717
36. Zou H, Zhao S, Zhang J, et al. Enhanced radiation-induced cytotoxic effect by 2-ME in glioma cells is mediated by induction of cell cycle arrest and DNA damage via activation of ATM pathways. Brain Res. 2007;1185:231–238. PMID: 17980860. doi:10.1016/j.brainres.2007.07.092
37. Massaro RR, Faião-Flores F, Rebecca VW, et al. Inhibition of proliferation and invasion in 2D and 3D models by 2-methoxyestradiol in human melanoma cells. Pharmacol Res. 2017;119:242–250. PMID: 28212889; PMCID: PMC5642284. doi:10.1016/j.phrs.2017.02.013
38. Kato S, Sadarangani A, Lange S, et al. 2-methoxyestradiol mediates apoptosis through caspase-dependent and independent mechanisms in ovarian cancer cells but not in normal counterparts. Reprod Sci. 2008;15(9):878–894. PMID: 19050321; PMCID: PMC9019574. doi:10.1177/1933719108324171
39. Liao C, Li Y, Tjong SC. Graphene nanomaterials: synthesis, biocompatibility, and cytotoxicity. Int J Mol Sci. 2018;19(11):3564. PMID: 30424535; PMCID: PMC6274822. doi:10.3390/ijms19113564
40. Wang K, Ruan J, Song H, et al. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6:8. doi:10.1007/s11671-010-9751-6
41. Ou L, Song B, Liang H, et al. Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part Fibre Toxicol. 2016;13(1):57. PMID: 27799056; PMCID: PMC5088662. doi:10.1186/s12989-016-0168-y
42. Cho JY, Kim JH, Yang HJ, et al. Tailored and highly efficient oxidation of various-sized graphite by kneading for high-quality graphene nanosheets. Carbon. 2020;157:663–669. doi:10.1016/j.carbon.2019.10.102
43. Zhu Y, Murali S, Cai W, et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater. 2010;22:3906–3924. doi:10.1002/adma.201001068
44. Chng E, Chua C, Pumera M. Graphene oxide nanoribbons exhibit significantly greater toxicity than graphene oxide nanoplatelets. Nanoscale. 2014;6:10792–10797. doi:10.1039/C4NR03608E
45. Gong P, Ji S, Wang J, et al. Fluorescence-switchable ultrasmall fluorinated graphene oxide with high near-infrared absorption for controlled and targeted drug delivery. Chem Eng J. 2018;348:438–446. doi:10.1016/j.cej.2018.04.193
46. Lei HZ, Mi LJ, Zhou XJ, et al. Adsorption of double-stranded DNA to graphene oxide preventing enzymatic digestion. Nanoscale. 2011;3:3888–3892. doi:10.1039/c1nr10617a
47. Wei G, Dong R, Wang D, et al. Functional materials from the covalent modification of reduced graphene oxide and [small beta].-cyclodextrin as a drug delivery carrier. New J Chem. 2014;38:140–145. doi:10.1039/C3NJ00690E
48. Georgakilas V, Otyepka M, Bourlinos AB, et al. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev. 2012;112(11):6156–6214. PMID: 23009634. doi:10.1021/cr3000412
49. Raslan A, Saenz Del Burgo L, Ciriza J, Pedraz JL. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int J Pharm. 2020;580:119226. PMID: 32179151. doi:10.1016/j.ijpharm.2020.119226
50. GO-2-ME and rGO 2-ME hybrids as part of the GO-2-ME and rGO 2-ME Hybrids project. Patent application no P.438737_14550.
51. Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80:1339. doi:10.1021/ja01539a017
52. Mombeshora ET, Muchuweni E. Dynamics of reduced graphene oxide: synthesis and structural models. RSC Adv. 2023;13(26):17633–17655. PMID: 37312999; PMCID: PMC10258683. doi:10.1039/d3ra02098c
53. Rhazouani A, Gamrani H, El Achaby M, et al. Synthesis and toxicity of graphene oxide nanoparticles: a literature review of in vitro and in vivo studies. Biomed Res Int. 2021;2021:5518999. PMID: 34222470; PMCID: PMC8213470. doi:10.1155/2021/5518999
54. Musial C, Knap N, Zaucha R, et al. Induction of 2-hydroxycatecholestrogens O-methylation: a missing puzzle piece in diagnostics and treatment of lung cancer. Redox Biol. 2022;55:102395. PMID: 3584. doi:10.1016/j.redox.2022.102395
55. Koopmans T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms[On the Assignment of Wave Functions and Eigenvalues to the Individual Electrons of an Atom]. Physica. 1933;1:104–113 German . doi:10.1016/S0031-8914(34)90011-2
56. Kohn W, Sham LJ. Self-consistent equations including exchange and correlation effects. Phys Rev B. 1965;140:A1133–A1138. doi:10.1103/PhysRev.140.A1133
57. Parr RG, Yang W. Density-functional theory of the electronic structure of molecules. Annu Rev Phys Chem. 1995;46:701–728. doi:10.1146/annurev.pc.46.100195.003413
58. Domingo LR, Rios-Gutierrez M, Perez P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules. 2016;21:748. doi:10.3390/molecules21060748
59. Maayah ZH, Levasseur J, Siva Piragasam R, et al. 2-Methoxyestradiol protects against pressure overload-induced left ventricular hypertrophy. Sci Rep. 2018;8(1):2780. PMID: 29426916; PMCID: PMC5807528. doi:10.1038/s41598-018-20613-9
60. Zhang MZ, Liu YF, Ding L, et al. 2-Methoxyestradiol inhibits the proliferation level in keloid fibroblasts through p38 in the MAPK/Erk signaling pathway. J Cosmet Dermatol. 2023;22(11):3135–3142. PMID: 37190848. doi:10.1111/jocd.15810
61. Zhu L, Song Y, Li M. 2-Methoxyestradiol inhibits bleomycin-induced systemic sclerosis through suppression of fibroblast activation. J Dermatol Sci. 2015;77(1):63–70. PMID: 25465161. doi:10.1016/j.jdermsci.2014.10.007
62. Zhang MZ, Liu YF, Ding N, et al. 2-Methoxyestradiol improves the apoptosis level in keloid fibroblasts through caspase-dependent mechanisms in vitro. Am J Transl Res. 2018;10(12):4017–4029. PMID: 30662647; PMCID: PMC6325513.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.









