Back to Journals » Drug Design, Development and Therapy » Volume 19
Comparison of the Dural Puncture Epidural and Conventional Epidural Analgesia Maintained Using Programmed Epidural Boluses for Labor Analgesia
Authors Mo X , Yu J , Qin Z, Ma J, Chen Y, Chen X
Received 9 February 2025
Accepted for publication 13 May 2025
Published 26 May 2025 Volume 2025:19 Pages 4373—4382
DOI https://doi.org/10.2147/DDDT.S521681
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Xiaofei Mo,* Jie Yu,* Zhimin Qin, Junyi Ma, Yueyue Chen, Xi Chen
Department of Anesthesiology, Guangzhou Medical University Affiliated Women and Children’s Medical Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi Chen, Department of Anesthesiology, Guangzhou Medical University Affiliated Women and Children’s Medical Center, Guangzhou Medical University, 9 Jinsui Road, Tianhe District, Guangzhou, 510623, People’s Republic of China, Tel +86 18620688567, Fax +86 020 38076243, Email [email protected]
Purpose: Research indicates that the dural puncture epidural (DPE) technique offers quicker analgesia onset compared to the conventional epidural (EP) technique. Programmed intermittent epidural bolus (PIEB) is superior to continuous epidural infusion (CEI) for maintaining labor analgesia, providing better pain relief and less motor block. Few studies have explored if combining DPE with the PIEB offers additional benefits in analgesia onset, maintenance, local anesthetic consumption, and side effects compared to DPE with EP. We hypothesized that DPE, when combined with PIEB, not only speeds up analgesia onset but also improves neuraxial analgesia maintenance over EP.
Patients and Methods: A total of 126 term nulliparous women with singleton pregnancies with a VAS pain score > 50 mm and cervical dilation < 5 cm were randomized to receive EP+PIEB or DPE+PIEB for labor analgesia, initiated with 15 mL of 0.0625% ropivacaine with 0.4 μg/mL of sufentanil using the EP or DPE technique (using 25-gauge Whitacre needle) technique and both maintained with the same solution for PIEB (fixed volume 10 mL, intervals 45 minutes, lockout interval 15 minutes) with labor analgesia. The primary outcome was time to achieving adequate analgesia, defined as a VAS pain score ≤ 30 mm. Secondary outcomes included pain scores, motor blockade, obstetric and neonatal outcomes, and satisfaction with analgesia.
Results: Adequate analgesia was achieved faster in the DPE+PIEB group than in the EP+PIEB group (hazard ratio 2.409; 95% CI 1.670 to 3.474, P< 0.001). The median time (interquartile range) to VAS pain score ≤ 30 mm was 10 (7 to 13) minutes for the DPE+PIEB group and 15 (11 to 19) minutes for the EP+PIEB group (P< 0.001). No differences in any of the secondary outcomes between the two groups were observed.
Conclusion: DPE with PIEB accelerated onset time but did not improve maintenance of neuraxial labor analgesia over DPE with EP.
Keywords: analgesia, epidural, labor pain, spinal puncture
Introduction
Dural puncture epidural (DPE) is a specific technique in which the dura mater is punctured with a spinal needle, but instead of delivering the drug directly into the intrathecal space, the epidural drug is allowed to enter the subarachnoid spaces through the puncture hole.1 This technique has been shown in several studies to reduce the time to onset of labor analgesia1,2 and improve the quality of the block with less maternal and fetal adverse effects.3 Compared with the conventional continuous epidural infusion (CEI) mode, programmed intermittent epidural bolus (PIEB) was demonstrated to be associated with better analgesia, fewer motor blocks, and less local anesthetic consumption for labor epidural analgesia maintenance.4,5 This is due to PIEB provides greater longitudinal extension of the local anaesthetic solution in the epidural space.6,7 While there are some studies8,9 on the optimal time and volume of DPE combined with PIEB, could this combination provides benefits not only for the onset of labor analgesia, but also for the additional benefits in maintaining analgesia? The evidence regarding the use of DPE in combination with PIEB for labor analgesia is still unclear, with no clear benefits, and extensive future research is needed.10,11 This study was conducted to evaluate the potential advantages of integrating DPE with PIEB over DPE with EP in terms of analgesia onset, maintenance, local anesthetic consumption, and associated side effects.
The concentration of local anaesthetic administered can affect the analgesic effect of labor analgesia pain control, and undesirable side effects (such as hypotension, motor blockade) can occur when high doses are used. The use of lower concentrations of local anesthetics has drastically reduced the risk of these complications. Previous studies by us12 and others13,14 have shown that 0.0625% ropivacaine provides excellent quality labor analgesia. Therefore, in our study, we chose 0.0625% ropivacaine for initiation and maintenance of labor analgesia.
Therefore, we hypothesized that neuraxial labor analgesia initiated with the DPE technique and maintained with PIEB may be superior to initiated with EP and maintained with PIEB in terms of time to onset of analgesia, motor blockade, local anesthetic consumption, adverse effects, pain scores and satisfaction and the primary outcome was the onset time to adequate analgesia.
Materials and Methods
Study Design and Participants
Inclusion criteria were: aged 18 to 45 years, singleton vertex fetuses at 37 to 42 weeks’ gestation, American Society of Anesthesiologists (ASA) physical status II or III, spontaneous onset of labor, active labor with a cervical dilation <5 cm, and baseline pain score >50 mm on a 100-mm visual analogue scale2 (VAS; where 0 mm indicates no pain and 100 mm indicates the worst pain imaginable) at the time of request for labor analgesia. Exclusion criteria were: severe pregnancy complications (heart disease, gestational hypertension, gestational diabetes, complete placenta previa, placental abruption, preeclampsia), body mass index (BMI) ≥ 40 kg/m2, participant received antipsychotic or hypnotic medications, alcohol or drug abuse, any contraindication to neuraxial analgesia, allergy or hypersensitivity to drugs used in the study, administration of opioids or sedatives within 2 hours prior to requesting for neuraxial analgesia, and known fetal abnormalities. Patients were excluded from the study after randomization if an epidural puncture was inadvertently caused by the use of an epidural puncture needle, if cerebrospinal fluid (CSF) could not be confirmed with a spinal needle at the time of the epidural puncture, or if delivery occurred within 1 hour of epidural catheter placement.
Ethics
This study protocol was approved by the by the local ethics committee, the Guangzhou Women and Children’s Medical Center Ethics Committee, Guangzhou, China on 10 November, 2023 (The reference number 2023–301A01). The protocol was registered prior to patient enrolment at https://www.chictr.org.cn/ (Chinese Clinical Trial Registration [ChiCTR] ChiCTR2300078488, Principal Investigator: Mo Xiaofei, registration date: 11 December, 2023). This trial adheres to the Consolidated Standards of Reporting Trials guidelines and conforms to the Declaration of Helsinki. All participants gave written informed consent before the start of the study. The study was conducted from 15 December 2023 to 20 June 2024.
Randomization and Blinding
After obtaining written informed consent, parturients were randomly assigned to either the EP+PIEB group or the DPE+PIEB group (in a 1:1 ratio) through a computer-generated randomization list. Confidentiality of the allocation results was maintained through sequentially numbered opaque sealed envelopes. The envelope containing the group assignment was opened by a staff at the time of request for labor analgesia. All procedures were performed by an attending anesthesiologist or senior anesthesia resident under the supervision of the attending anesthesiologist. During the neuraxial analgesia procedure, the outcome assessors waited outside the patient’s room. Parturients, obstetricians, outcome assessors, nurses, and anesthesia providers involved in labor analgesia management and data collection were blinded to the randomization.
Initiation of Labor Analgesia
Maternal baseline heart rate, noninvasive arterial blood pressure (average of 3 measurements between uterine contractions), and oxygen saturation were recorded. Participants were preloaded with 500 mL of lactated Ringer’s solution prior to induction of neuraxial analgesia using pulse oximetry, automated non-invasive blood pressure monitoring, electrocardiography or fetal tocodynamometry. The epidural was performed in a left lateral decubitus at the L3-L4 or L2-L3 interspace via a midline approach with an 18-gauge Tuohy needle using a loss of resistance to saline technique.
In subjects randomly assigned to the DPE+PIEB group, the spinal needle was withdrawn after confirming free flow of CSF using a needle-through-needle technique in which a 25-gauge pencil-point needle was inserted into the shaft of a previously placed epidural needle to create an dural hole. After negative aspiration for blood or CSF, 3 mL of 1% lidocaine was administered as a test dose. Approximately 3 minutes after a negative test dose, 15 mL of epidural medication (0.0625% ropivacaine with 0.4 µg/mL sufentanil) was injected via the epidural catheter over 2 minutes to initiate labor analgesia according to standard practice. The time point at the end of administration of the loading dose was recorded as “zero time”. At this time, the blinded investigator was called into the room to begin data collection.
Maintenance of Labor Analgesia
The maintenance solution utilized was a combination of 0.065% ropivacaine and 0.4 µg/mL sufentanil, which has been shown in clinical studies to provide effective analgesia during labor. In all subjects, the programmed intermittent bolus dose was fixed at 10 mL at regular intervals of 45 minutes with an epidural pump (Apon MC ZZB-IV; Jiangsu Apon Medical Technology, Jiangsu, China), and the first bolus was delivered 1 hour after the initial manual loading dose. The PIEB pump was programmed to deliver 8-mL patient-controlled epidural analgesia (PCEA) boluses with a 15-minute lockout interval and a maximum hourly volume of 42 mL. The patient was instructed to press the PCEA button if she felt uncomfortable.
Breakthrough Pain Management
If breakthrough pain occurred (defined as parturient requesting additional supplemental analgesia beyond the self-administered boluses), an additional epidural top-up of 10 mL of 0.2% ropivacaine was administered. After 15 minutes, if VAS score remained > 30 mm, an additional top-up was allowed. If the VAS score remained > 30 mm after the second top-up dose, the epidural catheter was considered unsatisfactory and the patient was withdrawn from the study.
Outcomes
Primary Outcome
The primary outcome was the onset time to adequate analgesia. This was defined as the end of the loading dose (time zero on the stopwatch) to a VAS score of ≤30 mm. The VAS score was used to assess uterine contraction pain when the patients requested for labor analgesia, and the baseline VAS score was recorded. Pain was assessed with each uterine contraction for 30 minutes or until a pain score of 30mm or less was reached, then assessments continued at 60-minute intervals until 5 hours or delivery, which ever occurred first.
Secondary outcomes
Secondary outcomes included: Sensory block level at 30 minutes after the end of the loading dose; motor block, which was assessed in both lower extremities using the modified Bromage score [0=no motor paralysis; 1=unable to raise the extended leg, but able to move knee and foot; 2=unable to raise the extended leg as well as flex knees, able to move foot; 3=unable to flex ankle, foot or knee (complete block)]15 at 30 minutes and during labor; mode of delivery; satisfaction score of analgesia; pruritus; nausea and vomiting; hypotension; mode of delivery; physician bolus during labor; the PCEA boluses; time to first PCEA bolus; the maternal satisfaction with labor analgesia, which was evaluated with a 5-point scale (1=very disappointed; 2=disappointed; 3=so-so; 4=satisfactory; 5=very pleased) and postpartum headache (followed up on postpartum day 1); Apgar scores at 1, 5 and 10 minutes; fetal bradycardia.
Sensory block level was evaluated bilaterally with ice chips starting at the S2 dermatome and moving in a caudad to cephalad direction. Thoracic dermatomal sensory levels were assessed along the midclavicular line, and lower extremity at the inguinal crease (L1), anterior thigh (L2), medial knee (L3), medial malleolus (L4), dorsal web between the great and second toes (L5), lateral heel (S1), and medial popliteal fossa (S2).2 Asymmetric block were defined as a difference in the sensory block level of >2 dermatomes between the left and right sides of the patient. Fetal bradycardia was defined as a rate of <110 bpm for >10 minutes. Hypotension was defined as systolic blood pressure 20% below baseline or less than 90 mmHg; fetal bradycardia within 30 minutes.
Sample Size Calculation
The sample size estimation was calculated based on the results of a pilot study showing that the mean of onset time to adequate analgesia (primary outcome) was 10 minutes in the EP+PIEB group and 8 minutes in the DPE+PIEB group, with a standard deviation (SD) of 3 minutes. A sample size of 52 subjects per group had 90% power at α=0.05 in a 2-sided 2-sample t-test to identify this difference calculated using PASS software (version 11. NCSS, LLC. Kaysville, Utah, USA). To account for 20% dropouts, 63 subjects were recruited in each group, for a total of 126 in both groups.
Statistical Analysis
Statistical analyses were performed using SPSS (version 25.0, IBM Corp., Armonk, NY, USA) and GraphPad Prism V.15.0 (GraphPad Software, San Diego, California, USA). The Kolmogorov–Smirnov test was used to assess the normal distribution of continuous variables. Normally distributed data were presented as the mean ± SD and analyzed using the Student’s t-test. Non-normally distributed data were presented as median (interquartile range, IQR) and analyzed using the Mann–Whitney U-test. Hodges-Lehmann location shift estimates and 95% confidence intervals (CIs) are presented for comparisons associated with differences in medians. Categories and proportions were analyzed using Pearson chi-squared test or Fisher exact test, as appropriate. Kaplan-Meier survival curves with log-rank (Mantel-Cox) test and Cox proportional hazards model were used to analyze the time to VAS≤30 mm and time to first PCEA.
Results
One hundred and twenty-six women were included and randomized to either the EP+PIEB group (n=63) or the DPE+PIEB group (n=63) between December 2023 and June 2024 (Figure 1). No patient was withdrawn from the DPE+PIEB group due to failure to locate CSF and all patients had adequate analgesia after the initial dose. Baseline characteristics are presented in Table 1.
 |
Table 1 Patient Characteristics |
 |
Figure 1 Flowchart of the study. Abbreviations: DPE, dural puncture epidural; EP, conventional epidural; PIEB, programmed intermittent epidural bolus. |
The DPE+PIEB group experienced a faster onset of adequate analgesia compared to the EP+PIEB group, as evidenced by a hazard ratio of 2.409 (95% CI 1.670 to 3.474, P<0.001). Analysis using the log-rank (Mantel-Cox) test showed that the median times (IQR) to VAS pain score ≤30 mm were 10 (7 to 13) minutes for that DPE+PIEB group and 15 (11 to 19) minutes for that EP+PIEB group (P<0.0001; Figure 2), with a Hodges-Lehmann estimate of the median difference of 5.000 (95% CI, 3.000 to 7.000) minutes.
Pain VAS scores (mm) at various time points are shown in Table 2.
 |
Table 2 Pain VAS Score (Mm) at Various Time Points |
The study found no significant difference in epidural ropivacaine consumption per hour between the EP+PIEB group [median (IQR), 5.2 (3.5 to 6.9) mg/h, n=52] and the DPE+PIEB group [5.7 (4.4 to 7.0) mg/h, n=54; P=0.163; Table 3], which is consistent with previous findings on the use of ropivacaine in epidural anesthesia. The associated Hodges-Lehmann estimate of the median difference was −7.500 (95% CI: −15.630 to 3.120). There were no significant differences in the percentage of patients requiring PCEA boluses (P=0.709; Table 3) and physician boluses (P=0.315; Table 3); the time to first PCEA request is shown in Figure 3. There was no significant difference in the time to first PCEA bolus in the EP+PIEB group 281 (154 to 481) minutes versus the DPE+PIEB group 322 (234 to 472) minutes, (P = 0.4239; Figure 3).
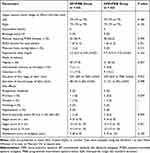 |
Table 3 Analgesia Characteristics and Labor Outcomes |
Side effects of neuraxial labor analgesia between groups are shown in Table 3. No patient developed a Bromage score >0 and asymmetric blocks during the study. No patient experienced postpartum headache, hypotension, or vomiting during the study. There was no difference in the incidence of pruritus [1/63 (1.6%) vs 2/63 (3.2%); P=0.559] and nausea [1/63 (1.6%) vs 1/63 (1.6%); P=1].
There were no significant differences between the groups in total epidural ropivacaine consumption, the percentage of patients who required PCEA boluses and physician bolus, the time to first PCEA request, duration of labor, mode of delivery, newborn Apgar scores, the incidence of fetal bradycardia within 30 minutes, patient satisfaction scores, side effects (Bromage score >0, asymmetric blocks, postpartum headache, hypotension, vomiting/ nausea, and pruritus).
Discussion
We observed that the DPE+PIEB group achieved adequate analgesia, defined as a VAS score of ≤30 mm from the end of the loading dose (time zero on the stopwatch), faster than the EP+PIEB group. This finding is consistent with the principles of double-blind trials, which are designed to minimize bias and ensure the reliability of the results. However, no additional benefit was found in the DPE+PIEB group during the subsequent maintenance period. We also observed no significant differences in side effects of analgesia between the two groups.
Neuraxial labor analgesia includes the initial period of loading dose to the adequate analgesia and maintenance period. There are conflicting opinions as to whether the DPE technique can shorten the onset time of adequate analgesia. Our study demonstrated that DPE resulted in a shorter onset of analgesia compared with EP technique, which is consistent with Wilson et al1 and Song et al.2 However, Anthony Chau et al3 and Tan et al16 showed that DPE technique did not provide a earlier analgesia onset when compared with EP. An in vitro study has shown that the diameter of the puncture needle correlates with the rate of drug transfer from the epidural cavity to the subarachnoid space through the puncture hole.17 Clinical studies have suggested that dural puncture epidural analgesia with 26-G18 or 25-G3 Whitacre spinal needles improve analgesia onset time than traditional epidural technique, but 27-G dose not.19 Dural puncture epidural analgesia with 25G spinal needles provides a shorter onset time than 27G.20,21 Wilson et al1 used a 26G spinal puncture needle, whereas we and three other studies used a 25G spinal puncture needle. In our study, we used a smaller volume (15 mL) and a more dilute (0.0625%) ropivacaine for the loading dose, whereas 0.125% bupivacaine 20 mL for Anthony Chau et al,3 this larger volume and more concentrated local anesthetics may have masked differences between the groups. We and Wilson et al1 used a shorter time (2 min and 3 min, respectively) to administer the initial dose, compared with 5 min and 6 min in Chau et al3 and Tan et al.16 The pressure generated increases with faster delivery speeds, which may result in more drug translocation from the epidural space to the subarachnoid space. We and Song et al2 chose pain score ≤30 mm as adequate analgesia score, while the other 3 studies chose pain score ≤1 (0–10 scale). Differences between us and other studies also included adequate analgesia score and inclusion population, Tang et al16 included obese parturients (BMI≥ 35 kg/m 2), and we and Song et al2 used pain score ≤30 mm as the criterion for adequate analgesia, while the other 3 studies used 10 mm.
Labor analgesia of DPE combined with the PIEB mode has not been well evaluated. Song et al2 showed that there was no significant difference in the consumption of ropivacaine between the DPE+CEI and EP+CEI groups, but the anesthetic consumption in the EP+PIEB group was reduced compared to the EP+CEI group. We found only one previous study by Yao et al22 on comparing DEP+PIEB and EP+PIEB, which was consistent with our study that there were no differences between groups in ropivacaine consumption, patients requiring PCEA, physician top-up interventions and the time to first PCEA request. Yao et al22 did not report the onset time of adequate analgesia.
Our findings suggest that, compared with the EP technique, the DPE technique does not increase the side effects of epidural analgesia, especially the risk of postdural puncture headache and hypotension, which is consistent with previous reports.2,3,22 Many studies have demonstrated that PIEB produced less motor block compared with CEI during maintenance of labor analgesia.5,23 Both groups in our study were maintained with a PIEB during labor analgesia, and no patient in either group had a Bromage score >0. However, Yao et al22 found that the incidence of Bromage score >0 in DEP+PIEB and EP+PIEB was 7.1% and 6.5%, respectively. The possible reason for the different results is that we used a lower concentration of local anesthetics (0.0625% ropivacaine) than Yao et al22 (0.1% ropivacaine) in the maintenance period of labor analgesia. In our study no patient developed asymmetric block in both groups, in the study by Song et al2 the incidence of asymmetric block in the PIEB+DPE group is 5.3% and in the study by Chau et al3 the DPE group has a lesser incidence of asymmetric blocks for the first 30 min (40%) and after 30 min (10%) initial dosing than the traditional epidural technique group. Our findings that no patients had postdural puncture headache were consistent with previous reports3,16,22 in the DPE group.
Our study has several limitations. First, we recorded only fetal heart tracings and Apgar scores, but did not record uterine contractions and fetal blood gases. Second, we recorded the sensory block levels 30 minutes after the end of the loading dose, limiting our ability to obtain sensory block levels at other time points. Finally, due to the absence of other studies on the combination of DPE and PIEB with 0.0625% ropivacaine, we chose a moderate volume of local anesthetic for initial and intermittent administration based on other8,9 and our studies12 and the routine of our institution.
Conclusion
In summary, when EP or DPE were combined with PIEB techniques, the DPE technique provided the early onset of analgesia. However, in the maintenance of labor analgesia, this mode was not shown to provide any additional benefit.
Data Sharing Statement
The data collected for this study can be shared with researchers in de-identified form after the publication date, and in the presence of a data transfer agreement, and if it complies with China legislation. Requests for data and study proposal should be directed to [email protected], including a proposal that must be approved by the trial’s steering committee.
Funding
This work was supported by the Guangzhou Health Science and Technology Project (20251A011030).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wilson SH, Wolf BJ, Bingham K, et al. Labor analgesia onset with dural puncture epidural versus traditional epidural using a 26-gauge whitacre needle and 0.125% bupivacaine bolus: a randomized clinical trial. Anesth Analg. 2018;126(2):545–551. doi:10.1213/ANE.0000000000002129
2. Song Y, Du W, Zhou S, et al. Effect of dural puncture epidural technique combined with programmed intermittent epidural bolus on labor analgesia onset and maintenance: a randomized controlled trial. Anesth Analg. 2021;132(4):971–978. doi:10.1213/ANE.0000000000004768
3. Chau A, Bibbo C, Huang CC, et al. Dural puncture epidural technique improves labor analgesia quality with fewer side effects compared with epidural and combined spinal epidural techniques: a randomized clinical trial. Anesth Analg. 2017;124(2):560–569. doi:10.1213/ANE.0000000000001798
4. Hussain N, Lagnese CM, Hayes B, et al. Comparative analgesic efficacy and safety of intermittent local anaesthetic epidural bolus for labour: a systematic review and meta-analysis. Br J Anaesth. 2020;125(4):560–579. doi:10.1016/j.bja.2020.05.060
5. Joyce C, Free R, Calpin P, et al. Programmed intermittent epidural bolus regimen vs continuous epidural infusion: a retrospective study of motor block and obstetric outcomes using the Robson’s Ten Group Classification System. Int J Obstet Anesth. 2024;59:104215.
6. Cole J, Hughey S. Bolus epidural infusion improves spread compared with continuous infusion in a cadaveric porcine spine model. Reg Anesth Pain Med. 2019;44(12):1080–1083. doi:10.1136/rapm-2019-100818
7. Mowat I, Tang R, Vaghadia H, et al. Epidural distribution of dye administered via an epidural catheter in a porcine model. Br J Anaesth. 2016;116(2):277–281. doi:10.1093/bja/aev432
8. Yao HQ, Huang JY, Deng JL, et al. Randomized assessment of the optimal time interval between programmed intermittent epidural boluses when combined with the dural puncture epidural technique for labor analgesia. Anesth Analg. 2023;136(3):532–539. doi:10.1213/ANE.0000000000006201
9. Xiao F, Yao HQ, Qian J, et al. Determination of the optimal volume of programmed intermittent epidural bolus when combined with the dural puncture epidural technique for labor analgesia: a random-allocation graded dose-response study. Anesth Analg. 2023;137(6):1233–1240. doi:10.1213/ANE.0000000000006451
10. Heesen M, Rijs K, Rossaint R, et al. Dural puncture epidural versus conventional epidural block for labor analgesia: a systematic review of randomized controlled trials. Int J Obstet Anesth. 2019;40:24–31. doi:10.1016/j.ijoa.2019.05.007
11. Layera S, Bravo D, Aliste J, et al. A systematic review of DURAL puncture epidural analgesia for labor. J Clin Anesth. 2019;53:5–10. doi:10.1016/j.jclinane.2018.09.030
12. Zhao B, Qian X, Wang Q, et al. The effects of ropivacaine 0.0625% and levobupivacaine 0.0625% on uterine and abdominal muscle electromyographic activity during the second stage of labor. Minerva Anestesiol. 2019;85(8):854–861. doi:10.23736/S0375-9393.19.13246-4
13. Su PY, Peniche A, Clelland E, et al. Comparison of programmed intermittent epidural bolus and continuous epidural infusion for post-operative analgesia after major abdominal surgery: a randomized controlled trial. J Clin Anesth. 2020;64:109850. doi:10.1016/j.jclinane.2020.109850
14. Mei Z, Wang Q, Song S, et al. Optimum programmed intermittent epidural bolus interval time of ropivacaine 0.0625% with dexmedetomidine 0.4 mug/mL at a fixed volume of 10 mL: a randomized controlled trial. Front Pharmacol. 2024;15:1368222. doi:10.3389/fphar.2024.1368222
15. Mo X, Zhao T, Chen J, et al. Programmed intermittent epidural bolus in comparison with continuous epidural infusion for uterine contraction pain relief after cesarean section: a randomized, double-blind clinical trial. Drug Des Devel Ther. 2022;16:999–1009. doi:10.2147/DDDT.S350418
16. Tan HS, Reed SE, Mehdiratta JE, et al. Quality of labor analgesia with dural puncture epidural versus standard epidural technique in obese parturients: a double-blind randomized controlled study. Anesthesiology. 2022;136(5):678–687. doi:10.1097/ALN.0000000000004137
17. Bernards CM, Kopacz DJ, Michel MZ. Effect of needle puncture on morphine and lidocaine flux through the spinal meninges of the monkey in vitro. Implications for combined spinal-epidural anesthesia. Anesthesiology. 1994;80(4):853–858. doi:10.1097/00000542-199404000-00019
18. Suzuki N, Koganemaru M, Onizuka S, et al. Dural puncture with a 26-gauge spinal needle affects spread of epidural anesthesia. Anesth Analg. 1996;82(5):1040–1042. doi:10.1097/00000539-199605000-00028
19. Thomas JA, Pan PH, Harris LC, et al. Dural puncture with a 27-gauge Whitacre needle as part of a combined spinal-epidural technique does not improve labor epidural catheter function. Anesthesiology. 2005;103(5):1046–1051. doi:10.1097/00000542-200511000-00019
20. Contreras F, Morales J, Bravo D, et al. Dural puncture epidural analgesia for labor: a randomized comparison between 25-gauge and 27-gauge pencil point spinal needles. Reg Anesth Pain Med. 2019;44(7):750–753. doi:10.1136/rapm-2019-100608
21. Abdelhaleem AR, Khalil AR, Elsayed IEI, et al. A randomized comparative study of 25-gauge vs. 27-gauge pencil-point spinal needles during dural puncture epidural anesthesia for elective cesarean section. Anaesthesiol Intensive Ther. 2025;57(1):18–28. doi:10.5114/ait.2025.147736
22. Yao HQ, Qian J, Dong FY, et al. Comparison of the dural puncture epidural and the standard epidural techniques in patients having labor analgesia maintained using programmed epidural boluses: a prospective double-blinded randomized clinical trial. Reg Anesth Pain Med. 2024.
23. Capogna G, Camorcia M, Stirparo S, et al. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg. 2011;113(4):826–831. doi:10.1213/ANE.0b013e31822827b8
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



