Back to Journals » International Journal of Nanomedicine » Volume 20
Competition Between Protein and DNA for Binding to Natural Sepiolite Nanofibers
Authors Adame Brooks D, Piétrement O, Dardillac E, Castro Smirnov FA, Aranda P , Ruiz-Hitzky E , Lopez BS
Received 24 July 2024
Accepted for publication 28 November 2024
Published 5 March 2025 Volume 2025:20 Pages 2711—2726
DOI https://doi.org/10.2147/IJN.S488353
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
David Adame Brooks,1,2 Olivier Piétrement,3 Elodie Dardillac,1 Fidel Antonio Castro Smirnov,4 Pilar Aranda,5 Eduardo Ruiz-Hitzky,5 Bernard S Lopez1
1Université de Paris Cité, INSERM U1016, UMR 8104 CNRS, Institut Cochin, Paris, France; 2Centro de Biofísica Médica (Cbiomed), Universidad de Oriente, Santiago de Cuba, Cuba; 3Université Bourgogne Europe, CNRS, Laboratoire interdiciplinaire Carnot de Bourgogne ICB UMR 630, Dijon, F-21000, France; 4Instituto Superior de Tecnologías Ciencias Aplicadas, Universidad de la Habana (Instec-UH), Quinta de Los Molinos, Ave. Salvador Allende Luaces, Habana, 6163, Cuba; 5Instituto de Ciencia de Materiales de Madrid (ICMM), CSIC, c/Sor Juana Inés de la Cruz 3, Madrid, 28049, Spain
Correspondence: Bernard S Lopez, Institut Cochin 24 rue du Faubourg St Jacques, Paris, 75014, France, Email [email protected]
Introduction: Sepiolite nanofibers, which are natural silicates belonging to the clay mineral family, could be promising potential nanocarriers for the nonviral transfer of biomolecules. The physicochemical characteristics of sepiolite make it capable of binding various types of biological molecules, including polysaccharides, lipids, proteins and viruses. Sepiolite nanofibers have also been shown to bind effectively to various types of DNA molecules through electrostatic interactions, hydrogen bonds, cationic bridges and van der Waals forces. In this study, we analyzed the adsorption of DNA and proteins to sepiolite by analyzing the competition among these biomolecules during the adsorption process.
Methods: To determine the binding of sepiolite to proteins, we used BSA and a monoclonal antibody (mAb) against the CD4 membrane antigen as a model. The binding efficiency was measured by adsorption isotherms. Zeta potential measurements of the suspensions were performed using a Brookhaven NanoBrook 90 Plus PALS instrument.
Results: We show here that the adsorption of proteins to sepiolite is increased in the presence of CaCl2 and is charge-dependent and that sepiolite can adsorb proteins even when their net charges are equal to those on its surface. Coating of sepiolite with DNA (Sep/DNA bionanocomposites) reduces the absorption efficiency of both BSA and mAb, and this can be rescued by CaCl2. Conversely, preincubation of sepiolite with BSA or the mAb decreased the efficiency of DNA binding; Ca2+ restored the binding efficiency for BSA but not for the mAb. Changes in pH result in changes in the net charge of proteins, influencing the amount of protein adsorbed.
Conclusion: Although various types of protein interactions with mineral clays have been described, our results confirm that electrostatic forces are among the primary interactions in the adsorption process. These results pave the way for the use of biohybrids as a new class of nanoplatform for gene transfer with potential clinical applications.
Keywords: bionanomaterials, sepiolite clay mineral, sepiolite-DNA interaction, sepiolite-protein interaction
Introduction
Sepiolite, a natural clay of fibrous morphology, belongs to a group of hydrated Mg-silicates that are used mainly for their adsorbent and rheological properties, from the support of pesticides to the development of polymer‒clay nanocomposites.1 This clay mineral has been used in diverse applications and is currently marketed for use in animal feed, fat thickeners and paints, catalyst and enzyme support, elimination of toxic organic compounds and heavy metals, and the preparation of nanocomposites of various types.1–6
The ideal formula of sepiolite is Si12Mg8O30(OH)4(OH2)4.8H2O, its structural arrangement consists of alternate magnesium silicate blocks and tunnels that grow in the crystallographic c-direction. Tunnels that reach the external surface of sepiolite fibers can be considered as channels. These structures are involved in the interaction between the silicate and many diverse compounds, in particular through silanol groups (Si−OH) located at the edges of the channels.7 Other active sites at the sepiolite interface include the negatively charged surface attributed to isomorphic substitution of Mg2+ ions by Al3+ ions and other trivalent cations in the octahedral layers. These two types of active sites are central to the adsorption mechanisms that occur at the external surfaces of fibrous clay minerals.7
Importantly, sepiolite holds great promise for biomedical applications. Indeed, in recent years, interest in the use of sepiolite in biomedicine has increased due to its ability to interact with a wide variety of biomolecules, and sepiolite has been proposed as a good candidate for the administration of drugs and co-adjuvants in vaccines. Sepiolite has been used to transport small molecules such as tetracyclines, nitric oxide, and antisense oligonucleotides into cells in combination with carbon nanotubes to detect DNA and DNA‒drug interactions.8–14 Moreover, the physicochemical particularities of sepiolite and its ability to bind various types of biological molecules, including DNA,15 polysaccharides,16 lipids,17 proteins18 and virus particles,19 make it a promising nanocarrier for the nonviral transfer of biomolecules. The adsorption of proteins to solid surfaces is important in the natural sciences, medicine and industry and has an important role in numerous applications such as the preparation of protein chips and biosensors, the food industry and medical implants.20,21 On another hand, sepiolite nanofibers efficiently and spontaneously bind different types of DNA molecules through electrostatic interactions, hydrogen bonding, cation bridges, and van der Waals forces.15 Because of this characteristic, sepiolite nanofibers can be used to transfer DNA into mammalian cells. DNA transfer of into mammalian cells is an essential issue in biotechnology and biomedical applications constituting the basis of new strategies aiming at developing elaborate model organisms for academic, agronomical or biomedical research and gene therapy.14,15,22 Mammalian cells spontaneously internalize sepiolite through endocytosis, facilitating the spontaneous transport and delivery of bound molecules.22 Conversely, sepiolite can be spontaneously expelled from the cell, limiting its toxicity.22
In some future applications, the cotransfer of DNA and proteins is a significant advantage. For example, future improvements would be to specifically address the therapeutic nucleic acids to pathological cells thanks to antibodies recognizing specific membrane antigens of the target cells. Therefore, the vector should be able to carry both nucleic acids and antibodies, which are proteins. Moreover, serum albumin is the most abundant protein in human blood. Knowledge of the adsorption of albumin to sepiolite surfaces is essential because, when sepiolite is introduced into organisms, proteins will adsorb nonspecifically to the particles, and this will ultimately affect their cellular uptake and/or lead to deleterious consequences for the organism.23 Saturation of the sepiolite/DNA (Sep/DNA) bionanocomposite with bovine serum albumin (BSA), which is widely used in molecular biology, would allow us to circumvent this problem and, in addition, would protect the DNA from extracellular components that could assault it. For all these reasons, it is vital to study the adsorption of these proteins to sepiolite already coated with DNA bionanocomposites.
Unfortunately, because the charges of proteins generally differ from those of DNA, it is not possible to cotransfer DNA and proteins via conventional methods. The ability of sepiolite to bind to both proteins18 and DNA15 opens new avenues for its use in carrying both nucleic acids and proteins as nanocargo. However, no studies addressed the question of adsorption efficiency when sepiolite is first coated with DNA or reciprocally when it is first coated with proteins. Indeed, the charges of proteins and DNA are different, and even opposite, therefore the presence of one type of molecule might affect the efficiency of binding of the other type of molecule. Moreover, the proteins charges differ between the different proteins, and thus might differently affect the subsequent binding of DNA.
While the binding of proteins or DNA on sepiolite have been studied separately, the competition and common binding of both proteins and DNA on the same sepiolite fibers remained to be addressed. The growing interest in the biomedical applications of sepiolite has required the performance of these studies.
In this study, we analyzed the competition between DNA and proteins for adsorption on common sepiolite fibers. To determine the binding of proteins to sepiolite, we used BSA and an antibody (mAb) raised against the CD4 membrane antigen as a model.
Here, we show that the adsorption of proteins can be charge-dependent and that proteins can be adsorbed even when their charges are equal to those on the surface. We also show how divalent cations (CaCl2) can be used to modulate the adsorption of proteins on Sep/DNA bionanocomposites, increasing the maximum adsorption capacity. pH variation causes a variation in protein charge, influencing the amount of protein adsorbed. Although various types of interactions of proteins with mineral clays have been described, our results emphasize that electrostatic forces are among the primary interactions that occur during the adsorption process.
Materials and Methods
Sepiolite Suspension
In this study, sepiolite (Sep) obtained from Vicálvaro-Vallecas deposits, Madrid, Spain, commercialized under the trade name Pangel®S9, was generously supplied by TOLSA, S.A. A 2 mg/mL sepiolite suspension was prepared in 10 mM Tris-HCl buffer, pH 7.5, using vigorous vortexing at maximum speed for a minimum of 10 min to properly disperse the clay. The sepiolite suspension was sonicated (sSep) 3 times at an amplitude of 30% for 10s each time using a Vibra-Cell 75042. Indeed, sonication of sepiolite increased disaggregation and dispersion, which can be important for wider spreading and the formation of stable and homogenous suspensions that are crucial steps to improve their performance, as we previously published.15,24
Preparation of sSep-Protein
Protein solutions were prepared at concentrations of 5, 10, 20, 50, 100, 150, and 200 µg/mL. The protein used was bovine serum albumin (BSA): 2 mg/mL stock solutions were prepared in 10 mM Tris-HCl buffer, pH 7.5. To each protein sample, 500 µL of sSep was added at a concentration of 100 µg/mL. Each sample was then brought to a volume of 1 mL with 10 mM Tris-HCl, pH 7.5. The sSep/protein mixtures were stirred overnight at 25 °C. Finally, the mixtures were centrifuged for 5 minutes at 5,000 rpm, and the protein concentrations in the supernatants were measured using a NanoDrop One spectrophotometer.
Preparation of sSep–DNA
First, a Tris-HCl/CaCl2 solution consisting of 25 mL of 20 mM Tris-HCl at pH 7.5, 5 mL of 100 mM CaCl2 and 20 mL of distilled water was prepared. Then, 1 mL of an sSep dispersion at 2 mg/mL was centrifuged for 5 minutes at 5,000 rpm. The resulting supernatant was removed, and the precipitate was resuspended in 10 mL of the Tris-HCl/CaCl2 mixture. Plasmid DNA was obtained by amplifying a bacterial culture and was purified via the NucleoBond® Plasmid Purification Protocol. To individual samples of plasmid DNA at different concentrations, 500 µL of sSep at a concentration of 100 µg/mL in Tris-HCl/CaCl2 was added. The samples were mixed with 10 mM Tris-HCl, pH 7.5, until a volume of 1 mL was obtained for each sample. These samples were incubated with stirring overnight at 25 °C. After the overnight incubation, the mixtures were centrifuged for 5 minutes at 5,000 rpm, and the DNA concentrations in the supernatants were measured using a NanoDrop One spectrophotometer.
AFM
Atomic force microscopy (AFM) imaging was carried out in Peak Force Mode with SCANASYST-Air probes (Bruker), with a Multimode system (Bruker) operating with a Nanoscope V controller (Bruker). All images were collected at a scan frequency of 1 Hz and a resolution of 1024×1024 pixels. Images were analyzed with Nanoscope V and Image J softwares. A third-order polynomial function was used to remove the background.
5µL of sepiolite solutions (with and without BSA) at 0.1 mg.mL−1 was deposited on a freshly cleaved mica surface (V1 quality, EMS) for 1 min and rinsed with 50 μL of ultra-pure water (Milli-Q water, Millipore). The samples were finally blotted and dried.
Calculation of Adsorption Isotherms
To determine the adsorption isotherms for the proteins and DNA used in our experiments, the concentration of the adsorbent phase 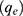 was calculated using Equation 1:25
was calculated using Equation 1:25
where C0 is the initial protein concentration (µg/mL), C is the protein concentration (µg/mL) in the supernatant, V denotes the volume (mL) and m (µg) is the amount of clay.
The adsorption performance was calculated as shown in Equation:25,26
Here, the adsorption process onto sepiolite nanofibres has been studied using adsorption isotherms and the Langmuir and Freundlich models. The objective was to determine if the phenomenon of adsorption of DNA and proteins on sepiolite fits any of these two models, both or neither, in order to analyze the thermodynamic parameters of these mathematical models, for the understanding of the adsorption process on sepiolite nanofibers.
Langmuir Model
The Langmuir isotherm is based on the assumption that the adsorbate will bind on the adsorbent forming a monolayer on its surface and that there will be no lateral interaction between the bound adsorbate molecules.27 Equation 3 represents the Langmuir model
Where Ce is the equilibrium concentration of protein; qe is the amount of adsorbed protein per unit weight of the adsorbent; qmax is the maximum adsorption capacity; b is the adsorption equilibrium constant related to the affinity of the binding sites. Equation 4 can be made linear as follows:
Freundlich Model
This model has an empirical origin and, as in the Langmuir model, adsorption is a function of equilibrium concentration, without taking into account the presence of other ions in solution. Freundlich’s model is based on multilayer adsorption on a heterogeneous surface, 28 and the equation that represents it is equation 5.
Where Ce is the equilibrium concentration of protein; qe is the amount of adsorbed protein per unit weight of the adsorbent; K and n are the constants of the system.
Equation 5 can be linearized by taking logarithms, so that equation 6 is obtained.
Where 1 / n gives a prediction of how favorable the adsorption process is, and K is the adsorption or distribution coefficient that represents the amount of protein adsorbed by sepiolite in equilibrium. The slope (1 / n) of the linearized equation indicates the intensity of adsorption or the heterogeneity of the surface. As 1 / n approaches zero, the adsorbing surface becomes more heterogeneous. 1 / n ˂ 1 indicates a normal Langmuir isotherm, while 1 / n ˃ 1 shows cooperative adsorption.
Z-Potential
One set of 1-mL samples of sepiolite was prepared in 10 mM Tris-HCl (pH 7.5, 100 μg/mL) in the presence of 10 mM CaCl2. A second set of samples was prepared similarly but incorporated DNA, BSA and mAb at the same concentration (50 μg/mL). Zeta potential measurements of the 1-mL suspensions were performed using a Brookhaven NanoBrook 90 Plus PALS instrument.
Point of Zero Charge
The point of zero charge (pHPZC) of the sepiolite used in our experiments was determined as previously described.29,30 The measurements included a set of 10 samples with 25 cm3 of a KNO3 solution at a concentration of 0.1 mol/dm3. The initial pH (pHi) was adjusted to a value between 3.5 and 8, and small volumes of 0.1 mol/dm3 HCl or KOH were added. Then, 0.050 g of sepiolite was added to each sample. Equilibrium was obtained after 24 hours at room temperature, and the final pH value was determined (pHf). The point of zero charge was obtained from the graph of pHf vs pHi. For the second stage, this procedure was repeated using KNO3 concentrations of 0.01 and 0.001 mol/dm3.
NMR Experiment
For NMR measurements, 500 μL samples were used and measurements were performed on a Tecmag LapNMR console (Tecmag Inc). T2 values were measured using the Carr–Purcell–Meiboom–Gill (CPMG) sequence, at a frequency of 4.0711 MHz (homogeneous permanent magnet, B0=0.095 T), 5 and 10 μs for 90° and 180° pulses respectively, and an echo time of 0.5 ms. All measurements were performed at 20 °C.
Statistical Analysis
The data presented in this paper are the mean values obtained in 3 to 5 independent experiments performed in triplicate. The error bars represent the mean squared standard deviation. The spline tool, an interpolation method that is used in several statistical software packages, was used to fit the data. Cubic spline interpolation was used. These adjustments provide information and are very useful for interpreting the adsorption processes represented by the isotherms. The statistical analysis was performed via Excel (Office 2019), OriginPro 8.5 and MATLAB R2015a.
Results and Discussion
Effect BSA on Sepiolite Morphology
We have previously shown that the binding of DNA did not affect the morphology of the sepiolite fibers.15 Here we show by AFM experiments that except the presence of BSA (see with arrows), no modification of sepiolite fibers was observed (Figure 1).
Effect of pH on the Efficiency of Binding of BSA, mAb and DNA to Sepiolite
Prior to performing the other experiments, we defined the optimal conditions for binding by analyzing the impact of pH on the adsorption of proteins and DNA to the sonicated sepiolite (sSep) surface. Because the charges on and the isoelectric points of the individual proteins might vary (in contrast to DNA, which is negatively charged), this might affect the efficiency with which they bind to sepiolite. Therefore, we compared the efficiency of binding of the two model proteins, BSA and the CD4 antibody (mAb), and that of DNA to sepiolite as a function of pH. The adsorption efficiency differed with pH, but the maximum adsorption percentages of BSA, mAb and DNA were 57%, 96%, and 82%, respectively (Figure 2).
The BSA adsorption experiment indicated that maximum absorption of BSA from the solution reached a maximum at pH 5 (Figure 2), close to the protein’s isoelectric point (iep).31,32 Consistent with this, it has been reported that adsorption of BSA to clays such as kaolinite and sepiolite, as well as to related solids such as diatomite, reaches a maximum at pH 5.5.33,34
Since BSA has a negative charge at pH values above 5, electrostatic repulsion between the solid surface and the BSA molecules can prevent the binding of the protein to sepiolite, which has an iep of pH 6.6.34–36 Therefore, protein adsorption at pH values between 7 and 9 decreased. These electrostatic repulsion forces also act at pH values ranging from 2 to 4, at which both BSA and sepiolite are positively charged.
The mAb used in our work (anti-CD4) has iep values in the range of 8−9.37,38 Therefore, at pH values below this range, it will be positively charged. Consistent with this, the antibody showed maximum adsorption at pH 7.5 (Figure 2), a value at which the charges of the mAb and sepiolite differ, favoring adsorption. Proteins are polypeptides made up of individual amino acid residues whose lateral chains contain hydrophobic or hydrophilic organic groups that can be negatively or positively charged or may bear no charge, depending on the pH of the solution.39 Dashman and Stotzky reported that positively charged amino acids were retained on clays more strongly than were neutral or negatively charged amino acids.40 These authors associated the observed adsorption patterns with the electrostatic interactions of different amino acids with the clay surface.
For DNA, which is negatively charged,41,42 maximum adsorption was obtained at pH values below 6 (Figure 2), a pH at which sepiolite is positively charged. Notably, prolonged contact with acid solutions can produce alterations in sepiolite due to the leaching of Mg2+ ions from its octahedral layer. For this reason, we have attempted to work at pH values that are not excessively low and to avoid very long treatment times to prevent these types of alterations.
The pH value at which the maximum adsorption of different molecules occurs can be considered to be the condition under which the electrostatic attraction is most favorable; in other words, a condition in which the charges of sepiolite and these molecules are different.
Zero-Charge Measurement Points
The results of the determination of the pHPZC for sepiolite are presented in Figure 3. From the curve of pHf vs pHi, the pHPZC value was determined as the pH value at which a plateau appears on the curve, ie, the inflection point of the curve. At this pH, the surface charge changes from positive to negative or vice versa. Thus, the common plateau obtained at a pH of 6.9 ± 0.1 corresponds to the pHPZC of sepiolite.
According to the literature,30 the specific adsorption of cations on a solid surface shifts the pHPZC value toward lower pH values. This shift is more pronounced as the amount of specifically adsorbed ions increases. The specific adsorption of cations on the surface of a solid phase reduces the number of sites available for the adsorption of H+ ions so that many H+ ions remain in solution, leading to lower pH values.
Figure 3 shows the dependence of pHf on pHi during the equilibration of sepiolite with a CaCl2 solution. The shift in the position of the plateau indicates that specific adsorption of these cations to sepiolite occurred, suggesting that Ca2+ ions have a high affinity for adsorption on sepiolite samples.
Binding of Proteins to Sepiolite in the Presence or Absence of Cations
Because the charges on the molecules in the system is an important parameter, we tested the effect of adding cations (Figures 4 and 5). Addition of cations has already been shown to improve the efficiency of DNA binding to sepiolite.15 In contrast to DNA, the presence of monovalent (K+) or divalent (Ca2+, Mg2+) cations did not significantly affect the efficiency of BSA adsorption to sepiolite at pH 7.5 (Figure 4A).
At pH 7.5, BSA is negatively charged, and although cations reduce the negative charge of sepiolite, they do not result in charge inversion; thus, the charge of sepiolite continues to be negative, and it varies only in magnitude.35,36
The effects of monovalent cations on the zeta potential of sepiolite over a wide range of concentrations are not pronounced.36 Alkan et al concluded that monovalent ions such as Li+, Na+ and K+ cannot be specifically adsorbed to sepiolite and are not capable of causing charge inversion.36
However, in the presence of divalent cations, the zeta potential of sepiolite decreased, becoming less negative. In the case of Ca2+ and Mg2+ ions, the zeta potential of sepiolite decreased with increasing ion concentration, but again, the presence of the ions did not lead to charge inversion.35 Therefore, multivalent cations exert a greater influence on the zeta potential of sepiolite than do monovalent cations.36
In contrast to BSA, the presence of cations strongly affects the efficiency of mAb adsorption by sepiolite at pH 7.5; greater adsorption is observed in the presence of divalent cations, whereas monovalent cations have a marginal influence on the efficiency of mAb binding (Figure 4B). This is consistent with the fact that the influence of monovalent cations on the charge of sepiolite is not pronounced.35,36 However, divalent cations decrease the zeta potential of sepiolite, which becomes less negative.31 At pH 7.5, the mAb has a positive charge, and the cations, although they reduce the negative charge, do not produce charge inversion; consequently, sepiolite and the mAb still have opposite charges, reducing the electrostatic forces of repulsion.
At pH 5.0, the adsorption of BSA and mAb was similar to that at pH 7.5 (compare Figures 4 and 5). The presence of monovalent or divalent cations did not significantly affect the efficiency of BSA adsorption by sepiolite, although it did affect the efficiency of mAb adsorption. However, variation in the adsorption with the protein concentration was observed. An increase in the adsorption efficiency was observed when albumin was present, whereas absorption efficiency decreased slightly for the mAb. This once again shows the influence of pH and how the adsorption of these molecules can be modulated by pH.
At this pH value, variations occur in the charges of sepiolite and albumin, and these variations cause variations in the adsorption efficiency. Greater adsorption will occur where electrostatic conditions favor these interactions.
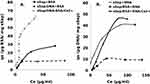
Competition of BSA and DNA for Binding to Sepiolite in the Presence or Absence of Ca 2+
Previous studies have addressed the adsorption of DNA to sepiolite.15 Here, we focused on whether this adsorption might further affect the adsorption efficiency of BSA. In this way, we observed that precoating sepiolite with DNA (Sep/DNA bionanocomposites) caused a decrease in the BSA adsorption efficiency (Figure 6A). The presence of DNA on sepiolite decreases the number of adsorption sites at which BSA molecules can bind. Moreover, both DNA and BSA are negatively charged at pH 7.5, and this should constitute an electrostatic barrier that prevents the BSA molecules from approaching closely enough for adsorption to occur through other mechanisms. Despite the equal charge, adsorption occurs. BSA molecules also have the ability to reversibly bind substances, especially negatively charged substances. For this reason, the BSA can itself assume the role of transport.31
When BSA was previously prepared with CaCl2, an increase in adsorption was observed (Figure 6A). The presence of Ca2+ reduces the electrostatic energy barrier between DNA and BSA, increasing the number of adsorbed BSA molecules. These results further confirm that electrostatic forces are the main interaction mechanism for BSA adsorption.36
The adsorption of DNA to the sSep/BSA bionanocomposite was then analyzed, and similar results were obtained (Figure 6B). Thus, the pre-adsorption of BSA to sepiolite reduces the efficiency of adsorption of DNA, and absorption could be increased with the use of divalent cations such as Ca2+.
Figure 2 shows that at pH 5.0, BSA has greater adsorption efficiency on the sepiolite surface than at pH 7.5. Similarly, the adsorption isotherms of BSA on Sep/DNA were more efficient at pH 5.0 than at pH 7.5 (Figure 7).
The decrease in the pH of the suspension results in a decrease in the negative potential of sepiolite due to the adsorption of H+ at the negatively charged centers.36 Under these conditions, negatively charged molecules such as DNA and BSA have better access to sepiolite adsorption centers. DNA binds to sepiolite through various adsorption sites located on the outer surface of the nanofibers,15 but BSA molecules can bind negatively charged substances.31 At pH 5.0, BSA is close to its iep and is less negatively charged than it is at higher pH values.
Conversely, the presence of BSA on sepiolite decreased the efficiency of DNA binding, but the DNA binding efficiency could be restored by incorporating Ca2+ (Figure 7B). Once again, the use of cations increases the adsorption capacity, causing the electrostatic forces of attraction to favor a closer approach between these molecules.
Competition of mAbs/DNA for Binding to Sepiolite (± Ca2+)
We then measured the impact of the presence of DNA on sepiolite on mAb adsorption. Similar to the results obtained for BSA, the presence of DNA reduced mAb adsorption by 50% (Figure 8A). Under these conditions, Sep/DNA has a negative charge, and the antibody has a positive charge. Therefore, the restrictions on absorption due to electrostatic forces should be reduced, but adsorption is still reduced because the DNA reduces the number of adsorption sites at which mAbs can be adsorbed. However, adding CaCl2 to the mAb increased the adsorption efficiency (Figure 8A). The presence of Ca2+ allows the mAb not only to adsorb to sepiolite but also to interact with negatively charged DNA.
Conversely, the presence of mAbs on sepiolite caused DNA adsorption to decrease by approximately 38% (Figure 8B). However, in this case, the presence of Ca2+ did not affect the efficiency of DNA adsorption by the Sep/mAb compound.
On the one hand, after prior adsorption of the mAb, the number of adsorption sites at which the DNA can interact is lower, reducing the amount of adsorbed DNA. On the other hand, the Sep/mAb compound will have a positive charge since the mAb achieves charge inversion (Table 1). Presumably, due to the difference in charge between the Sep/mAb and the DNA, an increase in DNA absorption should occur. However, it should be noted that electrostatic interactions favor a rapprochement between sepiolite and DNA, and this implies that other processes may occur. Such adsorption processes could operate through van der Waals forces in addition to hydrogen bonding with the silanol groups located on the outer surface of the sepiolite nanofibers.15 As these adsorption sites have already been taken up by the mAb, these other types of interactions are prevented from occurring, thus decreasing DNA adsorption to the compound. The presence of Ca2+ also did not favor this process, since the presence of charges equal to those of the Sep/mAb compound can cause repulsion. This confirms that the interaction between DNA and the sepiolite surface involves various mechanisms.
The adsorption of the mAb at pH 5 was similar to that observed at pH 7.5; there was a decrease in adsorption after prebinding of DNA and an increase in adsorption in the presence of Ca2+ ions (Figure 9A). At pH 5, the adsorption of DNA is impaired by the presence of mAb on sepiolite at the highest concentration, and this effect is slightly reduced by the addition of Ca2+ (Figure 9B).
Analysis of Proteins and DNA Adsorption on Sepiolite Using Mathematical Models
To mathematically analyze the results, the Langmuir and Freundlich models were used in this work to study the adsorption process of proteins and DNA on sepiolite nanofibers. The results show that the adsorption isotherms follow L and S type isotherms, according to the Giles classification.43 The following table (Table 2) shows some of the parameters obtained from the adjustments of the experimental data to the linear models.
 |
Table 2 Parameters of the Adjustments to the Langmuir and Freundlich Models |
The sSep/BSA and sSep/mAb composites fitted the Freundlich model best, indicating that multilayer adsorption is favored in these processes. The adsorption processes with the previous presence of one of the biomolecules used fitted the Langmuir model best, which is based on the assumption that the adsorbate will bind to the adsorbent forming a monolayer on its surface.
The parameter 1/n is related to the surface heterogeneity and an indicator of the adsorption intensity. As 1/n approaches zero, the adsorption surface becomes more heterogeneous. Taking this into account, it was observed that the adsorption surface was more heterogeneous when proteins were adsorbed on the sepiolite surface than on the sSep/DNA composite. The maximum values of this parameter were obtained for the bionanocomposites where the previous adsorption of DNA occurred. The parameter K is an approximate indicator of the adsorption capacity and showed a high correlation with the maximum adsorption capacity. The sSep/DNA and sSep/DNA/mAb bionanocomposites showed the highest adsorption capacity (35.3 and 26.4 µg/mg), which in turn showed the highest K values. A similar thing happened with the sSep/DNA/ASB composite which had the lowest adsorption capacity (4.2 µg/mg). The previous presence of DNA on the sepiolite surface decreased the adsorption of BSA, however, the adsorption capacity of mAb, under the same conditions, increased. The adsorption sites on the sepiolite surface are occupied by the previous presence of DNA, in addition, the presence of this biomolecule causes an increase in the electronegativity of the surface. Both BSA and mAb have negative and positive charges respectively, which shows once again that the maximum adsorption of these molecules will occur where the conditions of electrostatic attraction are more favorable. The adsorption energy is related to the adsorption constant b. The sSep/mAb/BSA compound showed the highest value of this parameter, which in turn had the highest surface heterogeneity. The lowest value of this parameter was shown by the sSep/DNA/mAb/BSA compound, which had one of the highest values of 1/n, indicating a lower surface heterogeneity. This indicates that surface heterogeneity is related to a higher or lower adsorption energy. The b values in the protein adsorption processes on sSep/DNA were higher than the same process, but on sSep. The differences in surface heterogeneity in both processes were observed.
Zeta Potential
The electrokinetic properties of sepiolite are very important for its variety of applications. The zeta potential is one of the most important electrokinetic properties of minerals since it allows an understanding of the adsorption/desorption processes that occur on the surfaces of these minerals.
To document our interpretations, we analyzed the variations in surface charge in sepiolite for the different bionanocomposites in a study of their zeta potentials (Table 1). We observed that the presence of the divalent cation Ca2+ in the medium reduced the electronegativity of sepiolite but did not result in charge inversion at the concentration used. BSA slightly increased the charge; however, when the sample was pretreated with CaCl2, a decrease in electronegativity was achieved. Similar behavior occurs in the case of the Sep/DNA bionanocomposite. The presence of the mAb resulted in charge inversion on the surface of the sepiolite; the charge ranged from negative to positive when the mAb was present. This confirms the positive charge of the mAb and the negative charge of the BSA at pH 7.5.
The zeta potential values of the bionanocomposites Sep/DNA, Sep/BSA and Sep/mAb at pH 7.5 and 5.0 were also evaluated (Table 3). At pH 5.0, the electronegativity of the Sep/DNA complex increased. This pH is below the isoelectric point of Sep, so it presents a positive charge, and this, as shown in Figure 2, favors an increase in DNA adsorption, resulting in a high negative charge value in the formed bionanocomposite. For Sep/BSA, a reversal of charge at pH 5.0 and pH 7.5 was observed. This behavior may be due in the first place to the fact that albumin is very close to its isoelectric point. Additionally, the presence of the Ca2+ cation, which at pH 7.5 decreased the electronegativity but did not produce charge reversal, plays a fundamental role in the reversal of the charge and, again, the positive value of Sep at this pH value.
The mAb maintained a positive charge at both pH values; however, at pH 7.5, this molecule inverted from negative to positive with respect to the surface charge of Sep, whereas at pH 5.0, it caused an increase in the positive charge.
Evaluation of Sepiolite-Proteins Interaction Using NMR
The presence of silanol (Si-OH) groups on the external surface of sepiolite, converts these hydroxyls into active centers of interaction with various biomolecules. One of the advantages of fibrous clays is the very high density of silanol groups, approximately 2 Si-OH groups/nm2, which allows the formation of hydrogen bonds in addition to van der Waals interactions. Sepiolite is a natural hydrated magnesium silicate.7 The tunnels and channels are filled with two types of water molecules, including a) coordinated water molecules, and b) zeolitic water, which is associated with the former ions through hydrogen bonding. The interactions of the water molecule with the silanol groups on the surface of silicates have been studied.44 The results show that these sites are the first to be covered when the water molecule approaches the surface.
Despite the small sizes of the tunnels and channels, the mobility of water can be significant and, at the same time, interactions such as restriction of rotational mobility of water molecules can occur. Nuclear magnetic resonance (NMR) operated at low fields is used to evaluate the magnetization carried by water molecules. The amount of magnetization gives the total number of hydrogen nuclei in the system, and the decay of magnetization is driven by nuclear interactions between hydrogen nuclei and the solid surface.45–48 In porous clay minerals, the relaxation process is the result of interactions between nuclear spins carried by water molecules exploring the tunnels and channel space and electron spins on the solid surface.45
In the present work, we performed a study using NMR to evaluate the interactions of these proteins with sepiolite.
The adsorption was evaluated for different concentrations of sepiolite (Figure 10). The adsorption sites and the population of bound water molecules increase with the concentration. The results showed that the T2 values decrease as the concentration of sepiolite increases. With the increase of the amount of nanofibers in the solution, the volume of water molecules bound to the surface increases, causing an increase in the restriction of the rotational mobility of the water molecules. In a second stage of the experiment, the same analysis was performed on solutions of sepiolite with proteins, experimentally observing an increase in the T2 parameter with the addition of proteins to the sepiolite solution. This indicates that the capacity of sepiolite to cause the decay of the magnetization of the water molecule decreases with the presence of proteins. The interactions between sepiolite and proteins remove water molecules from the protein/sepiolite region, which would result in the volume of bound water decreasing and more free water molecules being found. This causes a decrease in the restrictions of the rotational mobility of the water molecule, inducing an increase in the relaxation time T2.
Conclusion
Sepiolite-based nanocarriers offer a new and attractive nanoplatform for the transfer of nucleic acids into mammalian, and particularly human, cells, with potential applications in nanomedicine and nanobiotechnology. Here, we show that protein adsorption can be charge-dependent and that proteins can be adsorbed even when the charges on the protein are equal to those on the surface. The efficiency of adsorption of biological molecules on sepiolite is limited by prior adsorption of DNA or proteins. We also show that the presence of divalent cations (CaCl2) can enhance protein adsorption to Sep/DNA bionanocomposites by increasing the maximum adsorption capacity of the nanocomposites. Changes in the pH cause variations in sepiolite charge, influencing the amount of protein and DNA adsorbed. Although several types of protein interactions with clay minerals have been described, our results confirm that electrostatic forces are among the main interactions affecting the adsorption process. However, other mechanisms, such as hydrogen bonding involving the sepiolite surface (eg, external surface silanol groups) and the adsorbate, cannot be ruled out. Our results could improve the use of sepiolite in some biomedical applications, such as the delivery of nonviral vectors for various treatments.
Acknowledgments
This work was supported by the Institut National du Cancer (PLBIO21-072), La Ligue Contre le Cancer, and ITMO Cancer (PCSI 2022). P.A. & E.R.-H. thank the Agencia Estatal de Investigación, MCIN/AEI/10.13039/501100011033 (Spain) and “ERDF A way of making Europe” (EU) for financial support (project PID2022-137889OB-I00).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Can MF, Çinar M, Benli B, Özdemir O, Çelik MS. Determining the fiber size of nano structured sepiolite using Atomic Force Microscopy (AFM). Appl Clay Sci. 2010;47:217–222. doi:10.1016/j.clay.2009.10.010
2. Rodríguez-Beltrán J, Rodríguez-Rojas A, Yubero E, Blázquez J. The animal food supplement sepiolite promotes a direct horizontal transfer of antibiotic resistance plasmids between bacterial species. Antimicrob Agents Chemother. 2013;57:2651–2653. doi:10.1128/AAC.02363-12
3. Zayed MA, El-Begawy SEM, Hassan HES. Enhancement of stabilizing properties of double-base propellants using nano-scale inorganic compounds. J Hazard Mater. 2012;227–228:274–279. doi:10.1016/j.jhazmat.2012.05.050
4. Wang Z, Liao L, Hursthouse A, Song N, Ren B. Sepiolite-based adsorbents for the removal of potentially toxic elements from water: a strategic review for the case of environmental contamination in Hunan, China. Int J Environ Res Public Health. 2018;15:393–452.
5. Dabiri R, Amiri Shiraz E. Evaluating performance of natural sepiolite and zeolite nanoparticles for nickel, antimony, and arsenic removal from synthetic wastewater. J Min Environ. 2018;9:1049–1064.
6. Xie S, Zhang S, Wang F, Yang M, Séguéla R, Lefebvre Jm. et al. Preparation, structure and thermomechanical properties of nylon-6 nanocomposites with lamella-type and fiber-type sepiolite. Compos Sci Technol. 2007;67:2334–2341. doi:10.1016/j.compscitech.2007.01.012
7. Ruiz AI, Ruiz-García C, Ruiz-Hitzky E. From old to new inorganic materials for advanced applications: the paradigmatic example of the sepiolite clay mineral. Appl Clay Sci. 2023;235:106874. doi:10.1016/j.clay.2023.106874
8. Ruiz-Hitzky E, Aranda P, Álvarez A, Santarén J, Esteban-Cubillo A. Advanced materials and new applications of sepiolite and palygorskite. Developments in Palygorskite-Sepiolite Research. A New Outlook on These Nanomaterials. editorSinger AVol. 3 393–452 Elsevier, Oxford 2011
9. Erdem A, Kuralay F, Çubukçu HE, et al. Sensitive sepiolite-carbon nanotubes based disposable electrodes for direct detection of DNA and anticancer drug-DNA interactions. Analyst. 2012;137:4001–4004. doi:10.1039/c2an35181a
10. Fernandes AC, Antunes F, Pires J. Sepiolite based materials for storage and slow release of nitric oxide. New J Chem. 2013;37:4052. doi:10.1039/c3nj00452j
11. Mitsudome Y, Takahama M, Hirose J, Yoshida N. The use of nano-sized acicular material, sliding friction, and antisense DNA oligonucleotides to silence bacterial genes. AMB Express. 2014;4:70. doi:10.1186/s13568-014-0070-7
12. Gür E, Altinisik A, Yurdakoc K. Preparation and characterization of chitosan/sepiolite bionanocomposites for tetracycline release. Polym Compos. 2017;38:1810–1818. doi:10.1002/pc.23751
13. Olivato JB, Marini J, Yamashita F, et al. Sepiolite as a promising nanoclay for nano-biocomposites based on starch and biodegradable polyester. Mater Sci Eng C. 2017;70:296–302. doi:10.1016/j.msec.2016.08.077
14. Piétrement O, Castro‐Smirnov FA, Le Cam E, et al. Sepiolite as a new nanocarrier for DNA transfer into mammalian cells: proof of concept, issues and perspectives. Chem Rec. 2018;18:849–857. doi:10.1002/tcr.201700078
15. Castro-Smirnov FA, Piétrement O, Aranda P, et al. Physical interactions between DNA and sepiolite nanofibers, and potential application for DNA transfer into mammalian cells. Sci Rep. 2016;6:36341. doi:10.1038/srep36341
16. Alcântara ACS, Darder M, Aranda P, Ruiz-Hitzky E. Polysaccharide-fibrous clay bionanocomposites. Appl Clay Sci. 2014;96:2–8. doi:10.1016/j.clay.2014.02.018
17. Wicklein B, Ruiz-Hitzky E, Darder M. et al. Enzyme supporting phospholipid-sepiolite hybrids incorporated in polymer matrices for biosensor and bioreactor applications. 2010 SEA-CSSJ-CMS trilateral meet. Clays Gen Meet. 2010. 8548:2–9
18. Alcântara ACS, Darder M, Aranda P, Ruiz-Hitzky E. Zein-fibrous clays biohybrid materials. Eur J Inorg Chem. 2012;2012:5216–5224. doi:10.1002/ejic.201200582
19. Ruiz-Hitzky E, Darder M, Wicklein B. et al. Advanced biohybrid materials based on nanoclays for biomedical applications. Proc SPIE Nanosyst Eng Med. 2012;8548:85480D1–85480D8.
20. Zhdanov VP, Kasemo B. Protein adsorption and desorption on lipid bilayers. Biophys Chem. 2010;146:60–64. doi:10.1016/j.bpc.2009.10.005
21. Szewczuk-Karpisz K, Wiśniewska M. Impact of lysozyme on stability mechanism of nanozirconia aqueous suspension. Appl Surf Sci. 2016;379:8–13. doi:10.1016/j.apsusc.2016.04.031
22. Castro-Smirnov FA, Ayache J, Bertrand J-R, et al. Cellular uptake pathways of sepiolite nanofibers and DNA transfection improvement. Sci Rep. 2017;7:5586. doi:10.1038/s41598-017-05839-3
23. Chittur KK. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials. 1998;19:357–369. doi:10.1016/S0142-9612(97)00223-8
24. Brooks DA, Piétrement O, Dardillac E, et al. Impact of increased sonication-induced dispersion of sepiolite on its interaction with biological macromolecules and toxicity/ proliferation in human cells. ACS Omega. 2023;8:1026–1036. doi:10.1021/acsomega.2c06391
25. Dada AO, Olalekan AP, Olatunya AM, Dada OO. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn 2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem. 2012;3:38–45. doi:10.9790/5736-0313845
26. Kopac T, Bozgeyik K, Flahaut E, et.al. OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible this is an author s version published in, 2018. Available from: http://oatao.univ-toulouse.fr/21003.
27. Langmuir I. The adsorption of gases on plain surface of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–1405. doi:10.1021/ja02242a004
28. Freundlich H. Uber die adsorption in Íösungen, W. Engelmann. 1906.
29. Lazarević SS, Janković-Častvan IM, Jokić BM, Janaćković DT, Petrović RD. Sepiolite functionalized with N-[3-(trimethoxysilyl)propyl]-ethylenediamine triacetic acid trisodium salt. part I: preparation and characterization. J Serbian Chem Soc. 2015;80:1193–1202. doi:10.2298/JSC150219038L
30. Erovi LS, Milonji SK, Bahloul-Hourlier D, Doucey B. Surface properties of silicon nitride powders. Colloids Surf a Physicochem Eng Asp. 2002;197:147–156. doi:10.1016/S0927-7757(01)00863-9
31. Maleki MS, Moradi O, Tahmasebi S. Adsorption of albumin by gold nanoparticles: equilibrium and thermodynamics studies. Arab J Chem. 2017;10:S491–S502. doi:10.1016/j.arabjc.2012.10.009
32. Salgin S, Takaç S, Özdamar TH. Adsorption of bovine serum albumin on polyether sulfone ultrafiltration membranes: determination of interfacial interaction energy and effective diffusion coefficient. J Memb Sci. 2006;278:251–260. doi:10.1016/j.memsci.2005.11.008
33. Çalımlı MH, Demirbaş Ö, Aygün A, Alma Mh, Nas Ms, Şen F, et al. Immobilization kinetics and mechanism of bovine serum albumin on diatomite clay from aqueous solutions. Appl Water Sci. 2018;8:1–12. doi:10.1007/s13201-018-0858-8
34. Alkan M, Demirbaş Ö, Doğan M, Arslan O. Surface properties of bovine serum albumin -adsorbed oxides: adsorption, adsorption kinetics and electrokinetic properties. Microporous Mesoporous Mater. 2006;96:331–340. doi:10.1016/j.micromeso.2006.07.007
35. Lazarević S, Janković-Častvan I, Jovanović D, et al. Adsorption of Pb2+, Cd2+ and Sr2+ ions onto natural and acid-activated sepiolites. Appl Clay Sci. 2007;37:47–57. doi:10.1016/j.clay.2006.11.008
36. Alkan M, Demirbaş Ö, Doǧan M. Electrokinetic properties of sepiolite suspensions in different electrolyte media. J Colloid Interface Sci. 2005;281:240–248. doi:10.1016/j.jcis.2004.08.036
37. Goyon A, Excoffier M, Janin-Bussat M-C, et al. Determination of isoelectric points and relative charge variants of 23 therapeutic monoclonal antibodies. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1065–1066:119–128. doi:10.1016/j.jchromb.2017.09.033
38. Khawli LA, Goswami S, Hutchinson R, et al. Charge variants in IgG1: isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs. 2010;2:613–624. doi:10.4161/mabs.2.6.13333
39. Barral S, Villa-García MA, Rendueles M, Díaz M. Interactions between whey proteins and kaolinite surfaces. Acta Mater. 2008;56:2784–2790. doi:10.1016/j.actamat.2008.02.009
40. Dashman T, Stotzky G. Physical properties of homoionic montmorillonite and kaolinite complexed with amino acids and peptides. Soil Biol Biochem. 1985;17:189–195. doi:10.1016/0038-0717(85)90114-2
41. Fritz J, Cooper EB, Gaudet S, Sorger PK, Manalis SR. Electronic detection of DNA by its intrinsic molecular charge. Proc Natl Acad Sci U S A. 2002;99:14142–14146. doi:10.1073/pnas.232276699
42. Gao T, Zhang W, Wang Y, Yang G. DNA compaction and charge neutralization regulated by divalent ions in very low pH solution. Polymers. 2019;11:337. doi:10.3390/polym11020337
43. Giles CH, Macewans TH, Nakhwa N, D S. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc. 1960;3973–3993. doi:10.1039/jr9600003973
44. Saengsawang O, Remsungnen T, Fritzsche S, Haberlandt R, Hannongbua S. Structure and energetics of water-silanol binding on the surface of silicalite-1: quantum chemical calculations. J Phys Chem B. 2005;109:5684–5690. doi:10.1021/jp0452296
45. Fleury M, Soualem J. Quantitative analysis of diffusional pore coupling from T2-store-T2 NMR experiments. J Colloid Interface Sci. 2009;336:250–259.
46. Fleury M, Kohler E, Norrant F, Gautier S, M’hamdi J, Barré L. et al. Characterization and quantification of water in smectites with low-field NMR. J Phys Chem C. 2013;117:4551–4560. doi:10.1021/jp311006q
47. Fleury M, Canet D. Water orientation in smectites using NMR nutation experiments. J Phys Chem C. 2014;8:4733–4740. doi:10.1021/jp4118503
48. Fleury M, Kohler E, Barre L. et.al.Water mobility and structure in natural clay systems. Clay Minerals Soc Workshop Lect Series. 2016;117;79–86.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.