Back to Journals » Journal of Pain Research » Volume 18
Comparative Analysis of Muscle Elasticity and Pathological Characteristics Between Affected and Unaffected Sides in Postherpetic Neuralgia Patients: Protocol for a Pilot Cohort Trail
Authors Ji H , Ma J, Cui X , Huang Y
Received 5 November 2024
Accepted for publication 12 March 2025
Published 25 March 2025 Volume 2025:18 Pages 1597—1605
DOI https://doi.org/10.2147/JPR.S504823
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Michael A Ueberall
Heyu Ji,1 Jiangyu Ma,2 Xulei Cui,1 Yuguang Huang1
1Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Xulei Cui; Yuguang Huang, Department of Anesthesiology, Peking Union Medical College Hospital, Shuaifuyuan 1st, Dongcheng District, Beijing, 100730, People’s Republic of China, Email [email protected]; [email protected]
Background: Postherpetic neuralgia (PHN) is the most common condition that can develop as a complication after herpes zoster (HZ) infection, characterized by pain that persists for more than 3 months after the initial rash has resolved. In most patients with HZ, the rash appears unilaterally. While the treatment of PHN is primarily focused on neural mechanisms due to HZ’s neurotropism nature, recent evidence suggests that muscle tissues within the affected regions may also experience pathological changes that contribute to the pain. These changes could reveal novel therapeutic targets and enhance patient prognosis. This study aims to investigate these muscular changes and explore myogenic pain mechanisms in PHN patients. It employs ultrasound elastography to compare muscle elasticity between the affected and unaffected sides and conduct muscle biopsies for pathophysiological analysis to uncover the underlying mechanisms.
Materials and Methods: This comparative cross-sectional study aims to enroll 30 PHN patients. The primary outcome is the comparison of muscle elasticity on the affected sides with unaffected sides. The secondary outcome is from muscle biopsies, which are obtained and analyzed by histopathological techniques. Pain levels before and after therapy are assessed using the Numerical Rating Scale (NRS), with follow-up to evaluate outcomes and satisfaction. Statistical analysis will employ paired t-tests or Wilcoxon signed-rank tests to compare muscle elasticity, and correlation analysis to explore the relationship between elasticity and pathological findings.
Hypothesis: The study hypothesis is that muscle elasticity on the affected side is significantly higher than on the unaffected side, with the coexistence of myofascial pain. This myofascial pain may overlap with PHN pain and may be a source of discomfort in refractory PHN cases. Furthermore, muscle biopsies are conducted to clarify pathological changes. This study may pave the way for novel treatment strategies for PHN and establish a foundation for future research.
Study Registration: This study has obtained ethical approval from the Institutional Review Board of Peking Union Medical College Hospital on 28 August 2023 (I-23PJ1409) and is registered at ClinicalTrails.gov. Written informed consent has been obtained from all participants.
Keywords: postherpetic neuralgia, shear wave elastography, myofascial pain, muscle biopsy
Introduction
Postherpetic neuralgia (PHN) is the most common complication following herpes zoster (HZ) infection. PHN is characterized by persistent pain – often burning, stabbing, or throbbing pains in the skin where the rash had occurred, lasting for more than 3 months post rash resolution.1 The incidence of HZ rises significantly in individuals over 50 years of age, with the occurrence of PHN is being even higher in those over 60.2,3 Typically, HZ manifests unilaterally, with roughly half the patients developing the rash on their thoracic skin, and the face, particularly the trigeminal nerve region being the second most affected area.4 PHN is a prevalent cause of neuropathic pain which is driven by reactivation of the varicella-zoster virus (VZV) within the sensory ganglia causing neural inflammation, neural damage, central sensitization, and even demyelination.5 Such disruption in nerve function produces symptoms like allodynia and hyperalgesia. Conventional treatments for PHN target neuropathic pain, they include antiviral therapy, dorsal root ganglion block, intercostal nerve pulsed radiofrequency, and spinal cord stimulation.6 Despite these interventions, many patients develop refractory PHN, where pain persists even after aggressive neuropathic treatments. This causes a lot of suffering to both the patients and their families. There is thus an urgent need to develop more comprehensive and effective treatments for PHN patients.
Recent clinical observations and studies suggest that muscle-related factors may contribute to the persistence of pain in patients with refractory PHN.7 Evidence from certain case reports have shown that myofascial trigger point therapies—typically used for muscle pain—support the hypothesis that muscular changes, possibly related to myofascial dysfunction, may contribute to the pain experienced by these patients.8 These observations have prompted a reevaluation of the underlying mechanisms of PHN pain. This study thus proposes that PHN patients not only suffer from neural problems but may present with myofascial trigger point-like muscle pathology as well, which could be a key contributor to the persistence of their pain.
Elastography is a new ultrasound evaluation technique, effective in assessing and measuring muscle stiffness. It exhibits strong repeatability and reliability and is often used in clinical settings to assess conditions like myofascial pain syndrome, neck and shoulder pain, and low back pain.9 Shear wave elastography (SWE) is used to examine tissue stiffness by analyzing the propagation speed of shear waves, which travel faster in stiffer tissues than in softer ones.10 Previous studies have demonstrated the reliability of elastography in observing musculoskeletal changes related to pain caused by myofascial issues and for assessing muscle stiffness.11–14
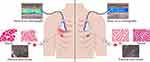
The objective of this study is to assess the disparity in muscle elasticity compared with the unaffected sides in patients with PHN. This is done using elastography ultrasound (EU) technology. The second objective is to elucidate the pathological changes in muscles of PHN patients. This is achieved through a comprehensive histopathological examination, ranging from morphological to microscopic assessments of tissue obtained using a microbiopsy technique.15 The anticipation is that this investigation will offer deeper insights on the effects of PHN on muscle tissue properties, offering valuable guidance into the clinical management of the condition.
Methods and Analysis
Study Design
This study is designed as a 24-month prospective cohort study and is based at a single center, the Peking Union Medical College Hospital. Given that HZ primarily manifests unilaterally, particularly in the thoracic region,16 SWE is to employed to measure the elasticity of the intercostal muscles bilaterally and thus allow the comparison of the affected and unaffected sides. To better understand the pathological muscle changes in PHN patients, a muscle biopsy is performed using a biopsy needle. The extracted tissue undergoes histopathological examination using hematoxylin and eosin (HE) staining to assess muscle morphology and the extent of inflammatory infiltration. Masson’s trichrome staining, on the other hand, is used to detect fibrosis within the muscle. Finally, electron microscopy is employed to identify ultrastructural changes in mitochondria and myofibril sarcomeres. This approach helps to identify coexisting pain triggers in patients and improves treatment outcomes. Table 1 illustrates the detailed timeline and data recording methods. Figure 1 depicts the procedural flow, highlighting the key interventions and assessments at each designated interval. Figure 2 provides an overview of the study design.
 |
Table 1 Assessment Schedule for PHN Muscle Elasticity and Pathology |
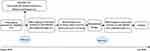 |
Figure 1 Flow chart. The horizontal axis represents the timeline, with corresponding procedures executed at designated intervals. The NRS scores are recorded at two specific points. |
Sample Size
A total of 30 patients will be included, with the unaffected side of each patient serving as the own control, which effectively doubles the sample size for comparisons. This selection is based on pilot study practices and similar studies.17–20 This paired analysis design enhances statistical power by reducing variability and directly assessing differences within each patient. The goal is to evaluate the study’s feasibility and refine protocols for future studies. Sensitivity analyses will be conducted to ensure the robustness and reliability of our findings despite the limited sample size.
Recruitment: Inclusion Criteria
Recruitment began in August 2023, with data collection expected to conclude by July 2025 (Figure 1). Patients aged 18 years and older, diagnosed with PHN that affects the thoracic nerves and experiencing severe, refractory pain despite pharmacological treatment, who were in need of surgical intervention, have been recruited. Written informed consent was obtained from all participants before inclusion. This study obtained ethical approval from the Institutional Review Board of Peking Union Medical College Hospital on 28 August 2023 (I-23PJ1409) and is registered with ClinicalTrials.gov (NCT05912738).
Exclusion Criteria
The exclusion criteria included patients with any of the following conditions: neuromuscular diseases; ongoing use of muscle relaxants and/or other medications that impact muscles; allergy to local anesthetic; a body mass index (BMI) over 30kg/m2; malignant tumors; and pregnancy. Patients who previously had surgery in the PHN-affected area or had another disease-causing muscle changes were considered ineligible. Additionally, patients currently using NSAIDs or anticoagulants who are unable to temporarily discontinue these medications at least 7 days, prior to the procedure will be excluded. Provisions have been made to facilitate any patients who chooses to withdraw from the study for any reason.
Shear Wave Elastography (SWE) Procedure
Patients were positioned in a relaxed supine state for lesions located on the anterior chest wall, ensuring adequate exposure of the thoracic intercostal lesion site for marking. The midline of the sternum served as the central reference point, with corresponding symmetrical positions on the healthy side identified and marked. For posterior chest lesions, the patient was positioned in a prone position to facilitate access to the back and ensure muscle relaxation during elastography. For lateral chest lesions, the patient was positioned in a lateral decubitus position with the lesion side facing upward, ensuring the affected muscles were relaxed for accurate imaging. SWE images of the intercostal muscles (located between the pleura and the overlying skin) on both the affected and unaffected sides were assessed using the Mindray Resona I9 ultrasound system (Shenzhen Mindray Biomedical Electronics Co., Ltd., Shenzhen, China). These images were assessed to evaluate the elasticity of the intercostal muscles, with special attention paid to potential changes indicative of myofascial trigger points or other pathological alterations. A small of ultrasonic gel was applied on the area to be examined, ensuring that the ultrasound probe exerted no pressure on the tissue under examination. A linear probe at L14-3Ws frequency was positioned parallel to the ribs on both sides of the lesion, targeting the intercostal muscles. The probe’s angle was adjusted to align parallel to the long axis of the muscle fibers, with the central part positioned perpendicularly to the fibers. The area of maximal pain, as described by the PHN patients, was identified, measured and documented with a ruler. The corresponding site was marked on the contralateral side. SWE mode was activated, with a rectangular region of interest (ROI) frame placed over the identified muscle. Within the ROI, three areas with the highest elasticity on elastography were selected for measurement. The system was then switched to penetration mode, with the focal area manually or automatically adjusted to the depth of the ROI. The image was then frozen once the patient held their breath and the elastography image stabilized, and the ROI was color-filled to reflect the Young’s modulus magnitude. Each muscle was measured at least three times, and the mean values of these measurements were recorded and analyzed in kilopascals (kPa). The elastography examination technique for the intercostal muscles on the healthy side was the same as that on the affected side. All elastography imaging procedures were performed by the same trained physician.
Muscle Biopsy and Histopathological Procedures
A local muscle biopsy is be performed using a MultiFire Disposable Biopsy Instrument (LEAPMED C1616, 16G*160 mm) in the area of maximal pain, which also has the highest elasticity, to obtain pathological insights. Under EU-guided imaging, the selected muscle is identified, and the puncture needle inserted into the selected-muscle, the device activated to retrieve the tissue sample. The same biopsy technique is used to obtain tissue samples on the unaffected side for comparison. To minimize these risks associated with the biopsy procedure, such as bleeding, infection, and local pain at the biopsy site, strict aseptic techniques are be applied during the procedure, and post-procedure monitoring is conducted to quickly identify and manage any adverse events. Participants are closely monitored for any signs of complications and provided with appropriate care if necessary. The samples are divided into several portions, for which some are stained with HE, others Masson's trichrome, and others observed under the electron microscope. Muscle samples are be fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5 µm thickness. The sections undergo staining using standard the HE and Masson’s trichrome protocols.21,22 Another section is fixed in 2.5% glutaraldehyde, post-fixed in 1% osmium tetroxide, dehydrated in ethanol, and embedded in epoxy resin. Ultrathin sections (70 nm) are stained with uranyl acetate and lead citrate for examination under an electron microscope. The HE and Masson’s trichrome staining will elucidate the cellular detail and help to detect fibrosis, respectively, while the electron microscope will clarify ultrastructural changes in mitochondria and myofibril sarcomeres, all of which aid to better understand the muscle changes in PHN patients.
Outcomes
Primary Outcome
Muscle elasticity of the affected side and the unaffected side in the PHN patients is the primary outcome. It is assessed using SWE which has recently emerged as a promising assessment tool with the potential for diagnosing and monitoring muscle changes in a noninvasive method.
Secondary Outcomes
The secondary outcomes include 1) a comparison of muscle elasticity between the affected and contralateral healthy sides across different PHN stages, 2) a comparison of the before and after the interventional therapy of SWE measurements, 3) a comparison SWE of measurements between groups receiving conventional treatment alone and those receiving conventional treatment plus targeted muscle relaxation therapy, 4) a comparison of the histopathological data of the muscle samples from the affected and the unaffected sides, using HE staining, Masson's trichrome staining and electron microscope to observe morphological changes, and electron microscopy to examine intracellular mitochondrial and other cellular alterations, and 5) a comparison of the NRS pain scores before and after the treatment, document the number of treatment sessions, and analyze the duration of PHN.
Statistical Analysis
Data analysis will be performed using the SPSS software, version 26.0. Patient demographics, including gender, age, height, weight, BMI and SWE, will be described by means ± standard deviations. The intraclass correlation coefficient (ICC) will be used to examine the feasibility of SWE by evaluating the consistency and reliability of repeated measurements. Spearman correlation analysis will assess the relationships between variables and elasticity. Differences in SWE measurements of the affected and unaffected sides muscle will be evaluated using the Mann Whitney U-test. Normally distributed continuous data will be presented as means ±standard deviations and assessed using Shapiro–Wilk test. Paired t-tests will be used to compare SWE measurements before and after interventional therapy, while unpaired t-tests will compare the SWE measurements of affected and unaffected sides. Pearson correlation coefficient will be used to evaluate the association between SWE elasticity before and after the interventional therapy, with statistical significance set at p <0.05. Differences in pathological metrics—including inflammatory cell counts, muscle fiber degeneration/necrosis percentages, mitochondrial density, and sarcomere and myofibril measurements—will be analyzed using paired t-tests or Wilcoxon signed-rank tests, depending on data normality. The Shapiro–Wilk test will be applied to determine normality, and the Bonferroni correction will be applied for multiple comparisons, with statistical significance considered at p <0.05.
Data Collection and Management
All data are recorded in the standardized case report forms (CRF) specifically designed for this study. For elastography, the three SWE measurements for both affected and unaffected muscle are entered directly into the CRF. Muscle biopsy samples are collected by experienced clinicians under sterile conditions and processed and analyzed in a certified pathology laboratory. Detailed findings from the biopsy analysis are also recorded in the CRFs. Signed informed consent forms and CRFs are be stored on a secure server with restricted access, whose access is available to only the authorized researchers. The data and documents will be retained for a minimum of 10 years.
Discussion
HZ infects unilateral nerves, often leading to neuropathic pain known as PHN.23 Various therapies for PHN have emerged, including antiviral agents, paravertebral block, spinal cord stimulation, pulsed radiofrequency, or dorsal root ganglion destruction,24,25 etc. The pain in PHN patients can be clinically manifested in as, neuropathic pain (eg, burning, throbbing, shooting) and myofascial pain (eg, trigger points, taut bands within the muscle).26,27 Despite the availability of numerous PHN treatment options, a subset of the patients remains unresponsive even after utilizing combined therapeutic approaches. This renders PHN to be a clinically challenging and refractory pain condition. Studies have found significant correlations between PHN and myofascial trigger points (MTrPs), which are particularly evident in cases where chronic pain following a HZ infection persists beyond one month. It is accompanied by hyperesthesia and distinct tender points in the intercostal muscles that correspond to the affected nerve.8 In another study, tender spots were found in the intercostal muscles of PHN patients, highlighting the association between MTrPs and PHN.8 In another, Volokitin et al found that osteopathic manipulative therapy (OMT), which releases muscle and fascia tension, was beneficial as an adjunct therapy in the cases of PHN.28 It is estimated that about 57% of PHN patients experience myofascial pain, which can be alleviated by dry and wet needling.7,29 The relationship between PHN and myofascial pain may be caused by protective posture model of PHN patients prompting the muscle under HZ become strained and stiff. HZ leads to central and peripheral sensitization of pain which may also exacerbate myofascial pain. Furthermore, elderly patients with degenerative scoliosis are more likely to experience spinal dorsal angle angulation or traction, making them more prone to myofascial pain. In summary, it is highly probable that PHN and myofascial pain interact and influence each other. Stimulation of the dorsal root ganglion may result in abnormal nerve discharge, causing abnormal muscle contraction and myofascial pain. Conversely, myofascial dysfunction may induce peripheral sensitization by activating muscle nociceptors, amplifying pain signals. Traditionally regarded as a neurological condition, PHN may also have a significant myofascial component.27 It thus only prudent to consider the complex pain derived from PHN as not only being neurological but myofascial as well.
Myofascial pain syndrome (MPS) is often characterized by localized muscle stiffness, tenderness, and pain at trigger points.30 SWE is a reliable method for assessing MPS13,31 and monitoring the muscle stiffness in clinical situations.32 SWE works by tracking pulse-echo ultrasound and calculating the tissue modulus quantitatively. The shear-wave speed correlates directly with the tissue stiffness.33 Being a non-invasive technique, SWE is capable of quantifying muscle elasticity and distinguishing subtle changes in soft tissues, thus providing a unique advantage in assessing muscle lesions in PHN patients. Although this study employs muscle biopsy, which is an invasive method, it is intended to validate the muscle histopathological changes and establish a correlation with SWE. The combination of histopathological techniques—including HE staining, Masson's trichrome staining, and electron microscopy—provides a robust framework for a investigating muscle tissue at both the cellular and subcellular levels. This multifaceted approach ensures a comprehensive data collection, which is crucial for correlating mechanical properties with underlying tissue changes. By comparing the muscle elasticity of the affected and unaffected sides and conducting detailed histopathological analyses, this study aims to develop a comprehensive understanding the pathological mechanisms of muscle involvement in PHN.
This protocol is significant as it addresses the gap in understanding the musculoskeletal contributions to PHN, a condition which has been primarily approached from a neural perspective. By integrating the analysis of muscle elasticity using ultrasound elastography with detailed histopathological examinations, this study seeks to provide a novel approach on PHN’s pathophysiology. This dual approach allows for a comprehensive assessment that could correlate previously overlooked factors contributing to PHN pain and potentially lead to more effective treatment strategies. Despite efforts to minimize bias were made, the clinical presentation of PHN patients was heterogeneous, with variations in pain intensity and disease duration. This may affect the external validity of the study results. Since the study’s participants are also limited to those requiring interventional therapy (patients with moderate-to-severe pain), its finding may not fully represent all PHN patients, thereby affecting the generalizability.
The study hypothesizes that the muscle elasticity of the affected side will be significantly higher than that of the unaffected side, possibly due to muscle fibrosis, inflammation, or neuromuscular changes associated with PHN. This study protocol sets the stage for future research exploring the relationship between muscle elasticity and histopathological changes in PHN. A promising future direction could integrate ultrasound elastography with sonomics, which involves the comprehensive analysis of ultrasound data using advanced computational methods. This integration could improve the ability to detect small changes in muscle tissue not visible to the naked eye, thus providing deeper insights into the musculoskeletal involvement in PHN. By advancing the understanding of both neural and muscular factors in PHN, this study aims to enhance diagnostic accuracy and develop targeted therapeutic interventions that can significantly improve patient outcomes and quality of life. The finding may also reveal new therapeutic targets, such as enhancing or restoring muscle function to alleviate pain symptoms in PHN, potentially shifting treatment from a purely neural mechanism to a neuro-muscular approach.
Strengths and Limitations of This Study
This is a well-designed, prospective cohort study, focusing on muscle-related changes in PHN patients.
This study utilizes an innovative, non-invasive technique, ultrasound elastography, to compare muscle stiffness in PHN patients.
By incorporating muscle biopsies, the study offers detailed pathophysiological insights, enhancing the understanding of muscle changes in PHN patients.
This study may not fully represent the general population, as PHN incidence is higher in patients over 50 years.
Conclusion
This single-center prospective cohort study aims to compare changes in the bilateral muscle elasticity in PHN patients using elastography. It seeks to elucidate the coexistence of altered muscle stiffness and myofascial pain, providing more insight into the issue of myofascial pain in PHN patients with poor responses to neuropathic pain treatments. Muscle biopsy is conducted to validate these findings. This protocol reveals a potential neuro-muscular pain mechanism and clarifies pathological changes. This has the potential to offer a new therapeutic direction for refractory PHN patients and provide additional diagnostic and treatment strategies for clinical practice.
Ethics and Dissemination
This study obtained ethical approval from the Institutional Review Board of Peking Union Medical College Hospital (I-23PJ1409) and is registered with ClinicalTrials.gov (NCT05912738). This study will comply with the Declaration of Helsinki. All participants have been informed about the study’s objectives, procedures, potential risks, and benefits. Written informed consent was obtained from each participant before any study-related activities were conducted. Participants’ privacy and confidentiality will be maintained throughout the study through anonymization and secure storage of data. The results of this study will be disseminated through peer-reviewed scientific journal publication and presentation at relevant national and international conferences. The findings and data will also be shared with the study participants upon reasonable request.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (Grant No.2022-PUMCH-B-007) and Peking Union Medical College Hospital Research Funding for Postdoc (kyfyjj202301).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Johnson RW, Rice AS. Clinical practice. postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. doi:10.1056/NEJMcp1403062
2. Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthc. 2016;9:447–454. doi:10.2147/jmdh.S106340
3. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi:10.1136/bmjopen-2014-004833
4. Johnson RW, Wasner G, Saddier P, Baron R. Postherpetic neuralgia: epidemiology, pathophysiology and management. Expert Rev Neurother. 2007;7(11):1581–1595. doi:10.1586/14737175.7.11.1581
5. Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9(1 Suppl 1):S10–8. doi:10.1016/j.jpain.2007.10.003
6. Rui M, Ni H, Xie K, Xu L, Yao M. Progress in radiofrequency therapy for zoster-associated pain about parameters, modes, targets, and combined therapy: a narrative review. Pain Ther. 2024;13(1):23–32. doi:10.1007/s40122-023-00561-7
7. Lu XH, Chang XL, Liu SL, Xu JY, Gou XJ. Ultrasound-guided inactivation of trigger points combined with muscle fascia stripping by liquid knife in treatment of postherpetic neuralgia complicated with abdominal myofascial pain syndrome: a prospective and controlled clinical study. Pain Res Manag. 2020;2020:4298509. doi:10.1155/2020/4298509
8. Chen SM, Chen JT, Kuan TS, Hong CZ. Myofascial trigger points in intercostal muscles secondary to herpes zoster infection of the intercostal nerve. Arch Phys Med Rehabil. 1998;79(3):336–338. doi:10.1016/s0003-9993(98)90016-8
9. Tsuchida W, Yamakoshi Y, Matsuo S, et al. Application of the novel estimation method by shear wave elastography using vibrator to human skeletal muscle. Sci Rep. 2020;10(1):22248. doi:10.1038/s41598-020-79215-z
10. Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7(5):1303–1329. doi:10.7150/thno.18650
11. Thanwisate T, Siriwanarangsun P, Piyaselakul S, Tharmviboonsri T, Chuckpaiwong B. Plantar fascia thickness and stiffness in healthy individuals vs patients with plantar fasciitis. Foot Ankle Int. 2024;10711007241274765. doi:10.1177/10711007241274765
12. David M, Devantéry K, Nauche B, et al. Ultrasound elastography of back muscle biomechanical properties: a systematic review and meta-analysis of current methods. Insights Imaging. 2024;15(1):206. doi:10.1186/s13244-024-01785-7
13. Valera-Calero JA, Sánchez-Jorge S, Buffet-García J, Varol U, Gallego-Sendarrubias GM, Álvarez-González J. Is shear-wave elastography a clinical severity indicator of myofascial pain syndrome? an observational study. J Clin Med. 2021;10(13):2895. doi:10.3390/jcm10132895
14. Jafari M, Bahrpeyma F, Mokhtari-Dizaji M, Nasiri A. Novel method to measure active myofascial trigger point stiffness using ultrasound imaging. J Bodyw Mov Ther. 2018;22(2):374–378. doi:10.1016/j.jbmt.2017.06.019
15. Townsend JR, Hoffman JR, Fragala MS, et al. A microbiopsy method for immunohistological and morphological analysis: a pilot study. Med Sci Sports Exerc. 2016;48(2):331–335. doi:10.1249/mss.0000000000000772
16. Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol. 2018;84(3):251–262. doi:10.4103/ijdvl.IJDVL_1021_16
17. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2010;4(4):287–291.
18. Li H, Yang CC, Bai T, et al. The impact of fu’s subcutaneous needling on lower limb muscle stiffness in knee osteoarthritis patients: study protocol for a pilot randomized controlled trial. J Pain Res. 2024;17:3315–3326. doi:10.2147/jpr.S482082
19. Yang X, Wang H, Sun J. Understanding tightened muscle in knee osteoarthritis and the impacts of Fu’s subcutaneous needling: a pilot trial with shear-wave elastography and near-infrared spectroscopy. Medicine. 2024;103(21):e38274. doi:10.1097/md.0000000000038274
20. Jhamb M, Devaraj SM, Alemairi M, et al. A Comprehensive Exercise (COMEX) intervention to optimize exercise participation for improving patient-centered outcomes and physical functioning in patients receiving hemodialysis: development and pilot testing. Kidney Med. 2023;5(11):100720. doi:10.1016/j.xkme.2023.100720
21. Wang C, Yue F, Kuang S. Muscle histology characterization using h&e staining and muscle fiber type classification using immunofluorescence staining. Biol Protoc. 2017;7(10). doi:10.21769/BioProtoc.2279
22. Calvi EN, Nahas FX, Barbosa MV, et al. An experimental model for the study of collagen fibers in skeletal muscle. Acta Cir Bras. 2012;27(10):681–686. doi:10.1590/s0102-86502012001000003
23. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811–826. doi:10.1016/j.idc.2017.07.016
24. Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96(10):656–663.
25. Lin CS, Lin YC, Lao HC, Chen CC. Interventional treatments for postherpetic neuralgia: a systematic review. Pain Physician. 2019;22(3):209–228. doi:10.36076/ppj/2019.22.209
26. Dworkin RH, Gnann JW, Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain. 2008;9(1 Suppl 1):S37–44. doi:10.1016/j.jpain.2007.10.008
27. Weiner DK, Schmader KE. Postherpetic pain: more than sensory neuralgia? Pain Med. 2006;7(3):243–249.discussion 250. doi:10.1111/j.1526-4637.2006.00151.x
28. Volokitin M, Izadi N, Myers R, Kane Diaw N, Milani S. Osteopathic manipulative treatment of herpes zoster ophthalmicus/postherpetic neuralgia. Cureus. 2021;13(5):e14906. doi:10.7759/cureus.14906
29. Huang Y, Gao M, Li Q, et al. Ultrasound-guided dry needling for trigger point inactivation in the treatment of postherpetic neuralgia mixed with myofascial pain syndrome: a prospective and controlled clinical study. Pain Res Manag. 2022;2022:2984942. doi:10.1155/2022/2984942
30. Urits I, Charipova K, Gress K, et al. Treatment and management of myofascial pain syndrome. Best Pract Res Clin Anaesthesiol. 2020;34(3):427–448. doi:10.1016/j.bpa.2020.08.003
31. Ertekin E, Kasar ZS, Turkdogan FT. Is early diagnosis of myofascial pain syndrome possible with the detection of latent trigger points by shear wave elastography? Pol J Radiol. 2021;86:e425–e431. doi:10.5114/pjr.2021.108537
32. Olchowy C, Więckiewicz M, Sconfienza LM, et al. Potential of using shear wave elastography in the clinical evaluation and monitoring of changes in masseter muscle stiffness. Pain Res Manag. 2020;2020:4184268. doi:10.1155/2020/4184268
33. Brandenburg JE, Eby SF, Song P, et al. Ultrasound elastography: the new frontier in direct measurement of muscle stiffness. Arch Phys Med Rehabil. 2014;95(11):2207–2219. doi:10.1016/j.apmr.2014.07.007
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.