Back to Journals » Drug Design, Development and Therapy » Volume 19
Combining Network Pharmacology, Molecular Docking and Experimental Validation to Explore the Effects and Mechanisms of Indirubin on Acute Lymphoblastic Leukemia
Authors Jin L, Guan Y, Li X, Wang M, Shen Y, Wang N, He Z
Received 13 November 2024
Accepted for publication 2 February 2025
Published 18 February 2025 Volume 2025:19 Pages 1083—1103
DOI https://doi.org/10.2147/DDDT.S500249
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Tamer Ibrahim
Lu Jin,1,2 Yunshuang Guan,3 Xue Li,3,4 Mingyue Wang,2 Ying Shen,1,2 Nianxue Wang,3 Zhixu He1,2,5
1Department of Pediatrics, School of Clinical Medicine, Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 2Department of Pediatric Hematology, Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 3Department of Immunology, College of Basic Medical Sciences, Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 4Department of Central Laboratory, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, People’s Republic of China; 5Tissue Engineering and Stem Cell Experiment Center, Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China
Correspondence: Nianxue Wang, Email [email protected]; Zhixu He, Email [email protected]
Purpose: To investigate the effects and underlying mechanisms of indirubin in treating ALL using network pharmacology and experimental validation.
Methods: Potential targets of indirubin- and ALL-related genes were identified using public databases. Core genes were filtered through protein-protein interaction analysis in Cytoscape. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were conducted to explore the potential mechanisms of indirubin against ALL. Drug-disease-functional annotation-signaling pathway network maps were constructed. Molecular docking between indirubin and core proteins was performed using AutoDock Vina software. Finally, both in vitro and in vivo experiments were performed to validate these findings.
Results: PPI network analysis identified eight potential core targets of indirubin in ALL: AKT1, CASP3, and the mammalian target of rapamycin. GO and KEGG enrichment analyses suggested that the mechanism of action of indirubin against ALL involves multiple biological functions and signaling pathways, with the PI3K-AKT pathway likely playing a central role. Molecular docking findings further confirmed the strong binding affinity of indirubin for the core targets. Both in vitro and in vivo experiments demonstrated that indirubin inhibited ALL cell proliferation and induced cell cycle arrest and apoptosis; the underlying mechanism may involve the PI3K-AKT signaling pathway.
Conclusion: The action and mechanism of indirubin in ALL through network pharmacology, as well as in vivo and in vitro experimental validation were elucidated, offering new insights and potential therapeutic avenues for the treatment of ALL.
Keywords: indirubin, acute lymphoblastic leukemia, network pharmacology, molecular docking, experimental validation
Graphical Abstract:

Introduction
Acute lymphoblastic leukemia (ALL) is characterized by the malignant clonal proliferation of hematopoietic stem or progenitor cells during differentiation from the normal hematopoietic system. ALL is primarily marked by the abnormal proliferation and aggregation of naïve lymphocytes (either B or T lineage) in the bone marrow and lymphoid tissues.1,2 The incidence of ALL exhibits a bimodal age distribution, with the highest occurrence among children aged 1 to 4 years and adults aged 45 years and older. Globally, the annual incidence of ALL is estimated to range between one and five cases per 100,000 individuals, with approximately 6,660 new cases and 1,560 mortalities reported in both children and adults, based on 2022 US statistics.3,4 Despite significant advances in multidrug combination therapy and hematopoietic stem cell transplantation, which have considerably improved the overall survival rate of patients with ALL, the disease continues to present challenges, including drug resistance, minimal residual disease, and relapse.5 Thus, the identification of novel therapeutic agents is imperative.
Traditional Chinese medicine has been utilized since a long time in the field of antitumor therapies, demonstrating low toxicity, substantial pharmacological activity, and minimal drug resistance.6 This study presents a novel perspective on the treatment of ALL. Indirubin, a primary active component of Indigofera tinctoria L. also known by various names such as Yuhong Tablet and Couraupitine B, is widely recognized for its clinical use in treating chronic granulocytic leukemia. Contemporary pharmacological studies have reported the antiviral, anti-inflammatory, and immunomodulatory properties of indirubin.7,8 In recent years, numerous studies have highlighted the ability of indirubin and its derivatives to inhibit tumor cell proliferation, regulate the cell cycle, and induce apoptosis in various human cancers. Studies have reported that indirubin modulates the STAT3 signaling pathway, inhibiting the proliferation of ovarian cancer cells.9 Additionally, indirubin has the potential to induce ferroptosis in the treatment of colon cancer.10 Indirubin, a bis-indole alkaloid, reportedly binds to tubulin and exhibits anti-mitotic activity against HeLa cells by synergizing with vinblastine.11 However, investigations into the effects and mechanisms of action of indirubin on ALL remain limited.
Recently, network pharmacology has rapidly emerged as a novel method for drug design and development rooted in modern pharmacological research. It integrates technologies and content from various disciplines including systems bioinformatics, computer science, and multidirectional pharmacology. Systems biology suggests that complex diseases such as cancer result from mutations in multiple genes rather than a single target gene, disrupting the balance of the biological network system. Network pharmacology aims to study the balance of biological networks through the systemic analysis of drug effects on the human body, aiding in the identification of therapeutic targets to enhance drug efficacy and reduce side effects, rendering it significant theoretical and practical value.12,13 Molecular docking is a computer simulation technique used to predict the binding mode and affinity between molecules and proteins, as well as to assess binding interactions. This technique is characterized by its high accuracy, cost-effectiveness, and predominant use in drug design and the elucidation of biochemical pathways.14 Several studies have integrated network pharmacology and molecular docking techniques. For example, the mechanism of action of resveratrol in the treatment of diabetic nephropathy was revealed through a combination of network pharmacology, molecular docking, and experimental validation.15 However, only a few network pharmacology studies have investigated the use of indirubin for the treatment of ALL.
Therefore, this study aimed to combine network pharmacology, molecular docking, and experimental approaches to analyze the potential targets and molecular mechanisms of indirubin for the treatment of ALL. Additionally, preliminary validation was conducted using cellular and animal experiments. The findings of this study may offer new insights and scientific evidence for the treatment of ALL. The workflow of the study is presented in Figure 1.
 |
Figure 1 Flowchart of the research process. |
Materials and Methods
Drug Targets of Indirubin
The PubChem database was searched using “indirubin” as the search term. The indirubin Structure Data File was obtained and imported into the PharmMapper database for prediction. Similarly, the 2D structure or Canonical SMILES of indirubin was obtained from the PubChem database and imported into the SwissTargetPrediction database to predict the target genes of indirubin. The targets of indirubin were also searched and collected from the DrugBank and STITCH databases. Finally, data from these databases were merged and duplicates were eliminated.
Screening Therapeutic Targets for ALL
Human genes associated with ALL were obtained from the DisGeNET, GeneCards, National Center for Biotechnology Information (NCBI), and Online Mendelian Inheritance in Man (OMIM) databases. These databases were searched using the search term “acute lymphoblastic leukemia.” Targets with scores greater than 10 were selected from the GeneCards database. All targets from the four databases were then merged, and duplicate values were removed to obtain a comprehensive list of ALL-related targets. Intersecting genes and Venn diagrams of indirubin and ALL targets were obtained using the SangerBox online platform. The potential targets of indirubin for the treatment of ALL were acquired for subsequent analyses.
Construction of Protein-Protein Interaction (PPI) Network and Hub Gene Analysis
The STRING database, a functional protein association network resource, was used to construct a PPI network for common targets of indirubin and ALL. The intersecting genes of indirubin and ALL were initially submitted to the STRING database, with the species type specified as “Homo sapiens”, a minimum interaction score set at 0.4, and isolated nodes hidden in the network. All other settings were left at their default values. Subsequently, the results were imported into Cytoscape 3.7.2 software to construct a PPI network and conduct further analyses of the core targets within the network. The core targets in the screening network were identified in Cytoscape using the CytoHubba plugin. The four most commonly used algorithms in the plugin—Degree, Maximum Neighborhood Component (MNC), Maximal Clique Centrality (MCC), and closeness—were employed to identify the top 10 genes in each algorithm. The intersections of these gene sets were used to determine the final core targets.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
To gain a more systematic and comprehensive understanding of the mechanism underlying indirubin treatment for ALL, we performed GO (covering biological processes, cellular components, and molecular functions) and KEGG pathway enrichment analyses. Intersecting genes were converted to Entrez IDs using Perl 5.3.8 software. Subsequent analyses were conducted using R 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria), along with related R packages, including clusterProfiler, org.Hs.eg.db, enrichplot, and ggplot2. Statistical significance was set at p<0.05. The top 10 genes were chosen for GO analysis, whereas the top 20 genes were selected for KEGG analysis. KEGG classification maps were generated using the Bioinformatics website (http://www.bioinformatics.com.cn/). Finally, the drug-disease-target-function annotation-signaling pathway network was constructed using Cytoscape software to elucidate the relationships between drugs, diseases, targets, and functions.
Molecular Docking
Molecular docking of indirubin to its core target proteins was performed. The 2D structure of indirubin was obtained from the PubChem database and converted into a 3D structure using Chem3D software. The 3D structure of the core target protein was obtained from UniProt and Protein Data Bank (PDB) databases. Water molecules, primitive ligands, and peptides associated with the core protein were removed using Pymol software. The active pocket of the core protein was identified using AutoDock Tools 1.5.7 software. Molecular docking of indirubin with the core protein was then performed using AutoDock Vina software to determine the optimal binding mode and free binding energy. Lower binding energy values indicated stronger binding activity. Finally, the docking images were visualized using PyMOL software.
Cell Lines and Cell Culture
The BALL-1 and Jurkat cell lines used in the experiments were obtained from the American Tissue Culture Collection (Manassas, Virginia, United States of America). The culture medium consisted of Roswell Park Memorial Institute 1640 (Gibco) supplemented with 10% fetal bovine serum (Gibco), 100 U/mL streptomycin, and 100 U/mL penicillin (Gibco). Cell was cultured at 37°C in a humidified atmosphere with, 5% CO2.
Cck8
The effect of indirubin (MCE, HY-N0117) on the viability of ALL cells was assessed using the Cholecystokinin-8 (CCK-8) kit (NCM Biotech). ALL cells were initially grown in 96-well plates at a density of 1×104 cells/well. Subsequently, indirubin solutions at concentrations of 1, 5, and 10 µM were added, along with a blank control group (BC) and a negative control group (DMSO). At 24, 48, and 72 h post-treatment, 10 µL of CCK-8 solution was added to each well. Following a 3-h incubation period, the absorbance at 450 nm was measured using an enzyme marker (BioTek, Vermont, USA), and the cell proliferation rate was calculated based on the following formula: absorbance of (a control group – experimental group)/absorbance of (a control group – blank group)×100%. Each group comprised five subwells (n=5), and the experiment was independently repeated three times (n=3 independent experiments).
EdU
The effect of indirubin on the proliferative capacity of ALL cells was evaluated using an EdU kit (Beyotime,C0075S), following the manufacturer’s instructions. Initially, the cells were seeded at a density of 3×105 cells/well in six-well plates, maintaining the same dosing concentrations and groupings as in the CCK-8 experiment. After 48 h of treatment, each well received 50 µmol/L EdU solution, which was incubated for 2 h. Subsequently, the cells were harvested by centrifugation and washed with phosphate-buffered saline (PBS). The cells were fixed with 4% paraformaldehyde and permeabilized with 0.3% Triton X-100. The nuclei were then stained with Hoechst 33342 (Beyotime,C0075S), and the cells were resuspended in PBS and deposited onto slides. Finally, images were observed and captured using a fluorescence microscope (Olympus, Japan), and the data were processed and analyzed using ImageJ software (National Institute of Health, Bethesda, MD, USA). Each experiment was repeated independently three times (n=3 independent experiments).
Cell Apoptosis Assay
Apoptosis was assessed using an Annexin V-FITC/PI kit (Beyotime,C1383M). ALL cells were seeded in six-well plates at a density of 3×105 cells/well and exposed to indirubin for 48 h. The indirubin concentrations and groupings were consistent with those in the CCK-8 assay. Subsequently, the cells were harvested, washed twice with PBS, and stained with Annexin V and propidium iodide (PI). The stained cells were incubated at room temperature for 15 min in darkness before analysis using flow cytometry (BD Biosciences, San Jose, USA), and analyzed using Flowjo10. Each set of experiments was independently repeated three times (n=3 independent experiments).
Cell Cycle Assay
The effect of indirubin on ALL cell cycles was assessed using a Cell Cycle Kit (Beyotime,C1052). Cells were cultured and treated following the same procedure used for the apoptosis assay. After 48 h of treatment, the cells were harvested, washed with pre-cooled PBS, and fixed in chilled 70% ethanol. Subsequently, the cells were washed twice with PBS and incubated with PI staining solution for 30 min at room temperature in the dark. Finally, the assay was performed using flow cytometry (BD Biosciences, San Jose, USA), and analyzed using Flowjo10. Each set of experiments was independently repeated three times (n=3 independent experiments).
Western Blotting
The concentration and grouping of drug-treated ALL cells were consistent with those used in previous experiments. Initially, the cells were collected and washed with PBS, followed by the addition of radioimmunoprecipitation assay lysates and protease inhibitors. The mixture was lysed on ice and sonicated. Afterward, the supernatant was collected by centrifugation at 4°C at 15,000 rpm for 15 min. The protein concentration was determined using a bicinchoninic acid kit (Beyotime,P0010) according to the manufacturer’s protocol. Next, the proteins were separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes(Millipore). Blocking was performed with 5% skim milk for 1 h at room temperature. The membrane was subsequently incubated with the primary antibody overnight at 4°C followed by the corresponding secondary antibody for 2 h at room temperature. Finally, enhanced chemiluminescence imaging was performed. The antibodies and dilution rates for the relevant proteins are listed in Supplementary Table 1. All original blots are shown in the Supplementary Material. Each set of experiments was independently repeated three times (n=3 independent experiments).
Extraction of Bone Marrow Mononuclear Cells
Bone marrow samples were obtained from six patients diagnosed with ALL (n=6) for this trial.The bone marrow was thoroughly mixed with an equal volume of saline or PBS solution The diluted bone marrow fluid was slowly added to a 15 mL centrifuge tube preloaded with 5 mL of cell separation medium, ensuring that the fluid levels remained distinctly separated. After centrifugation at 2000 rpm for 25 min at room temperature, the samples were separated into four distinct layers (from top to bottom): plasma, mononuclear cells, separation medium, and red blood cells. The mononuclear cell layer was carefully aspirated and transferred to a 15 mL centrifuge tube, resuspended in 10 mL of saline or PBS, and centrifuged at 2000 rpm for 10 min at room temperature. The washing step was repeated after discarding the supernatants. The final cell pellet was collected for subsequent experiments. The use of ALL bone marrow samples in this study was approved by the Guizhou Medical University Human Experimentation Ethics Committee.
Xenograft Tumor Model
Ten female NOD SCID gamma mice 6–8 weeks were used for subcutaneous tumor formation experiments to investigate the effects of indirubin on ALL cells in vivo. The right side of each mouse was injected subcutaneously with 100 μL of serum-free culture containing a suspension of 1×107 BALL-1 cells. After 3 weeks, the tumor-bearing mice were randomly divided into two groups of five mice each (n=5 per group). One group was gavaged with indirubin (25 mg/kg), while the other group was gavaged with 0.1% dimethyl sulfoxide once daily for 3 weeks. Tumor volume was measured every 5 d. After 3 weeks, the mice were euthanized, and the tumors were removed for photography and weighing. All animal experimental procedures were conducted in accordance with the guidelines of the Animal Ethics Committee and were approved by the Animal Ethics Committee of Guizhou Medical University.
Statistical Analyses
Values are expressed as mean ± standard deviation (SD). Statistical comparisons were conducted using the t-test and one- or two-way analysis of variance with Turkey’s post-hoc tests. Statistical analyses were performed using GraphPad Prism 8.0 software (San Diego, CA, USA). Statistical significance was set at p<0.05.
Results
Potential Targets of Indirubin Action in ALL and Construction of PPI Network
The 2D and 3D structures of indirubin are obtained from the PubChem database (Figure 2A and B). The targets of indirubin are identified from the DrugBank, SwissTargetPrediction, PharmMapper, and STITCH databases, which yielded 375 drug targets after merging and deduplicating the data. Similarly, 2,257 target genes associated with ALL are identified by searching the Therapeutic Target Database, PharmGKB, DrugBank, OMIM, NCBI, GeneCards (relevance score >10), and DisGeNET databases. The intersection of the indirubin targets with ALL-related targets identifies 170 potential anti-ALL targets (Figure 2C). Network maps illustrating drug-target relationships (Figure 2D) and intersecting genes (Figure 2E) are generated using STRING and Cytoscape. As shown in Figure 2E, genes represented by larger and darker circles are more central in the network. All Uniform Resource Locators used in the database are listed in Supplementary Table 2.
Enrichment Analysis and Construction of The Total Network Diagram
GO and KEGG enrichment analyses of the 170 intersecting genes are performed using R software, and the results are visualized as follows: In the GO enrichment analysis, the top 10 significantly enriched terms from the categories of Biological Process (BP), Cellular Component (CC), and Molecular Function (MF) are selected for display (Figure 3A). In the GO analysis, BP primarily includes terms such as “cellular response to chemical stress”, “positive regulation of kinase activity”, and “protein autophosphorylation”; CC mainly includes “membrane raft”, “membrane microdomain”, and “vesicle lumen”; while MF includes “protein serine/threonine kinase activity”, “ligand-activated transcription factor activity”, and “transmembrane receptor protein kinase activity”. In the KEGG enrichment analysis, the top 20 significantly enriched pathways are shown (Figure 3B). KEGG enrichment analysis revealed that the primary signaling pathways targeted by indirubin in anti-ALL treatment include the PI3K-Akt signaling pathway, the FoxO signaling pathway, and the Ras signaling pathway. The top 20 KEGG pathway target genes are classified (Figure 3C), with most genes categorized under “Human Diseases”, particularly in ‘Hematological and Solid Tumors’ and ‘Organismal Systems’. Additional targets are classified under “Environmental Information Processing”. Based on the KEGG analysis results, we focused on the PI3K-Akt signaling pathway and visualized its details (Supplementary Figure 1). A PPI network is constructed for genes in the PI3K-Akt signaling pathway (Supplementary Figure 2), with larger circles and darker colors representing the importance of the genes. Finally, a comprehensive network diagram of drug-disease-functional annotation-signaling pathways is constructed (Figure 3D). In this diagram, purple, orange, and yellow represent the top 10 terms for BP, CC, and MF, respectively, from the GO analysis, while blue represents the top 20 terms from the KEGG analysis.
Core Gene Screening
Core gene analysis is performed on the 170 intersecting genes using the CytoHubba plugin in Cytoscape software. Core targets are identified using four algorithms: MCC, MNC, Degree, and Closeness. Figure 4A–D shows the top 10 core genes obtained from these algorithms, with node colors transiting from light yellow to red, indicating increasing core importance. The top 10 core genes from each algorithm result in eight core genes (Figure 4E).
 |
Figure 4 Core gene screening. Top 10 core targets identified by (A) MCC, (B) Closeness, (C) MNC and (D) Degree. (E) Venn diagram of the common core targets of MCC, MNC, Degree, and Closeness. |
Molecular Docking
We evaluated the interaction patterns between the drugs and their core genes using molecular docking techniques. The protein crystal structures of the eight core genes are obtained from the PDB, and molecular docking analysis is performed using AutoDock Vina. The binding energy reflects the strength of the interaction between the ligand and receptor; lower binding energy indicates stronger interaction and higher stability of the ligand-receptor complex. Typically, binding energy below −5.0 kcal/mol indicates medium affinity, while below −7.0 kcal/mol indicates high affinity. The binding energy data for indirubin and the eight core genes are displayed in Supplemental Table 3. We visualized the highest-binding-energy complexes for each target with indirubin and analyzed their binding mechanisms using PyMOL 2.5 (Figure 5). The results revealed that indirubin exhibited a strong binding affinity for these eight core genes, suggesting that they may be potential targets of indirubin. Subsequently, we analyzed the expression of these eight core genes in ALL using data from The Cancer Genome Atlas and Genotype-Tissue Expression databases (Supplementary Figure 3). Significant differences in the expression of these genes in ALL are observed (p<0.05).
 |
Figure 5 3D mapping of docking models with minimum binding energies between indirubin and eight core targets. |
Indirubin Inhibits ALL Cell Proliferation
CCK-8 experiments are performed to determine the effects of different time points and concentrations of indirubin on the proliferative capacity of ALL cells. The findings revealed that the inhibition rate of ALL cells gradually increased with increasing concentrations of indirubin (Figure 6A and B), indicating that indirubin significantly inhibited the proliferation of ALL cells. Regarding the time gradient, the inhibition rate of cells did not increase with time after 48 h (Figure 6A and B). Based on the findings of the CCK-8 experiments, we select 1 μM, 5 μM, and 10 μM concentrations of indirubin to treat the cells over 48 h in subsequent experiments. EdU experiments demonstrate that the proliferative capacity of ALL cells gradually decreased with increasing concentrations of indirubin (Figure 6C and D). Each experiment is repeated thrice.
Indirubin Induces Apoptosis in ALL Cells
Apoptosis is detected by Annexin V-FITC/PI double staining, and the findings reveal that the apoptotic rate of both ALL cell lines increases significantly with increasing concentrations of indirubin (Figure 7A and B). Subsequently, we analyzed the expression of apoptosis-related proteins by Western blotting, indicating that the expression of caspase 3 remains unchanged, whereas the expression of Bax and cleaved-caspase 3 increased, and the expression of Bcl-2 decreased (Figure 7C). These findings suggest that indirubin induces apoptosis in ALL cells.
Indirubin Induces ALL Cell Cycle Arrest in The G2/M Phase
To further investigate the effects of indirubin on ALL cells, we examined its effects on cell cycle regulation. The findings revealed that The proportion of cells in the G2/M phase gradually increased with increasing concentrations of indirubin, suggesting that indirubin induces cell cycle arrest in the G2/M phase (Figure 8A and B). Consistent with this, Western blotting findings indicate that the expression levels of CDK1 and Cyclin B1 decrease after indirubin treatment (Figure 8C). These findings demonstrate that indirubin induces cell cycle arrest in the G2/M phase by inhibiting the expression of CDK1 and Cyclin B1, consequently inhibiting the proliferation of ALL cells.
In vivo Experimental Verification of The Action of Indirubin on ALL Cells
The effects of indirubin on ALL are evaluated in vivo (Figure 9A). We observe that the subcutaneous tumors in the control nude mice grew progressively over time, while the tumor volume and weight in the treatment group are significantly reduced compared to those in the control group (Figure 9B–D). There is no significant difference in body weight between the two groups throughout the experiment (Figure 9E). Additionally, we isolated mononuclear cells from the bone marrow of 6 patients with ALL and exposed them to 10 μM indirubin for 48 h. The findings revealed that apoptosis increased following indirubin treatment. These findings further confirm the tumor growth-inhibiting effects of indirubin (Figure 9F).
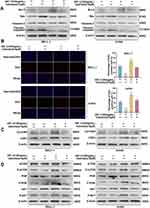
Indirubin Inhibits Activation of The PI3K/Akt/mTOR Signaling Pathway
To further explore the anti-ALL mechanism of indirubin, we selected the PI3K-AKT signaling pathway for validation experiments based on the results of GO and KEGG analyses, as well as molecular docking. Western blotting is performed to evaluate the expression of PI3K/AKT pathway-related proteins (PI3K/p-PI3K, p-AKT/AKT, and p-mTOR/mTOR). The findings indicate that indirubin dose-dependently inhibits the phosphorylation of PI3K, AKT, and mTOR in ALL cells (Figure 10). Additionally, we investigated the role of the PI3K/AKT/mTOR pathway in mediating the anti-ALL effects of indirubin by using a PI3K/AKT agonist (IGF-1). The findings reveal that IGF-1 diminishes the indirubin-mediated regulation of apoptosis-related proteins (Figure 11A) and reverses the inhibitory effects of indirubin on ALL cell proliferation (Figure 11B). Additionally, IGF-1 weakened the inhibitory effects of indirubin on cell cycle-related proteins and the PI3K/AKT/mTOR signaling pathway (Figure 11C and D). These findings indicate that the PI3K/AKT/mTOR signaling pathway plays a key role in the anti-ALL effects of indirubin.
 |
Figure 10 Western blot analysis was used to evaluate the expression of PI3K/AKT pathway-related proteins (PI3K/p-PI3K, p-AKT/AKT, and p-mTOR/mTOR). |
Discussion
ALL is a common hematological malignancy. Patients undergoing chemotherapy often experience relapses and severe side effects, highlighting the critical need for highly effective treatments with low toxicity.16 Isolated from Indigofera tinctoria L. and commonly used in the treatment of chronic granulocytic leukemia, indirubin possesses antitumor, anti-inflammatory, and neuroprotective activities, and it inhibits a number of kinases.17 Network pharmacology has become a pivotal field in drug research. In this study, we investigated the anti-ALL mechanism of indirubin using network pharmacology, molecular docking, and experimental validation.
In this study, 170 overlapping targets of ALL and indirubin were identified by analyzing several databases, and a PPI network was constructed from these targets. Eight key genes were identified using the CytoHubba algorithm: mTOR, CASP3, AKT1, Matrix metalloproteinase 9 (MMP9), albumin (ALB), HSP90AA1, ESR1, and PTGS2. These genes play key roles in the regulation of apoptosis, proliferation, signaling, and the cell cycle. AKT1 is a key kinase involved in cell signaling that regulates cell proliferation and apoptosis. In leukemia, phosphorylated AKT1 inhibits apoptosis and promotes cell proliferation.18 Our findings revealed that indirubin significantly reduced the expression of phosphorylated AKT1. ALB is the most abundant protein in the blood and functions as a carrier protein with esterase activity and broad substrate specificity. Studies have shown that serum ALB levels correlate with the prognosis and therapeutic outcomes of patients with leukemia.19 HSP90AA1, a cancer-associated chaperone protein, supports tumor protein activation and protects cancer cells from environmental stress. Its high expression is associated with poor prognosis in patients with leukemia.20,21 Caspase-3 is a core gene in the apoptotic pathway, with cleaved caspase-3 ultimately causing apoptosis. In ALL, activated caspase-3 levels are correlated with patient prognosis.22,23 Our findings revealed that indirubin significantly increased the expression of cleaved caspase-3. ESR1, an estrogen-activated transcription factor, regulates DNA binding and downstream genes, potentially acting through the PI3K/AKT or Mitogen-Activated Protein Kinase pathways, making it an effective target for leukemia therapy.24 MMP9 is crucial for tumor invasion and metastasis. Studies have shown differences in MMP9 expression in the bone marrow of patients with ALL, indicating that ALL cells can induce MMP9 expression through the release of specific factors, thereby enhancing their invasiveness.25,26 Prostaglandin-endoperoxide synthase 2 (PTGS2) is closely associated with tumorigenesis and the development of ALL. It is upregulated in ALL and inhibits ALL cell growth, making it a potential therapeutic target.27 mTOR plays a key role in signaling networks that initiate and control cellular activities, including messenger ribonucleic acid translation, cell cycle progression, gene transcription, apoptosis, and metabolism. Evidence from the literature suggests that high expression and activation of mTOR are associated with a poor prognosis in ALL.28–30 Collectively, these core genes play important roles in the onset, progression, and prognosis of ALL. We also explored the variation in the expression of these core genes in ALL using publicly available databases. Molecular docking findings revealed that these core genes have significant binding potential to indirubin, suggesting that indirubin may inhibit the progression of ALL by targeting these key genes.
GO and KEGG enrichment analyses of the 170 overlapping targets were performed to systematically understand the mechanism of indirubin treatment in ALL. GO enrichment analyses revealed that indirubin primarily regulated cellular responses to chemical stress, positive regulation of kinase activity, response to oxidative stress, and other biological processes. This suggests that the treatment of ALL with indirubin involves the synergistic effects of multiple pathways. Further KEGG pathway analyses revealed that indirubin treatment of ALL mainly involved the PI3K-AKT, RAS, and FoxO signaling pathways, all of which are closely associated with the onset and development of ALL. Based on the number of targets corresponding to each pathway, we focused on the PI3K-AKT signaling pathway, which may be one of the most critical pathways in the mechanism of indirubin therapy for ALL. The PI3K-AKT is a well-known intracellular signaling pathway, that regulates key biological processes such as oxidative stress, inflammatory response, cell growth, proliferation, and survival.31–33 This signaling pathway can be activated by a variety of extracellular stimuli, including receptor tyrosine kinases, integrins, B and T cell receptors, and G protein-coupled receptors.34,35 The PI3K-AKT signaling pathway plays a central role in hematopoietic cells, regulating both normal and malignant hematopoiesis. In 88% of patients with ALL, the PI3K-AKT signaling pathway is over-activated, and this activation is strongly associated with poor prognosis and chemotherapy resistance.36–38 Numerous recent studies have demonstrated a significant role of the PI3K-AKT signaling pathway in ALL. For instance, some studies have reported that CASZ1 upregulates PI3K-AKT-mTOR signaling and promotes T cell ALL.39 In addition, B. javanica seed oil induces apoptosis through the PI3K/Akt signaling pathway in acute lymphocytic leukemia Jurkat cells.40 Thus, the PI3K-AKT signaling pathway represents a promising target for the treatment of ALL. Our findings also reveal that the core targets are primarily focused on the PI3K-AKT signaling pathway.
The predicted findings were verified through further experiments. In vitro experiments revealed that indirubin inhibited ALL cell proliferation, promoted apoptosis, and induced G2/M phase arrest. In vivo experiments revealed that indirubin significantly inhibited ALL progression. Detection of relevant core genes in the PI3K-AKT signaling pathway indicated that indirubin affects their expression. Our findings suggest that indirubin exerts an anti-ALL effect, possibly by modulating the PI3K-AKT signaling pathway. However, this study has some limitations. First, we did not compare indirubin with other anti-ALL drugs, nor did we assess combination therapies to further evaluate their efficacy against ALL. Second, the anti-ALL mechanism of indirubin involves multiple pharmacological mechanisms. Although we identified several important targets and pathways, further pharmacological studies are required to elucidate these complex mechanisms.
Conclusion
This study systematically investigated the therapeutic effects of indirubin on ALL from a mechanistic perspective. Through network pharmacology, molecular docking, and experimental validation, our findings revealed that indirubin exerted its anti-ALL effects through multiple targets and pathways, including the PI3K-AKT signaling pathway (Figure 12). This study provides important evidence for the clinical use of indirubin as a potential therapeutic agent for ALL.
 |
Figure 12 Mechanistic illustration of indirubin’s therapeutic effects on ALL. Image created with BioRender.com, with permission. |
Abbreviations
ALL, Acute lymphoblastic leukaemia; PPI, protein-protein interaction; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; NCBI, National Center for Biotechnology Information; OMIM, Online Mendelian Inheritance in Man; PDB, Protein Data Bank; BC, blank control; PBS, phosphate-buffered saline; PI, propidium iodide; BP, Biological Process; CC, Cellular Component; MF, Molecular Function; DMSO, Dimethyl sulfoxide; EdU, 5-ethynyl-2-deoxyuridine; P-PI3K, phospho-PI3K; P-AKT, phospho-AKT; P-mTOR, phospho-mTOR.
Data Sharing Statement
The datasets in this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Animal experiments in the study protocol were reviewed and approved by the Animal Ethics Committee of Guizhou Medical University (No. 2400569). The use of ALL bone marrow samples in this study was approved by the Guizhou Medical University Human Experimentation Ethics Committee. This study was conducted in accordance with the principles of the Declaration of Helsinki.Written informed consent was obtained from all patients prior to the collection of samples.
Acknowledgment
We express our gratitude to the Children’s Blood Centre at Guizhou Medical University Hospital for kindly providing the bone marrow specimen.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (No. 32270848,U23A20498), and the Guizhou Province Science and Technology Project (LC[2022]001, Qian Ke He[2019]5406).
Disclosure
The authors declare no conflict of interest.
References
1. Gower M, Tikhonova AN. Under the surface: scratching the acute lymphoblastic leukemia niche. Haematologica. 2023;108(5):1210–1212. doi:10.3324/haematol.2022.282191
2. Pagliaro L, Chen SJ, Herranz D, et al. Acute lymphoblastic leukaemia. Nature Reviews Disease Primers. 2024;10(1):41. doi:10.1038/s41572-024-00525-x
3. Dong Y, Shi O, Zeng Q, et al. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol. 2020;9(1):14. doi:10.1186/s40164-020-00170-6
4. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. Ca a Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
5. Hunger SP, Raetz EA. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. 2020;136(16):1803–1812. doi:10.1182/blood.2019004043
6. Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15(5):283–298. doi:10.5582/bst.2021.01318
7. Yang L, Li X, Huang W, Rao X, Lai Y. Pharmacological properties of indirubin and its derivatives. Biomed Pharmacothe. 2022;151:113112. doi:10.1016/j.biopha.2022.113112
8. Wang H, Wang Z, Wei C, et al. Anticancer potential of indirubins in medicinal chemistry: biological activity, structural modification, and structure-activity relationship. Eur J Med Chem. 2021;223:113652. doi:10.1016/j.ejmech.2021.113652
9. Chen L, Wang J, Wu J, Zheng Q, Hu J. Indirubin suppresses ovarian cancer cell viabilities through the STAT3 signaling pathway. Drug Des Devel Ther. 2018;12:3335–3342. doi:10.2147/dddt.S174613
10. Zhu JM, Chen C, Kong M, et al. Discovery and optimization of indirubin derivatives as novel ferroptosis inducers for the treatment of colon cancer. Eur J Med Chem. 2023;261:115829. doi:10.1016/j.ejmech.2023.115829
11. Mohan L, Raghav D, Ashraf SM, Sebastian J, Rathinasamy K. Indirubin, a bis-indole alkaloid binds to tubulin and exhibits antimitotic activity against HeLa cells in synergism with vinblastine. Biomed Pharmacothe. 2018;105:506–517. doi:10.1016/j.biopha.2018.05.127
12. Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. doi:10.1016/j.jep.2023.116306
13. Zhang P, Zhang D, Zhou W, et al. Network pharmacology: towards the artificial intelligence-based precision traditional Chinese medicine. Briefings Bioinf. 2023;25(1). doi:10.1093/bib/bbad518
14. Agu PC, Afiukwa CA, Orji OU, et al. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci Rep. 2023;13(1):13398. doi:10.1038/s41598-023-40160-2
15. Chen S, Li B, Chen L, Jiang H. Uncovering the mechanism of resveratrol in the treatment of diabetic kidney disease based on network pharmacology, molecular docking, and experimental validation. J Transl Med. 2023;21(1):380. doi:10.1186/s12967-023-04233-0
16. Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105(11):2524–2539. doi:10.3324/haematol.2020.247031
17. Lee JH, Shin JE, Kim W, et al. Discovery of indirubin-3’-aminooxy-acetamide derivatives as potent and selective FLT3/D835Y mutant kinase inhibitors for acute myeloid leukemia. Eur J Med Chem. 2022;237:114356. doi:10.1016/j.ejmech.2022.114356
18. Astolfi A, Milano F, Palazzotti D, et al. From Serendipity to Rational Identification of the 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one Core as a New Chemotype of AKT1 Inhibitors for Acute Myeloid Leukemia. Pharmaceutics. 2022;14(11):2295. doi:10.3390/pharmaceutics14112295
19. Zhang Y, Chen Q, Lu C, Yu L. Prognostic role of controlling nutritional status score in hematological malignancies. Hematology. 2022;27(1):653–658. doi:10.1080/16078454.2022.2078040
20. Wang Z, Fan L, Xu H, et al. HSP90AA1 is an unfavorable prognostic factor for hepatocellular carcinoma and contributes to tumorigenesis and chemotherapy resistance. Transl Oncol. 2024;50:102148. doi:10.1016/j.tranon.2024.102148
21. Wu YW, Chao MW, Tu HJ, et al. A novel dual HDAC and HSP90 inhibitor, MPT0G449, downregulates oncogenic pathways in human acute leukemia in vitro and in vivo. Oncogenesis. 2021;10(5):39. doi:10.1038/s41389-021-00331-0
22. Montiel-Cervantes LA, Reyes-Maldonado E, Garcia-Chavez J, et al. Prognostic Value of CD95, Active Caspase-3, and Bcl-2 Expression in Adult Patients with De Novo Acute Lymphoblastic Leukemia. Archiv Med Res. 2018;49(1):44–50. doi:10.1016/j.arcmed.2018.04.006
23. Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discovery. 2020;6(1):112. doi:10.1038/s41420-020-00349-0
24. Liu Q, Liu Y, Li X, et al. Perfluoroalkyl substances promote breast cancer progression via ERα and GPER mediated PI3K/Akt and MAPK/Erk signaling pathways. Ecotoxicol Environ Saf. 2023;258:114980. doi:10.1016/j.ecoenv.2023.114980
25. Verma D, Zanetti C, Godavarthy PS, et al. Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia. Leukemia. 2020;34(6):1540–1552. doi:10.1038/s41375-019-0674-7
26. Augoff K, Hryniewicz-Jankowska A, Tabola R, Stach K. MMP9: a Tough Target for Targeted Therapy for Cancer. Cancers. 2022;14(7):1847. doi:10.3390/cancers14071847
27. Cruz-Miranda GM, Olarte-Carrillo I, Bárcenas-López DA, et al. Transcriptome Analysis in Mexican Adults with Acute Lymphoblastic Leukemia. Int J mol Sci. 2024;25(3):1750. doi:10.3390/ijms25031750
28. Simioni C, Martelli AM, Zauli G, Melloni E, Neri LM. Targeting mTOR in Acute Lymphoblastic Leukemia. Cells. 2019;8(2):190. doi:10.3390/cells8020190
29. Ge Z, Song C, Ding Y, et al. Dual targeting of MTOR as a novel therapeutic approach for high-risk B-cell acute lymphoblastic leukemia. Leukemia. 2021;35(5):1267–1278. doi:10.1038/s41375-021-01132-5
30. Glaviano A, Foo ASC, Lam HY, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. mol Cancer. 2023;22(1):138. doi:10.1186/s12943-023-01827-6
31. Yu L, Wei J, Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semi Cancer Biol. 2022;85:69–94. doi:10.1016/j.semcancer.2021.06.019
32. He Y, Sun MM, Zhang GG, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduc Target Ther. 2021;6(1):425. doi:10.1038/s41392-021-00828-5
33. Huang J, Chen L, Wu J, et al. Targeting the PI3K/AKT/mTOR Signaling Pathway in the Treatment of Human Diseases: current Status, Trends, and Solutions. J Med Chem. 2022;65(24):16033–16061. doi:10.1021/acs.jmedchem.2c01070
34. Li Q, Li Z, Luo T, Shi H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. mol Biomed. 2022;3(1):47. doi:10.1186/s43556-022-00110-2
35. Liu R, Chen Y, Liu G, et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11(9):797. doi:10.1038/s41419-020-02998-6
36. Nepstad I, Hatfield KJ, Grønningsæter IS, Reikvam H. The PI3K-Akt-mTOR Signaling Pathway in Human Acute Myeloid Leukemia (AML) Cells. Int J mol Sci. 2020;21(8):2907. doi:10.3390/ijms21082907
37. Wiese W, Barczuk J, Racinska O, et al. PI3K/Akt/mTOR Signaling Pathway in Blood Malignancies-New Therapeutic Possibilities. Cancers. 2023;15(21):5297. doi:10.3390/cancers15215297
38. Sanchez VE, Nichols C, Kim HN, Gang EJ, Kim YM. Targeting PI3K Signaling in Acute Lymphoblastic Leukemia. Int J mol Sci. 2019;20(2):412. doi:10.3390/ijms20020412
39. Cardoso BA, Duque M, Gírio A, et al. CASZ1 upregulates PI3K-AKT-mTOR signaling and promotes T-cell acute lymphoblastic leukemia. Haematologica. 2024;109(6):1713–1725. doi:10.3324/haematol.2023.282854
40. Zhang H, Yin SL, Wang LH, et al. Seed oil of Brucea javanica induces apoptosis through the PI3K/Akt signaling pathway in acute lymphocytic leukemia Jurkat cells. Chinese J Nat Med. 2021;19(8):608–620. doi:10.1016/s1875-5364(21)60060-2
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.