Back to Journals » Journal of Inflammation Research » Volume 18
Combining Experimental Validation and Network Pharmacology to Reveal the Action Mechanism of Panax Notoginseng-Radix Salviae on Atherosclerosis
Authors Song N , Zhang X, Yin H, Zhang L, Li C , Li J, Li Y, Wu J
Received 2 December 2024
Accepted for publication 28 March 2025
Published 9 April 2025 Volume 2025:18 Pages 4929—4945
DOI https://doi.org/10.2147/JIR.S508025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Subhasis Chattopadhyay
Ningning Song,1,2 Xinrong Zhang,3 Honglin Yin,3 Lei Zhang,1 Chao Li,4 Jian Li,5 Yunlun Li,4,* Jibiao Wu1,*
1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China; 2Qilu Hospital of Shandong University, Jinan, People’s Republic of China; 3First College of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China; 4Department of Cardiovascular, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China; 5Department of Medical Imaging, The Fifth People’s Hospital of Jinan City, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jibiao Wu, College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, 250355, People’s Republic of China, Tel +86-15169005698, Email [email protected] Yunlun Li, Department of Cardiovascular, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, 250014, People’s Republic of China, Tel +86-13869102760, Email [email protected]
Purpose: Few studies have evaluated the mechanisms of the specific efficacy of Panax notoginseng-Radix Salviae couplet medicines (PN-RS) in the treatment of atherosclerosis (AS). This study aims to explore the potential bioactive ingredients and molecular mechanisms of PN-RS in the treatment of AS.
Materials and Methods: C57BL/6J mice were fed a common diet as the control group. ApoE−/− mice were randomly divided into the model, PN-RS treatment (0.75 mgPN+3.75 mgRS/g/day via gavage), and positive drug groups (rosuvastatin, 1.25 mg/kg/day via gavage). After two months of drugs intervention, H&E and Masson staining, the serum lipid concentration and atherosclerotic index were conducted to verify the PN-RS efficacy. Network pharmacology analysis was carried out to identify the pathways and targets of PN-RS. The signaling pathway-related targets and cytokines were tested via enzyme-linked immunosorbent assay (ELISA), Western blotting and real-time polymerase chain reaction (PCR).
Results: Network pharmacology analysis identified the JAK2-STAT3 signaling pathway as a key pathway through which PN-RS exerts its therapeutic effects. Results from animal experiments demonstrated that PN-RS significantly reduced the atherosclerotic lesion area and decreased serum levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and atherogenic index (AI). Mechanistic studies further revealed that PN-RS inhibited the JAK2-STAT3 signaling pathway and reduced the concentrations of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and monocyte chemoattractant protein-1 (MCP-1).
Conclusion: These findings suggest that PN-RS could be a promising treatment for atherosclerosis, applicable across diverse populations. Future research should investigate its efficacy in various demographic groups and in combination with other therapies.
Keywords: traditional Chinese medicine, atherosclerosis, lipid metabolism, inflammatory reaction, network pharmacology
Introduction
Cardiovascular disease (CVD) is a major barrier to sustainable human development due to its high morbidity and mortality worldwide.1,2 Atherosclerosis (AS) is a chronic inflammatory artery disease characterized by the accumulation of lipids and fibrous elements in the large arteries, which is the primary cause of CVD and stroke.3 As a slowly progressing disease, AS remains the leading cause of mortality worldwide despite its declining incidence in some countries. Although the pathogenesis of AS is not clear, according to different causative factors, there are several recognized hypotheses, such as lipid infiltration, endothelial injury, oxidative stress, and inflammatory response.4 Plasma lipoproteins are complexes of lipids with proteins being transported through the circulation, the levels of which constitute the major risk factors for AS. In the early stages of AS, hyperlipidemia can lead to endothelial damage and induce cytokine release and adhesion molecule expression. Then, macrophages move to sites of injury, where they promote the secretion of inflammatory factors, plaque formation and even rupture. Therefore, the therapeutic strategy should be focused on intervention in dyslipidemia, reducing the inflammatory response and protecting endothelial function. As the rate-limiting enzyme in cholesterol synthesis, statins are widely used to treat AS by inhibiting 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase to effectively lower low-density lipoprotein (LDL) levels,5 and statins can inhibit high-fat diet-induced lipid metabolism disorders in rats by inhibiting bacteroides reduction and improving metabolism.6 However, statins have significant side effects, such as muscle pain and weakness.7 In recent years, traditional Chinese medicine (TCM) has been widely used to treat AS.8 Rauf et al9 pointed out that combination therapy with TCM regulates blood lipids has great prospects.
Panax notoginseng (PN) (SAN QI), a perennial herb, is the dried root and rhizome of the Araliaceae plant Panax notoginseng (Burk). F.H.Chen. It was first recorded in the Compendium of Materia Medica (Ben Cao Gang Mu) compiled by Li Shizhen in the Ming dynasty and has been used for more than 400 years. PN is often used to promote blood circulation and dispel blood stasis in traditional applications. Accumulating evidence suggests that the main chemical ingredients of PN, including saponins, flavonoids, volatile oils and polysaccharides, have good treatment effects on AS and other CVDs. Panax notoginseng saponin R1 mediates its anti-atherogenic effects through the multiple targeting effects of anti-inflammatory, oxidative stress, lipid metabolism, and miRNA expression.10,11 Therefore, the pharmacological actions of PN are mainly manifested in its effect on circulatory systems and cerebrovascular systems.
Radix Salviae (RS) (DAN SHEN), the root and rhizome of Salvia miltiorrhiza Bge., a plant of the labiate family, has been widely used to treat various diseases, such as cardiovascular and immune system diseases.12 Clinical studies show that RS has anti-inflammatory effects because of the tanshinones and rich phenolic acids in the stems and leaves, and more evidence shows that RS can enhance recovery after myocardial ischemia and decrease adhesion molecule expression.13,14 In addition, the main pharmacological effects of RS include protecting vascular endothelial cells, lowering blood lipids, improving microcirculation and exerting antitumor effects.15 Panax notoginseng and Radix Salviae have been widely used clinically in the treatment of CVD. However, few studies have evaluated the mechanisms of the specific efficacy of Panax notoginseng-Radix Salviae couplet medicines (PN-RS) in the treatment of AS.
Network pharmacology was proposed by Andrew L Hopkins in 2007. It is a systematic research method of study that uses omics and network visualization techniques to reveal complex biological network relationships among drugs, active ingredients, diseases, and targets and then to predict and analyze the action targets and mechanisms of drugs.16 Given the complexity of the composition and function, network pharmacology is able to evaluate the multichannel regulation of signaling pathways and is particularly suited to elucidating the intrinsic link between bioactive ingredients of Chinese herbals and disease treatment targets.17 Therefore, the network pharmacology approach has important guiding significance to clarify the pharmacological mechanisms of Chinese herbs.18
In this study, we first conducted animal experiments to verify the therapeutic effect of PN-RS on AS. Then, we used network pharmacology methods to predict the potential targets and signal transduction pathways of PN-RS treatment in AS and further verified the mechanism of PN-RS through experiments, which provides a basis for subsequent pharmacological experimental research and clinical transformation research.
Materials and Methods
Animal Experiments
Drug Preparation
PN and RS were manufactured by Beijing Kangrentang Pharmaceutical Co., Ltd (Beijing, China), and authenticated by Hospital of Shandong University of Traditional Chinese Medicine (Jinan, Shandong, China).
Animals and Groups
Male C57BL/6 mice and ApoE−/− mice (both 8 weeks old) under specific pathogen-free (SPF) laboratory conditions were purchased from Beijing Weitonglihua Experimental Animal Technology Co., Ltd (Animal Qualification License No. SCXK (Beijing) 2021–0006). Animal experiments were approved by Animal Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine (Approval number 2021–66) and carried out according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The animals were tested after one week of adaptive feeding under SPF laboratory conditions. ApoE−/− mice were fed continuously for 12 weeks with a high-fat diet including 0.15% cholesterol and 21% pork fat, and then randomly divided into model group, which received an equal amount of distilled water by gavage every day; the PN-RS treatment group, 0.75 mgPN+3.75 mgRS/g/day; and the positive drug group, rosuvastatin, 1.25 mg/kg/day. C57BL/6J mice were fed with common diet as control group. All animals received treatment continuously by gavage for eight weeks.
Specimen Collection
Blood samples were drawn from the orbital cavity. Serum was centrifuged at 1300×g for 10 min at 4°C. After the thoracic cavity and abdominal cavity opened, the myocardial tissue and aortic root were removed and quickly fixed in 4% paraformaldehyde (PFA). Parts of the aorta samples were stored at −80°C for subsequent experiments.
Oil Red O Staining
After removal of the outside connecting tissue and fat, the entire aorta was opened longitudinally from the aortic root to the renal artery and fixed with 4% PFA solution for 24 h. Then, the entire aorta was stained with 0.5% Oil-red O working solution at 37°C in a biochemical incubator. After 1 h of staining, the entire aorta was immersed in 70% ethanol for destaining. The stained aorta was scanned, and the total morphometric lesion area was evaluated with En face method by using Image-Pro Plus 6.0 software.
Hematoxylin-Eosin and Masson Staining
The aortic arch tissue was sliced into 5 µm serial paraffin sections. After deparaffinization and rehydration, the sections of the aortic arch were stained with hematoxylin and eosin (H&E) and Masson staining to determine aortic lipid plaque areas and collagen fiber content, respectively. Images were collected under a microscope and measured by Image-Pro Plus 6.0 software. After H&E staining and photography, the ratio of plaque area and lumen area was calculated by Image-Pro Plus 6.0. Relative plaque area = plaque area/lumen area × 100%. Section collagen fibers are colored blue after Masson staining. The total area of positive staining and the area of the intima, media were determined by Image-Pro Plus to calculate the percentage of vascular collagen fiber area.
Determination of the Serum Lipids
Serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels in mice were determined by a Chemray 240 Automatic Biochemistry Analyzer (Shenzhen Ledu Life Technology, Shenzhen, China). The blood lipid levels were determined by calculating the AS index (AI) (AI = (TC-HDL-C)/HDL-C).
Network Pharmacology Analysis
Based on the theory of network pharmacology, this topic studied the public resource database and applied for exemption to the Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine. According to the ethics committee requirements, exemption application is allowed for the collection or research of previously archived data, documents, records, pathological specimens, or diagnostic specimens, and these resources are public resources. After the approval of the Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine, the project passed the application for exemption.
Collection of BioactiveiIngredients and Targets of PN and RS
We used the TCMSP database19 (https://old.tcmsp-e.com/tcmsp.php) and the ETCM database20 (http://www.tcmip.cn/ETCM/) to collect bioactive ingredients of PN and RS. The screening conditions were oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18. In addition, we reviewed the pertinent literature to supplement the bioactive ingredients. The targets of the ingredients were searched through the TCMSP database, ETCM database and SwissTargetPrediction database (http://www.swisstargetprediction.ch/). After screening, the UniProt ID of the protein target was then searched through the UniProt database (https://www.uniprot.org) with species defined as Homo sapiens. Finally, the obtained potential targets were collated, and a diagram was constructed after removing duplicate targets.
Collection of Targets of AS
The targets of AS were collected through the DisGeNET database21 (https://www.disgenet.org/), GeneCards database22 (https://www.genecards.org/) and DrugBank database23 (https://go.drugbank.com/). We screened out targets of AS using a relevance score greater than the median as the criterion. The intersection targets between drugs and diseases were visualized by BioVenn24 (http://www.biovenn.nl).
Construction and Analysis of Ingredient and Target Network
Via Cytoscape software (v3.9.1),25 network-built editing and analysis software, drugs and ingredients were used to construct a relationship network. Then, ingredients and intersection targets were uploaded to Cytoscape software to generate a network and analyzed by the Network Analyzer plug-in of Cytoscape. In the network, the degree value of a node reflected the importance of ingredients or targets.
Construction of the Protein-Protein Interaction Network (PPI) and Screening of Core Clusters and Key Targets
We imported the intersection targets into the STRING database (https://string-db.org/) with a minimum needed interaction score ≥0.900 to obtain the protein interaction relationship. Then, the results were uploaded into Cytoscape software to construct the interaction network. Under the following filter conditions: degree cutoff 2 and k-core 2, the MCODE plug-in of Cytoscape was applied for cluster analysis to select the core cluster with the closest relationship in the network. Then, the CytoHubba plug-in was used to carry out network topology analysis for the PPI network and to screen the key targets based on MCC.
GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analyses
Enrichment analysis of GO function for the intersection targets was performed using the Metascape database (https://Metascape.org/) according to a P value cutoff < 0.01, and enrichment analysis of KEGG pathways was performed using the R language clusterprofiler computer package. Via tools on the Bioinformatics website, the significant terms and pathways were used to generate diagrams.
Mechanism Research
According to the above animal experiments and network pharmacology studies, we further explored the mechanism of PN-RS in AS. To further validate the combination effect of PN and RS, we added a single-drug treatment group. ApoE−/− mice were randomly divided into four groups: the model group, PN treatment group (0.75 mgPN/g/day), RS treatment group (3.75 mgRS/g/day) and PN-RS treatment group (0.75 mgPN+3.75 mgRS/g/day). C57BL/6J mice were used as the control group.
Enzyme-Linked Immunosorbent Assay
According to the manufacturer’s instructions, the concentrations of vascular endothelial growth factor (VEGF), monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-6) were examined by enzyme-linked immunosorbent assay (ELISA) kits (Wuhan Servicebio Technology Co., Ltd., Wuhan, China).
Protein Extraction and Western Blotting
The levels of Janus kinase 2 (JAK2) and signal transduction and activator of transcription 3 (STAT3) in mice were detected by Western blot. Tissue homogenate was prepared using phenylmethanesulfonyl fluoride (PMSF) lysis buffer. The protein concentration was determined using a bicinchoninic acid (BCA) kit (Dalian Meilun Biotechnology Co., Ltd., Dalian, China). The isolated protein (30 μg) was separated by 10% sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking with TBST containing 5% skim milk for 1 h, the membranes were incubated overnight at 4°C with specific primary antibodies. Then, the membranes were washed and incubated with secondary antibodies for 1.5 h at room temperature. Finally, the blot signal was detected by using ECL (Millipore Corporation, USA) with β-actin as the internal reference, and data were analyzed with Image-Pro Plus 6.0 software.
RNA Extraction and Quantitative Real-Time PCR Analysis
Following the kit instructions, total RNA was isolated from mouse tissue using an RNA extraction kit (Omega, USA) under RNase-free conditions. Then, SweScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (Wuhan Servicebio Technology Co., Ltd., Wuhan, China) was used to reverse transcribe total RNA (2 μg) samples into cDNA. qRT‒PCR was performed with 2×SYBR Green qPCR Master Mix (Wuhan Servicebio Technology Co., Ltd., Wuhan, China). The sequences of gene-specific primers were synthesized as follows:
JAK2: CTTATTCATGGGAATGTGTGTGC (F), ATCCAGAGCACTCAGGGGCTTA (R);
Stat3: TGCGGAGAAGCATTGTGAGTG (F), TCTTAATTTGTTGGCGGGTCT (R);
GAPDH: CCTCGTCCCGTAGACAAAATG (F), TGAGGTCAATGAAGGGGTCGT (R).
Statistical Analysis
We used GraphPad Prism 9.0.0 software (San Diego, CA, USA) to analyze and visualize the data. Statistical differences among more than two groups were calculated using analysis of variance (ANOVA) followed by Tukey’s post hoc test. A p value <0.05 was considered to indicate statistical significance.
Results
PN-RS Significantly Attenuated Atherosclerotic Pathology
We evaluated the inhibitory effect of PN-RS on the AS lesion process by studying aortic pathological section changes in animals. Oil red O staining showed that the atherosclerotic plaque-positive area in the model group was significantly higher than that in the control group (Figure 1A). After PN-RS treatment, the size of the atherosclerotic plaque in the whole aorta was significantly reduced, and the treatment effect of PN-RS was similar to that of rosuvastatin (Figure 1A).
H&E and Masson staining can be used to assess the severity of AS. H&E staining showed that the arterial vascular structure of the control group was regular, with smooth intima and uniform media thickness, and intact lumen. In the model group, the vascular intima was thickened, and the significant formation of atherosclerotic plaques with a large number of foam cells and cholesterol crystals was observed. The relative plaque area was significantly higher than that in the control group (Figure 1B). Masson staining showed that the arterial collagen tissue of the control group was evenly distributed. The arterial intima and media collagen fibers were significantly increased in the model group. Compared to the control group, the arterial collagen fiber area percentage increased significantly (Figure 1C). However, the relative plaque area and the percentage of the collagen fiber area were significantly decreased after PN-RS and rosuvastatin treatment (Figure 1B and C).
PN-RS Significantly Reduced Serum Lipid Levels
We assessed the effect of PN-RS on AS lipid metabolism by measuring serum lipid levels in mice. The levels of TC, TG, LDL-C, and AI were significantly higher while HDL-C was decreased in ApoE−/− mice compared to the control group. However, PN-RS improved the lipid index to varying degrees, and the effect was similar to that of rosuvastatin treatment. These results suggested that PN-RS significantly regulated dyslipidemia (Figure 2A–E).
Ingredient and Target Collection and Network Analysis
Screening through the TCMSP, ETCM, and SwissTargetPrediction databases and pertinent literature, 10 bioactive ingredients of PN and 306 ingredient-related targets were selected, as well as 66 bioactive ingredients of RS and 251 ingredient-related targets. The network relationship of ingredients and drugs is shown in Figure 3. With “atherosclerosis” as the keyword, after deleting duplicates, a total of 2412 AS disease targets were identified from the DisGeNET and the GeneCards databases under the condition of a relevance score greater than the median. There were 222 intersection targets of ingredient-related and AS-related targets using the BioVenn diagram (Figure 4). Among the total intersection targets, PN and RS jointly shared 112 disease-associated intersection targets, and each had specific distinct intersection targets (79 for PN and 31 for RS) (Figure 4), which indicated that these two Chinese herbs may have synergistic effects on AS.
 |
Figure 3 Network diagram of bioactive ingredients and herbs. The hexagon nodes represent Panax notoginseng and Radix Salviae, and the diamond nodes represent the bioactive ingredients. |
 |
Figure 4 Venn diagram of PN and RS targets and AS targets. PN, Panax notoginseng; RN, Radix Salviae; AS, atherosclerosis. |
Using Cytoscape software and the Network Analyzer plug-in, a network relationship diagram of ingredients and intersection targets was constructed including 291 nodes and 1029 interaction edges, and the diagram revealed the main ingredients by the degree value (Figure 5). Table 1 shows the top 10 bioactive ingredients of PN and RS that bind to key regulatory targets. The results showed that not only could one ingredient of PN and RS act on multiple target proteins, but multiple different ingredients could also act on the same target.
 |
Table 1 Top 10 Ingredients of PN and RS |
 |
Figure 5 Network diagram of ingredients and targets. The purple node represents the ingredient. The gray node represents the intersection target. |
Construction of the PPI Network and Functional Enrichment Analysis
Using STRING and Cytoscape with a confidence of 0.900, the PPI network of intersection targets was created, including 221 nodes and 973 interaction edges (Figure 6A). Through the analysis of CytoHubba and MCODE, the core clusters were obtained from the PPI network (Figure 6B), and then the core targets were screened based on the MCC according to core clusters (Figure 6C). In Figure 6, a darker color and a larger diameter of nodes indicate a larger degree value. The top 10 targets affecting each other were RELA, JUN, TNF, IL6, IL1B, IL1A, CCL2, CXCL8, IL4 and STAT3 (Table 2).
 |
Table 2 Top 10 Targets in the PPI Network |
 |
Figure 6 (A) PPI network diagram of intersection targets, (B) Core clusters, (C) Core targets. |
The KEGG pathway enrichment analysis of the top 10 targets showed that the most relevant signaling pathways were the AGE-RAGE signaling pathway in diabetic complications, the IL-17 signaling pathway, the chemokine signaling pathway and the JAK-STAT signaling pathway (Figure 7).
 |
Figure 7 Top 10 targets in the GO enrichment analysis of biological processes. |
The intersection targets of the PPI network were then used for GO and KEGG enrichment analysis. The GO analysis results revealed that biological processes (BP) were mainly related to cellular response to nitrogen compound, lipid, lipopolysaccharide and oxygen levels and positive regulation of cell migration. Cellular components (CC) were mainly connected with membrane rafts, transcription regulator complexes, side of membranes, receptor complexes and neuronal cell bodies. The relevant molecular functions (MF) included kinase binding, DNA-binding transcription factor binding, nuclear receptor activity, oxidoreductase activity, and protein homodimerization activity. The top 10 BP, MF and CC subcategories are shown in Figure 8.
 |
Figure 8 GO functional analysis of BP, MF and CC. Abbreviations: BP, biological process; MF, molecular function; CC, cellular component. |
According to the P value, the KEGG pathway enrichment analysis screened the top 10 signaling pathways, as shown in the bubble diagram (Figure 9A), and the relationship between pathways and targets is shown in Figure 9B. The size and color of each bubble represent the number of enriched genes and the degree of enrichment in the pathway, respectively. The results indicated that PN-RS inhibited the AS pathophysiological process by modulating the AGE-RAGE signaling pathway in diabetic complications, fluid shear stress and atherosclerosis, lipid and AS, hepatitis B and endocrine resistance. On the other hand, these results also showed that PN-RS might have a multichannel synergistic effect on AS by participating in lipid metabolism, the oxidative stress response, the regulation of endothelial cell function, and the inflammatory response.
PN-RS Inhibited the JAK2-STAT3 Signaling Pathway and Attenuated Inflammation in Mice
Combined with the results of network pharmacology analysis, based on the JAK-STAT signaling pathway, the mechanism of PN-RS in AS was further explored, and the inflammatory response was used as the entry point.
The JAK2-STAT3 signaling pathway is closely related to the development of AS. By Western blotting detection, the protein expression levels of JAK2, p-JAK2, STAT3, and p-STAT3 in the model group were significantly increased compared with those in the control group. However, after drug treatment, especially after PN-RS treatment, the protein expression levels of JAK2, p-JAK2, STAT3, and p-STAT3 were significantly decreased (Figure 10A). Similar to the protein expression, compared with the control group, the mRNA expression of JAK2 and STAT3 was significantly increased in the model group, which was significantly reduced in the drug treatment group, especially in the PN-RS treatment group (Figure 10B).
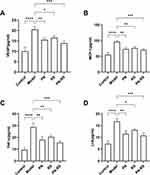
The expression of the multiplication factor VEGF, chemotactic factor MCP-1 and the inflammatory cytokines TNF-α and IL-6 was measured by ELISA. Compared with the control group, the contents of plasma VEGF, MCP-1, TNF-α and IL-6 were significantly increased in the model group. PN, RS and PN-RS significantly decreased the levels of VEGF, MCP-1, TNF-α and IL-6 in mice. PN-RS provided the best treatment results (Figure 11A–D).
Discussion
Atherosclerosis (AS), accompanied by fragile plaque and altered structure and function of the vessel wall, has become a frequent disease worldwide. To date, the detailed mechanism of AS is not fully understood, as the activation mechanism involves multiple factors and many genes. Ross et al26 proposed the hypothesis that AS results from damage to vascular endothelial structures. AS mainly results from a series of inflammatory reactions and complex endothelial dysfunction after endothelial injury, which is an important cause of cardiovascular and cerebrovascular diseases. Therefore, it is important to develop drugs that effectively control the development of AS. TCM has a significant therapeutic effect on AS through the synergistic effect of various ingredients and various targets. Moreover, it has fewer toxic and side effects, which has unique therapeutic advantages for AS.
In this study, we explored the potential mechanisms of PN-RS in AS using network pharmacology and animal experiments. First, aortic histopathological changes and serum lipid levels in ApoE−/− mice were detected through experiments to observe the effect of PN-RS on lipid metabolism. Pathological tissue staining and serum lipid detection showed that PN-RS could significantly improve aortic intimal damage, lipid deposition and serum lipid levels, and the treatment effect was the same as the effect of rosuvastatin (Figures 1 and 2). To further explore the potential targets and signal transduction pathways of PN-RS treatment in AS, a network pharmacology analysis was performed based on the data of this animal experiment.
Through screening from multiple databases, a network consisting of 76 bioactive ingredients of PN-RS and 222 intersection targets was generated (Figure 5). Based on the ingredient-target-disease network and the degree values, we screened the top 10 ingredients binding to key regulatory targets, including quercetin, luteolin, tanshinone IIA, β-sitosterol, stigmasterol, 2-isopropyl-8-methylphenanthrene-3,4-dione, dihydrotanshinlactone, methylenetanshinquinone, 4-methylenemiltirone, and dan-shexinkum D (Table 1). Among the top 10 ingredients, quercetin could act on 155 targets and probably played the most extensive and important role in therapy. Quercetin can prevent cell infiltration in atherosclerotic plaques and reduce the risk of stroke by inhibiting THP-1 monocyte migration and increasing cholesterol efflux.27 Luteolin has many pharmacological effects, such as anti-inflammatory, antitumor, antiviral and CVD effects. Luteolin inhibits abdominal aortic plaque development and lipid accumulation by reducing macrophage inflammation in AS, and its mechanism involves the AMPK-SIRT1 signaling pathway.28 β-sitosterol is a plant sterol that can inhibit blood lipids by inhibiting exogenous absorption, endogenous synthesis, reverse transport and excretion, and some animal experiments have proven that β-Sitosterol has anti-inflammatory effects.29 Tanshinone IIA can achieve anti-AS effects by promoting autophagy in macrophages, attenuating the inflammatory damage caused by ox-LDL, and regulating IL-6 expression.30 In summary, the bioactive ingredients of PN and RS screened by network pharmacology have a wide range of pharmacological effects and strong anti-AS effects.
After obtaining the bioactive ingredients of PN-RS, PPI and functional enrichment analyses were performed on these 222 ingredient targets. The GO enrichment analysis showed that the BP included response to nitrogen compound, lipid, lipopolysaccharide, and regulation of cell migration (Figure 8). BP was compatible with the process of occurrence and development of AS, which was that the vascular intima was damaged by stimulation factors, monocytes adhered and migrated, macrophages invaded and formed foam cells, and lipid deposition eventually resulted in AS. The KEGG pathway enrichment analysis showed that PN-RS inhibited AS by modulating the AGE-RAGE signaling pathway in diabetic complications, fluid shear stress and atherosclerosis, lipid and atherosclerosis, hepatitis B, endocrine resistance and human cytomegalovirus infection (Figure 9). In the AGE-RAGE signaling pathway, AGEs can interact with RAGE to activate NF-kappa-B, thus inducing the oxidative stress response, promoting inflammation and the accumulation of extracellular matrix, enhancing the formation of atherosclerotic plaques and accelerating the development of atherosclerosis.31 The second KEGG pathway of enrichment analysis was fluid shear stress and atherosclerosis. The fluid shear stress is a tangential stress parallel to the tube wall caused by the friction between the blood flow and the tube wall, and it has been confirmed to be closely related to the occurrence and development of AS.32 Abnormal changes in fluid shear stress, such as too low of fluid shear stress or oscillation, will lead to the disturbance of endothelial cell function and promote the development of AS.33 For lipid and atherosclerosis, the imbalance in lipid metabolism is a central aspect of disease progression, and dysfunction of lipid metabolism has been demonstrated as an independent risk factor for AS-associated CVD.34 The results of enrichment analysis and animal experiments support each other that PN-RS can exert its anti-AS effect by intervening in lipid metabolism (Figures 2 and 9).
According to the PPI network map, the top 10 core targets involved in the treatment of PN-RS for AS were screened, including RELA, JUN, TNF, IL6, IL1B, IL1A, CCL2, CXCL8, IL4 and STAT3 (Table 2). RELA (NF-κ-B) can participate in immune and inflammatory responses and is also involved in the regulation of cell differentiation, proliferation, and apoptosis processes. A study reported that inhibiting STAT3/NF-kappa-B and endoplasmic reticulum stress signaling pathways can protect endothelial cells and alleviate atherosclerotic inflammation.35 The STAT3 protein mediates the expression of multiple genes in response to cell stimulation and thus plays a key role in many cellular processes, such as cell growth, apoptosis, and carcinogenesis. In addition, STAT3, TNF, IL-6 and CXCL8 proteins are all associated with the inflammatory response. The core targets of PN-RS are involved in the whole process of AS development, and the mechanisms are related to the AGE-RAGE signaling pathway in diabetic complications, the IL-17 signaling pathway, the chemokine signaling pathway and the JAK-STAT signaling pathway through KEGG pathway enrichment analysis (Figure 7).
The JAK-STAT signaling pathway is a common pathway by which various cytokines and growth factors transmit signals inside cells, participating in various biological activities, such as the immune response, vascular cell migration, proliferation and apoptosis.36 There are four isoforms of JAK, namely, JAK 1, JAK 2, JAK 3, and JAK 4. As the target protein of JAK, STAT has seven isoforms, including STAT 1, STAT 2, STAT 3, STAT 4, STAT5a, STAT5b, and STAT 6.37,38 The signal transduction process of the JAK-STAT pathway is that cytokines first combine with their specific receptor to form receptor dimers and recruit JAK family members. Nearby JAKs are phosphorylated by each other and provide anchor sites for STAT in the cytoplasm. Subsequently, STAT phosphorylation and dimerization occur, and activated STAT is transferred to the nucleus to interact with other transcription factors to regulate gene transcription and protein expression.39 Many studies have confirmed that the JAK-STAT signaling pathway acts on various aspects of AS formation, including dysfunction of vascular endothelial cells (ECs), proliferation and migration of smooth muscle cells (SMCs) and inflammatory cells, and overexpression of inflammatory cytokines. The activation of the JAK-STAT protein in cells can cause increased expression of various inflammatory mediators, such as MCP-140 and VCAM-1,41 which can result in increased cell proliferation and migration and promote the adhesion and infiltration of inflammatory cells to the vascular endothelium. MCP-1 is one of the most important monocyte chemokines that can mediate the migration of monocytes to the blood vessel wall. VEGF is the major factor inducing the proliferation and migration of ECs. TNF-α and IL-6 inflammatory cytokines can promote AS development by enhancing the inflammatory response. As an inducer of the acute phase inflammatory response, IL-6 promotes the phosphorylation of STAT3 and the transfer of STAT3 to the nucleus, promotes the activation of various downstream inflammatory factors, such as TNF-α and IL-6, and finally mediates the inflammatory damage of cells. Moreover, Watanabe et al42 experiments demonstrated that IL-6 could promote the proliferation of vascular smooth muscle cells (VSMCs) and the release of MCP-1 through the JAK-STAT signaling pathway.
Based on the results of the GO and KEGG pathway enrichment analysis and previous animal experiment data, we hypothesized that PN-RS might exert anti-AS effects by regulating the JAK-STAT signaling pathway. The JAK2-STAT3 signaling pathway, as a classical inflammatory pathway, is closely related to AS. Therefore, we conducted further mechanistic studies based on the JAK2-STAT3 signaling pathway using the inflammatory response as the entry point. In this study, to further verify the effect of PN-RS drugs on AS treatment, the drug treatment groups were divided into PN, RS and PN-RS treatment groups. The expression levels of JAK2-STAT3 pathway-related proteins and mRNA were determined by Western blot and real-time PCR. The results showed that each treatment group, especially the PN-RS treatment group, could effectively inhibit the protein and mRNA expression of JAK 2 and STAT3 in AS mice (Figure 10). The expression of cytokines was measured by ELISA, and the experimental results showed that each treatment group could inhibit the secretion of TNF-α and IL-6 inflammatory cytokines, and the best anti-inflammatory effect of PN-RS was achieved (Figure 11C and D). In addition, PN-RS significantly reduced the serum VEGF and MCP-1 concentrations in ApoE−/− mice, which was better than the monotherapy group (Figure 11A and B). The above series of experimental results suggest that PN-RS can inhibit the JAK2-STAT3 signaling pathway, thus reducing inflammatory factor and cytokine expression, inhibiting cell proliferation and migration and inflammatory cell infiltration, and reducing the inflammatory response to effectively exert anti-AS effects.
We combined network pharmacology and animal experiments to verify that the anti-AS mechanism of PN-RS may be related to the regulation of the JAK2-STAT3 signaling pathway, inflammatory response and abnormal lipid metabolism regulation, which provides a theoretical basis and research direction for further experimental verification.
Conclusion
Based on the network pharmacological data analysis, the potential effective mechanism of PN-RS treatment for AS relates to the regulation of targets and pathways in various biological processes, especially lipid metabolism and inflammatory reactions. Animal experiments confirmed the anti-AS effect of PN-RS in ApoE−/− mice. PN-RS significantly reduced the atherosclerotic area and lipid levels in ApoE−/− mice. Further experimental results showed that PN-RS could significantly inhibit the JAK2-STAT3 signaling pathway and reduce the concentrations of the inflammatory factors TNF-α and IL-6, the growth factor VEGF, and the chemokine MCP-1 in mice. In conclusion, PN-RS can protect against AS through multiple targets and pathways.
Abbreviations
AI, AS index; ANOVA, Analysis of variance; AS, Atherosclerosis; BCA, Bicinchoninic acid; BP, Biological Processes; CC, Cellular Components; CVD, Cardiovascular disease; DL, Drug-likeness; ECs, Endothelial cells; ELISA, Enzyme-linked immunosorbent assay; GO, Gene Ontology; H&E, Hematoxylin and Eosin; HDL-C, High-density lipoprotein cholesterol; HMG-CoA, 3-Hydroxy-3-methyl glutaryl coenzyme A reductase; IL-6, Interleukin-1; JAK2, Janus kinase 2; KEGG, Kyoto Encyclopedia of Genes and Genomes; LDL, Low-density lipoprotein; LDL-C, Low-density lipoprotein cholesterol; MCP-1, Monocyte chemotactic protein-1; MF, Molecular Functions; OB, Oral bioavailability; PFA, Paraformaldehyde; PMSF, Phenylmethanesulfonyl fluoride; PN, Panax Notoginseng; PN-RS, Panax Notoginseng-Radix Salviae couplet medicines; PPI, Protein-protein interaction network; PVDF, Polyvinylidene fluoride; RS, Radix Salviae; SDS-PAGE, Sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SMCs, Smooth muscle cells; SPF, Specific pathogen-free; STAT3, Signal transduction and activator of transcription 3; TC, Total cholesterol; TCM, Traditional Chinese medicine; TG, Triglyceride; TNF-α, Tumor necrosis factor-α; VEGF, Vascular endothelial growth factor; VSMC, Vascular smooth muscle cell.
Data Sharing Statement
The datasets and all data in this study are available from the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This article is supported by the National Natural Science Foundation of China (81974566), Taishan Scholars, Shandong Province.
Disclosure
The authors declare that the study was conducted in the absence of any commercial or financial relationship that could be interpreted as a potential conflict of interest.
References
1. Clark H. NCDs: a challenge to sustainable human development. Lancet. 2013;381(9866):510–511. doi:10.1016/s0140-6736(13)60058-6
2. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi:10.1016/j.jacc.2017.04.052
3. Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi:10.1038/35025203
4. Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185:1630–1645. doi:10.1016/j.cell.2022.04.004
5. Razavi AC, Mehta A, Sperling LS. Statin therapy for the primary prevention of cardiovascular disease: pros. Atherosclerosis. 2022;356:41–45. doi:10.1016/j.atherosclerosis.2022.07.004
6. Li H, Wang S, Wang S, et al. Atorvastatin inhibits high-fat diet-induced lipid metabolism disorders in rats by inhibiting bacteroides reduction and improving metabolism. Drug Des Devel Ther. 2022;Volume 16:3805–3816. doi:10.2147/dddt.S379335
7. Lindsay C, Musgaard M, Russell AJ, Sitsapesan R. Statin activation of skeletal ryanodine receptors (RyR1) is a class effect but separable from HMG‐CoA reductase inhibition. Br J Pharmacol. 2022;179:4941–4957. doi:10.1111/bph.15893
8. Pan Y, Feng X, Song W, et al. Effects and potential mechanism of zhuyu pill against atherosclerosis: network pharmacology and experimental validation. Drug Des Devel Ther. 2023;Volume 17:597–612. doi:10.2147/dddt.S398808
9. Rauf A, Akram M, Anwar H, et al. Therapeutic potential of herbal medicine for the management of hyperlipidemia: latest updates. Environ Sci Pollut Res Int. 2022;29(27):40281–40301. doi:10.1007/s11356-022-19733-7
10. Fu C, Yin D, Nie H, Sun D. Notoginsenoside R1 protects HUVEC against oxidized low density lipoprotein (Ox-LDL)-induced atherogenic response via down-regulating miR-132. Cell Physiol Biochem. 2018;51:1739–1750. doi:10.1159/000495677
11. Jia C, Xiong M, Wang P, et al. Notoginsenoside R1 attenuates atherosclerotic lesions in ApoE deficient mouse model. PLoS One. 2014;9:e99849. doi:10.1371/journal.pone.0099849
12. Wang Y, Zhou C, Gao H, et al. Therapeutic effect of cryptotanshinone on experimental rheumatoid arthritis through downregulating p300 mediated-STAT3 acetylation. Biochem Pharmacol. 2017;138:119–129. doi:10.1016/j.bcp.2017.05.006
13. Chen W, Chen G. Danshen (Salvia miltiorrhiza Bunge): a prospective healing sage for cardiovascular diseases. Curr Pharm Des. 2017;23:5125–5135. doi:10.2174/1381612823666170822101112
14. Li F, Han G, Wu K. Tanshinone IIA alleviates the AD phenotypes in APP and PS1 transgenic mice. Biomed Res Int. 2016;2016:7631801. doi:10.1155/2016/7631801
15. Liu M, Li Z, Ouyang Y, et al. Material basis and integrative pharmacology of danshen decoction in the treatment of cardiovascular diseases. Phytomedicine. 2023;108:154503. doi:10.1016/j.phymed.2022.154503
16. Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi:10.1038/nchembio.118
17. Lee M, Shin H, Park M, Kim A, Cha S, Lee H. Systems pharmacology approaches in herbal medicine research: a brief review. BMB Reports. 2022;55:417–428. doi:10.5483/BMBRep.2022.55.9.102
18. Wu J, Luo Y, Shen Y, et al. Integrated metabonomics and network pharmacology to reveal the action mechanism effect of shaoyao decoction on ulcerative colitis. Drug Des Devel Ther. 2022;Volume 16:3739–3776. doi:10.2147/dddt.S375281
19. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi:10.1186/1758-2946-6-13
20. Xu H, Zhang YQ, Liu ZM, et al. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47(D1):D976–D982. doi:10.1093/nar/gky987
21. Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48(D1):D845–D855. doi:10.1093/nar/gkz1021
22. Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54(1):301–13033. doi:10.1002/cpbi.5
23. Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi:10.1093/nar/gkx1037
24. Hulsen T, de Vlieg J, Alkema W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional venn diagrams. BMC Genomics. 2008;9:488. doi:10.1186/1471-2164-9-488
25. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi:10.1101/gr.1239303
26. Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol. 1977;86(3):675–684.
27. Huwait EA, Saddeek SY, Al-Massabi RF, Almowallad SJ, Pushparaj PN, Kalamegam G. Antiatherogenic effects of quercetin in the THP-1 macrophage model in vitro, with insights into its signaling mechanisms using in silico analysis. Front Pharmacol. 2021;12:698138. doi:10.3389/fphar.2021.698138
28. Li J, Dong JZ, Ren YL, et al. Luteolin decreases atherosclerosis in LDL receptor‑deficient mice via a mechanism including decreasing AMPK‑SIRT1 signaling in macrophages. Exp Ther Med. 2018;16:2593–2599. doi:10.3892/etm.2018.6499
29. Liu R, Hao D, Xu W, et al. β-sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm Biol. 2019;57:161–168. doi:10.1080/13880209.2019.1577461
30. Chen W, Li X, Guo S, et al. Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int Immunopharmacol. 2019;70:486–497. doi:10.1016/j.intimp.2019.02.054
31. Fukami K, Yamagishi S, Okuda S. Role of AGEs-RAGE system in cardiovascular disease. Curr Pharm Des. 2014;20:2395–2402. doi:10.2174/13816128113199990475
32. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi:10.1038/ncpcardio1397
33. Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol. 2017;219:382–408. doi:10.1111/apha.12725
34. Guimaraes RC, Goncalves TT, Leiria LO. Exploiting oxidized lipids and the lipid-binding GPCRs against cardiometabolic diseases. Br J Pharmacol. 2021;178:531–549. doi:10.1111/bph.15321
35. Jin Y, Nguyen TLL, Myung C-S, Heo K-S. Ginsenoside Rh1 protects human endothelial cells against lipopolysaccharide-induced inflammatory injury through inhibiting TLR2/4-mediated STAT3, NF-κB, and ER stress signaling pathways. Life Sci. 2022;309:120973. doi:10.1016/j.lfs.2022.120973
36. Harrison DA. The JAK/STAT pathway. Cold Spring Harb Perspect Biol. 2012;4:a011205–a011205. doi:10.1101/cshperspect.a011205
37. Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285(1–2):1–24. doi:10.1016/s0378-1119(02)00398-0
38. Ihle JN. The stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211–217. doi:10.1016/s0955-0674(00)00199-x
39. Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. doi:10.1038/s41392-021-00791-1
40. Lv L, Zhang J, Huang X, Zhao Y, Zhou Z, Zhang H. Lentivirus-mediated RNA interference targeting STAT4 inhibits the proliferation of vascular smooth muscle cells. Arch Med Res. 2008;39:582–589. doi:10.1016/j.arcmed.2008.06.001
41. Nizamutdinova IT, Kim YM, Jin H, et al. Tanshinone IIA inhibits TNF-α-mediated induction of VCAM-1 but not ICAM-1 through the regulation of GATA-6 and IRF-1. Int Immunopharmacol. 2012;14:650–657. doi:10.1016/j.intimp.2012.09.017
42. Watanabe S, Mu W, Kahn A, et al. Role of JAK/STAT pathway in IL-6-induced activation of vascular smooth muscle cells. Am J Nephrol. 2004;24(4):387–392. doi:10.1159/000079706
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Metabolomics Combined with Network Pharmacology-Based Strategy to Reveal the Underlying Mechanism of Zhenhuang Submicron Emulsion in Treating Oropharyngeal Mucositis Complications of Radiation Therapy for Head and Neck Cancer
Chen W, Li C, Jin D, Shi Y, Zhang M, Bo M, Qian D, Wang M, Li G
Drug Design, Development and Therapy 2022, 16:3169-3182
Published Date: 17 September 2022
Study on the Anti-Atherosclerotic Mechanisms of Xin-Tong-Tai Granule Through Network Pharmacology, Molecular Docking, and Experimental Validation

Zhu J, Wang Z, Liu C, Shi M, Guo Z, Li Y, Yu R, Wei J
Journal of Inflammation Research 2024, 17:8147-8164
Published Date: 4 November 2024
Integrated Network Pharmacology, Machine Learning and Experimental Validation to Identify the Key Targets and Compounds of TiaoShenGongJian for the Treatment of Breast Cancer

Ying H, Kong W, Xu X
OncoTargets and Therapy 2025, 18:49-71
Published Date: 16 January 2025
Mechanism Exploration of Dunyeguanxinning in the Treatment of Atherosclerosis Based on UPLC-Q-Orbitrap HRMS Technology, Network Pharmacology, Molecular Docking and Experimental Validation
Wang D, Jin Q, Zhang J, Shi X, Wang X
Journal of Inflammation Research 2025, 18:4857-4878
Published Date: 9 April 2025
Integration of Network Pharmacology, Transcriptomics, and Metabolomics Strategies to Uncover the Mechanism of Chaihuang Qingfu Pill in Treating Sepsis-Induced Liver Injury
Zhang C, Chen F, Jiang Y, Deng J, Yan X, Yin X, Su B, Liu W
Drug Design, Development and Therapy 2025, 19:4665-4688
Published Date: 2 June 2025