Back to Journals » Drug Design, Development and Therapy » Volume 19
Comparison of Esketamine/Propofol and Sufentanil/Propofol on Intraoperative Hypoxemia During Bronchoscopy: A Randomized Trial
Authors Huang X , Li X, Sun Y, Wu A , Ai P
Received 7 August 2024
Accepted for publication 13 May 2025
Published 27 May 2025 Volume 2025:19 Pages 4429—4436
DOI https://doi.org/10.2147/DDDT.S490423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Prof. Dr. Georgios Panos
Xiao Huang,1 Xueyang Li,1 Yuan Sun,2 Anshi Wu,1 Pan Ai1
1Department of Anesthesiology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Pharmacy, Peking University Third Hospital, Beijing, People’s Republic of China
Correspondence: Yuan Sun, Department of Pharmacy, Peking University Third Hospital, No. 49 huayuan North Road, Haidian District, Beijing, 100191, People’s Republic of China, Email [email protected] Anshi Wu, Department of Anesthesiology, Beijing Chao-Yang Hospital, Capital Medical University, No. 8 Workers’ Stadium South Road, Chaoyang Distirct, Beijing, 100020, People’s Republic of China, Email [email protected]
Purpose: Propofol and sufentanil are the most commonly used anesthetics during bronchoscopy. Esketamine is an s-enantiomer of ketamine racemate and has both sedative and analgesic effects, it does not inhibit respiration and maintains hemodynamic stability. We aimed to compare the intraoperative hypoxemia risk of esketamine/propofol with sufentanil/propofol for patients in bronchoscopy.
Methods: This study was an investigator-initiated, single-center, randomized, double-blind clinical trial. Patients undergoing bronchoscopy were randomly assigned to receive either sufentanil group (n = 33; sufentanil: 0.2 μg/kg) or esketamine group (n = 33; esketamine: 0.2 mg/kg) for sedation and analgesia. Clinical data, anesthetics usage, incidence of intraoperative hypoxemia, total time of hypoxemia, recovery time, and adverse events were recorded.
Main Results: The incidence of intraoperative hypoxemia was significantly lower in the esketamine group than in the sufentanil group (27.2% vs 66.7%, P=0.001, OR=5.333, 95% CI=1.859– 15.301). Propofol usage was significantly higher in the esketamine group than in the sufentanil group (t=2.952, P=0.004). The duration of hypoxia was significantly lower in the esketamine group than in the sufentanil group (Z=− 3.445, P< 0.001), and the minimum oxygen saturation (SpO2) was significantly higher than in the sufentanil group (Z=− 2.682, P=0.007). Recovery time from anesthesia was significantly lower in the esketamine group than in the sufentanil group (Z=− 2.709, P=0.007). No difference was found in adverse reactions between the two groups.
Conclusion: Esketamine combined with propofol reduced the incidence of intraoperative hypoxemia compared with sufentanil in bronchoscopy. Our results offer the possibility for a novel recommendation for the prevention of intraoperative hypoxemia during bronchoscopy. However, we mentioned the higher propofol use in the esketamine group. Additional clarification is necessary on the indications and the optimal dose of esketamine.
Trial Registration: Chinese clinical trial registry: ChiCTR2200058990.
Keywords: bronchoscopy, esketamine, hypoxemia, propofol, sufentanil
Introduction
Flexible bronchoscopy is a well-established tool for the diagnosis and interventional treatment of respiratory diseases. It is widely used by physicians for safety, portability, and effectiveness. This procedure is usually performed under sedation and analgesia.1 Generally, bronchoscopy is considered a low-risk procedure for healthy patients or those with mild, stable systemic diseases.2 However, patients with confirmed hypoxic respiratory insufficiency present a unique challenge for the bronchoscopist. Hypoxemia is common during bronchoscopy, with risk heightened in patients with hypoxic respiratory failure.3 Statistically, hypoxemia is observed in 67% of cases during therapeutic rigid bronchoscopy when intravenous general anesthesia and autonomous ventilation are employed.4
Esketamine, the S-enantiomer of the ketamine racemate, exhibits a higher affinity for the NMDA receptor than the R-enantiomer. This results in a reduction of the dose-dependent dissociative properties typically associated with ketamine.5 Esketamine demonstrates twice the affinity for NMDA receptors as ketamine, as well as double the sedative and analgesic effects. Both the European Medicines Agency (EMA) and the National Medicines and Medical Devices Agency (NMPA) have approved the use of esketamine for general anesthesia in Europe and China, respectively. Compared to ketamine, esketamine has a higher clearance rate, faster metabolism, rapid recovery from sedation, and potent analgesic effects. The required dose of esketamine is approximately half that of ketamine. It has a minimal effect on the respiratory center, allowing for the maintenance of spontaneous respiration. In recent years, esketamine has become increasingly established in clinical practice.6–8
Opioids are generally used to relieve pain, but they are highly addictive with adverse effects such as respiratory depression, histamine release, hemodynamic changes and hypersensitivity reactions. Respiratory depression is one of the most severe symptoms of acute opioid intoxication.9 It is regulated by opioid receptors expressed on the respiratory neurons of the CNS.10 Neurons in the lateral parabrachial nucleus expressing μ-opioid receptors are involved in the pathogenesis of opioid-induced respiratory depression.11 Sufentanil, a potent opioid analgesic, is a thiophene derivative of fentanyl that was first synthesized in the 1970s. It is the basic drug for the intravenous component of balanced general anesthesia.12 Given its non-negligible respiratory depression, the search for new anesthetic adjuncts is necessary to reduce the potential risks.
Several recent studies have demonstrated that esketamine is effective in reducing hypoxemia compared to sufentanil. For instance, Jonkman et al showed that esketamine effectively mitigates remifentanil-induced respiratory depression.13 Deng et al found that a low dose of 0.25 mg/kg esketamine provided safe and effective anesthesia during gastrointestinal endoscopy and reduced the incidence of intraoperative hypoxemia. When combined with propofol, low-dose esketamine helps maintain stable respiratory and circulatory status while providing sedative and paralytic effects.14 Additionally, a randomized controlled study revealed that esketamine enhances hemodynamic stability and decreases the incidence of hypotension during tracheoscopic drug injection, compared to sufentanil.15
Although clinical research on esketamine in humans is ongoing, the strength of evidence for its efficacy remains low. This study aimed to examine the available data on esketamine and sufentanil and determine the comparative efficacy of these two drugs in patients undergoing bronchoscopy. The primary outcome was the incidence of intraoperative hypoxemia. Secondary outcomes included hemodynamic stability and safety, recovery time from anesthesia, severe intraoperative hypoxemia risk and adverse outcomes.
Materials and Methods
Study Design
This was a single-center, randomized, double-blind study conducted at Beijing Chao-Yang Hospital, Capital Medical University from April 2022 to August 2022. The study was performed by clinical practice standards and the principle of the Declaration of Helsinki 2002 and was approved by the ethical committees of Beijing Chao-Yang Hospital, Capital Medical University, and was registered on the Chinese clinical trial registry (ChiCTR2200058990). All patients provided written informed consent before study participation. There have been no significant changes since the start of this study trial. This report adheres to the Comprehensive Reporting Standards for Randomized Trials (CONSORT) reporting guidelines.
Study Inclusion and Exclusion Criteria
To identify patients’ eligibility, an independent clinical reviewer confirmed the qualifications. Briefly, all patients who were scheduled for bronchoscopy under general anesthesia were screened for qualification. The inclusion criteria were the following: patients aged ≥18 years or older; planning for elective bronchoscopy under general anesthesia without intubation; American Society of Anesthesiologists (ASA) classification I–III. The exclusion criteria were: SpO2 < 90% after preoperative oxygenation; history of depression or taking antidepressants, neuropsychiatric disorders, hyperthyroidism or drug abuse; inability to communicate; abnormal liver and kidney function; serious adverse reactions such as cardiac arrest and cardiopulmonary resuscitation during bronchoscopy; severe hypertension, high intraocular pressure or high intracranial pressure patients.
Outcomes
Primary outcomes
The primary outcome was the incidence of intraoperative hypoxemia, which was diagnosed as the rate of SpO2 < 90% lasting for 30s during the bronchoscopy.
Secondary Outcomes
- Hemodynamic stability and safety were assessed by non-invasive blood pressure and electrocardiogram, which included cardiovascular adverse events such as hypotension. It was measured as a change >25% from baseline and/or necessitating an intervention.
- Recovery time from anesthesia was defined as the time from the end of anesthesia to Aldrete score of 9.
- Severe intraoperative hypoxemia was defined as SpO2 < 75% at any time during the bronchoscopy.
- The total duration of intraoperative hypoxemia was assessed as the total duration of SpO2 < 90% during the bronchoscopy.
- Adverse events indicated accidental or adverse reactions to sedatives that threaten or cause patient injury.
- Patient satisfaction consisted of very unsatisfied, unsatisfied, satisfied, and very satisfied.
Anesthesia
Bronchoscopy was performed in the outpatient bronchoscopy room. All patients were not allowed to eat or drink for one night before the procedure. Preoxygenation was performed 5 minutes before induction of anesthesia. All patients received standardized surgical and anesthesiological care. Routine monitoring was performed on arrival in the operating room, including electrocardiogram, pulse oximetry, and noninvasive blood pressure measurement. Patients received either sufentanil (0.2 μg/kg) and propofol (1.5–2.5 mg/kg) in the sufentanil group or esketamine (0.2 mg/kg) and propofol (1.5–2.5 mg/kg) in the esketamine group during induction of anesthesia. After loss of consciousness (LOC), propofol (4–12 mg·kg−1· h−1) was used to maintain anesthesia. Oxygen (3–6 L/min via nasal cannula) was administered to all patients during bronchoscopy. Five mL of 2% lidocaine (Tianjin Jinyao Pharmaceutical Co., Tianjin, China) was injected into the trachea by the surgeon through the tracheoscope after the tracheoscope was inserted into the trachea. In patients with SpO2 below 90%, fresh oxygen was initially administered to achieve adequate ventilation and compensate for airway leakage. If this did not work, we offered the patient jaw support or asked the surgeon to interrupt the operation and ventilate the patient for a few minutes until the SpO2 was above 95%. Intraoperative vasopressor medications were used to maintain mean arterial blood pressure within 20% of baseline. Adverse events were monitored throughout the study.
Time points for recording hemodynamics were listed as the following:
t0: before the start of surgery; t1: start of surgery; t2: 3 minutes after the start of surgery; t3: 6 minutes after the start of surgery; t4: 9 minutes after the start of surgery; and t5: at the end of the surgery.
Randomization, binding were in line with the protocol presented in a previously published study protocol.16
Statistical Analysis
The sample size for this trial was determined based on the results of pretrial. In the pretrial, the incidence of intraoperative hypoxemia was approximately 64% in the sufentanil group and 30% in the esketamine group. Considering a 10% dropout rate, 33 individuals per treatment group were randomly assigned to ensure a significance level of 5% and a study power of 80%.
For continuous variables, the Shapiro–Wilk test was used to estimate the distribution. Normally distributed variables were presented as means, and two-tailed unpaired t-tests were used for comparisons between groups. Non-normally distributed variables were depicted as medians, and the Mann–Whitney U-test was used for comparisons between groups. Categorical variables were analyzed using the χ2 test and expressed as numbers (percentages). Two-way repeated ANOVA was applied for the continuous variables at different time points.
For the primary outcome, between-group differences in categorical data were expressed as odds ratio (OR) and 95% CI. Regarding data management, variables with missing data exceeding 20% of the total observations were excluded from the final analysis. No imputation methods were applied to address the remaining missing values to preserve data integrity and avoid potential bias from speculative substitutions. Statistical analyses were conducted using SPSS 26.0 software (IBM SPSS Statistics, Armonk, NY) and GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). P-values less than 0.05 were regarded as statistically significant.
Results
The flowchart of the study is shown in Figure 1. Finally, a total of 66 patients were randomly assigned to the esketamine group or the sufentanil group (33 in the esketamine plus propofol group and 33 in the sufentanil plus propofol group). The two groups were comparable in demographic and baseline clinical characteristics (Table 1). The average age in the esketamine and sufentanil groups was 58.4 ±13.9 and 58.0 ±17.2 years old, respectively. The average dose of esketamine and sufentanil used by patients in the esketamine group and sufentanil group was 15.2±11.2 mg and 12.6±2.1μg, respectively.
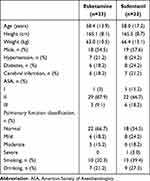 |
Table 1 Baseline Characteristics of the Patients |
 |
Figure 1 Flowchart of the study. |
There were 9 and 22 patients who had intraoperative hypoxemia in the esketamine group and sufentanil group, respectively. The incidence of intraoperative hypoxemia was significantly lower in the esketamine group than in the sufentanil group (27.3% vs 66.7%, P=0.001, OR=5.333, 95% CI=1.859–15.301). The duration of intraoperative hypoxemia in the esketamine group was significantly lower than that in sufentanil group (Z=−3.445, P<0.001). Intraoperative lowest SpO2 in the esketamine was also lower than that in the sufentanil group (Z=−2.682, P=0.007). The usage of propofol in the esketamine group was significantly higher than that in the sufentanil group (t=2.952, P=0.004). Recovery time from anesthesia (Z=−2.709, P=0.007) and time from the end of surgery to a score of 9 of ALDRETE (Z=−2.401, P=0.016) in the esketamine group was significantly shorter than that in the sufentanil group. There was no serious intraoperative or postoperative complication in the two groups. No significant difference was found in patient satisfaction between the two groups. (Table 2)
 |
Table 2 Details of Study Drug Administered and Outcomes in the Two Groups |
Two-way repeated ANOVA revealed that SpO2 levels were significantly higher in the esketamine group compared to the sufentanil group (F=16.073, P<0.001), and a significant time effect was observed (F=8.443, P<0.006). Significant differences were also found in the time effect for systolic blood pressure (F=11.431, P<0.001), diastolic blood pressure (F=7.122, P<0.001), and heart rate (F=4.477, P<0.006). However, no significant differences were observed for group effects or time*group interactions (both P>0.05). (Figure 2)
Discussion
Our study showed that respiratory depression was significantly reduced in the esketamine group compared with the sufentanil group. To our knowledge, this was the first RCT that has compared esketamine with sufentanil for the risk of intraoperative hypoxemia in the setting of bronchoscopy.
In this study, patients receiving esketamine/propofol had a significantly lower rate of intraoperative hypoxemia (27.2% versus 66.7%). Similar to our results, Chen et al also showed that subclinical doses of esketamine lowered the incidence of apnea and intraoperative asphyxia.17 Of note, patients were at high risk for hypoxemia during bronchoscopy. For example, Van et al found a 56% incidence of hypoxemia in patients undergoing bronchoscopy.18 Esketamine complements opioids in pain management19 and counteracts opioid-induced respiratory depression, indicating a possible profound interaction between esketamine and opioid neurotransmitter systems.13 Esketamine has a stimulating effect on respiration, it stabilizes the respiratory center by blocking NMDA receptors and increasing carbon dioxide sensitivity, thereby decreasing the incidence of respiratory depression.20
In our hospital, anesthesia for bronchoscopy is mainly performed with propofol in combination with sufentanil. However, cumulative doses of opioids have a detrimental effect on respiratory saturation. Surgically induced hypoxia, such as endobronchial hemorrhage, bronchial secretions, or tissue debris obstructing the airway, can be relieved by repositioning the bronchoscope, secretion inhalation and oxygenation. In this study, although intraoperative hypoxemia occurred in a significant number of cases, it was easily reversible and rarely life-threatening. No serious adverse events such as myocardial infarction, cardiac arrest or death occurred in this study. The majority of our patients were in ASA2, who may be able to avoid transient hypoxemia.
Interestingly, the use of propofol was significantly higher in the esketamine group compared to the sufentanil group. However, a randomized, controlled multicenter study conducted in the Netherlands demonstrated that low-dose esketamine reduced the total amount of propofol required for sedation during endoscopic retrograde cholangiopancreatography, compared to alfentanil, although recovery time was not affected.21 Similarly, the results of a meta-analysis showed a reduction in propofol consumption with the use of the combination of esketamine and propofol when compared to other drug combinations.22 The discrepancy in findings may be attributed to differences in patient selection. The increased propofol use in the esketamine group and the lack of long-term outcome data warrant further investigation.
As an NMDA receptor antagonist, esketamine produces analgesic, sedative, and anesthetic effects by selectively blocking NMDA receptors involved in pain transmission and mood regulation. Esketamine has dose-dependent effects on the respiratory center. It does not induce significant respiratory depression at routine doses, and voluntary respiratory function is maintained.23 Additionally, esketamine has a bronchodilating effect that relaxes bronchial smooth muscles, further reducing its potential risks to the respiratory system. These properties may help explain the lower incidence of hypoxemia observed in the esketamine group, as compared to sufentanil’s opioid-related respiratory depression.24
Limitation
The results of this study are valuable for improving airway care during bronchoscopy and for training or instructing anesthesiologists. Although this study has several advantages, several limitations deserve expansion here. First, we did not observe postoperative SpO2. Therefore, our results on the reduction of hypoxemia by esketamine are limited to the perioperative period and cannot be generalized to the postoperative period. Second, the participants were only from Beijing Chao-Yang Hospital, Capital Medical University. The single-center design and fixed esketamine dose limit generalizability, and further exploration of dosing regimens and long-term effects would strengthen the findings. Further large-scale multicenter clinical trials are needed. Third, a fixed dose of esketamine (0.25 mg/kg) was used in this study. Lower doses of esketamine might also be useful and have fewer adverse effects. However, further studies are needed to clarify the indications and effects of esketamine supplementation in this patient group. Fourth, we could not demonstrate specific side effects such as postoperative depression, anxiety, cognition, etc. Intraoperative monitoring of end-expiratory carbon dioxide information was not possible in the present study. Furthermore, the higher propofol use in the esketamine group and the lack of long-term outcome data may influence the interpretation of results. Finally, a notable methodological limitation arises from the initiation of patient recruitment prior to formal trial registration. Although ethical approval was obtained in March 2022, a pilot study was first conducted during this period to determine sample size requirements, evaluate drug dosage safety, and assess protocol feasibility. Subsequent formal enrollment of participants occurred between April and August 2022.
Conclusions
In this study, esketamine was found to have a significantly lower risk of intraoperative hypoxemia compared to sufentanil. Our results provide an opportunity for a new recommendation to avoid intraoperative hypoxemia during bronchoscopy using esketamine/propofol. Further elaboration is needed on the implications of higher propofol use in the esketamine group. Additionally, the lack of data on long-term outcomes and the single-center design limit the balanced interpretation of the study’s applicability. Further prospective studies and adequate follow-up are required to investigate the effects of esketamine on long-term clinical outcomes after bronchoscopy.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors (Yuan Sun and Anshi Wu) upon reasonable request.
Acknowledgments
We thank all the patients and nurses who participated in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gasparini S. It is time for patients to undergo bronchoscopy without discomfort. Eur Resp J. 2011;38(3):507–509. doi:10.1183/09031936.00047311
2. Depuydt PO, Benoit DD, Vandewoude KH, Decruyenaere JM, Colardyn FA. Outcome in noninvasively and invasively ventilated hematologic patients with acute respiratory failure. Chest. 2004;126(4):1299–1306. doi:10.1378/chest.126.4.1299
3. Chumpathong S, Tscheikuna J, Boonsombat T, Muangman S, Luansritisakul C. Incidence and risk factors of hypoxemia during interventional rigid bronchoscopy under spontaneous-assisted ventilation. J Bronchology Interv Pulmonol. 2017;24(4):268–274. doi:10.1097/LBR.0000000000000387
4. Murgu S, Laxmanan B, Stoy S, et al. Evaluation of safety and short-term outcomes of therapeutic rigid bronchoscopy using total intravenous anesthesia and spontaneous assisted ventilation. Respiration. 2020;99(3):239–247. doi:10.1159/000504679
5. Correia-Melo FS, Leal GC, Carvalho MS, et al. Comparative study of esketamine and racemic ketamine in treatment-resistant depression: protocol for a non-inferiority clinical trial. Medicine. 2018;97(38):e12414. doi:10.1097/MD.0000000000012414
6. Chen Y, Guo Y, Wu H, et al. Perioperative Adjunctive Esketamine for Postpartum Depression Among Women Undergoing Elective Cesarean Delivery: A Randomized Clinical Trial. JAMA Netw Open. 2024;7(3):e240953. doi:10.1001/jamanetworkopen.2024.0953
7. Qiu D, Wang X-M, Yang -J-J, et al. Effect of intraoperative esketamine infusion on postoperative sleep disturbance after gynecological laparoscopy: a randomized clinical trial. JAMA Netw Open. 2022;5(12):e2244514. doi:10.1001/jamanetworkopen.2022.44514
8. Reif A, Bitter I, Buyze J, et al. Esketamine nasal spray versus quetiapine for treatment-resistant depression. New Engl J Med. 2023;389(14):1298–1309. doi:10.1056/NEJMoa2304145
9. Kiyatkin EA. Respiratory depression and brain hypoxia induced by opioid drugs: morphine, oxycodone, heroin, and fentanyl. Neuropharmacology. 2019;151:219–226. doi:10.1016/j.neuropharm.2019.02.008
10. Baldo BA. Toxicities of opioid analgesics: respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch Toxicol. 2021;95(8):2627–2642. doi:10.1007/s00204-021-03068-2
11. Liu S, Kim D-I, Oh TG, et al. Neural basis of opioid-induced respiratory depression and its rescue. Proc Natl Acad Sci USA. 2021;118(23). doi:10.1073/pnas.2022134118
12. Niemegeers CJ, Schellekens KH, Van Bever WF, Janssen PA. Sufentanil, a very potent and extremely safe intravenous morphine-like compound in mice, rats and dogs. Arzneimittelforschung. 1976;26(8):1551–1556.
13. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117–1127. doi:10.1016/j.bja.2018.02.021
14. Song N, Shan X-S, Yang Y, et al. Low-dose esketamine as an adjuvant to propofol sedation for same-visit bidirectional endoscopy: protocol for a multicenter randomized controlled trial. Int J Gen Med. 2022;15:4733–4740. doi:10.2147/IJGM.S365068
15. Zhao X, Zhou Z, Li Z, Hu Z, Yu Y. Use of esketamine for tracheoscopic drug injection: a randomized controlled trial. Front Med. 2024;11:1479741. doi:10.3389/fmed.2024.1479741
16. Huang X, Ai P, Wei C, Sun Y, Wu A. Comparison of the effects of esketamine/propofol and sufentanil/propofol on the incidence of intraoperative hypoxemia during bronchoscopy: protocol for a randomized, prospective, parallel-group trial. J Clin Med. 2022;11(15). doi:10.3390/jcm11154587
17. Chen H, Ding X, Xiang G, et al. Analysis of the efficacy of subclinical doses of esketamine in combination with propofol in non-intubated general anesthesia procedures - a systematic review and meta-analysis. BMC Anesthesiol. 2023;23(1):245. doi:10.1186/s12871-023-02135-8
18. van Schaik EPC, Blankman P, Van Klei WA, et al. Hypoxemia during procedural sedation in adult patients: a retrospective observational study. Can J Anaesthesia. 2021;68(9):1349–1357. doi:10.1007/s12630-021-01992-6
19. Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2017;6(6):CD003351. doi:10.1002/14651858.CD003351.pub3
20. Zheng L, Wang Y, Ma Q, et al. Efficacy and safety of a subanesthetic dose of esketamine combined with propofol in patients with obesity undergoing painless gastroscopy: a prospective, double-blind, randomized controlled trial. Drug Des Devel Ther. 2023;17:1347–1356. doi:10.2147/DDDT.S408076
21. Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37(5):394–401. doi:10.1097/EJA.0000000000001134
22. Huang X, Lin F, Chen Q, Hu X. Safety and efficacy of the combination of esketamine and propofol in procedural sedation/analgesia: a systematic review and meta-analysis. Minerva Anestesiol. 2023;89(7–8):680–689. doi:10.23736/S0375-9393.23.17100-8
23. Suleiman A, Wongtangman K, Eikermann M, Stucke AG. Neuroanatomical and pharmaco-physiological effects of hypoxia and esketamine on breathing, the sympathetic nerve system, and cortical function. Br J Anaesth. 2025;134(2):277–280. doi:10.1016/j.bja.2024.11.011
24. Gui Y-K, Zeng X-H, Xiao R, et al. The effect of dezocine on the median effective dose of sufentanil-induced respiratory depression in patients undergoing spinal anesthesia combined with low-dose dexmedetomidine. Drug Des Devel Ther. 2023;17:3687–3696. doi:10.2147/DDDT.S429752
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Low-Dose Esketamine as an Adjuvant to Propofol Sedation for Same-Visit Bidirectional Endoscopy: Protocol for a Multicenter Randomized Controlled Trial
Song N, Shan XS, Yang Y, Zheng Z, Shi WC, Yang XY, Li Y, Tan AP, Liu H, Peng K, Ji FH
International Journal of General Medicine 2022, 15:4733-4740
Published Date: 6 May 2022
A Comparative Study of Esketamine-Propofol and Sufentanil-Propofol for Analgesia and Sedation During Breast Minimally Invasive Rotary Resection with Local Anesthesia: A Randomized Double-Blind Clinical Trial
Li N, Qi X, Bao J, Gu Y, Zhou X, Wang T, Jiang N, Wang Y, Ye Q
Drug Design, Development and Therapy 2024, 18:5397-5407
Published Date: 25 November 2024


