Back to Journals » Infection and Drug Resistance » Volume 18
Community-Acquired Necrotizing Pneumonia with Bronchopleural Fistulas Due to Streptococcus pneumoniae in an Adult
Received 12 December 2024
Accepted for publication 29 March 2025
Published 12 April 2025 Volume 2025:18 Pages 1831—1836
DOI https://doi.org/10.2147/IDR.S511714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Pei-Jun Li,1 Ming-Jing Yu,1 Dan Liu1– 3
1Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 2Department of Institute of Respiratory Health, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 3Department of Precision Medicine Key Laboratory, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China
Correspondence: Dan Liu, Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, No. 37 Guoxue Road, Chengdu, Sichuan, 610041, People’s Republic of China, Tel +86 18980605965, Email [email protected]
Background: Necrotizing pneumonia with subsequent bronchopleural fistula attributable to Streptococcus pneumoniae infection has not been reported in adults.
Case Presentation: We here firstly report a case of 23-year-old female with severe community-acquired multiple lung abscesses due to Streptococcus pneumoniae in the left lower lung, which merged and ruptured, further leading to the development of bronchopleural fistulas. After adequate anti-infective treatment and repairing the fistulas under thoracoscopy, the pulmonary infection was under control, and the fistulas were closed. A follow-up chest CT demonstrated the complete resolution of lung abscesses.
Conclusion: This case shows the possibility that necrotizing pneumonia due to Streptococcus pneumoniae infection can lead to bronchopleural fistula in adults. Early diagnosis and management of bronchopleural fistula are crucial for improving patients outcomes.
Keywords: Streptococcus pneumoniae, necrotizing pneumonia, lung abscess, bronchopleural fistula
Introduction
Streptococcus pneumoniae infection is a common cause of community-acquired pneumonia. Streptococcus pneumoniae pneumonia typically resolves without leaving destructive damage, although pneumococcal infection is accompanied by significant inflammation.1 Necrotizing pneumonia caused by Streptococcus pneumoniae infection is rare in adults.2,3 To our knowledge, there have been no reported cases of Streptococcus pneumoniae causing bronchopleural fistula in adults to date. This is the first reported case of severe necrotizing pneumonia with subsequent bronchopleural fistulas due to Streptococcus pneumoniae infection in an adult.
Case Presentation
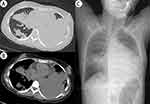
A 23-year-old female was admitted to our hospital with fever, cough, purulent sputum, and dyspnea for 2 weeks. She had a history of exposure to cold prior to the onset of this illness, with no underlying medical conditions reported. She had received intravenous moxifloxacin for 10 days prior to admission, but the condition was progressively worsening. One day before admission, she presented to our emergency ward with respiratory distress and subsequently underwent tracheal intubation with invasive mechanical ventilation. Meropenem was empirically administered for anti-infective treatment. Then she was transferred to our respiratory intensive care unit for hospitalization. On examination, she was under sedation and analgesia, with temperature 39.2°C, and SpO2 95% with an oxygen concentration of 50%. Laboratory tests showed increased white blood cell (17,050 cells/µL with 93.5% neutrophils), evaluated C-reactive protein (24.5 mg/dL), and increased procalcitonin (exceeded 100 ng/mL). The fungal β-D-glucan and galactomannan antigen tests were negative. The T-cell release assay for tuberculosis infection was negative. Anti-neutrophil cytoplasmic antibody and antinuclear antibody were normal. Chest CT (Figure 1A and B) and chest X-ray (Figure 1C) performed in the emergency ward one day prior to admission revealed consolidation in both lungs, with a notably extensive area of consolidation in the lower left lung. Bronchoscopy showed purulent secretions in the left lower lobe bronchus with no visible fistulas. Bronchoalveolar lavage fluid (BALF) identified 4078 sequences of Streptococcus pneumoniae with a relative abundance of 68.35% by next-generation sequencing (NGS), although BALF culture was negative. No other pathogens were detected by NGS of BALF. The acid-fast staining, Xpert MTB/RIF assay, mycobacterium tuberculosis DNA, and fungal culture of BALF were all negative. Blood culture further confirmed the presence of Streptococcus pneumoniae. Therefore, meropenem (500mg every 8 hours) plus ceftriaxone (4g per day) were administered for anti-infective therapy.
However, on the fourth day of hospitalization, continuous cardiac monitoring revealed a sudden increase in the patient’s heart rate from approximately 90 beats per minute to 160 beats per minute, accompanied by a decline in SpO2 from 97% to 88% under 60% oxygen supplementation. An urgent bedside chest X-ray revealed a left-sided loculated pneumothorax (Figure 2). Following the placement of left thoracic drainage, the patient’s heart rate and SpO2 gradually returned to baseline levels.
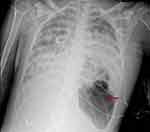 |
Figure 2 Chest X-ray on the fourth day of hospitalization revealed a left-sided loculated pneumothorax (red arrow). |
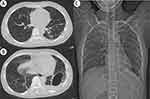
Two weeks after admission, the patient’s respiratory distress improved, and the tracheal tube was removed. The anti-infection regimen was de-escalated from meropenem combined with ceftriaxone to ceftriaxone alone. The patient was transferred to the general ward. But the patient still had a fever of around 38°C, and the chest tube showed significant gas leakage. Chest CT and X-ray on the fifteenth day of hospitalization indicated multiple cavitations and patchy consolidations in both lungs, loss of normal structure in the left lower lung (Figure 3A), and left pleural gas with small pleural effusion (Figure 3B and C). Thoracoscopy revealed multiple fistulas on the visceral pleura with significant necrotic material (Figure 4A). Subsequently, methylene blue injected through bronchoscopy into the left lower lobe bronchus flowed out from the fistulas under thoracoscopy (Figure 4B). Debridement of necrotic tissue was performed under direct vision, along with argon plasma coagulation cauterization of the fistulas, and spraying of fibrin glue and thrombin (Figure 4C). Postoperatively, temperature gradually returned to normal, and gas leakage from the tube significantly reduced.
After a total of one month of hospitalization, the patient’s respiratory symptoms resolved, with SpO2 95% without supplemental oxygen. The patient was discharged with the tube, and continued antibiotic therapy with oral amoxicillin/clavulanate (875 mg/125 mg tablets every 8 hours) for four weeks. Seven weeks post-discharge, the tube showed no gas leakage and was subsequently removed. A follow-up chest CT and X-ray demonstrated complete resolution of the lung abscesses (Figure 5A), with small amount of encapsulated pleural gas and effusion (Figure 5B and C).
Discussion
Necrotizing pneumonia is characterized by pulmonary infection-induced parenchymal damage and necrosis of consolidated lung tissue, leading to the formation of multiple cavitary lesions.4,5 In our case, Streptococcus pneumoniae infection initially caused extensive pulmonary consolidation, which subsequently developed necrotic changes with multiple cavities. Multiple cavities in the left lower lung further merged and ruptured, ultimately resulted in the development of bronchopleural fistulas. Streptococcus pneumoniae infection rarely causes necrotizing pneumonia in adults.2,3 A systematic search in PubMed was conducted by using the search strategy “(Streptococcus pneumoniae OR S. pneumoniae OR pneumococcus) AND (necrotizing pneumonia OR cavitary pneumonia OR lung abscess OR bronchopleural fistula)”. No case reports of necrotizing pneumonia complicated by bronchopleural fistula due to Streptococcus pneumoniae in adults were identified.
Necrotizing pneumonia represents an uncommon but severe complication of bacterial pneumonia, with an estimated incidence of 1% among bacterial pneumonia cases.6 In adults, necrotizing pneumonia is commonly associated with Staphylococcus aureus, Klebsiella pneumoniae, and Streptococcus pneumoniae.4,7,8 Panton-Valentine leukocidin (PVL) detected in Staphylococcus aureus (especially methicillin-resistant Staphylococcus aureus) exhibits particular virulence, inducing rapid tissue necrosis through neutrophil lysis and subsequent release of proinflammatory cytokines, even in immunocompetent hosts.6,9 Hypervirulent variants of Klebsiella pneumoniae with K1/K2 capsular polysaccharides demonstrate enhanced antiphagocytic properties and resistance to complement-mediated clearance.10,11 These strains are linked to severe necrotizing pneumonia and hepatic abscess, particularly in patients with chronic alcoholism, diabetes mellitus, or structural lung disease. Among pneumococcal strains, non-vaccine serotype 3 predominates in necrotizing cases.2 The primary pathogenic mechanism is likely related to the anti-phagocytic properties of its capsular polysaccharides.8,12 Streptococcus pneumoniae can also produce pneumolysin, a pore-forming toxin disrupting alveolar-epithelial and endothelial membrane integrity.13 Compared to PVL, pneumolysin exhibits relatively weaker virulence in driving tissue destruction. Besides, other pathogens such as Pseudomonas aeruginosa, Escherichia coli, and anaerobic species may also rarely contribute to necrotizing pneumonia.7,8,14
Hematogenous dissemination, diabetes mellitus, excessive alcohol consumption, aspiration, immunosuppression, and malignancy are high-risk factors for the development of necrotizing pneumonia.15 Streptococcus pneumoniae infection can trigger systemic hyperinflammatory responses, and some highly invasive serotypes may enter blood. Bacteria via bloodstream may cause septic pulmonary emboli, ultimately resulting in lung necrosis.16 In our case, Streptococcus pneumoniae was detected in blood culture, which may be the potential high-risk factor for severe necrotizing pneumonia and bronchopleural fistulas.
The culture of BALF was negative in this patient, but NGS of BALF detected a large number of Streptococcus pneumoniae sequences. One reason is that BALF culture typically has low sensitivity in detecting Streptococcus pneumoniae.17 Streptococcus pneumoniae is difficult to culture because it can trigger an autolysin and cause its own death upon reaching a certain density. Another reason may be that the patient had been treated with antibiotics prior to the collection of BALF specimen, which could inhibit bacterial proliferation. Therefore, for critically ill patients with pneumonia of unknown etiology, it is necessary to collect respiratory specimens as early as possible for NGS detecting. In addition, routine blood culture should be performed to increase the culture positive rate for severe pneumococcal pneumonia.17
Bronchopleural fistula is a rare and severe complication of necrotizing pneumonia, which can increase the risk of recurrent infections, prolong the duration of antibiotic use, and elevate mortality rate.18,19 There is currently no standardized treatment protocol for bronchopleural fistula.18 The patient in our case showed improvement in infection following thoracoscopic debridement of necrotic material and repair of the fistulas. Although there was still small amount of gas leakage postoperatively, which was considered related to residual small fistulas, they eventually closed spontaneously. This case suggests that for patients with multiple and complex bronchopleural fistulas, thoracoscopic fistulas repair can improve their condition, but it is challenging to completely repair the fistulas during the infection period. Residual small fistulas after adequate infection control may heal on their own. Therefore, it is important to allow sufficient observation time and avoid premature surgical intervention.
Conclusion
In conclusion, we should consider the possibility that community-acquired necrotizing pneumonia due to Streptococcus pneumoniae infection can lead to bronchopleural fistula in adults. Early diagnosis and management of bronchopleural fistula are crucial for improving patients outcomes.
Abbreviations
BALF, bronchoalveolar lavage fluid; NGS, next-generation sequencing; PVL, Panton-Valentine leukocidin.
Data Sharing Statement
The original data in the study have been provided in the article. Further reasonable request can be available from the corresponding author.
Ethics and Consent
Informed written consent for the use of case details for the publication was obtained from the patient. The Ethics Committee of West China Hospital of Sichuan University approved the waiver in this case report.
Funding
This article was supported by Science and Technology Department of Sichuan Province-International Science and Technology Innovation Cooperation Project, 2024YFHZ0273.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Musher DM. Streptococcus pneumoniae. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier Churchill Livingstone; 2005:2392–2411.
2. Pande A, Nasir S, Rueda AM, et al. The incidence of necrotizing changes in adults with pneumococcal pneumonia. Clin Infect Dis. 2012;54:10–16. doi:10.1093/cid/cir749
3. Nicolini A, Cilloniz C, Senarega R, Ferraioli G, Barlascini C. Lung abscess due to Streptococcus pneumoniae: a case series and brief review of the literature. Pneumonol Alergol Pol. 2014;82:276–285. doi:10.5603/PiAP.2014.0033
4. Kapania EM, Cavallazzi R. Necrotizing pneumonia: a practical guide for the clinician. Pathogens. 2024;13:984. doi:10.3390/pathogens13110984
5. Gillet Y, Vanhems P, Lina G, et al. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing panton-valentine leukocidin. Clin Infect Dis. 2007;45:315–321. doi:10.1086/519263
6. Li HT, Zhang TT, Huang J, Zhou YQ, Zhu JX, Wu BQ. Factors associated with the outcome of life-threatening necrotizing pneumonia due to community-acquired Staphylococcus aureus in adult and adolescent patients. Respiration. 2011;81:448–460. doi:10.1159/000319557
7. Krutikov M, Rahman A, Tiberi S. Necrotizing pneumonia (aetiology, clinical features and management). Curr Opin Pulm Med. 2019;25:225–232. doi:10.1097/MCP.0000000000000571
8. Tsai YF, Ku YH. Necrotizing pneumonia: a rare complication of pneumonia requiring special consideration. Curr Opin Pulm Med. 2012;18:246–252. doi:10.1097/MCP.0b013e3283521022
9. Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus panton-valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi:10.1126/science.1137165
10. Harada S, Ishii Y, Saga T, Aoki K, Tateda K. Molecular epidemiology of Klebsiella pneumoniae K1 and K2 isolates. Diag Micro Biol Infect Dis. 2018;91:354–359. doi:10.1016/j.diagmicrobio.2018.03.010
11. Lin YC, Lu MC, Tang HC, et al. Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: compara tive virulence analysis with hypermucoviscosity-negative strain. BMC Micro Biol. 2011;11:50–58. doi:10.1186/1471-2180-11-50
12. Hsieh YC, Hsueh PR, Lu CY, et al. Clinical manifestations and molecular epidemiology of necrotizing pneumonia and empyema caused by streptococcus pneumoniae in children in Taiwan. Clin Infect Dis. 2004;38:830–835. doi:10.1086/381974
13. Witzenrath M, Gutbier B, Hocke AC, et al. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med. 2006;34:1947–1954. doi:10.1097/01.CCM.0000220496.48295.A9
14. Maharaj S, Isache C, Seegobin K, Chang S, Nelson G. Necrotizing Pseudomonas aeruginosa community-acquired pneumonia: a case report and review of the literature. Case Rep Infect Dis. 2017;2017:1717492. doi:10.1155/2017/1717492
15. Hammond JM, Potgieter PD, Hanslo D, Scott H, Roditi D. The etiology and antimicrobial susceptibility patterns of microorganisms in acute community-acquired lung abscess. Chest. 1995;108:937–941. doi:10.1378/chest.108.4.937
16. Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21:305–333. doi:10.1128/CMR.00060-07
17. Dion CF, Ashurst JV. Streptococcus pneumoniae. Treasure Island (FL): StatPearls Publishing; 2024.
18. Dugan KC, Laxmanan B, Murgu S, Hogarth DK. Management of persistent air leaks. Chest. 2017;152:417–423. doi:10.1016/j.chest.2017.02.020
19. Jin L, Li Y. Bronchoscopic interventions for bronchopleural fistulas. Ther Adv Respir Dis. 2023;17:17534666231164541. doi:10.1177/17534666231164541
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.