Back to Journals » Infection and Drug Resistance » Volume 18
Compassionate Use of Omadacycline in a Down Syndrome Pre-Schooler With Critically Ill Atypical Pneumonia Caused by Macrolide-Resistant Mycoplasma Pneumoniae
Authors Wu D , Xie FJ, Wang YJ, Jiang XH, Zhang GL, Zhang H, Zhu YC, Zhang Y, Tang YJ, Lin YL , Xu JX, Zhang JN, Liu BW, Kang K, Gao Y
Received 15 October 2024
Accepted for publication 16 January 2025
Published 21 January 2025 Volume 2025:18 Pages 391—400
DOI https://doi.org/10.2147/IDR.S500982
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Di Wu,1,* Feng-Jie Xie,2,* Ya-Jun Wang,3,* Xiao-Hui Jiang,1,* Guo-Li Zhang,3,* Hong Zhang,2 Yu-Cheng Zhu,4 Yan Zhang,4 Yu-Jia Tang,1 Yi-Lu Lin,1 Jia-Xi Xu,5 Jia-Ning Zhang,5 Bo-Wen Liu,5 Kai Kang,5 Yang Gao1
1Department of Critical Care Medicine, The Sixth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China; 2Department of Critical Care Medicine, The Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, Heilongjiang Province, People’s Republic of China; 3Department of Pediatrics, The Sixth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China; 4Department of Critical Care Medicine, The Hongxinglong Hospital of Beidahuang Group, Shuangyashan, Heilongjiang Province, People’s Republic of China; 5Department of Critical Care Medicine, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kai Kang, Department of Critical Care Medicine, The First Affiliated Hospital of Harbin Medical University, 23 Youzheng Street, Nangang District, Harbin, Heilongjiang Province, 150001, People’s Republic of China, Tel +86-13904618016, Email [email protected] Yang Gao, Department of Critical Care Medicine, The Sixth Affiliated Hospital of Harbin Medical University, 998 Aiying Road, Songbei District, Harbin, Heilongjiang Province, 150027, People’s Republic of China, Tel +86-13045160709, Email [email protected]
Background: Rapid and accurate identification of causative organisms and prompt initiation of pathogen-targeted antibiotics are crucial for managing atypical pneumonia. The widespread application of targeted next-generation sequencing (t-NGS) in clinical practice demonstrates significant targeted advantages in rapid and accurate aetiological identification and antimicrobial resistance genes detection, particularly for difficult-to-culture, rare, or unexpected pathogens. An alarming surge of acquired macrolide resistance (MR) in Mycoplasma pneumoniae (MP) presents a substantial challenge for the clinical selection of pathogen-targeted antibiotics worldwide, especially for fluoroquinolone-restricted pediatric patients with limited options available.
Case Presentation: In this case report, we present for the first time the compassionate use of omadacycline (OMC) in a Down syndrome pre-schooler with critically ill atypical pneumonia caused by macrolide-resistant MP. The treatment achieved a favourable therapeutic effect without any related adverse events (AEs) during hospitalization and follow-up.
Conclusion: In clinical practice, rapid and accurate identification of causative organisms should be a priority for prompt initiation of pathogen-targeted antibiotics, in which tNGS possesses enormous potential, particularly for difficult-to-culture MP. At present, OMC is not recommended in the package insert for clinical application in pediatric patients under 8 years of age due to potential age-specific AEs on tooth colour and development as well as bone growth. The superior efficacy and safety of OMC in the management of critically ill atypical pneumonia caused by macrolide-resistant MP were comprehensively documented in this Down syndrome pre-schooler, which merits future well-designed studies to validate our findings, enhance understanding of the features of OMC, and further expand its clinical application in preschool-aged patients.
Keywords: omadacycline, pre-schooler, down syndrome, macrolide-resistant mycoplasma pneumoniae, atypical pneumonia, targeted next generation sequencing, aetiological identification, antimicrobial resistance gene detection
Introduction
Atypical pneumonia is typically caused by atypical pathogens that evade detection and cultivation by Gram staining and conventional microbiological approaches, among which the most predominant causative organisms include Mycoplasma pneumoniae (MP), Chlamydia pneumoniae, Chlamydia psittaci, Legionella species, and Coxiella burnetii.1 Rapid and accurate identification of these organisms and prompt initiation of pathogen-targeted antibiotics are crucial for managing atypical pneumonia.2 The widespread application of targeted next-generation sequencing (t-NGS) in clinical practice can compensate for the limitations of traditional diagnostic means for atypical pathogens, and demonstrate targeted superiority in rapid and accurate aetiological identification and antimicrobial resistance genes detection, particularly for difficult-to-culture, rare, or unexpected pathogens.3–5 Currently, the predominant form of macrolide resistant (MR) is attributed to A2063G mutation in domain V of the 23S rRNA gene, which can further decrease the affinity of macrolide with MP ribosome, as is the cyclic outbreak of macrolide-resistant MP epidemic in China since June 2023.6–10 An alarming surge of acquired MR in MP presents a substantial challenge for the clinical selection of pathogen-targeted antibiotics worldwide, which is more severe in East Asia, especially for fluoroquinolone-restricted pediatric patients with limited options available.11,12
Omadacycline (OMC), a novel broad-spectrum aminomethylcycline antibiotic, received approval from the United States Food and Drug Administration in October 2018. It is used to treat community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections caused by sensitive causative organisms in adult patients through a regimen of intravenous or oral administration once a day.13,14 Due to its potent antibacterial activity against atypical pathogens in vitro, OMC may serve as an effective and safe alternative or salvage option for the management of macrolide resistant MP infection, particularly in fluoroquinolone-restricted pediatric patients.15–17 However, OMC is not recommended in the package insert for clinical application in pediatric patients under 8 years of age due to potential age-specific adverse effects (AEs) on tooth colour and development as well as bone growth. Consequently, the clinical efficacy and safety of OMC in pediatric patients under 8 years of age have not been established.
To address this issue, this case report introduces the compassionate use of OMC for the first time in a Down syndrome pre-schooler with critically ill atypical pneumonia caused by macrolide-resistant MP. The treatment achieved a favourable therapeutic effect without any related AEs during hospitalization and follow-up. As a salvage option for inadequate clinical response to initial empirical antibiotics against macrolide resistant MP, OMC has exhibited superior efficacy and safety in the management of critically ill atypical pneumonia in this preschool-aged patient, indicating a promising new option in such scenarios that needs to be further explored and validated in the following studies.
Case Presentations
Chief Complaints
On April 2, 2024, a 5-year-and-10-month-old boy was admitted to the Department of Critical Care Medicine at the Sixth Affiliated Hospital of Harbin Medical University due to worsening atypical pneumonia caused by macrolide resistant MP.
History of Present Illness
Fifteen days before admission, the pre-schooler experienced intermittent fever with a maximum body temperature of 38.9°C, which responded temporarily to oral antipyretics but recurred within a few hours. Thirteen days prior to admission, he developed paroxysmal cough with sputum and occasional wheezing, leading to his admission to the local hospital. Eight days before admission, the initial lung computed tomography (CT) scan revealed multiple patchy shadows on both sides with the right side being more prominent (Figure 1), and therefore the diagnosis of pneumonia was confirmed. Seven days before admission, the pathogens were identified as macrolide resistant MP with A2063G mutation in domain V of the 23S rRNA gene and rhinovirus (RV) type C by t-NGS of pharyngeal swab, with homogenized sequence counts of 28,203 and 15,412 × 106, respectively. During his hospitalization at the local hospital, he underwent treatment including intravenous infusion of cefoperazone/sulbactam sodium for 10 days, meropenem for 3 days, azithromycin and methylprednisolone (40 mg once daily for the first three days, halved for the next four days) for 7 days, as well as oral cough suppressant and antiviral drug, and aerosol inhalation. One day before admission, the rechecked lung CT scan showed a significant deterioration (Figure 1), prompting his transfer to the Department of Critical Care Medicine at the Sixth Affiliated Hospital of Harbin Medical University for further management.
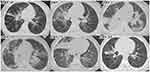 |
Figure 1 The lung CT scans before admission and during hospitalization and follow-up. Abbreviations: CT, computed tomography. |
History of Past Illness
The pre-schooler was diagnosed with Down syndrome shortly after birth and subsequently developed an allergy to mangoes over time.
Personal and Family History
There was no related personal or family history.
Physical Examination
Upon admission, the following physical examination were noted: height 1.18 meters, weight 21.5 kg, body mass index 15.44 kg/m², body temperature 36.7°C, heart rate 160 beats/min, blood pressure 90/61 mmHg, respiratory rate 35 breaths/min, and pulse oxygen saturation 92%. Additionally, the child exhibited listlessness, inability verbal communication, a dull expression, a low and flat nose bridge, small external ears, nasal flaring, shortness of breath, rough breathing sounds and audible moist rales in both lungs, a short penis, and short and thick fingers on both hands.
Laboratory Parameters
Laboratory parameters before admission and during hospitalization are detailed in Table 1 and illustrated in Figure 2. Arterial blood gas analysis results showed pH 7.492, carbon dioxide partial pressure 26.9 mmHg, oxygen partial pressure 72.6 mmHg, potassium ion 2.8 mmol/L, sodium ion 133 mmol/L, free calcium ion 1.12 mmol/L, and lactic acid 2.0 mmol/L. The counts of total T cells, T8 cells, T4 cells, and natural killer lymphocytes were 372,116,254, and 30 cells/µL, respectively, all of which were significantly lower than the normal values. Immunoglobulin A and M levels were 1.98 g/L and 2.61 g/L, respectively, exceeding the upper limit of the normal values. Tests for hepatitis B virus antigen and antibodies for hepatitis C virus, syphilis, and human immunodeficiency virus were all negative. The Pediatric Critical Illness Score upon admission was 84 points.
 |
Table 1 Timeline of Disease Progression (March 20 to April 24, 2024) |
Imaging Findings
Upon admission, echocardiography revealed the presence of left ventricular false tendons and trace pericardial effusion. Chest ultrasound indicated consolidation in the lower lobe of the right lung accompanied by minimal pleural effusion, while gastrointestinal ultrasound displayed flatulence in the digestive tract. Furthermore, ultrasound examinations of the liver, gallbladder, pancreas, spleen, and urinary system showed no significant abnormalities. Lung CT scans conducted before admission, and during hospitalization and follow-up are depicted in Figure 1.
Diagnosis Upon Admission
The pre-schooler was diagnosed with atypical pneumonia caused by macrolide-resistant MP and RV type C, along with Down syndrome, based on current and past medical history, clinical presentations, physical examination, laboratory parameters, and imaging findings upon admission. The resistance mechanism of MP was identified as A2063G mutation in domain V of the 23S rRNA gene by t-NGS of pharyngeal swab.
Treatment and Follow-up
After admission to intensive care unit, comprehensive therapies were promptly initiated, including mask oxygen inhalation, oral doxycycline as initial empirical antibiotic (47 mg twice daily), intravenous immunoglobulin pulse therapy (400 mg/kg), LiDCO rapid V3-directed fluid management, appropriate sedation, and aerosol inhalation.18 Subsequently, induced sputum was collected for tNGS. A central venous catheter was inserted into the right femoral vein under ultrasound guidance and central venous pressure was measured as 8 cmH2O. On the second day post-admission, due to worsening respiratory status and pulse oxygen saturation, the pre-schooler received high-flow nasal cannula (HFNC) oxygen therapy. The pathogens were still identified as macrolide-resistant MP with A2063G mutation in domain V of the 23S rRNA gene and homogenized sequence counts of 23,426 × 106, as well as human adenovirus (HAdV) type 3 and RV type C by tNGS of induced sputum. By the third day, the rechecked lung CT scan revealed multiple patchy shadows and partial consolidation on both sides (Figure 1). Given the progression of lung infection and the limited self-cleaning ability of the airway in Down syndrome, the patient underwent orotracheal intubation and invasive mechanical ventilation. Lung-protective ventilation strategies, lateral decubitus position on both sides, methylprednisolone (40 mg once daily), appropriate analgesia, prophylactic anti-coagulation, enteral nutrition, and probiotics were implemented. Oral doxycycline was replaced with intravenous infusion of OMC (50 mg once daily, doubled on the first day). By the fourth day post-admission, the serum levels of 12 cytokines, including interleukin (IL)-2, IL-4, tumor necrosis factor-α, IL-10, IL-17A, IL-6, interferon (IFN)-γ, IFN-α, IL-5, IL-8, IL-12p70, and IL-1β, showed significant elevation. Notably, the serum levels of IL-10, IL-17A, IL-6, IL-8, and IL-12p70 were 92.26 pg/mL,238.19 pg/mL,350.70 pg/mL,270.78 pg/mL, and 95.87 pg/mL, respectively, all of which exceeded the upper limit of the normal values by more than tenfold. By the tenth day post-admission, the pre-schooler’s condition and lung CT scan showed significant improvement, allowing for the transition from invasive mechanical ventilation to HFNC oxygen therapy and cessation of methylprednisolone. Lower respiratory tract specimens were collected for tNGS again before extubation, and subsequent result remained macrolide-resistant MP with a significant decrease in homogenized sequence counts to 396 × 104, as well as HAdV type 3, herpes simplex virus type 1, and RV type C. As the condition improved and the requirements of respiratory support decreased, the intensity of respiratory support was progressively reduced. By the twelfth day post-admission, HFNC oxygen therapy was replaced with nasal catheter oxygen inhalation. On the fourteenth day post-admission, the serum levels of all 12 cytokines significantly decreased, among which IFN-α and IL-12p70 had returned to normal levels, and only the serum level of IL-6 still exceeded the upper limit of the normal value by more than tenfold. The pre-schooler was in stable condition and transferred to the pediatric ward after thorough evaluation. On the fifteenth day post-admission, the rechecked lung CT scan displayed that multiple consolidation and inflammatory exudation in both lungs were gradually relieved and absorbed. On the eighteenth day post-admission, OMC was discontinued, and empiric treatment with cefoperazone/sulbactam sodium was initiated.
Outcome and Follow-up
The pre-schooler was discharged from the hospital on the 22nd day following admission. A follow-up lung CT scan conducted 32 days post-discharge revealed scattered ground-glass high-density patches in both lungs and localized consolidation in the right lung (Figure 1).
Discussion
MP is a major airway-invasive pathogen and responsible for approximately 15–40% of community-acquired pneumonia, with significant heterogeneity in geographical regions, time frames, diagnostic samples and methods, age distribution, and disease severity.19–22 However, there is a lack of specific clinical manifestations, physical examination, laboratory parameters, microbial diagnostic approaches, and imaging features for accurate identification of MP pneumonia (MPP), posing a considerable challenge for clinicians to make rapid and accurate aetiological diagnoses.23,24 tNGS, as a sophisticated molecular level technique, holds substantial potential for early detection of infection-causing MP and identification of antimicrobial resistance genes to guide subsequent optimization of targeted antibiotics and minimize overconsumption of macrolides.25 MP is a type of paracellular bacteria without a peptidoglycan cell wall susceptible to destruction of β-lactam antibiotics, and therefore its morphological peculiarity leads to natural resistance to them.26,27 Due to low minimum inhibitory concentration and superior safety profile in pediatric patients with atypical pneumonia, macrolides stand out in competition with tetracyclines and fluoroquinolones as the first-line recommended option in clinical practice.27,28 However, the rapid increase in acquired MR caused by overconsumption of macrolides has shaken this option and pushed pediatric clinicians to a dead end.29 Currently, developing and newly developed pathogen-targeted antibiotics are highly expected to address this clinical dilemma.30
OMC, an improved semisynthetic derivative of the tetracycline class, exerts antibacterial activity by binding to the 30S ribosomal subunit to inhibit protein synthesis in causative organisms.31 Importantly, OMC retains efficacy against common acquired tetracycline resistance mechanisms through chemical modifications of structure at the C9 and C7 positions of the tetracycline core ring, which may be one of the underlying reasons for the successful management in this case after the switch from oral doxycycline to intravenous infusion of OMC.32,33 Moreover, in healthy adult subjects, the concentrations of OMC in epithelial lining fluid and alveolar macrophages are greater than/equal to those in plasma at systemic exposure, indicating its preferred targeting of lung tissue during clinical application.34 OMC has a favourable safety profile in pharmacokinetics, as demonstrated by a minimal potential for known drug-drug interactions and unnecessary dose adjustments for age, hepatic or renal injury, or even end-stage renal disease.35,36 A well-tolerated clinical property of OMC is usually manifested in the major AEs being only mild-to-moderate and transient central nervous system and gastrointestinal intolerances, none of which were observed during the management and follow-up in this pre-schooler.37,38
The clinical features of critically ill atypical pneumonia caused by macrolide-resistant MP and the superior efficacy and safety of OMC were comprehensively documented in this case. Firstly, multiple positive results from tNGS as well as the significant decrease in homogenized sequence counts and clinical improvement following OMC administration strongly indicated that macrolide-resistant MP with A2063G mutation in domain V of the 23S rRNA gene was the main culprit for the deterioration in this pre-schooler’s condition. Secondly, the significant reduction in various lymphocyte counts upon admission underscores the pivotal role of host immune responses, particularly the intrinsic immune response, in the progression of MPP.39 The immune deficiency in this pre-schooler may be attributed to Down syndrome diagnosed after birth, which is also our main concern for not increasing dose and prolonging duration of methylprednisolone.40 In addition to host immune responses, co-infection with macrolide-resistant MP and HAdV contributed to the occurrence and development of refractory MPP and exacerbated clinical features in this pediatric patient, highlighting that refractory MPP may be a multifactorial condition.25,41 Thirdly, the off-label use of OMC was a critical turning point in managing this pre-schooler’s condition, emphasizing the importance of appropriate selection of pathogen-targeted antibiotics in clinical management of critically ill atypical pneumonia. However, the lack of an available oral formulation in clinical practice precluded the step-down transition of OMC from intravenous infusion to oral administration in this case. Furthermore, the unique immunomodulatory activity of OMC suppresses the early over-activated systemic inflammatory responses, which is conducive to the successful management in this pre-schooler.42,43 Finally, the follow-up lung CT scan on the 32nd day post-discharge revealed scattered ground-glass high-density patches in both lungs and localized consolidation in the right lung, indicating that it may take a longer time to repair critically ill lung injury caused by macrolide-resistant MP in preschool-aged patients.
The major limitation of this case is the insufficient follow-up time to fully establish the absence of OMC-related AEs in this pre-schooler, and further follow-up is ongoing. Additionally, the aetiological diagnosis of macrolide resistant MP relied primarily on three tNGS results from pharyngeal swab, induced sputum, and lower respiratory tract specimens, respectively, without associated serological evidence. Lastly, the off-label use of OMC in the successful management of this pre-schooler with critically ill atypical pneumonia caused by macrolide resistant MP represents only an isolated case, and therefore further well-designed studies are warranted to definitively assess the efficacy and safety of OMC in this patient population.
Conclusion
In clinical practice, rapid and accurate identification of causative organisms should be a priority for prompt initiation of pathogen-targeted antibiotics, in which tNGS possesses enormous potential, particularly for difficult-to-culture MP. Avoiding overconsumption of macrolides may be one of the targeted measures to minimize acquired MR in MP. The superior efficacy and safety of OMC in the management of critically ill atypical pneumonia caused by macrolide resistant MP were comprehensively documented in this Down syndrome pre-schooler, which merits future well-designed studies to validate our findings, enhance understanding of the features of OMC, and further expand its clinical application in preschool-aged patients.
Data Sharing Statement
The original contributions discussed in this study are detailed in the article. For additional inquiries, please contact the corresponding authors directly.
Ethics Statement
The study involving human participants was approved by the Ethics Committee of the Sixth Affiliated Hospital of Harbin Medical University (IRB number: LC2024-057) and authorized to publish case details. The medical data required for this study were obtained by our research team from the Department of Medical Records of the Sixth Affiliated Hospital of Harbin Medical University. The study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent to have the case details and any accompanying potentially identifiable images or data published has been obtained by the patient’s parent.
Acknowledgments
The authors extend their gratitude to all individuals who provided valuable assistance, guidance, and support for this article.
Author Contributions
Di Wu, Feng-Jie Xie, Ya-Jun Wang, Xiao-Hui Jiang, Guo-Li Zhang, Kai Kang and Yang Gao were involved in the literature search, conception, and preparation, editing, and review of the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design and execution, acquisition, analysis and interpretation of data, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Di Wu, Feng-Jie Xie, Ya-Jun Wang, Xiao-Hui Jiang and Guo-Li Zhang have contributed equally to this work.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study received financial support from the following sources: the National Natural Science Foundation of China (No.82372172), the Key Research and Development Plan Project of Heilongjiang Province (No.GA23C007), the Heilongjiang Province Postdoctoral Start-up Fund (No.LBH-Q20037), the Research Project of Heilongjiang Provincial Health Commission (No.20231717010461), the Special Fund for Clinical Research of Wu Jie-Ping Medical Foundation (No.320.6750.2022-02-16), the 2024 Artificial Liver Special Fund (No.iGandanF-1082024-RGG021 and iGandanF-1082024-RGG063), and the Scientific Research Innovation Fund of the First Affiliated Hospital of Harbin Medical University (No.2021M08 and 2018L006).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Miyashita N. Atypical pneumonia: pathophysiology, diagnosis, and treatment. Respir Investig. 2022;60(1):56–67. doi:10.1016/j.resinv.2021.09.009
2. Gao Y, Wang HL, Zhang ZJ, et al. A standardized step-by-step approach for the diagnosis and treatment of sepsis. J Intensive Care Med. 2022;37(10):1281–1287. doi:10.1177/08850666221085181
3. Lin R, Xing Z, Liu X, et al. Performance of targeted next-generation sequencing in the detection of respiratory pathogens and antimicrobial resistance genes for children. J Med Microbiol. 2023;72(11). doi:10.1099/jmm.0.001771
4. Liu HY, Bi XF, Wang YJ, et al. Compassionate use of contezolid in a toddler with severe community-acquired pneumonia induced by staphylococcus aureus: a case report and follow-up. Front Pediatr. 2024;12:1321447. doi:10.3389/fped.2024.1321447
5. Yang SL, Gao Y, Han ZY, et al. Successful treatment of near-fatal pulmonary embolism and cardiac arrest in an adult patient with fulminant psittacosis-induced severe acute respiratory distress syndrome after veno-venous extracorporeal membrane oxygenation rescue: a case report and follow-up. Heliyon. 2023;9(10):e20562. doi:10.1016/j.heliyon.2023.e20562
6. Jiang Y, Dou H, Xu B, et al. Macrolide resistance of mycoplasma pneumoniae in several regions of China from 2013 to 2019. Epidemiol Infect. 2024:152:e75. doi:10.1017/S0950268824000323
7. Xu M, Li Y, Shi Y, et al. Molecular epidemiology of mycoplasma pneumoniae pneumonia in children, Wuhan, 2020-2022. BMC Microbiol. 2024;24(1):23. doi:10.1186/s12866-024-03180-0
8. Xing FF, Chiu KH, Deng CW, et al. Post-COVID-19 pandemic rebound of macrolide-resistant mycoplasma pneumoniae infection: a descriptive study. Antibiotics. 2024;13(3):262. doi:10.3390/antibiotics13030262
9. Dekyi XY, Wang X, Feng S, et al. Predominance of A2063G mutant strains in the mycoplasma pneumoniae epidemic in children: a clinical and epidemiological study in 2023 in Wuhan, China. Int J Infect Dis. 2024;145:107074. doi:10.1016/j.ijid.2024.107074
10. Morozumi M, Hasegawa K, Kobayashi R, et al. Emergence of macrolide-resistant mycoplasma pneumoniae with a 23S rRNA gene mutation. Antimicrob Agents Chemother. 2005;49:2302–2306. doi:10.1128/AAC.49.6.2302-2306.2005
11. Oishi T, Ouchi K. Recent trends in the epidemiology, diagnosis, and treatment of macrolide-resistant mycoplasma pneumoniae. J Clin Med. 2022;11(7):1782. doi:10.3390/jcm11071782
12. Yen MH, Yan DC, Wang CJ, et al. The clinical significance of and the factors associated with macrolide resistance and poor macrolide response in pediatric mycoplasma pneumoniae infection: a retrospective study. J Microbiol Immunol Infect. 2023;56(3):634–640. doi:10.1016/j.jmii.2023.01.010
13. Jahanbakhsh S, Howland J, Ndayishimiye Uwineza MO, et al. Evaluation of omadacycline against intracellular Mycobacterium abscessus in an infection model in human macrophages. JAC Antimicrob Resist. 2023;5(5):dlad104. doi:10.1093/jacamr/dlad104
14. Stets R, Popescu M, Gonong JR, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med. 2019;380(6):517–527. doi:10.1056/NEJMoa1800201
15. Karlowsky JA, Steenbergen J, Zhanel GG. Microbiology and preclinical review of omadacycline. Clin Infect Dis. 2019;69(Suppl 1):S6–S15. doi:10.1093/cid/ciz395
16. Cilloniz C, Torres A. The pharmacokinetic evaluation of omadacycline (Oral Only Dosing Regimen) for the treatment of community-acquired bacterial pneumonia (CABP). Expert Opin Drug Metab Toxicol. 2023;19(9):569–576. doi:10.1080/17425255.2023.2261376
17. Xiao M, Huang JJ, Zhang G, et al. Antimicrobial activity of omadacycline in vitro against bacteria isolated from 2014 to 2017 in China, a multi-center study. BMC Microbiol. 2020;20:350. doi:10.1186/s12866-020-02019-8
18. Lee H, Choi YY, Sohn YJ, et al. Clinical efficacy of doxycycline for treatment of macrolide-resistant mycoplasma pneumoniae pneumonia in children. Antibiotics. 2021;10(2):192. doi:10.3390/antibiotics10020192
19. Noori Goodarzi N, Pourmand MR, Rajabpour M, Arfaatabar M, Mosadegh M, Syed Mohamad SA. Frequency of Mycoplasma pneumoniae, Legionella pneumophila and Chlamydia spp. among patients with atypical pneumonia in Tehran. New Microbes New Infect. 2020;37:100744. doi:10.1016/j.nmni.2020.100744
20. Otheo E, Rodríguez M, Moraleda C, et al. Viruses and mycoplasma pneumoniae are the main aetiological agents of community-acquired pneumonia in hospitalized pediatric patients in Spain. Pediatr Pulmonol. 2022;57(1):253–263. doi:10.1002/ppul.25721
21. Suh JH, Ahn B, Song SH, et al. Etiology and clinical characteristics of community-acquired pneumonia in Korean children during the pre-COVID-19 period, 2015-2020. J Korean Med Sci. 2023;38(43):e339. doi:10.3346/jkms.2023.38.e339
22. Chi H, Huang YC, Liu CC, et al. Characteristics and etiology of hospitalized pediatric community-acquired pneumonia in Taiwan. J Formos Med Assoc. 2020;119(10):1490–1499. doi:10.1016/j.jfma.2020.07.014
23. Kumar S, Kumar S. Mycoplasma pneumoniae: among the smallest bacterial pathogens with great clinical significance in children. Indian J Med Microbiol. 2023;46:100480. doi:10.1016/j.ijmmb.2023.100480
24. Copete AR, Vera C, Herrera M, Aguilar YA, Rueda ZV, Vélez LA. Mycoplasma pneumoniae in children with and without community-acquired pneumonia. what do PCR and serology say? Pediatr Infect Dis J. 2020;39(7):e104–e108. doi:10.1097/INF.0000000000002636
25. Lin L, Zhang R, Zhang Z, et al. Clinical value of metagenomics next-generation sequencing in antibiotic resistance of a patient with severe refractory mycoplasma pneumoniae pneumonia: a case report. Infect Drug Resist. 2023;16:4593–4597. doi:10.2147/IDR.S419873
26. Matsui H, Sugimura M, Inoue-Tsuda M, Iwabuchi K, Hanaki H. Development of an immunochromatographic test for the detection of mycoplasma pneumoniae GroES antigen. J Microbiol Methods. 2021;191:106359. doi:10.1016/j.mimet.2021.106359
27. Shim JY. Current perspectives on atypical pneumonia in children. Clin Exp Pediatr. 2020;63(12):469–476. doi:10.3345/cep.2019.00360
28. Cai F, Li J, Liang W, Wang L, Ruan J. Effectiveness and safety of tetracyclines and quinolones in people with mycoplasma pneumonia: a systematic review and network meta-analysis. EClinicalMedicine. 2024;71:102589. doi:10.1016/j.eclinm.2024.102589
29. Tsai TA, Tsai CK, Kuo KC, Yu HR. Rational stepwise approach for mycoplasma pneumoniae pneumonia in children. J Microbiol Immunol Infect. 2021;54(4):557–565. doi:10.1016/j.jmii.2020.10.002
30. Xu L, Fang C. Case report: omadacycline in the treatment of macrolide-unresponsive mycoplasma pneumoniae pneumonia in an adolescent patient. Front Cell Infect Microbiol. 2023;13:1244398. doi:10.3389/fcimb.2023.1244398
31. Zhanel GG, Esquivel J, Zelenitsky S, et al. Omadacycline: a novel oral and intravenous aminomethylcycline antibiotic agent. Drugs. 2020;80(3):285–313. doi:10.1007/s40265-020-01257-4
32. Rodvold KA, Burgos RM, Tan X, Pai MP. Omadacycline: a review of the clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2020;59(4):409–425. PMID: 31773505. doi:10.1007/s40262-019-00843-4
33. Barber KE, Bell AM, Wingler MJB, Wagner JL, Stover KR. Omadacycline enters the ring: a new antimicrobial contender. Pharmacotherapy. 2018;38:1194–1204. doi:10.1002/phar.2185
34. Rodvold KA, Pai MP. Pharmacokinetics and pharmacodynamics of oral and intravenous omadacycline. Clin Infect Dis. 2019;69(Suppl 1):S16–S22. doi:10.1093/cid/ciz309
35. Gallagher JC. Omadacycline: a Modernized Tetracycline. Clin Infect Dis. 2019;69(Suppl 1):S1–S5. doi:10.1093/cid/ciz394
36. Leviton IM, Amodio-Groton M. Omadacycline oral dosing and pharmacokinetics in community-acquired bacterial pneumonia and acute bacterial skin and skin structure infection. Clin Drug Investig. 2022;42(3):193–197. doi:10.1007/s40261-022-01119-9
37. O’Riordan W, Cardenas C, Shin E, et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a Phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis. 2019;19(10):1080–1090. doi:10.1016/S1473-3099(19)30275-0
38. Kovacs SJ, Ting L, Praestgaard J, et al. An open-label study of the impact of hepatic impairment on the pharmacokinetics and safety of single oral and intravenous doses of omadacycline. Antimicrob Agents Chemother. 2020;64(11):e01650–20. doi:10.1128/AAC.01650-20
39. Zhu Y, Luo Y, Li L, et al. Immune response plays a role in mycoplasma pneumoniae pneumonia. Front Immunol. 2023;14:1189647. doi:10.3389/fimmu.2023.1189647
40. Liu J, He R, Zhang X, et al. Clinical features and ”early” corticosteroid treatment outcome of pediatric mycoplasma pneumoniae pneumonia. Front Cell Infect Microbiol. 2023;13:1135228. doi:10.3389/fcimb.2023.1135228
41. Zhou Y, Wang J, Chen W, et al. Impact of viral coinfection and macrolide-resistant mycoplasma infection in children with refractory mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2020;20(1):633. doi:10.1186/s12879-020-05356-1
42. Bryant AE, Stevens DL. Investigating the immunomodulatory activities of omadacycline. J Antimicrob Chemother. 2022;78(1):78–83. doi:10.1093/jac/dkac356
43. Sakoulas G. Linezolid versus omadacycline in diabetic soft tissue infections: a signal of different adjunctive immunological properties? J Antimicrob Chemother. 2022;77(6):1503–1505. doi:10.1093/jac/dkac030
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
An Atypical Pneumonia Case of Quinolone-Refractory Chlamydia Pneumoniae Successfully Treated With Omadacycline
Tong J, Zhou L, Chen Y, Xu L, Wang J
Infection and Drug Resistance 2025, 18:2357-2363
Published Date: 7 May 2025


