Back to Journals » International Journal of Nanomedicine » Volume 19
Comparison of Solid Self-Nanoemulsifying Systems and Surface-Coated Microspheres: Improving Oral Bioavailability of Niclosamide
Authors Baek K, Woo MR, ud Din F , Choi YS, Kang MJ, Kim JO, Choi HG, Jin SG
Received 13 October 2024
Accepted for publication 26 November 2024
Published 24 December 2024 Volume 2024:19 Pages 13857—13874
DOI https://doi.org/10.2147/IJN.S494083
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Kyungho Baek,1 Mi Ran Woo,2 Fakhar ud Din,3 Yong Seok Choi,4 Myung Joo Kang,4 Jong Oh Kim,5 Han-Gon Choi,2 Sung Giu Jin1
1Department of Pharmaceutical Engineering, Dankook University, Cheonan, South Korea; 2College of Pharmacy, Hanyang University, Ansan, South Korea; 3Department of Pharmacy, Quaid-I-Azam University, Islamabad, Pakistan; 4College of Pharmacy, Dankook University, Cheonan, South Korea; 5College of Pharmacy, Yeungnam University, Gyeongsan, South Korea
Correspondence: Sung Giu Jin; Han-Gon Choi, College of Pharmacy, Hanyang University, 55 hanyangdaehak-ro, Sangnok-gu, Ansan, 15588, South Korea, Tel +82 41 550-3558 ; +82 31 400-5802, Fax +82 41 559 7945 ; +82 31 400 5958, Email [email protected]; [email protected]
Purpose: This study aimed to develop a solid self-nanoemulsifying drug delivery system (SNEDDS) and surface-coated microspheres to improve the oral bioavailability of niclosamide.
Methods: A solubility screening study showed that liquid SNEDDS, prepared using an optimized volume ratio of corn oil, Cremophor RH40, and Tween 80 (20:24:56), formed nanoemulsions with the smallest droplet size. Niclosamide was incorporated into this liquid SNEDDS and spray-dried with calcium silicate to produce solid SNEDDS. Surface-coated microspheres were prepared using sodium alginate and poloxamer 407 and optimized through solubility and dissolution tests. Scanning electron microscopy, differential scanning calorimetry, and X-ray diffraction were used to evaluate the physicochemical properties of the prepared solid SNEDDS, surface-coated microspheres, and the drug alone. The solubility, dissolution, and oral bioavailability were also assessed.
Results: Physicochemical evaluation demonstrated that niclosamide was converted to an amorphous state in the Solid SNEDDS formulation, with enhanced solubility and oral bioavailability. In comparison to niclosamide alone, solid SNEDDS exhibited an increase in drug solubility (approximately 2500-fold vs 158-fold) and oral bioavailability (approximately 10-fold vs 1.65-fold), significantly outperforming surface-coated microspheres.
Conclusion: This solid SNEDDS formulation may be an excellent candidate for niclosamide with improved oral bioavailability for repurposing.
Keywords: niclosamide, microsphere, solid SNEDDS, dissolution, crystallinity, oral bioavailability
Graphical Abstract:

Introduction
Niclosamide is composed of yellow pale crystals, practically insoluble in water, and moderately soluble in ethanol, chloroform, and ether.1 Niclosamide, an FDA-approved anthelmintic, is included in the World Health Organization’s (WHO) model list of essential medicines.2 Niclosamide has been shown to have beneficial effects in the treatment of various diseases beyond its original use. It has shown promise in the treatment of Parkinson’s disease, diabetes, and cancer, attracting attention for its potential for repurposing.3–5 Over the past decade, there has been a significant surge in related research, and the broad therapeutic potential of niclosamide has sparked interest in its repurposing, which has led to numerous clinical trials. However, the repurposing niclosamide is limited by its low water solubility, limited formulation design, and low oral bioavailability, which present significant barriers.6,7
To overcome this low water solubility, several studies have reported the use of nanodrug delivery systems such as liposomes, micro/nanoemulsions, micelles, solid lipid nanoparticles, polymeric nanoparticles, inclusion complex, and nanocrystals.8–11 Drug delivery systems that increase water solubility may differ depending on the original characteristics of the drug. Nanoemulsions, including self-nanoemulsifying drug delivery systems (SNEDDS), are prominent systems for improving the bioavailability of orally administered drug candidates by increasing solubility and permeability. These approaches are particularly effective in enhancing the delivery and efficacy of drugs with poor water solubility.12 SNEDDS are homogeneous mixtures of oils, surfactant, co-surfactant, and drugs. They spontaneously form a fine oil-in-water nanoemulsion with minimal agitation upon introduction into aqueous environments.13 Upon oral administration, SNEDDS interacts with gastrointestinal (GIT) fluids during peristalsis, spontaneously forming an oil-in-water (o/w) emulsion. This process results in the formation of nanosized droplets with an average size ranging from 20 to 200 nm, which enhance drug solubility and absorption.14 The SNEDDS formulation aims to enhance oral systemic availability by improving intestinal membrane permeability, mitigating or eliminating food effects, reducing droplet size, and increasing drug dissolution.15
Solid SNEDDS incorporates the advantages of its liquid counterparts, such as improved solubility and bioavailability. Solid dosage forms improve stability, patient compliance, and accurate dosing.16 Solid SNEDDS can be formulated through spray drying, freeze drying, or direct nanoemulsion preconcentrate adsorption onto microporous carriers.17
In solid dispersions, drugs dispersed at the molecular level within a polymer exhibit significantly higher solubility or dissolution rates than their crystalline counterparts. This method effectively improves drug bioavailability.18 Transformation of the liquid phase into dry powdered microspheres was efficiently accomplished by spray drying. This technique is well known for its effectiveness in producing microspheres.19 Within the framework of traditional microencapsulation, solvent-evaporation techniques are utilized to produce microspheres.20,21 When fabricating these microspheres via solvent evaporation, organic solvents are employed to dissolve water-insoluble pharmaceuticals and compounds completely.22 The traditional approach to microencapsulation involves the generation of polymeric microparticles that encapsulate dissolved medications and compounds via spray drying. This procedure not only reduces the size of the particles but also changes the state of the drug from crystalline to amorphous, inducing supersaturation and, in turn, improving its solubility.23 However, the approaches involving solvent evaporation have certain drawbacks. The application of organic solvents poses potential toxicity risks because of solvent remnants. Furthermore, a high drug-to-carrier ratio requires larger therapeutic doses to be administered to patients.24 Emerging as a potent solution to overcome the limitations of traditional drug delivery methodologies, this novel strategy of surface-modified microencapsulation addresses the challenges associated with employing water-based solvents and minimal proportions of the drug to the carrier material.25,26
In this study, a systematic evaluation of how hydrophilic polymers, oils, and surfactants influence the water solubility of niclosamide, led to the selection of optimal components. Subsequently, spray drying was used to prepare solid SNEDDS and microspheres with a surface coating. The finalized solid SNEDDS formulation possessed the most diminutive nanoemulsion droplets, whereas the selected surface-coated microspheres were noted for their enhanced solubility and dissolution profiles. Advanced analytical methods, including differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and scanning electron microscopy (SEM), were employed to determine the physicochemical properties of niclosamide within solid SNEDDS and surface-coated microspheres, thereby uncovering the various mechanisms of supersaturation. Additionally, this investigation included a pharmacokinetic comparison of niclosamide in its powdered form, encapsulated within solid SNEDDS, and surface-coated microspheres in a rat model. This comparison aimed to shed light on the differences in oral bioavailability observed by exploring the unique physicochemical attributes and supersaturation mechanisms underlying each drug delivery strategy.
Materials and Methods
Raw Materials
Niclosamide was purchased from Elppe Chemical Co. PVT. LTD (Maharashtra, India), and Tween 80 (polysorbate 80), polyethylene glycol (PEG) (400, 600) and soybean oil were purchased from Daejeong Chemicals, Gyeonggi-do, Korea. Labrafil® M2125CS, Labrafil® M1944CS, Peceol™ (Type 40 Glyceryl Monooleate) and Labrasol® were supplied by Gattefosse, Saint-Priest Cedex, France. Cremophor RH40, Solutol HS15, and poloxamer 407 were purchased from BASF, Ludwigshafen, Germany. D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) was obtained from Sigma-Aldrich, St. Louis, MO, USA. Hanmi Pharmaceutical (Suwon, Korea) supplied polyvinyl alcohol (PVA), sodium alginate (Na-alginate), hydroxypropyl beta-cyclodextrin (HP-β-CD), gelatin, carbopol 934 and calcium silicate. Ashland Inc. (Hopewell, Virginia, USA) supplied Klucel® hydroxypropylcellulos (HPC-LF). Samchun Chemical Co., Ltd. (Pyeongtaek, Korea) supplied corn, coconut, castor, cotton seed, and peanut oil. Reagent-grade chemicals and solvents used in the study were used as received without further purification.
Animal Experiments
Eighteen healthy male Sprague-Dawley rats, each with a body weight ranging from 280–320 g and at the age of 8 weeks, were acquired from Koatech, Inc. (Pyeongtaek, Korea). The rats were kept under 20–26 °C temperature, 30–70% relative humidity, and ammonia levels under 20 ppm. Rats received food restriction (18–24 h) before pharmacokinetic testing and drug administration but were allowed free access to water. Pharmacokinetic experiments and animal management were performed in compliance with NIH guidelines. The Institutional Animal Care and Use Committee (IACUC) of Hanyang University (Seoul, Korea) approved the study protocol (IACUC No. 2019–0120A).
Solubility Study
Aqueous solutions containing a 1% (w/v) hydrophilic polymer, pure oil, and 10% (w/v) surfactant were prepared for solubility testing. Subsequently, approximately 10 mg of niclosamide was introduced into each solution (1 mL). These mixtures were subjected to a temperature-regulated shaking water bath (Daihan Scientific, Wonju, South Korea), maintained at 25°C with a shaking speed of 100 rpm, for 7 days to ensure complete equilibration of the concentrations.12,13 After incubation, the supernatant was separated by centrifuging the samples for 10 min at 15,000 × g using an Eppendorf 5430 R centrifuge (Eppendorf, Hamburg, Germany). For quantitative analysis of niclosamide, high-performance liquid chromatography (HPLC) was performed using an Agilent 1260 Infinity system equipped with a VWD VL detector (G1314B 1260). The study was conducted using an Inertsil ODS-2 column (4.6 mm internal diameter × 250 mm length, 5 μm particle size), held at a constant temperature of 25°C. The mobile phase flow rate was set at 1 mL/min. Peak areas were analyzed and calculated using ChemStation software. The absorbance of the separated solution was monitored at 254 nm. The mobile phase was a solution comprising 0.05M ammonium phosphate buffer (adjusted to pH 3.6 by phosphoric acid) and methanol in a volume ratio of 10:90.27
Construction of a Pseudo-Ternary Phase Diagram
Ternary mixtures of various compositions have been prepared using combinations of selected surfactants, co-surfactants, and oils.28 Corn oil (oil), Cremophor RH40 (surfactant), and Tween 80 (co-surfactant) were chosen as suitable ingredients to formulate liquid SNEDDS based on solubility tests. Drug-free liquid SNEDDS formulation (0.2 mL) was placed in 300 mL of distilled water in a glass beaker while stirring at 300 rpm (37°C) using a magnetic bar.13,26 The self-emulsifying ability of the liquid SNEDDS formulation to form nanoemulsions was determined by measuring transmittance (%) using a UV spectrophotometer (Ultrospec 7000, Biochrom, Cambridge, UK). A wavelength of 640 nm was passed through the sample, and distilled water was used as a blank. Areas with a wavelength transmittance of over 90% were classified as “good” and displayed on the ternary phase diagram.29 Pseudo-ternary phase diagrams were constructed to identify the self-nanoemulsification region. Once consistent visual standards were achieved, all experiments were performed in triplicate.
Preparation of Niclosamide-Loaded Solid SNEDDS
The ideal mixture of oil, surfactant, and co-surfactant was identified using a pseudo-ternary diagram as a guide, and the liquid SNEDDS formulation that exhibited the smallest emulsion droplet size was selected. This solution was composed of oil, surfactant, and auxiliary surfactant mixed in the order of 20%, 24%, and 56% by volume, respectively. The mixing process utilized a vortex mixer to ensure uniform distribution of the ingredients. A porous carrier, calcium silicate, was chosen to convert liquid SNEDDS loaded with niclosamide into a solid state. Calcium silicate (1 g) was suspended in liquid SNEDDS (1 mL) loaded with niclosamide in distilled water, using a Büchi mini spray dryer (B-290; Plawil, Switzerland).26,28 Stirring was continued throughout the spray drying process at 300 rpm using a magnetic bar to prevent precipitation. Solidification was carried out with an inlet temperature of 140°C, an outlet temperature of 70°C, 100% aspiration, 600 L/h airflow, and a 15.0 mL/min suspension feed rate.
Preparation of Niclosamide-Loaded Surface-Coated Microspheres
Based on the solubility screening results, sodium alginate (Na-alginate) and poloxamer 407 were identified as suitable polymers and surfactants. Distilled water was used to dissolve various ratios of Na-alginate to Poloxamer 407. Niclosamide, which was processed into a fine powder by passing through a 100-mesh sieve, was successfully dispersed in each aqueous solution listed in Table 1. Niclosamide-loaded surface-coated microspheres were prepared using a Büchi mini spray dryer.18,21 To ensure a stable suspension during the spray-drying process, continuous stirring with a magnetic bar was maintained to prevent clumping or settling of the solution. During the solidification procedure, the temperatures at the outlet and inlet were configured to 56°C and 90°C, respectively. The aspiration level was adjusted to capacity (100%), coupled with an airflow rate of 600 L/h, and the suspension feed rate was carefully controlled at 20.0 mL/min.
 |
Table 1 Formulation of Niclosamide-Loaded Surface-Coated Microspheres |
Characterization of Physical and Chemical Properties
Measurement of Nano-Emulsion Droplet Size
The droplet diameters within the SNEDDS formulations were evaluated using the Nano ZS particle size analysis system (Malvern Instruments, Malvern, UK).12,13 The evaluation was performed at a wavelength of 635 nm utilizing a 90° scattering angle and maintained at a constant temperature of 25°C. To assess the nanoemulsion droplets, Automeasure software (Malvern Instruments, Malvern, UK) was used to provide the z-average particle size in nm. This measurement reflects the average hydrodynamic diameter of the droplet, which is determined using a method based on intensity-weighted harmonics.
Evaluation of Physicochemical Properties
The thermal properties of the niclosamide-loaded surface-coated microspheres and niclosamide-loaded solid SNEDDS were analyzed using differential scanning calorimetry (DSC) (DSC Q200, TA Instruments, New Castle, DE, USA).19 DSC was used to investigate potential interactions between the drugs and substances used in the formulations. Dry nitrogen was used as the experimental exhaust gas, and all samples designated as test subjects were measured at a temperature increase rate of 10°C/min in the temperature range of 60 to 200°C. Solid-state properties of the formulations were investigated using PXRD (Ultima IV, Rigaku Corporation, Tokyo, Japan) at the core facility of the Biomedical Engineering Research Center (Dankook University).23 Measurements were performed over 2θ angles from 3° to 50° with parameters set to an angular advance of 0.02° per second at ambient temperature.
Analysis of Morphological Features
Samples, including niclosamide alone, sodium alginate, poloxamer 407, niclosamide-loaded surface-coated microspheres, and niclosamide-loaded solid SNEDDS, were mounted on brass plates with double-sided adhesive tape.23 After mounting, the samples were coated with platinum at a deposition rate of 6 nm/min and a current setting of 15 mA for 4 min, all carried out in a vacuum set to 0.8 Pa.25 This was performed using an EmiTeck sputter coater (model K575 K; Quorum Technologies, Lewes, UK).26 After platinum coating was applied, the detailed morphological structures of niclosamide alone, sodium alginate, poloxamer 407, calcium silicate, surface-coated microspheres, and solid SNEDDS were examined using a scanning electron microscope (S-4800; Hitachi, Tokyo, Japan).
Dissolution Testing
Dissolution tests were performed using the paddle technique using a USP Dissolution Apparatus II. The dissolution medium consisted of 900 mL of distilled water, and the temperature was maintained at 37 ± 0.5°C. The paddle rotational speed was kept constant at 50 rpm.12,13 The niclosamide-loaded surface-coated microspheres niclosamide-loaded solid SNEDDS, and niclosamide alone (5 mg) were tested using a dissolution tester (Vision Classic 6TM, Hanson Research Co., Los Angeles, CA, USA). Approximately 3 mL of eluate was collected from each sample at specific intervals. Samples were filtered through a glass fiber membrane filter (0.45 μm) and analyzed by HPLC to determine niclosamide concentration.27
Pharmacokinetic Studies
Three groups of six male Sprague Dawley rats were meticulously assigned to the experiment to ensure a precise and reliable study. A heparin-coated polyethylene tube (50 IU/mL) was carefully inserted into the right femoral artery of the rat to prevent tube blockage and was securely held in place.29 For oral delivery, the niclosamide-loaded surface-coated microspheres, niclosamide-loaded solid SNEDDS, and niclosamide alone were each suspended in 1% sodium carboxymethyl cellulose (Na-CMC) at a dose of 30 mg/kg and orally administered to rats using an oral zonde needle. The blood collection schedule was meticulously planned, including time points of 0, 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 12, 24, and 48 h for the 12 collection points. Blood samples (0.4 mL) were collected using a 1 mL syringe precoated with heparin to ensure accurate and consistent sample collection. After collection, plasma was immediately centrifuged at 15,000 × g for 15 min. Plasma samples were separated and stored at −20°C to maintain the integrity of samples until further analysis.
Quantitative Determination of Niclosamide Concentrations in Plasma
Niclosamide concentrations in rat plasma were determined using the protein precipitation method. This involved taking a 100 μL plasma sample, adding 200 μL internal standard solution (10 μg/mL indomethacin in acetonitrile), and vortex mixing for 1 min. Next, the mixture was centrifuged at 14,000 g for 10 min at 25°C, and 160 μL of the supernatant was collected and analyzed. For HPLC analysis, 50 μL of this supernatant was injected into a SHISEIDO Capcell Pak C18 column (4.6 mm I.D. × 150 mm, 5 μm) maintained at 25°C. The mobile phase consisted of 5 mm phosphate buffer (KH2PO4, pH adjusted to 3.5 with phosphoric acid) that was degassed before use in an ultrasonic bath. Analysis was performed at a flow rate of 0.5 mL/min, and detection was performed at a wavelength of 332 nm.27,30,31 The method demonstrated robust reproducibility, with a correlation coefficient (R2) of 0.99, and both intra- and inter-day variabilities were within acceptable ranges.
Analysis of Pharmacokinetic Parameters
For the pharmacokinetic assessment, parameters such as AUC (area under the curve), Tmax (time to reach maximum plasma concentration), Cmax (maximum observed plasma concentration), t1/2 (half-life), and Kel (elimination rate constant) were calculated using WinNonlin software (Pharsight Corp., Mountain View, CA, USA).19,29 The results were expressed as mean ± standard deviation, and the test results were considered statistically significant at p<0.05. These findings are of considerable importance in pharmacokinetics and drug development and provide valuable insights for future research and clinical applications.
Results
Selection of Appropriate Components
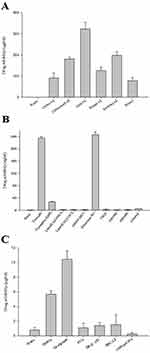
Extensive investigations of SNEDDS have been conducted to examine the formation of fine nanoemulsions when interacting with aqueous environments.29 The strategic use of oils and surfactants in SNEDDS formulations enables the transformation of drugs with low solubility into oil-in-water (o/w) emulsions upon dilution with water. The simultaneous addition of co-surfactants further increased the stability of the emulsions.32 The niclosamide-loaded SNEDDS formulation was optimized by evaluating the solubility of niclosamide in various excipients, including polymers, oils, and surfactants. The results of the drug solubility screening are shown in Figure 1. Among the oil samples, corn oil exhibited the highest solubility at 323.9 ± 31.6 µg/mL (Figure 1A). Owing to its high stability and low toxicity, corn oil was deemed the most suitable oil component and was selected for the SNEDDS formulation. In Figure 1B, Tween 80 (234.8 ± 5.6 µg/mL) and Cremophor RH40 (27.2 ± 2.6 µg/mL) were identified as the surfactant and co-surfactant, respectively. Tween 80 and Cremophor RH40 are non-ionic surfactants known for their lower toxicity than ionic surfactants. Previous studies reported their widespread use in drug solubilization studies to form stable and homogeneous emulsions in SNEDDS. Hydrophilic polymers and surfactants were used to prepare surface-coated microspheres using a spray dryer. Poloxamer 407, with a solubility of 246.4 ± 8.8 μg/mL (Figure 1B), was selected as the surfactant due to its excellent solubility. The polymer screening results in Figure 1C showed that Na-alginate (10.4 ± 1.1 μg/mL) significantly enhanced the drug solubility. Consequently, Na-alginate and poloxamer 407 were selected as the polymer and surfactant, respectively, to formulate surface-coated microspheres.
Preparation of Solid SNEDDS
To optimize the composition of the liquid SNEDDS formulation, different formulations, excluding niclosamide, were prepared and assessed for their self-emulsifying ability. This evaluation was facilitated by constructing a pseudo-ternary phase diagram illustrated in Figure 2A. The pseudo-ternary phase diagram assessed the relationships among the components in liquid SNEDDS formulations of different compositions. Optimal self-emulsification occurred when the oil component contained less than 30% (v/v) of the formulation. Above this percentage level, coalescence of oil droplets was observed when liquid SNEDDS was added to distilled water, resulting in a cloudy solution and phase separation, indicating that the self-emulsification ability was limited outside the preferred region of the ternary diagram. The critical role of emulsion droplet size in SNEDDS formulations has been highlighted, particularly because it significantly improves the solubility of poorly water-soluble drugs. Because of its increased surface area, a finer emulsion droplet size can enhance the solubility of poorly water-soluble drugs.33 The investigation of how the oil, surfactant, and co-surfactant ratios affect the emulsion droplet size within the self-emulsifying region revealed a decrease in the droplet size with increasing corn oil concentration from 5 to 20% v/v, as shown in Figure 2B. Nevertheless, the stability of the emulsion droplet size was compromised when the corn oil concentration exceeded 25% v/v; therefore, the corn oil was maintained at a concentration of 20% v/v. The effect of the surfactant/co-surfactant ratio on the emulsion droplet size was evaluated next, as shown in Figure 2C. The largest emulsion droplet size was observed when the surfactant (Cremophor RH40) to co-surfactant (Tween 80) ratio was 1:9, whereas no significant difference was observed at a 2:8 ratio. The smallest emulsion size and lowest variation in the polydispersity index (PDI) values were observed at a 3:7 ratio. Considering the smallest emulsion droplet size and minor variations in PDI across the tested formulations, a liquid SNEDDS composition using corn oil, Cremophor RH40, and Tween 80 with a volume ratio of 20/24/56 was selected. Comparative studies with niclosamide-loaded liquid SNEDDS showed comparable nanoemulsion droplet sizes, indicating that drug encapsulation did not negatively impact the self-emulsification process. Subsequently, the niclosamide-containing liquid SNEDDS was mixed with distilled water and this solution was spread within a porous calcium silicate matrix. The solidification process was performed using a spray dryer by applying the optimized parameters.
Development of Surface-Coated Microspheres
Traditional methods of microencapsulation, such as solvent evaporation, typically require high polymer to drug ratios, leading to an increased dosage for patients.34 In contrast, the surface-modified microsphere creation technique adopted in this study reduced the need for excipients by forming a solubilized layer on the surface of the drug, presenting a more effective strategy than solvent evaporation methods.35 Moreover, unlike conventional microsphere fabrication processes that use organic solvents to convert hydrophobic drugs into amorphous forms, the surface-coated microspheres in this study were produced using distilled water, thus preserving their hydrophobic properties in a crystalline state. This crystalline form is known for its enhanced stability in the amorphous form. Additionally, surface-modified microspheres can improve biocompatibility because they generally do not require high levels of surfactants. Several formulations were developed to determine the most effective ratio of Na-alginate to Poloxamer 407 for surface-coated microspheres, as summarized in Table 1. Interestingly, as the ratio of Na-alginate to niclosamide increased, the relationship between the viscosity and solubility of the formulations became more complex. Contrary to initial expectations, the solubility decreased beyond a certain point, as illustrated in Figure 3A. Formulation IV (1:0.75:0.25; drug: Na-alginate: Poloxamer ratio) exhibited significantly higher solubility than the other formulations and niclosamide alone. Furthermore, as illustrated in Figure 3B, Formulation IV achieved the highest dissolution rate, similar to the solubility evaluation results, and outperformed Formulations I, II, III, and niclosamide alone. In conclusion, Formulation IV with a ratio of niclosamide/Na-alginate/poloxamer 407 of 1/0.75/0.25 significantly improved the solubility of niclosamide, achieving an improvement of approximately 158-fold compared to niclosamide alone (0.8±0.3 vs 127.0±18.4 μg/mL). The dissolution evaluation demonstrated that there was hardly any release from niclosamide alone; in contrast, approximately 10% was released from Formulation IV. These results confirmed that optimizing the ratio of active ingredients to excipients is essential for achieving significantly improved solubility and dissolution profiles.
Comparison of Physical and Chemical Properties
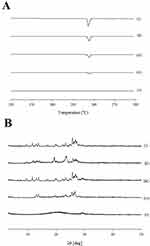
Figure 4A shows the differential scanning calorimetry (DSC) profiles for niclosamide alone, niclosamide-loaded surface-coated microspheres, and niclosamide-loaded solid SNEDDS. The DSC analysis revealed a pronounced endothermic peak at approximately 230°C for niclosamide (Figure 4A ⅰ), indicating its melting point and confirming its crystalline nature. Similarly, a subtle endothermic peak at the same temperature region, around 230°C, was observed in the physical mixtures of the two formulations (Figure 4A ii) and (Figure 4A iii), suggesting the melting point of niclosamide remains unaffected in these mixtures. These observations confirm that there are no compatibility issues between niclosamide and the selected excipients, ensuring the integrity of the drug’s crystalline structure in the formulations.36, DSC evaluation of the surface-coated microspheres (Figure 4A iv) showed an endothermic peak consistent with the melting point of niclosamide. These results suggest that the drug within the microspheres was crystalline.37 In contrast to the formulations discussed above, solid SNEDDS (Figure 4A v) lacked the characteristic peaks of native niclosamide, indicating that the drug could transform from a crystalline to an amorphous state.
The crystallinity was assessed using powder X-ray diffraction (PXRD) analysis, as shown in Figure 4B. Niclosamide alone (Figure 4B i) showed a distinct crystalline pattern characterized by specific peaks. These peaks were also present in the physical mixtures corresponding to surface-coated microspheres (Figure 4B ii) and solid SNEDDS (Figure 4B ii), suggesting that niclosamide did not interact with the excipients, which is consistent with the DSC results. Notably, the unique crystalline pattern of niclosamide was preserved in the surface-coated microspheres (Figure 4B iv), demonstrating that the crystalline pattern of the drug was not altered.38 In contrast, no distinct crystalline character of niclosamide was observed in solid SNEDDS (Figure 4B v).39, Similar to the DSC results, the PXRD results indicated that the drug transformed from a crystalline to an amorphous state. This transformation suggests that the drug in solid SNEDDS may have enhanced solubility and bioavailability in biological systems.
The structural morphologies of niclosamide alone, poloxamer 407, calcium silicate, surface-coated microspheres, and solid SNEDDS were evaluated by using scanning electron microscopy (SEM). The images in Figure 5 provide visual evidence of the transformation and interactions between these components. Niclosamide alone (Figure 5A) exhibited irregular rectangular crystals, whereas poloxamer 407 (Figure 5B) showed rectangular shapes with round corners. Calcium silicate exhibited a porous structure (Figure 5C). Solid SNEDDS was observed to cover the calcium silicate surface with liquid SNEDDS containing niclosamide, indicating that niclosamide was encapsulated in liquid SNEDDS and then adsorbed onto the rough surface of calcium silicate (Figure 5E). Additionally, the surface-coated microspheres showed that the polymer was attached to the surface of the undissolved drug (Figure 5D). This observation confirmed that the crystallinity of the drug did not change in surface-coated microspheres, and was supported by the DSC and PXRD results which demonstrated the crystalline nature of the drug.40
 |
Figure 5 Morphological examination using scanning electron microscopy (SEM): (A) niclosamide alone; (B) Poloxamer 407; (C) calcium silicate; (D) surface-coated microspheres; (E) solid SNEDDS. |
Comparison of Solubility and Dissolution
As shown in Figure 6A, the solubility of niclosamide in surface-coated microspheres and solid SNEDDS was quantitatively evaluated. The results showed that both surface-coated microspheres and solid SNEDDS significantly improved the solubility of niclosamide compared with the niclosamide alone (127.0 ± 18.4 vs 2,000.0 ± 220.4 μg/mL vs 0.8 ± 0.3, respectively). These differences were statistically significant (p<0.05). Notably, the solubility of solid SNEDDS was 15.7 times higher than that of the surface-coated microspheres, indicating a significant improvement (p<0.05).
During dissolution testing, solid SNEDDS and surface-coated microspheres exhibited excellent drug release within 60 min, significantly increasing the release rate compared with niclosamide alone (Figure 6B). The order of dissolution efficiency was: solid SNEDDS > surface-coated microspheres > niclosamide alone. Niclosamide alone was barely dissolved, with concentrations in the dissolution media below the method quantification limit, making it challenging to measure its dissolution. In contrast, approximately 10% of the drug encapsulated in the surface-coated microspheres was released after 60 min, whereas solid SNEDDS achieved 100% release. Niclosamide was encapsulated in a solid SNEDDS formulation using the liquid SNEDDS method, bypassing the dissolution step that is typically necessary for solid crystalline substances.41 When introduced into water, solid SNEDDS form a nanoemulsion by establishing an interface between the water and oil droplets. This process requires a low free energy to achieve supersaturation, promoting the swift release of the drug.42 The solid SNEDDS demonstrated a dissolution rate at 60 min that was 12.5 times higher than that of the surface-coated microspheres. This phenomenon supports the hypothesis that the creation of an oil-in-water emulsion enables niclosamide, a drug with limited water solubility, to achieve supersaturation in an aqueous environment more effectively than in the hydrophilic surroundings provided by surface-coated microspheres. This mechanism likely accounts for the observed differences in solubility and dissolution rates, with solid SNEDDS displaying a 15.7-fold increase in solubility and a 12.5-fold increase in dissolution rate compared to the surface-coated microspheres.
Comparison of Pharmacokinetics
To determine the pharmacokinetic profile of niclosamide, 40 mg/kg niclosamide was orally administered to rats. The results, detailed in Table 2 and Figure 7, outline the pharmacokinetic parameters and plasma concentrations of niclosamide following oral administration of surface-coated microspheres, solid SNEDDS, and niclosamide alone. Notably, compared to the administration of niclosamide alone in its powder form, the solid SNEDDS and surface-coated microsphere formulations exhibited a significant enhancement in pharmacokinetic parameters (p < 0.05). These formulations, developed through solubilization techniques, not only improved the solubility and dissolution rate of niclosamide, but also effectively increased its plasma concentration in rats, confirming the beneficial impact of these drug delivery systems on the bioavailability of niclosamide. Both solid SNEDDS and surface-coated microspheres demonstrated significantly higher Cmax and AUC values (p < 0.05), indicating a substantial improvement in oral bioavailability compared to niclosamide alone. Specifically, for solid SNEDDS, the AUC and Cmax increased by 9.9 and 18.7 fold respectively, compared to niclosamide alone. In the case of surface-coated microspheres, the AUC and Cmax increased 1.6 and 4.4 fold, respectively. Solid SNEDDS also exhibited higher AUC (approximately 7-fold) and Cmax (approximately 4-fold) values than the surface-coated microspheres (p < 0.05). Comparative analysis of blood concentrations following oral administration demonstrated a clear hierarchy: solid SNEDDS achieved the highest concentration, followed by surface-coated microspheres; niclosamide alone exhibited the lowest concentration. However, when examining other pharmacokinetic parameters, including the time to reach maximum concentration (Tmax), half-life (t1/2), and elimination constant (Kel), no significant differences were observed between the formulations and niclosamide alone. This suggests that although the solubilization techniques employed in the solid SNEDDS and surface-coated microspheres significantly enhanced the bioavailability of niclosamide by increasing its plasma concentration, they did not markedly alter the overall pharmacokinetic profile of the drug in terms of absorption speed, elimination rate, or duration of action within the body. The improved oral bioavailability of niclosamide in solid SNEDDS via the dissolution technology indicated that nanoemulsions were formed in the gastrointestinal tract, which enhanced its absorption.43 As a result, it shows superior drug absorption compared to niclosamide alone and surface-coated microspheres. Improved drug absorption in solid SNEDDS formulation could be a crucial factor contributing to the successful repositioning niclosamide. In conventional anthelmintic applications, niclosamide absorption was not a significant consideration. However, improving bioavailability and solubility is essential for clinical drug repositioning.
 |
Table 2 Parameters Related to the Drug Kinetics |
Conclusion
This study demonstrates significantly improved oral bioavailability, solubility, and dissolution rates in the formulations of niclosamide-loaded solid SNEDDS and surface-coated microspheres compared to the administration of the drug alone. These results highlight the potential of advanced drug delivery technologies to overcome the limitations of poorly water-soluble drugs and provide a promising approach for repositioning niclosamide, a biopharmaceutical classification system (BCS) class II drug with poor aqueous solubility, to improve therapeutic efficacy and patient outcomes. The observed trends in dissolution and solubility were consistent with the improved oral bioavailability. Furthermore, pharmacokinetic studies demonstrated that the final blood drug concentrations followed the order: solid SNEDDS > surface-coated microspheres > niclosamide alone. These results were confirmed by physicochemical characterization, which suggested that the changes in oral bioavailability, solubility, and dissolution rates of niclosamide were due to a change in the crystalline form of the drug. Surface-coated microspheres and solid SNEDDS have different dissolution mechanisms, explaining the differences in solubility and dissolution rate improvements. In the case of niclosamide in solid SNEDDS, nanoemulsions are formed, leading to efficient supersaturation and surface modification. It was confirmed that the solubility, dissolution, and oral bioavailability in solid SNEDDS were enhanced compared to the hydrophilic microenvironments of the manufactured microspheres.
Acknowledgments
This work was supported by the National Research Foundation of South Korea (NRF) grants funded by the South Korean government (MEST) (No. 2022R1A2C2004197, RS-2023-00208448, and RS-2024-00407053) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1A6A1A03043283).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barbosa EJ, Löbenberg R, De Araujo GLB, Bou-Chacra NA. Niclosamide repositioning for treating cancer: challenges and nano-based drug delivery opportunities. Eur J Pharma Biopharm. 2019;141:58–69. doi:10.1016/j.ejpb.2019.05.004
2. Mattheolabakis G, Rigas B, Constantinides PP. Nanodelivery strategies in cancer chemotherapy: biological rationale and pharmaceutical perspectives. Nanomed. 2012;7(10):1577–1590. doi:10.2217/nnm.12.128
3. Xu J, Shi P, Li H, Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis. 2020;6:909–915. doi:10.1021/acsinfecdis.0c00052
4. Li Y, Li P, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett. 2014;349(1):8–14. doi:10.1016/j.canlet.2014.04.003
5. Chen W, Mook RA, Premont RT, Wang J. Niclosamide: beyond an antihelminthic drug. Cell Signal. 2018;41:89–96. doi:10.1016/j.cellsig.2017.04.001
6. Tam J, Hamza T, Ma B, et al. Host-targeted niclosamide inhibits C. difficile virulence and prevents disease in mice without disrupting the gut microbiota. Nat Commun. 2018;9(1):5233. doi:10.1038/s41467-018-07705-w
7. Alhalaweh A, Alzghoul A, Kaialy W, Mahlin D, Bergström CAS. Computational predictions of glass-forming ability and crystallization tendency of drug molecules. Mol Pharm. 2014;11(9):3123–3132. doi:10.1021/mp500303a
8. Schweizer MT, Haugk K, Mckiernan JS, et al. A Phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS One. 2018;13(6):e0198389. doi:10.1371/journal.pone.0198389
9. Pal A, Roy S, Kumar A, et al. Physicochemical characterization, molecular docking, and in vitro dissolution of glimepiride–captisol inclusion complexes. ACS omega. 2020;5(32):19968–19977. doi:10.1021/acsomega.0c01228
10. Ghosal K, Augustine R, Zaszczynska A, et al. Novel drug delivery systems based on triaxial electrospinning based nanofibers. React Funct Polym. 2021;163:104895. doi:10.1016/j.reactfunctpolym.2021.104895
11. Ghosal K, Das A, Das SK, et al. Synthesis and characterization of interpenetrating polymeric networks based bio-composite alginate film: a well-designed drug delivery platform. Int J Biol Macromol. 2019;130:645–654. doi:10.1016/j.ijbiomac.2019.02.117
12. Choi MJ, Kim JS, Yu H, et al. Comparison of the physicochemical properties, aqueous solubility, and oral bioavailability of rivaroxaban-loaded high-pressure homogenised and Shirasu porous glass membrane emulsified solid self-nanoemulsifying drug delivery systems. J Mol Liq. 2021;346:117057. doi:10.1016/j.molliq.2021.117057
13. Choi JE, Kim JS, Choi MJ, et al. Effects of different physicochemical characteristics and supersaturation principle of solidified SNEDDS and surface-modified microspheres on the bioavailability of carvedilol. Int J Pharm. 2021;597:120377. doi:10.1016/j.ijpharm.2021.120377
14. Choi SA, Park EJ, Lee JH, et al. Preparation and characterization of pazopanib hydrochloride-loaded four-component self-nanoemulsifying drug delivery systems preconcentrate for enhanced solubility and dissolution. Pharmaceutics. 2022;14:1875. doi:10.3390/pharmaceutics14091875
15. Wang Z, Sun J, Wang Y, et al. Solid self-emulsifying nitrendipine pellets: preparation and in vitro/in vivo evaluation. Int J Pharm. 2009;383(1–2):1–6. doi:10.1016/j.ijpharm.2009.08.014
16. Kim JS, Choi YJ, Woo MR, et al. New potential application of hydroxypropyl-β-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr Polym. 2021;271:118433. doi:10.1016/j.carbpol.2021.118433
17. Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomed. 2010;5(10):1595–1616. doi:10.2217/nnm.10.126
18. Kim W, Kim JS, Choi HG, Jin SG, Cho CW. Novel ezetimibe-loaded fibrous microparticles for enhanced solubility and oral bioavailability by electrospray technique. J Drug Deliv Sci Technol. 2021;66:102877. doi:10.1016/j.jddst.2021.102877
19. Woo MR, Bak YW, Cheon S, et al. Modification of microenvironmental pH of nanoparticles for enhanced solubility and oral bioavailability of poorly water-soluble celecoxib. Int J Pharm. 2024;659:124179. doi:10.1016/j.ijpharm.2024.124179
20. Sahoo A, Kumar NSK, Suryanarayanan R. Crosslinking: an avenue to develop stable amorphous solid dispersion with high drug loading and tailored physical stability. J Control Release. 2019;311:212–224. doi:10.1016/j.jconrel.2019.09.007
21. Choi JE, Kim JS, Kim J, et al. A novel acidic microenvironment microsphere for enhanced bioavailability of carvedilol: comparison of solvent evaporated and surface-attached system. J Drug Deliv Sci Technol. 2022;76:103803. doi:10.1016/j.jddst.2022.103803
22. Knopp MM, Wendelboe J, Holm R, Rades T. Effect of amorphous phase separation and crystallization on the in vitro and in vivo performance of an amorphous solid dispersion. Eur J Pharm Biopharm. 2018;130:290–295. doi:10.1016/j.ejpb.2018.07.005
23. Choi MJ, Woo MR, Choi HG, Jin SG. Effects of polymers on the drug solubility and dissolution enhancement of poorly water-soluble rivaroxaban. Int J Mol Sci. 2022;23(16):9491. doi:10.3390/ijms23169491
24. Park JH, Cho JH, Kim DS, et al. Revaprazan-loaded surface-modified solid dispersion: physicochemical characterization and in vivo evaluation. Pharm Dev Technol. 2019;24(6):788–793. doi:10.1080/10837450.2019.1597114
25. Choi MJ, Woo MR, Baek K, et al. Novel rivaroxaban-loaded microsphere systems with different surface microstructure for enhanced oral bioavailability. Drug Delivery Trans Res. 2024;14:655–664. doi:10.1007/s13346-023-01420-w
26. Kim JS, ud Din F, Lee SM, et al. Comparison of three different aqueous microenvironments for enhancing oral bioavailability of sildenafil: solid self-nanoemulsifying drug delivery system, amorphous microspheres and crystalline microspheres. Int J Nanomed. 2021;16:5797–5810. doi:10.2147/IJN.S324206
27. Baek K, Woo MR, Choi YS, et al. Engineering sodium alginate microparticles with different crystallinities for niclosamide repositioning and solubilization to improve solubility and oral bioavailability in rats. Int J Biol Macromol. 2024;283:137471. doi:10.1016/j.ijbiomac.2024.137471
28. Kim JS, ud Din F, Lee SM, et al. Comparative study between high-pressure homogenisation and Shirasu porous glass membrane technique in sildenafil base-loaded solid SNEDDS: effects on physicochemical properties and in vivo characteristics. Int J Pharm. 2021;592:120039. doi:10.1016/j.ijpharm.2020.120039
29. Woo MR, Woo S, Bak YW, et al. Comparison of two self-nanoemulsifying drug delivery systems using different solidification techniques for enhanced solubility and oral bioavailability of poorly water-soluble celecoxib. Colloids Surf B Biointerfaces. 2024;241:114044. doi:10.1016/j.colsurfb.2024.114044
30. Lee HI, Woo MR, ud Din F, et al. Development of a novel apixaban-loaded solid self-emulsifying drug delivery system for oral administration: physicochemical characterization and pharmacokinetics in rats. J Pharm Investig. 2024. doi:10.1007/s40005-024-00709-3
31. ud Din F, Lee HI, Kim JS, et al. Physicochemical characterization and in vivo assessment of novel apixaban-loaded polymeric nano-aggregates. J Pharm Investig. 2024. doi:10.1007/s40005-024-00712-8
32. Kim JS, ud Din F, Cho HJ, et al. Impact of carrier hydrophilicity on solid self nano-emulsifying drug delivery system and self nano-emulsifying granule system. Int J Pharm. 2023;648:123578. doi:10.1016/j.ijpharm.2023.123578
33. Karavasili C, Andreadis II, Tsantarliotou MP, et al. Self-nanoemulsifying drug delivery systems (SNEDDS) containing rice bran oil for enhanced fenofibrate oral delivery: in vitro digestion, ex vivo permeability, and in vivo bioavailability studies. AAPS Pharm Sci Tech. 2020;21(6):1–10. doi:10.1208/s12249-020-01765-2
34. Mendes C, Andrzejewski RG, Pinto JMO, et al. Impact of drug-polymer interaction in amorphous solid dispersion aiming for the supersaturation of poorly soluble drug in biorelevant medium. AAPS Pharm Sci Tech. 2020;21(6):1–10. doi:10.1208/s12249-020-01737-6
35. Chavan RB, Lodagekar A, Yadav B, Shastri NR. Amorphous solid dispersion of nisoldipine by solvent evaporation technique: preparation, characterization, in vitro, in vivo evaluation, and scale up feasibility study. Drug Delivery Trans Res. 2020;10(4):903–918. doi:10.1007/s13346-020-00775-8
36. Park JH, Kim DS, Mustapha O, et al. Comparison of a revaprazan-loaded solid dispersion, solid SNEDDS and inclusion compound: physicochemical characterisation and pharmacokinetics. Colloids Surf B. 2018;162:420–426. doi:10.1016/j.colsurfb.2017.12.017
37. Choi MJ, Woo MR, Baek K, et al. Enhanced oral bioavailability of rivaroxaban-loaded microspheres by optimizing the polymer and surfactant based on molecular interaction mechanisms. Mol Pharm. 2023;20(8):4153–4164. doi:10.1021/acs.molpharmaceut.3c00281
38. Kim JS, ud Din F, Choi YJ, et al. Hydroxypropyl-β-cyclodextrin-based solid dispersed granules: a prospective alternative to conventional solid dispersion. Int J Pharm. 2022;628:122286. doi:10.1016/j.ijpharm.2022.122286
39. Kim DS, Kim JS, Lim SJ, et al. Comparison of 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol-loaded self-emulsifying granule and solid self-nanoemulsifying drug delivery system: powder property, dissolution and oral bioavailability. Pharmaceutics. 2019;11(8):415. doi:10.3390/pharmaceutics11080415
40. Sun R, Shen C, Shafique S, et al. Electrosprayed polymeric nanospheres for enhanced solubility, dissolution rate, oral bioavailability and antihyperlipidemic activity of bezafibrate. Int J Nanomed. 2020;15:705–715. doi:10.2147/IJN.S235146
41. Jain S, Dongare K, Nallamothu B, et al. Enhanced stability and oral bioavailability of erlotinib by solid self nano emulsifying drug delivery systems. Int J Pharm. 2022;622:121852. doi:10.1016/j.ijpharm.2022.121852
42. Tian J, Meng TT, Ma S, et al. Spatial-thermodynamic understanding of stabilization mechanism using computational approaches and molecular-level elucidation of the mechanism of crystal transformation in polymorphic irbesartan nanosuspensions. Int J Pharm. 2022;612:121350. doi:10.1016/j.ijpharm.2021.121350
43. Bravo-Alfaro DA, Ochoa-Rodríguez LR, Villaseñor-Ortega F, Luna-Barcenas G, García HS. Self-nanoemulsifying drug delivery system (SNEDDS) improves the oral bioavailability of betulinic acid. J Mol Liq. 2022;364:119946. doi:10.1016/j.molliq.2022.119946
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.