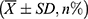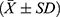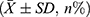Back to Journals » Cancer Management and Research » Volume 16
Comparison of Totally Laparoscopic Total Gastrectomy and Laparoscopy-Assisted Total Gastrectomy on Short-Term Outcomes, Inflammatory Response Markers, and Glucose and Lipid Metabolism in Gastric Cancer Patients
Received 5 June 2024
Accepted for publication 7 October 2024
Published 17 October 2024 Volume 2024:16 Pages 1435—1443
DOI https://doi.org/10.2147/CMAR.S479025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Chien-Feng Li
Jun Du,1,* Zijie An,2,* Kun Zhu2
1Department of Oncological Surgery, The First Affiliated Hospital of Bengbu Medical University, Bengbu, People’s Republic of China; 2Department of Orthopaedics, The First Affiliated Hospital of Bengbu Medical University, Bengbu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kun Zhu, Department of Orthopaedics, The First Affiliated Hospital of Bengbu Medical University, Bengbu, People’s Republic of China, Email [email protected]
Objective: To investigate the therapeutic efficacy of totally laparoscopic total gastrectomy (TLTG) versus laparoscopy-assisted total gastrectomy (LATG) in gastric cancer (GC) treatment, as well as their effects on postoperative inflammation and glucose and lipid metabolic status.
Methods: Clinical data of 68 individuals with GC who underwent LATG (n=31) and TLTG (n=37) from January 2020 to December 2022 were procured. This included intraoperative blood loss, operative time, incision length, number of lymph nodes dissected, postoperative complication rates, and recovery indicators, such as inflammation, glucose metabolism, and lipid metabolism.
Results: The TLTG cohort demonstrated significant advantages in intraoperative blood loss, operative time, and incision length compared to the LATG cohort. Furthermore, TLTG was superior in reducing the incidence of complications. Nevertheless, no substantial variation was observed in the quantity of lymph nodes dissected. Additionally, TLTG showed benefits in postoperative recovery, including better control of the inflammatory response, reduction of complication risks, shorter hospital stay, and alleviation of postoperative pain. TLTG also exhibited a reduced impact on inflammation and demonstrated greater effectiveness in improving postoperative glucose and lipid levels.
Conclusion: TLTG surgery is associated with superior clinical outcomes in the treatment of GC compared to LATG, particularly in reducing surgical trauma and accelerating postoperative recovery. Furthermore, TLTG facilitates the resolution of postoperative inflammatory responses and the amelioration of metabolic disorders. The findings from this investigation advocate for the broader adoption of TLTG in the surgical treatment of GC.
Keywords: totally laparoscopic total gastrectomy, laparoscopy-assisted total gastrectomy, gastric cancer, inflammatory markers, glucose and lipid metabolism
Introduction
Gastric cancer (GC) ranks as the fourth most prevalent malignant neoplasm worldwide and is the second leading cause of cancer-associated deaths. The pathophysiological processes of GC and associated treatments can disrupt systemic glucose and lipid metabolism through the production of inflammatory cytokines during tumor growth.1 These cytokines can impair pancreatic β-cell function and decrease insulin synthesis and secretion, leading to hyperglycemia. Additionally, tumor growth consumes a significant amount of energy, increasing lipid breakdown and elevating blood lipid levels. Both hyperglycemia and hyperlipidemia can promote tumor growth, thereby exacerbating the progression of GC.2 Surgical intervention can mitigate these inflammatory responses and ameliorate metabolic disturbances.3,4
In recent years, the development of laparoscopic technology popularized the use of laparoscopy-assisted total gastrectomy (LATG) and totally laparoscopic total gastrectomy (TLTG) as surgical interventions for managing GC.5 LATG is a procedure that combines laparoscopic techniques with a semi-open approach wherein certain steps are conducted within the abdominal cavity, followed by the removal of the stomach and extracorporeal anastomosis.6 In contrast, TLTG is performed entirely within the abdominal cavity through small incisions and, while challenging, this technique eliminates the need for open surgery.7 Despite the extensive use of LATG and TLTG in treating GC, the therapeutic efficacy of either surgical method remains controversial.8 This study provides evidence for the optimal surgical treatment of GC by comparing the efficacy of LATG and TLTG, as well as their effects on inflammatory markers and glucose and lipid metabolism.
Materials and Methods
Patients and Methodologies
We retrospectively collected clinical data from 68 individuals who received either LATG (n=31) or TLTG (n=37) at the First Affiliated Hospital of Bengbu Medical University from January 2020 to December 2022. Inclusion and exclusion criteria were based on the “Laparoscopic Gastric Cancer Surgery Operation Guidelines (2007)”9 Additional inclusion criteria were as follows: (1) individuals aged 18–80 years; (2) total gastrectomy was required; (3) Karnofsky Performance Scale (KPS) score >70;10 and (4) no history of diabetes. Additional exclusion criteria were as follows: (1) history of upper abdominal open surgery; (2) history of upper abdominal radiotherapy; (3) emergency surgery was required; and (4) transfer laparotomy and palliative surgery was required. The research protocol received approval from the Ethics Committee of the First Affiliated Hospital of Bengbu Medical University.
All individuals were evaluated by gastroscopy, enhanced abdominal and pelvic CT, plain chest CT, and supraclavicular lymph node ultrasonography. All patients had histologically confirmed advanced gastric adenocarcinoma. Preoperative TNM staging was performed according to the 2012 National Comprehensive Cancer Network (NCCN) clinical practice guidelines for GC.11
Surgical Techniques
Both TLTG and LATG cohorts strictly adhered to the Japanese Gastric Cancer Classification, 3rd edition,12 and the Japanese Gastric Cancer Treatment Guidelines 2014, 4th edition, performing standard D2 or D2+ lymph node dissections, including removal of lymph nodes at stations 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, and 12a.13 All procedures were executed by an identical cohort of experienced surgeons.
In the TLTG cohort, digestive tract reconstruction was carried out intra-abdominally using a linear stapler post-lymphadenectomy, with a small vertical incision below the navel for specimen extraction.
In the LATG cohort, part or all of the reconstruction was executed externally via a midline laparotomy incision. In both cohorts, total gastrectomy reconstruction was performed using standard Roux-en-Y.
Data Collection
Patient characteristics were collected, including age, gender, BMI, hypertension, history of abdominal surgery, smoking history, drinking history, tumor location, and TNM stage. Intraoperative data included operation time, blood loss, number of lymph nodes cleared, anesthesia duration, and incision length. Postoperative recovery included average time to first flatus, time to start on a liquid diet, time to first mobilization, average hospital stay, and Visual Analog Scale (VAS) assessment of pain intensity on postoperative days one and three.14 Postoperative complications were characterized as any unfavorable occurrences within 30 days post-operation and were classified according to the Clavien-Dindo system,15 with grade 3 or above deemed a significant complication. Perioperative mortality was described as any fatality resulting from any cause within 30 days postoperatively.
White blood cell (WBC) count, and serum C-reactive protein (CRP) levels were assessed on the first and third postoperative days as indicators of the patient’s immediate inflammatory responses. Fasting blood glucose and glycated hemoglobin (GHb) levels (mmol/L) were evaluated at one and three months postoperatively to assess glucose metabolism. Triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL) (mmol/L) were measured at one and three months postoperatively to evaluate lipid metabolism.
Statistical Analysis
The sample size was estimated using PASS software and the “two independent means” process, with blood loss serving as the main index for sample size calculation. The mean and standard deviation of the estimated blood loss of the two cohorts were obtained from the literature.16 Utilizing a statistical power of 0.8 and a type I error of 0.05, the minimum sample size required for each cohort was 26 cases, totaling 52 cases. All statistical analyses were performed utilizing SPSS version 25.0. Quantitative data were denoted as mean ± standard deviation (±SD). For data exhibiting normal distribution and variance homogeneity, one-way analysis of variance (ANOVA) was employed, whereas the Mann–Whitney U-test was utilized for data not meeting these criteria. Categorical information was examined employing the chi-square test, with pairwise comparisons conducted via the SNK test. Correlation analysis was performed utilizing the Pearson approach. P values below 0.05 were deemed statistically significant.
Results
Patient Characteristics
A sum of 68 individuals participated in the investigation, 37 of whom underwent TLTG and 31 underwent LATG. Detailed clinical data for both cohorts are presented in Table 1.
 |
Intraoperative Comparison
The TLTG cohort demonstrated markedly reduced surgical duration (244.76±6.74 minutes) than the LATG cohort (289.94±5.94 minutes; P<0.001). The intraoperative blood loss was markedly less in the TLTG cohort compared to the LATG cohort (92.41±3.52 vs 97.84±4.66 mL; P<0.001). The quantity of lymph nodes dissected did not differ markedly between the TLTG and LATG cohorts (31.70±1.56 vs 32.26±1.75; P=0.171). The incision length in the TLTG cohort (2.78±0.79 cm) was markedly shorter than the LATG cohort (7.29±1.62 cm; P<0.001). There were no operative mortalities (Table 2).
 |
Table 2 Intraoperative Comparison Between TLTG and LATG Groups |
Postoperative Recovery Comparison
The time to first flatus for the TLTG cohort (2.97±0.80 days) was markedly less than the LATG cohort (3.94±0.98 days; P<0.001). Individuals in the TLTG cohort started a liquid diet sooner compared to the LATG cohort (4.46±1.04 days vs 0.81±1.11 days; P<0.001). The TLTG cohort also had a markedly shorter time to mobilization (2.03±0.90 days) compared to the LATG cohort (4.42±1.18 days; P<0.001). The duration of hospital stay was markedly shorter for the TLTG cohort (8.92±0.95 days) than that for the LATG cohort (12.48±1.18 days; P<0.001). Two patients (5.4%) experienced postoperative complications in the TLTG cohort, including one patient with Anastomosis leakage and one patient with Delayed emptying. This is markedly fewer relative to the seven patients (22.5%) in the LATG cohort (P<0.05), of which 2 had Anastomosis leakage, 3 had Delayed emptying, 1 had Anastomosis stenosis and 1 had Wound infection (P<0.05) (Table 3 and Supplementary Table 1).
 |
Table 3 Postoperative Recovery Indicators Comparison Between TLTG and LATG Groups |
Changes in Inflammatory Response and Metabolism
Inflammatory levels were measured on the first and third days post-operation. On the first day, the WBC count for the TLTG cohort (10.42±1.20×109/L) was markedly lower than that of the LATG cohort (P<0.001), indicating a milder inflammatory response. However, by the third postoperative day, no significant differences in WBC counts were observed between the two cohorts (P=0.564). The levels of CRP, a more sensitive indicator of inflammation, showed significant differences on both the first and third postoperative days, with markedly lower levels in the TLTG cohort (P<0.001). In terms of glucose metabolism, fasting blood glucose and GHb levels one and three months postoperatively were slightly higher in the TLTG cohort compared to the LATG cohort (P <0.05), suggesting a potential advantage of TLTG surgery in modulating blood sugar. Regarding lipid metabolism, TG levels at one and three months postoperatively showed no significant difference between the TLTG and LATG cohorts (P>0.05). However, TC, LDL, and HDL levels at three months post-operation were lower in the TLTG cohort compared to the LATG cohort (P <0.001), indicating a potential advantage of TLTG surgery in improving postoperative lipid metabolism (Table 4).
 |
Table 4 Changes in Inflammation Levels and Glucose-Lipid Metabolism in Patients After Two Types of Surgery |
Discussion
GC represents a significant global health burden, and treatment strategies are continuously evolving. Surgical intervention plays a crucial role in the management of GC, particularly through two primary approaches: LATG and TLTG.17,18 LATG is widely used due to its mixed operation, which integrates the advantages of both laparoscopy and open surgery.19 In contrast, TLTG is a fully laparoscopic procedure that represents a more technologically advanced surgical approach and concept.20 However, the distinct clinical advantages and disadvantages of each technique have been the subject of significant attention and debate.21 In this study, we analyzed the efficacy of LATG and TLTG. Our results suggest that TLTG demonstrates significant improvements in intraoperative performance, postoperative recovery, and associated complications compared to LATG.
Our findings indicate that TLTG markedly outperformed LATG in reducing surgical duration and minimizing intraoperative bleeding, both critical factors that influence postoperative recovery and complication rates.16,22 These results demonstrate the intraoperative advantages of TLTG. However, there was no significant difference between the number of lymph nodes harvested, indicating that both surgical methods were equally effective. Additionally, TLTG resulted in faster postoperative gas passage and ambulation times. Early recovery of intestinal function and mobility can effectively prevent postoperative complications and facilitate patient recovery.23 Patients undergoing TLTG demonstrated a markedly shorter time to initiate liquid intake compared to LATG. However, this observation may be unrelated to surgical intervention, as other factors influencing postoperative recovery, such as pain control and subjective recovery experiences, may affect the patient’s ability to intake fluids. The average length of time spent in the hospital in the TLTG cohort was markedly shorter than that in the LATG cohort. This result is likely attributable to the ability of TLTG surgery to improve postoperative exhaustion and activity time. Patients in the TLTG cohort exhibited reduced postoperative pain scores and WBC counts, demonstrating the advantages of TLTG in mitigating pain and eliciting a milder inflammatory response. Reducing these factors may directly influence the incidence of complications, an important metric for assessing surgical outcomes.12,24 The results of this study indicate that the complications associated with surgery, such as pulmonary, incisional, cardiac, and gastrointestinal complications, were markedly lower in the TLTG cohort compared to the LATG cohort. This may be related to the minimally invasive nature of TLTG, smaller incision length, shorter operation time, reduced intraoperative blood loss, and other factors associated with TLTG. Additionally, the reduced incidence of postoperative complications not only facilitates patient recovery but also has the potential to shorten hospital stays and lower treatment costs.
The progression of GC is often accompanied by an enhanced systemic inflammatory response, including increased WBC and elevated CRP levels. The levels of these inflammatory markers are closely associated with patient prognosis.25 In the present study, it was observed that, immediately after surgery, the WBC and CRP levels in the TLTG cohort were markedly lower than those in the LATG cohort. This difference may be attributed to the minimally invasive nature of TLTG. Minimally invasive surgery has been shown to reduce tissue damage, subsequently ameliorating systemic inflammation immediately after surgery. However, three days post-operation, WBC counts were no different between the cohorts, whereas CRP levels were lower in the TLTG cohort. This observation may be attributed to the fact that CRP is a more sensitive inflammatory marker that more accurately reflects the systemic inflammatory response induced by surgery.26
In addition to increased inflammatory responses, recent studies have shown that patients with GC may exhibit abnormalities in glucose metabolism. Glucose metabolic disorders are prevalent issues among GC patients, primarily characterized by hyperglycemia.27 These disturbances can markedly impact patient outcomes. Hyperglycemia has been shown to damage the vascular endothelium, reduce vascular elasticity, and increase the risk of arteriosclerosis, leading to insufficient tumor blood supply, disruption of the tumor microenvironment, and further malignant progression. In the present study, postoperative fasting glucose and GHb levels were slightly higher in the TLTG cohort compared to the LATG cohort. This may be due to the amelioration of systemic inflammation after TLTG surgery, which subsequently improved perioperative risk factors, enhanced pancreatic β-cell function, and increased insulin synthesis and secretion, thereby positively influencing the metabolic health of GC patients.
Hyperlipidemia is a lipid metabolic disorder prevalent in GC patients and also represents a significant factor that influences cancer progression. Lipid peroxidation products may directly cause DNA damage, contributing to the onset of cancer. Additionally, hyperlipidemia can induce a chronic low-grade inflammatory state that stimulates tumor development, modulates the tumor microenvironment, and hampers treatment outcomes.28 In this study, the TLTG cohort showed superior postoperative lipid metabolism levels, with markedly lower TC, LDL, and HDL three months post-surgery compared to the LATG cohort. Similar to the observed alterations in glucose metabolism, this may be due to the ability of TLTG surgery to improve systemic inflammatory responses, which in turn regulates lipid metabolism.4 No significant difference was found in TG levels between the TLTG and LATG cohorts one and three months post-surgery. However, a comprehensive assessment of lipid metabolism should consider TG, TC, LDL, and HDL levels in combination to provide a more accurate understanding of a patient’s metabolic health.
Overall, this study found that TLTG has certain advantages over LATG in regulating postoperative inflammatory responses and improving glucose and lipid metabolism. These findings provide new insights into the treatment of GC and a basis for further optimizing treatment strategies. However, clinical practice cannot rely solely on evident data, and additional factors and potential future prospects should be considered. While current research has demonstrated the advantages of TLTG in several aspects,29 its surgical complexity and technical demands have also increased.30 Moreover, with advancements in laparoscopic technology, future applications of LATG may also improve in terms of surgery duration and blood loss, minimizing the differences between LATG and TLTG. The optimal choice should be made based on specific circumstances and technical conditions.27,30 In clinical practice, we recommend that physicians first evaluate the patient’s specific circumstances, such as lesion status and physical condition, before choosing the surgical approach based on the medical team’s expertise and equipment availability. Additionally, to improve surgical efficiency and safety, healthcare providers should actively adopt new technologies and concepts to determine the most suitable option between TLTG and LATG. Furthermore, it is crucial to fully consider the patient’s postoperative recovery and quality of life, opting for the least invasive surgical method whenever possible.
This study has several limitations. Firstly, this study did not explore the differences in disease prognosis and quality of life between TLTG and LATG patients, which is undoubtedly an important research direction. Secondly, there are challenges in quantifying and incorporating postoperative recovery into the clinician decision-making process which requires further exploration. Additionally, the cost-effectiveness ratio of TLTG and LATG was not considered, which is especially important in resource-limited healthcare settings. Further studies should focus on enhancing surgical efficiency and safety, thereby benefiting more patients, particularly those in resource-limited environments, while maintaining quality.
Conclusions
In conclusion, we demonstrated that TLTG surgery is superior in terms of short-term clinical outcomes compared to LATG for the treatment of GC. Specifically, TLTG exhibited markedly reduced surgical trauma, accelerated postoperative recovery, milder postoperative inflammatory responses, and the alleviation of metabolic disorders. The results of this study support the broader adoption of TLTG in the surgical treatment of GC. Nevertheless, further studies are required to elucidate the long-term effects.
Data Sharing Statement
All data generated or analysed during this study are included in this manuscript.
Ethics Approval
This study complied with the Declaration of Helsinki. Because of the retrospective nature of the study, patient consent for inclusion was waived.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Natural Science Research Project of Anhui Educational Committee (2024AH051283, 2024AH051233). Health Research Program of Anhui (AHWJ2023A30070). Medical Innovation Foundation from Spinal deformity clinical and research center of Anhui province (AHJZJX-GG2023-004).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Liu W, Zeng N, Pope ZC, et al. Acute effects of immersive virtual reality exercise on young adults’ situational motivation. J Clin Med. 2019;8(11):1947. doi:10.3390/jcm8111947
2. Yang Y, Chen Z, Zhou L, et al. In silico development and validation of a novel glucose and lipid metabolism-related gene signature in gastric cancer. Transl Cancer Res. 2022;11(7):1977–1993. doi:10.21037/tcr-22-168
3. Natsume T, Kawahira H, Hayashi H, et al. Low peritoneal and systemic inflammatory response after laparoscopy-assisted gastrectomy compared to open gastrectomy. Hepato-Gastroenterology. 2011;58(106):659–662.
4. Lee SJ, Kim JY, Ha TK, et al. Changes in lipid indices and body composition one year after laparoscopic gastrectomy: a prospective study. Lipids Health Dis. 2018;17(1):113. doi:10.1186/s12944-018-0729-1
5. Wang FH, Shen L, Li J, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. 2019;39(1):10. doi:10.1186/s40880-019-0349-9
6. Wang Z, Xing J, Cai J, et al. Short-term surgical outcomes of laparoscopy-assisted versus open D2 distal gastrectomy for locally advanced gastric cancer in North China: a multicenter randomized controlled trial. Surg Endosc. 2019;33(1):33–45. doi:10.1007/s00464-018-6391-x
7. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American association of clinical endocrinologists, the obesity society, and American society for metabolic & bariatric surgery. Obesity. 2013;21(Suppl 1(1)):S1–27.
8. Liu J, Zhou H, Qin H, et al. Comparative study of clinical efficacy using three-dimensional and two-dimensional laparoscopies in the treatment of distal gastric cancer. Onco Targets Ther. 2018;11:301–306. doi:10.2147/OTT.S153520
9. Antonakis PT, Ashrafian H, Isla AM. Laparoscopic gastric surgery for cancer: where do we stand? World J Gastroenterol. 2014;20(39):14280–14291. doi:10.3748/wjg.v20.i39.14280
10. Liu T, Liu J, Wang G, et al. Circulating tumor cells: a valuable indicator for locally advanced nasopharyngeal carcinoma. Europ Archiv Oto-Rhino-Laryngol. 2024;281:4963–4972. doi:10.1007/s00405-024-08714-w
11. Wood DE. National comprehensive cancer network (NCCN) clinical practice guidelines for lung cancer screening. Thorac Surg Clin. 2015;25(2):185–197. doi:10.1016/j.thorsurg.2014.12.003
12. Bosma E, Veen EJ, de Jongh MA, et al. Variable impact of complications in general surgery: a prospective cohort study. Can J Surg. 2012;55(3):163–170. doi:10.1503/cjs.027810
13. Tian Y, Yang P, Lin Y, et al. Assessment of carbon nanoparticle suspension lymphography-guided distal gastrectomy for gastric cancer. JAMA Netw Open. 2022;5(4):e227739. doi:10.1001/jamanetworkopen.2022.7739
14. Sung YT, Wu JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): a new technique for psychological measurement. Behavior Res Methods. 2018;50(4):1694–1715. doi:10.3758/s13428-018-1041-8
15. Guissé NF, Stone JD, Keil LG, et al. Modified Clavien-Dindo-sink classification system for adolescent idiopathic scoliosis. Spine Deform. 2022;10(1):87–95. doi:10.1007/s43390-021-00394-4
16. Chen Z, Chen G, Li Y, et al. Comparison of totally laparoscopic and laparoscopic-assisted approach in gastrectomy with D2 lymphadenectomy for advanced gastric cancer after neoadjuvant chemotherapy: a retrospective comparative study. Ann Surg Treat Res. 2024;106(4):218–224. doi:10.4174/astr.2024.106.4.218
17. Lin M, Zheng CH, Huang CM, et al. Totally laparoscopic versus laparoscopy-assisted Billroth-I anastomosis for gastric cancer: a case-control and case-matched study. Surg Endosc. 2016;30(12):5245–5254. doi:10.1007/s00464-016-4872-3
18. Zhao Y, Bai ZX, Wang T, et al. Application of self-pulling and latter transection in totally laparoscopic total gastrectomy. J Minim Access Surg. 2023. doi:10.4103/jmas.jmas_57_23
19. Wu Q, Wang Y, Peng Q, et al. Safety and effectiveness of totally laparoscopic total gastrectomy vs laparoscopic-assisted total gastrectomy: a meta-analysis. Int J Surg. 2024;110(2):1245–1265. doi:10.1097/JS9.0000000000000921
20. Xiao F, Qiu XF, You CW, et al. Influence of liver function after laparoscopy-assisted vs totally laparoscopic gastrectomy. World J Gastrointestinal Surg. 2023;15(5):859–870. doi:10.4240/wjgs.v15.i5.859
21. Hao Y, Yu P, Qian F, et al. Comparison of laparoscopy-assisted and open radical gastrectomy for advanced gastric cancer: a retrospective study in a single minimally invasive surgery center. Medicine. 2016;95(25):e3936. doi:10.1097/MD.0000000000003936
22. Seo JW, Park KB, Kim EY, et al. Surgical outcomes and prognosis of intracorporeal versus extracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer: a propensity score-matching study. Sci Rep. 2024;14(1):17793. doi:10.1038/s41598-024-67681-8
23. Rossi CR, Mozzillo N, Maurichi A, et al. Number of excised lymph nodes as a quality assurance measure for lymphadenectomy in melanoma. JAMA Surg. 2014;149(7):700–706. doi:10.1001/jamasurg.2013.5676
24. International Surgical Outcomes Study (ISOS) Group. Prospective observational cohort study on grading the severity of postoperative complications in global surgery research. Br J Surg. 2019;106(2):e73–e80. doi:10.1002/bjs.11025
25. Zhang R, Hu C, Zhang J, et al. Prognostic significance of inflammatory and nutritional markers in perioperative period for patients with advanced gastric cancer. BMC Cancer. 2023;23(1):5. doi:10.1186/s12885-022-10479-6
26. Han WH, Oh YJ, Eom BW, et al. A comparative study of the short-term operative outcome between intracorporeal and extracorporeal anastomoses during laparoscopic total gastrectomy. Surg Endosc. 2021;35(4):1602–1609. doi:10.1007/s00464-020-07539-y
27. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68(6):394–424. doi:10.3322/caac.21492
28. Hammer M, Storey S, Hershey DS, et al. Hyperglycemia and cancer: a state-of-the-science review. Oncol Nurs Forum. 2019;46(4):459–472. doi:10.1188/19.ONF.459-472
29. Xing J, Wang Y, Shan F, et al. Comparison of totally laparoscopic and laparoscopic assisted gastrectomy after neoadjuvant chemotherapy in locally advanced gastric cancer. Eur J Surg Oncol. 2021;47(8):2023–2030. doi:10.1016/j.ejso.2021.02.002
30. Dong C, Zhou W, Zang Y, et al. Totally laparoscopic gastrectomy with natural orifice (vagina) specimen extraction in gastric cancer: introduction of a new technique. J Minim Access Surg. 2022;18(3):484–486. doi:10.4103/jmas.JMAS_328_20
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.